Abstract
Psychiatric conditions marked by impairments in cognitive control often emerge during adolescence, when the prefrontal cortex (PFC) and its inputs undergo structural and functional maturation and are vulnerable to disruption by external events. It is not known, however, whether there exists a specific temporal window within the broad range of adolescence when the development of PFC circuitry and its related behaviors are sensitive to disruption. Here we show, in male mice, that repeated exposure to amphetamine during early adolescence leads to impaired behavioral inhibition, aberrant PFC dopamine connectivity, and reduced PFC dopamine function in adulthood. Remarkably, these deficits are not observed following exposure to the exact same amphetamine regimen at later times. These findings demonstrate that there is a critical period for the disruption of the adolescent maturation of cognitive control and PFC dopamine function and suggest that early adolescence is particularly relevant to the emergence of psychopathology in humans.
Keywords: amphetamine, cognitive control, dopamine, neurodevelopment, prefrontal cortex
The mammalian prefrontal cortex (PFC) is essential to decision-making and cognitive control, including the ability to evaluate and interpret environmental cues so as to appropriately respond, or inhibit responses, to ongoing opportunities and challenges. It is not surprising then, that adaptive behavior requires the proper functioning of the PFC and that deficits in PFC function, and subsequent impairments in cognitive control, are a common denominator in psychiatric disorders ranging from schizophrenia and depression to drug addiction (McTeague et al. 2016). In humans, PFC synaptic connectivity undergoes a prolonged period of dynamic refinement, stabilizing only in the third decade of life following dramatic changes across adolescence (Sowell et al. 2003; Gogtay et al. 2004; Petanjek et al. 2011). Likewise, the establishment of PFC circuitry is protracted in both rodents and nonhuman primates, with the maturation of cellular phenotype (Tseng and O’Donnell 2005; 2007), architecture (Lambe et al. 2000; Koss et al. 2014), and functional and structural connectivity continuing throughout adolescence (Manitt et al. 2011; Reynolds et al. 2018). This prolonged period of maturation renders the normal trajectory of PFC development and function vulnerable to disruption by external events experienced at this age (Makinodan et al. 2012; Baarendse et al. 2013; Reynolds et al. 2015; Baker and Reichelt 2016; Abbas et al. 2017; Manduca et al. 2017; Yamamuro et al. 2017). It is not known, however, whether there exists a specific temporal window within the broad range of adolescence during which the development of the PFC and its related behaviors are particularly sensitive to disruption.
One neurochemical system of the brain known to have influence over the function of the PFC in decision-making and cognitive control is the mesocortical dopamine system (Robbins et al. 1994; Chudasama and Robbins 2004; Floresco and Magyar 2006; Stefani and Moghaddam 2006; Simon and Moghaddam 2017). The density of mesocortical dopamine innervation continues to increase until early adulthood (Kalsbeek et al. 1988; Benes et al. 2000; Manitt et al. 2011; Naneix et al. 2012; Willing et al. 2017) due to the ongoing growth of dopamine axons during adolescence (Hoops et al. 2018; Reynolds et al. 2018). Although dopamine varicosities in the adult PFC are sparse, nearly all form functional synapses (Séguéla et al. 1988), suggesting that adolescent dopamine axon pathfinding is tightly regulated to correctly establish essential connections. Indeed, subtle changes to the organization of PFC dopamine connectivity during adolescence produce profound alterations in the morphology of postsynaptic neurons, response to abused drugs, and in cognitive control in adulthood (Manitt et al. 2013; Pokinko et al. 2015; Reynolds et al. 2018).
In fact, the developmental timeline of mesocortical dopamine circuitry appears to mirror the maturation of cognitive control. In humans, performance on behavioral inhibition tasks peaks in adulthood, following a gradual improvement across adolescence (Casey et al. 1997; Davidson et al. 2006; Luna 2009; Luna et al. 2010; Ordaz et al. 2013), and evidence suggests that the development of behavioral inhibition in rodents follows a similar developmental trajectory (Andrzejewski et al. 2011; Naneix et al. 2012). It is therefore plausible that external events experienced during adolescence that alter the trajectory of mesocortical dopamine development have the unique potential to impact cognitive control in an enduring and significant way. We have previously shown that exposure to the stimulant drug amphetamine from postnatal day (PND) 22–31 enduringly alters the establishment of mesocortical dopamine connectivity (Reynolds et al. 2015). What remains unknown is whether experience with amphetamine during adolescence has a corresponding lasting effect on cognitive control, and whether these effects are restricted to a particular temporal domain within the adolescent period.
We therefore designed our study to test whether there exists a critical period within the broad range of adolescence during which the maturation of inhibitory behavior and mesocortical dopamine innervation are particularly sensitive to disruption. Early adolescent, mid-adolescent, and adult male mice were administered the same amphetamine treatment regimen as used in our previous studies (Yetnikoff et al. 2007; 2011; 2013; Reynolds et al. 2015). After a 6-week interval, once all adolescent animals reached adulthood, we examined age-dependent disruptions in the maturation of cognitive control by measuring behavioral inhibition using a Go/No-Go task. In parallel, separate groups of age-matched mice were used to assess disruptions in mesocortical dopamine function and organization in adulthood.
Materials and Methods
Animals
All experiments and procedures were performed in accordance with the guidelines of the Canadian Council of Animal Care and the McGill University/Douglas Mental Health University Institute Animal Care Committee. Mice were bred in the Douglas Mental Health University Institute Neurophenotyping center, maintained on a 12-h light–dark cycle (light on at 08:00 h) and given ad libitum access to food and water unless noted. Pups were weaned at PND 21 ± 1 and housed with same-sex littermates. DATCre mice, which do not differ from wild-type controls, were used for axon-tracing experiments so that dopaminergic fibers could be labeled with a Cre-dependent eYFP virus. While sex differences in the structure and function of reward-related circuitries exist and are likely to emerge during adolescence (Walker et al. 2017), the current study focused only on male mice. Future studies will explore potential sex differences in the enduring effects of amphetamine exposure in early adolescence.
Amphetamine Treatment
Drugs
d-Amphetamine sulfate (AMPH; Sigma-Aldrich) was dissolved in 0.9% saline. All amphetamine injections were administered i.p. at a volume of 0.1 mL/10 g.
Treatment Regimen
Figure 1 shows the drug treatment regimen used in all the experiments. Mice received one injection of amphetamine (4 mg/kg) or vehicle (saline), once every other day for a total of 5 treatment days. This pretreatment regimen was given either during early adolescence (from PND 22 ± 1 to PND 31 ± 1), mid-adolescence (PND 35 ± 1 to PND 44 ± 1), or during adulthood (from PND 75 ± 15 to 84 ± 15). Locomotor activity was measured 15 min prior to and 90 min after each saline or amphetamine injection. This noncontingent 4 mg/kg dose of amphetamine was selected based on evidence that it 1) produces robust behavioral sensitization after repeated administration in wild-type mice (Karper et al. 2002; Flores et al. 2005), 2) regulates Dcc expression in the VTA in an age-dependent manner (Yetnikoff et al. 2007, 2011, 2013), and 3) achieves plasma concentrations of >400 ng/mL in mice, within the range reported in the plasma of human amphetamine abusers (Kramer et al. 1967; Anggård et al. 1970; Riffee et al. 1978; Gustavsen et al. 2006; Van Swearingen et al. 2013).
Figure 1.
Treatment timeline for all experiments. Mice received 5 injections of amphetamine (4 mg/kg) or saline, every other day, for a total of 5 treatment days. Behavioral, neurochemical, or neuroanatomical experiments were performed 6 weeks after treatment.
Behavior
Go/No-Go
The Go/No-Go task was performed as previously (Reynolds et al. 2018). Briefly, mice were food restricted to 1.5 g food per day for the duration of the task in order to maintain a body weight that was 85% of the initial free feeding weight. Mice were trained to nose poke for chocolate-flavored dustless precision pellets (BioServ, Inc.) in MedAssociates operant boxes (see Supplementary Material for full training information). After training, mice underwent 10 daily sessions of the Go/No-Go task. This task required the mice to respond to a lighted “Go” cue or inhibit their response to this cue when presented in tandem with an auditory “No-Go” cue. In the “Go” trials, mice had to respond to the illuminated nose poke hole in the 3-s timeframe during which the cue light was on in order to receive a reward. This was counted as a “Hit” in our analysis. In the “No-Go” trials, an 80-dB tone was paired with the 3-s cue light to signal that the mouse should withhold from responding. If mice responded during the 3-s “No-Go” trial, an ITI was initiated and no reward was dispensed. This was counted as a “Commission Error” in our analysis. However, if mice withheld from responding for the 3 s duration of the tone/light “No-Go” cue, a reward was dispensed. A randomized, variable pretrial period of 3–9 s preceded each trial and the number of premature responses was recorded. Within each session, the number of “Go” and “No-Go” trials were given in an approximately 1:1 ratio and presented in a randomized order. Each session lasted 30 min and consisted of approximately 30–50 “Go” and 30–50 “No-Go” trials.
High-Performance Liquid Chromatography
Mice were returned to the locomotor boxes one month after the last pretreatment day, administered a saline injection and killed by decapitation 90 min later. Brains were dissected from the skull cavities and immersed in 2-methylbutane, chilled with dry ice, and stored at −80 C. Bilateral punches of the medial PFC and nucleus accumbens (NAcc) from 600-µm-thick coronal sections were resuspended in 100 µL 0.1 M phosphate buffer, pH 7.0 and filtered using 0.45-µm syringe filters. A 10 µL volume of this filtrate was loaded onto a 15-cm C-18 reverse-phase column (15 cm × 0.46 cm Spherisorb-ODS2, 5 m; Higgins Analytical) via manual injection ports (Rheodyne 7125; Rheodyne LLC, Rhonert Park; 20-µL loop). Dual-channel coulometric III detectors (model 5100A; ESA, Inc.) were used to measure the reduction and oxidation currents for dopamine and dopamine metabolites (3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA)). Concentrations were obtained by comparing the peak heights for each compound against peak heights of previously injected standards containing known concentrations of each compound. The mobile phase (17% acetonitrile 40 mg, 0.076 M SDS, 0.1 M EDTA, 0.058 M NaPO4, 0.03 M citric acid, pH3.35) was circulated at a flow rate of 1.2 mL/min by Waters 515 HPLC pumps. The peaks corresponding to dopamine, DOPAC, and HVA were quantified and analyzed ysing the EZChrom Data Chromatography Data System (Scientific Software, Inc.). Two HPLC–ED systems were used in parallel, and the dialysate samples from a given mouse were always analyzed using the same system.
Dopamine Axon Architecture
The fine structure of PFC dopamine axon terminals was assessed as previously (Reynolds et al. 2018). Briefly, adult DATCre mice that received saline or amphetamine at each of our 3 treatment ages received unilateral stereotaxic infusions of the Cre-dependent fluorophore virus DIO-eYFP (AAV-EF1a-DIO-EYFP; UNC Vector Core) into the VTA. The following coordinates were used: −3.2 mm (anterior/posterior), +1.0 mm (lateral), and −4.6 mm (dorsal/ventral) relative to Bregma, and at a 10° angle. A total of 0.5 μL of purified virus was delivered on each side over an 8-min period followed by a pause of 6 min. Four to 5 weeks after virus injections, mice were intracardially perfused with 4% paraformaldehyde and their brains prepared for immunofluorescence. Sections of brain 35 μm thick were incubated with a polyclonal anti-GFP raised in chicken (1:500, antibody #1020, Aves labs) and immunostaining was visualized with Alexa Fluor 488-conjugated secondary antibodies raised in goat. Sections were mounted on slides and coverslipped with DAPI. Regions of interest were delineated according to the mouse brain atlas (Paxinos and Franklin 2008) at 5× magnification with a Leica DM4000B microscope. Only eYFP+ axons with intact arbors, defined as having all of the tips of their terminal branches within the section, were included in the analyses. Individual axon arbors were identified at 20× magnification. Neurolucida software (MicroBrightField) was used to trace the terminal arbors of selected axons at high magnification (40×), and to quantify axon arbor length, branch order, and varicosity density of each axon. Axon complexity was determined by computing the Axon Complexity Index (ACI) as previously (Marshak et al. 2007; Reynolds et al. 2018). Two-way mixed-design ANOVAs with genotype as a between-subjects factor and subregion as a within-subjects factor were used to analyze axon arbor length, complexity, and varicosity density.
Data Analysis
Planned comparisons were made between treatment groups within each treatment age for each experiment. For the Go/No-Go task, mice were grouped by treatment and the number of Commission Errors and Hits (correct responses to the “Go” cue) were analyzed over 10 testing days using a two-way mixed-design ANOVA with treatment as a between-subjects factor and day as a within-subjects factor. The performance of each mouse was averaged over the last 2 days of the task (Days 9–10) for Commission Errors and grouped by treatment. Students t-tests were performed on group means. For HPLC analysis, mice were grouped by treatment and the levels of dopamine and HVA were analyzed using a two-way mixed-design ANOVA with treatment as a between-subjects factor and metabolite as a within-subjects factor. Following a significant interaction in the ANOVA, we used the post hoc Bonferroni Multiple Comparison Test to assess differences. All statistical analyses for neurochemical experiments were performed using the raw data (i.e., actual concentration values for dopamine and HVA). Data in the figures are expressed as percentages of the saline group. Neuroanatomical data were analyzed using two-way mixed-design ANOVAs with treatment as a between-subjects factor and subregion as a within-subjects factor. All statistical analyses were carried out using Prism software (GraphPad).
Results
Amphetamine Exposure Only During the Earliest Part of Adolescence Leads to Deficits in Behavioral Inhibition in Adulthood
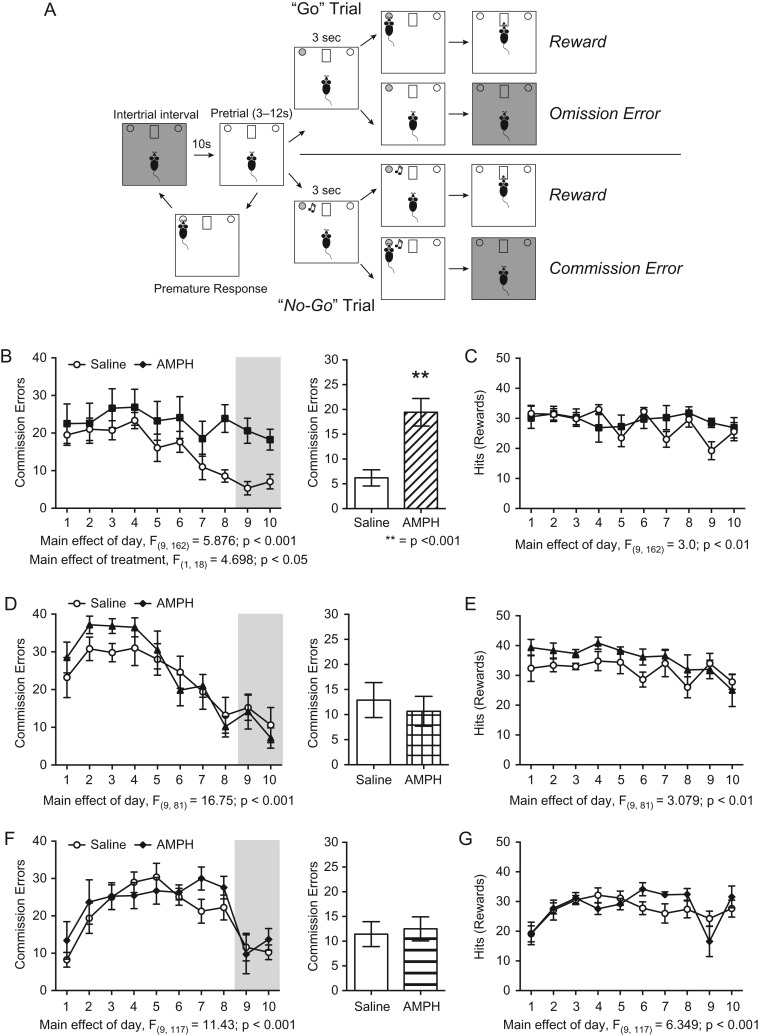
We treated male mice with a regimen of 5 injections of 4 mg/kg amphetamine or volume-matched saline, every other day for 5 treatment days during early adolescence (PND 22 ± 1–PND 31 ± 1), mid-adolescence (PND 35 ± 1–PND 44 ± 1), or adulthood (PND 75 ± 15–PND 84 ± 15) (Fig. 1). While the timing of adolescence in rodents is not well defined, these age groups were selected based on previous work from our lab and others suggesting that they represent discrete periods for 1) PFC dopamine fiber development and receptor expression (Kalsbeek et al. 1988; Andersen et al. 2000; 2008; Naneix et al. 2012; Reynolds et al. 2015; Hoops and Flores 2017; Pokinko et al. 2017; Walker et al. 2017; Willing et al. 2017), 2) differential regulation of proteins involved in axon growth and plasticity in response to stimulant drug exposure (Yetnikoff et al. 2007; 2011; 2013), and 3) vulnerability to the long-term effects of abused drugs or dopamine receptor activation (Gulley and Juraska 2013; Naneix et al. 2013; Reynolds et al. 2015; DePoy et al. 2016; Abbas et al. 2017). Six weeks after amphetamine exposure, when all adolescent-treated mice reached adulthood, we assessed behavioral inhibition using a Go/No-Go task (Fig. 2A, (Reynolds et al. 2018)). Remarkably, only adult mice that were treated with amphetamine in early adolescence, and not those treated in mid-adolescence or adulthood, showed an impairment in behavioral inhibition, evidenced by their maintenance of a high number of commission errors throughout the Go/No-Go task (Fig. 2B, left panel: Two-way mixed-design ANOVA, significant main effect of treatment, F(1,18) = 4.698, P = 0.0439; significant main effect of day, F(9,162) = 5.876, P < 0.0001; no interaction, F(9,162) = 1.731, P = 0.09. Amphetamine n = 8, Saline n = 12). This impairment is most evident in later Go-No/Go sessions (Fig. 2B, right panel: t(18) = 4.392, P = 0.0004). In contrast, adult mice treated with saline during early adolescence show a robust reduction in commission errors over the course of the Go/No-Go task, demonstrating that they are capable of effectively inhibiting disadvantageous behaviors, in line with previous findings from untreated, adult wild-type mice (Fig. 2B, (Reynolds et al. 2018)). Adult mice treated with amphetamine in mid-adolescence (Fig. 2D) or in adulthood (Fig. 2F) do not have deficits in behavioral inhibition in comparison to saline-treated controls when tested 6 weeks later (mid-adolescent treatment: left panel, two-way mixed-design ANOVA, no effect of treatment, F(1,9) = 0.2549, P = 0.63; significant main effect of day, F(9,81) = 16.75, P < 0.0001; no interaction, F(9,81) = 0.9303, P = 0.50. Right panel, t(9) = 0.49, P = 0.64. Amphetamine n = 6, Saline n = 5. Adult treatment: left panel, two-way mixed-design ANOVA, no effect of treatment, F(1,13) = 0.4422, P = 0.52; significant main effect of day, F(9,117) = 11.43, P < 0.0001; no interaction, F(9,177) = 0.9656, P = 0.47. Right panel, t(13) = 0.30, P = 0.77. Amphetamine n = 7, Saline n = 8).
Figure 2.
Behavioral inhibition is specifically impaired in adult mice exposed to amphetamine early in adolescence, delimiting a critical period for the maturation of inhibitory behavior. (A) Diagram of the Go/No-Go task to measure behavioral inhibition in adult mice. Adapted from Reynolds et al. (2018). (B) Adult mice that received amphetamine during early adolescence make more commission errors across the 10 days of the Go/No-Go task. Over the final 2 days of the task (shaded area), adult mice that received amphetamine during early adolescence make significantly more commission errors than their saline-treated littermates. (C) There are no significant differences in the number of correct responses (Hits) between treatment groups. (D) Adult mice that received amphetamine during mid-adolescence do not show impaired performance in the Go/No-Go task when tested as adults. (E) There are no significant differences in the number of correct responses (Hits) between treatment groups. (F) Mice that received amphetamine during adulthood do not have altered performance in the Go/No-Go task (G) There are no significant differences in the number of correct responses (Hits) between treatment groups.
It is important to note that because of the experimental design, there are qualitative differences in the pattern of commission errors over the course of the 10-day Go/No-Go task. These differences most likely result from the fact that 1) we tested mice that were treated at each experimental age separately, leading to subtle variations in response pattern and 2) we tested mice 6 weeks after the drug treatment to allow the group treated in early adolescence to reach adulthood. Therefore the different experimental groups were tested at different ages in adulthood.
Amphetamine treatment in early adolescence impairs behavioral inhibition but does not alter operant responding to the “Go” cue. In fact, there is no difference between saline- and amphetamine-treated mice in the number of correct responses within any treatment age (Hits, Fig. 2C,E,G. Early adolescent treatment: Two-way mixed-design ANOVA, no effect of treatment, F(1,18) = 0.3145, P = 0.58; significant main effect of day, F(9,162) = 3.0, P = 0.0025; no interaction, F(9,162) = 1.935, P = 0.0504. Mid-adolescent treatment: Two-way mixed-design ANOVA, no effect of treatment, F(1,9) = 2.509, P = 0.15; significant main effect of day, F(9,81) = 3.079, P = 0.0032; no interaction, F(9,81) = 0.7510, P = 0.66. Adult treatment: Two-way mixed-design ANOVA, no effect of treatment, F(1,13) = 0.1806, P = 0.68; significant main effect of day, F(9,117) = 6.349, P < 0.0001; no interaction, F(9,117) = 1.886, P = 0.06.). Furthermore, performance during training conditions was not different between amphetamine- and saline-treated mice at any exposure age (Supplementary Fig. 1). This indicates that the increase in the number of commission errors we observe in the Go/No-Go task following early adolescent amphetamine exposure does not result from a deficit in learning the task. Together, our results establish early adolescence as a defined critical period for the development of inhibitory control in mice.
Dopamine Turnover in the PFC is Enduringly Decreased Following Exposure to Amphetamine During Early Adolescence, But Not at Other Ages
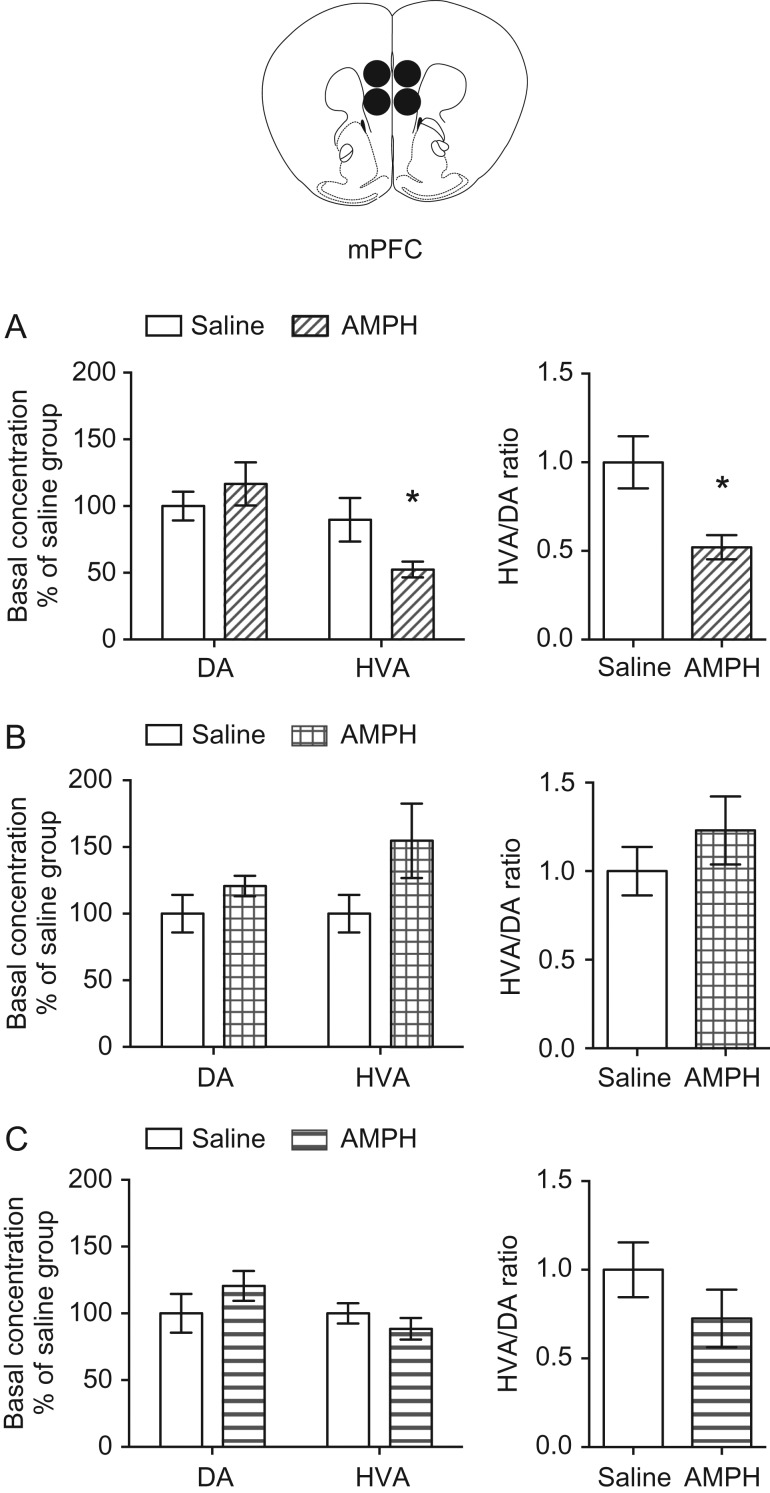
Mesocortical dopamine function plays an important role in regulating inhibitory behavior in rodents (Sokolowski and Salamone 1994; Grégoire et al. 2012; Bari and Robbins 2013; Willing and Wagner 2015a; Abraham et al. 2017). Thus, we next assessed whether amphetamine treatment produces enduring age-of-exposure dependent changes in mesocortical dopamine turnover. We exposed mice to amphetamine in early adolescence (PND 22 ± 1–PND 31 ± 1), mid-adolescence (PND 35 ± 1–PND 44 ± 1), or adulthood (PND 75 ± 15–PND 84 ± 15) (Fig. 1), and 4 weeks later we processed PFC tissue samples for HPLC to measure the ex vivo tissue content of dopamine and its metabolite HVA. While reuptake by the dopamine transporter is the primary method for terminating dopamine signals in the striatum (Giros et al. 1996), the dopamine transporter is only sparsely expressed in the PFC (Sesack et al. 1998). Instead, dopamine in the PFC is catabolized by Catechol-O-methyltransferase (COMT), which is bound to the membranes of postsynaptic neurons and glial cells (Käenmäki et al. 2010; Chen et al. 2011; Laatikainen et al. 2013). Unlike other brain regions, disabling COMT function in the PFC eliminates detectable levels of HVA, suggesting that the ratio of HVA/dopamine content is a measure of dopamine turnover for the PFC that primarily relies on the extracellular breakdown of synaptically released dopamine (Moghaddam et al. 1993; Gogos et al. 1998; Castner et al. 2005; Kellendonk et al. 2006; Käenmäki et al. 2010).
Dopamine concentration in the PFC of adult mice that received amphetamine in early adolescence is not different from that of their saline-treated littermates. However, the concentration of HVA is significantly lower in amphetamine- versus saline-treated mice (Fig. 3A, left panel: two-way mixed-design ANOVA, significant treatment × metabolite interaction, F(1,9) = 7.224, P = 0.025; significant main effect of metabolite, F(1,9) = 41.43, P = 0.0001; no main effect of treatment, F(1,9) = 3.478, P = 0.10. * = P < 0.05, post hoc Bonferroni’s multiple comparisons test. Amphetamine n = 6, saline n = 5). In fact, dopamine turnover, expressed as the ratio of HVA/DA content, is significantly reduced in mice treated with amphetamine in early adolescence (Fig. 3A, right panel: t(9) = 3.13, P = 0.012). In line with our behavioral results, neither PFC dopamine, HVA content, nor dopamine turnover is altered in adult mice that were exposed to amphetamine in mid-adolescence or adulthood (Fig. 3B,C. mid-adolescent treatment: left panel, two-way mixed-design ANOVA, significant main effect of metabolite, F(1,10) = 13.24, P = 0.0045; no effect of treatment, F(1, 10) = 3.306, P = 0.10; no interaction, F(1,10) = 2.124, P = 0.18. right panel, t(10) = 0.97, P = 0.35. Amphetamine n = 6, saline n = 6. Adult treatment: left panel, two-way mixed-design ANOVA, significant main effect of metabolite, F(1,12) = 58.72, P < 0.0001; no effect of treatment, F(1,12) = 0.1436, P = 0.71; no interaction, F(1,12) = 1.712, P = 0.22. Right panel, t(12) = 1.2, P = 0.24. Amphetamine n = 7, saline n = 7). Furthermore, this enduring effect of amphetamine exposure in early adolescence on dopamine turnover is limited to the PFC, as dopamine turnover in the nucleus accumbens is unaffected by exposure at any age (Supplementary Fig. 2). These findings indicate that early adolescence is a critical period in which amphetamine can alter mesocortical dopamine function.
Figure 3.
Exposure to amphetamine in early adolescence, but not mid-adolescence or adulthood, leads to blunted PFC dopamine turnover in adulthood. (A) Mice that received amphetamine during early adolescence have reduced baseline HVA content in the PFC. Dopamine turnover, as assessed by the ratio of HVA/dopamine content, is significantly reduced in adult mice that received amphetamine in early adolescence. (B) Baseline dopamine and HVA content are not altered in adult mice that received amphetamine during mid-adolescence. There are no significant changes in dopamine turnover in the PFC of mice that received amphetamine in mid-adolescence. (C) Baseline dopamine and HVA content are not altered in adult mice that received amphetamine during adulthood. There are no significant changes in dopamine turnover in the PFC of mice that received amphetamine in adulthood.
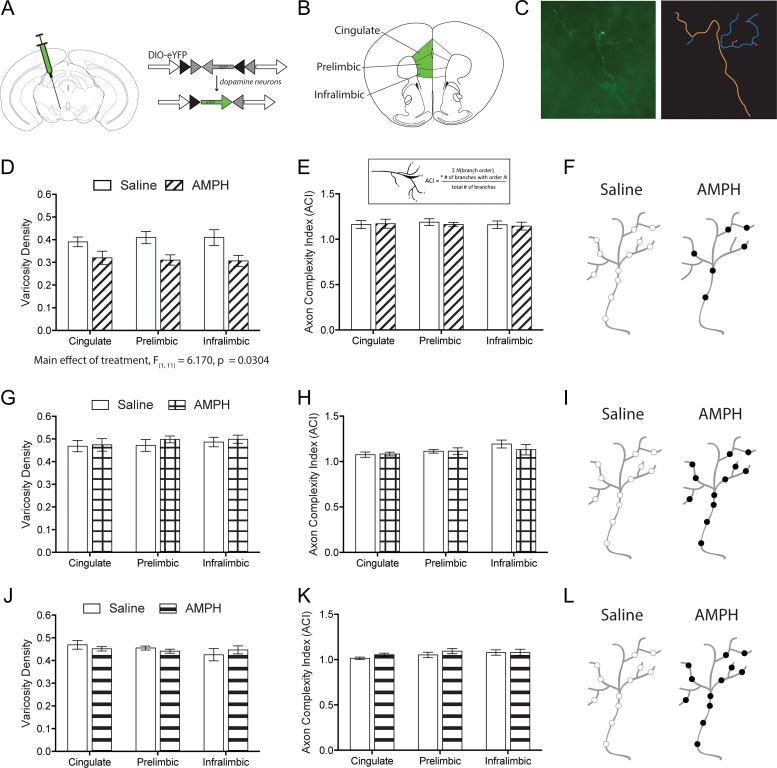
Exposure to Amphetamine Specifically in Early Adolescence Results in Reduced Density of Presynaptic Sites Along Mesocortical Dopamine Axons
In contrast to subcortical regions, dopamine axons innervate the PFC sparsely and strategically, with nearly all of their presynaptic sites forming functional synapses (Séguéla et al. 1988; Manitt et al. 2013; Pokinko et al. 2015; Reynolds et al. 2018). The decrease in PFC dopamine turnover following early adolescent exposure to amphetamine may be associated with a disruption in the establishment of synapses by dopamine axons. Previously, we have found that exposure to amphetamine in early adolescence, but not in adulthood, reduces the density of dopamine varicosities across the inner layers of the PFC (Reynolds et al. 2015). However, it remains unknown whether there exists a critical period for this effect. Therefore, we next determined whether exposure to amphetamine in early adolescence (PND 22 ± 1–PND 31 ± 1), mid-adolescence (PND 35 ± 1–PND 44 ± 1), or adulthood (PND 75 ± 15–PND 84 ± 15) impacts the fine architecture and varicosity density of PFC dopamine axon arbors. We infected dopamine neurons of DATCre mice with a Cre-dependent eYFP virus microinjected into the ventral tegmental area (Fig. 4A). We then used Neurolucida to quantify the complexity and varicosity density of individual eYFP-labeled PFC dopamine axon arbors in the cingulate, prelimbic, and infralimbic subregions of the PFC (Fig. 4B,C)(Reynolds et al. 2018). We found that adult mice exposed to amphetamine early in adolescence have a reduced density of varicosities along individual dopamine axon arbors in their PFC (Fig. 4D, two-way mixed-design ANOVA, significant main effect of treatment, F(1,11) = 6.17, P = 0.0304; no effect of subregion, F(2,22) = 0.1420, P = 0.86; no interaction. F(2,22) = 1.934, P = 0.17. Amphetamine n = 7, saline n = 6), without alterations in arbor complexity (Fig. 4E, two-way mixed-design ANOVA, no effect of treatment, F(1,11) = 0.06623, P = 0.80; no effect of subregion, F(2,22) = 0.2058, P = 0.81; no interaction, F(2,22) = 0.1128, P = 0.89). This is in line with our previous results where we assessed presynaptic site density across the PFC as a whole using stereology (Reynolds et al. 2015). In contrast, exposure to amphetamine during mid-adolescence or adulthood did not alter dopamine varicosity density, nor the complexity of dopamine axon arbors (Fig. 4G–L. Mid-adolescent treatment: varicosity density, two-way mixed-design ANOVA, no effect of treatment, F(1,10) = 0.2502, P = 0.63; no effect of subregion, F(2,20) = 2.239, P = 0.13; no interaction, F(2,20) = 0.5332, P = 0.60. Complexity, two-way mixed-design ANOVA, no effect of treatment, F(1,10) = 0.2627, P = 0.62; no effect of subregion, F(2,20) = 3.090, P = 0.07; no interaction, F(2, 20) = 0.6430, P = 0.54. Amphetamine n = 6, saline n = 6. Adult treatment: varicosity density, two-way mixed-design ANOVA, no effect of treatment, F(1,6) = 0.05995, P = 0.81; no effect of subregion, F(2,12) = 1.196, P = 0.34; no interaction, F(2,12) = 0.9554, P = 0.41. Complexity, two-way mixed-design ANOVA, no effect of treatment, F(1,6) = 0.8775, P = 0.39; no effect of subregion, F(2,12) = 1.707, P = 0.22; no interaction, F(2,12) = 0.4107, P = 0.67. Amphetamine n = 5, saline n = 3). These results show that early adolescence marks the time during which exposure to amphetamine disrupts both the development of PFC dopamine presynaptic sites and dopamine turnover. Early adolescence is therefore a critical period for the establishment of adult PFC dopamine connectivity and function. Whether the amphetamine treatment during this early adolescent critical period also affects the maturation of other neuronal or hormonal systems, including pubertal onset, remains to be determined (Yetnikoff et al. 2014; Kang et al. 2016).
Figure 4.
Only amphetamine treatment during early adolescence enduringly disrupts the distribution of presynaptic sites along PFC dopamine axons. (A) Viral recombination strategy. (B) Subregions of the PFC where dopamine axons were analyzed. (C) Representative micrograph and example tracing of a PFC dopamine axon. (D) PFC dopamine axons of adult mice that received amphetamine early in adolescence have a significant reduction in varicosity density.(E) Quantitative assessment reveals that dopamine axons in the PFC of adult mice that received amphetamine early in adolescence do not have differences in their complexity. Inset: ACI calculation. (F) Schematic of PFC dopamine axons of adult mice that received saline or amphetamine in early adolescence. (G) Amphetamine exposure in mid-adolescence does not change the varicosity density of PFC dopamine axons in adulthood. (H) Dopamine axons in the PFC of adult mice that received amphetamine in mid-adolescence do not have differences in their complexity. (I) Schematic of PFC dopamine axons of adult mice that received saline or amphetamine in mid-adolescence. (J) Amphetamine exposure in adulthood does not change the varicosity density of PFC dopamine axons. (K) Dopamine axons in the PFC of adult mice that received amphetamine in adulthood did not have differences in their complexity. (L) Schematic of PFC dopamine axons of adult mice that received saline or amphetamine in adulthood.
Discussion
In this study, we report that early adolescence is the critical period for the maturation of behavioral inhibition and mesocortical dopamine function in male mice. Only during this time non-contingent repeated exposure to amphetamine, at a dose corresponding to what it is used recreationally in humans, leads to adult (a) impaired performance in a Go/No-Go task, (b) reduced PFC dopamine turnover, and (c) reduced number of varicosities. It is well established that dopamine in the PFC regulates cognitive control, including maintaining appropriate levels of behavioral inhibition, working memory, attentional set shifting, and cue–reward associations (Sokolowski and Salamone 1994; Jentsch et al. 1997; Phillips et al. 2004; Stefani and Moghaddam 2006; Goldstein and Volkow 2011; Grégoire et al. 2012; Bari and Robbins 2013; Willing and Wagner 2015b; Abraham et al. 2017; Ellwood et al. 2017; Reynolds et al. 2018). The disruption of mesocortical dopamine development by amphetamine in early adolescence may therefore underlie impaired cognitive processing in adulthood.
Our previous work on the guidance cue receptor DCC provides insight into why amphetamine exposure during early adolescence, but not later, alters mesocortical dopamine development. DCC receptors delimit the adolescent growth of dopamine axons into the PFC (Manitt et al. 2013; Hoops et al. 2018; Reynolds et al. 2018) and, in turn, drive the maturation of PFC pyramidal neurons and behavioral inhibition (Reynolds et al. 2018). Remarkably, exposure to amphetamine in early adolescence, but not in mid-adolescence or adulthood, downregulates DCC expression in dopamine neurons (Yetnikoff et al. 2007; 2011; 2013; Cuesta et al. 2018). Furthermore, amphetamine-induced downregulation of DCC expression in dopamine neurons is necessary for its enduring effects on PFC dopamine connectivity and salience attribution to drug-paired contexts (Reynolds et al. 2015). Together, these results suggest that the early adolescent critical period of vulnerability to amphetamine exposure results from a DCC-mediated mechanism.
It is remarkable that the disruption of the development of both dopamine function and behavioral inhibition by repeated amphetamine exposure occurs during the exact same period, namely, early adolescence. The fact that the changes in dopamine function are so perfectly tied to the changes in cognitive control suggest that the critical period is delimited by the developmental timeline of mesocortical dopamine innervation. Combined with our recent report that DCC is involved in the formation of mesocorticolimbic circuitry and related behavioral traits in humans (Vosberg et al. 2018), our current preclinical findings suggest that the greater risk for addiction during adulthood imparted by initiation of drug use during early adolescence (Grant and Dawson 2003; McCabe et al. 2007; Jordan and Andersen 2017) may arise from an age-specific disruption of mesocortical dopamine circuitry and inhibitory control by drugs of abuse.
Supplementary Material
Note
Conflict of Interest: None declared.
Authors’ Contributions
L.M.R., L.Y., A.A., and C.F. conceived and designed the experiments. L.M.R., L.Y., M.P., M.W., J.G.E., L.C.L., and M-P.C. performed the experiments. L.M.R., L.Y., M.P., M.W., J.G.E., L.C.L., A. A., and C.F. analyzed the results. L.M.R., L.Y., A.A., and C.F. wrote the manuscript.
Funding and disclosure
The National Institute on Drug Abuse at the National Institutes of Health (R01DA037911 to C.F.; F31DA041188 to L.M.R.), the Natural Sciences and Engineering Research Council of Canada (2982226 to C.F.; 249848 to A.A.), the Canadian Institutes of Health Research (MOP-74709 to C.F.). C.F. is a research scholar of the Fonds de Recherche du Québec–Santé. L.M.R. was supported by predoctoral fellowships from The Djavad Mowafaghian Foundation and Fulbright Canada.
References
- Abbas Z, Sweet A, Hernandez G, Arvanitogiannis A. 2017. Adolescent exposure to methylphenidate increases impulsive choice later in life. Front Behav Neurosci. 11:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AD, Fontaine HM, Song AJ, Andrews MM, Baird MA, Kieffer BL, Land BB, Chavkin C. 2017. Kappa opioid receptor activation in dopamine neurons disrupts behavioral inhibition. Neuropsychopharmacology. 43(2):362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Napierata L, Brenhouse HC, Sonntag KC. 2008. Juvenile methylphenidate modulates reward-related behaviors and cerebral blood flow by decreasing cortical D3 receptors. Eur J Neurosci. 27:2962–2972. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. 2000. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 37:167–169. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Schochet TL, Feit EC, Harris R, McKee BL, Kelley AE. 2011. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behav Neurosci. 125:93–105. [DOI] [PubMed] [Google Scholar]
- Anggård E, Gunne LM, Jönsson L-E, Niklasson F. 1970. Pharmacokinetic and clinical studies on amphetamine dependent subjects. Eur J Clin Pharmacol. 3:3–11. [Google Scholar]
- Baarendse PJJ, Counotte DS, O’Donnell P, Vanderschuren LJMJ. 2013. Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology. 38:1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, Reichelt AC. 2016. Impaired fear extinction retention and increased anxiety-like behaviours induced by limited daily access to a high-fat/high-sugar diet in male rats: implications for diet-induced prefrontal cortex dysregulation. Neurobiol Learn Mem. 136:127–138. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. 2013. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 108:44–79. [DOI] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. 2000. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 10:1014–1027. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, et al. . 1997. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J Cogn Neurosci. 9:835–847. [DOI] [PubMed] [Google Scholar]
- Castner SA, Vosler PS, Goldman-Rakic PS. 2005. Amphetamine sensitization impairs cognition and reduces dopamine turnover in primate prefrontal cortex. Biol Psychiatry. 57:743–751. [DOI] [PubMed] [Google Scholar]
- Chen J, Song J, Yuan P, Tian Q, Ji Y, Ren-Patterson R, Liu G, Sei Y, Weinberger DR. 2011. Orientation and cellular distribution of membrane-bound catechol-O-methyltransferase in cortical neurons: implications for drug development. J Biol Chem. 286:34752–34760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. 2004. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 29:1628–1636. [DOI] [PubMed] [Google Scholar]
- Cuesta S, Restrepo-Lozano JM, Silvestrin S, Nouel D, Torres-Berrío A, Reynolds LM, Arvanitogiannis A, Flores C. 2018. Non-contingent exposure to amphetamine in adolescence recruits miR-218 to regulate Dcc expression in the VTA. Neuropsychopharmacology. 43:900–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. 2006. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 44:2037–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy LM, Zimmermann KS, Marvar PJ, Gourley SL. 2016. Induction and blockade of adolescent cocaine-induced habits. Biol Psychiatry. 81:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood IT, Patel T, Wadia V, Lee AT, Liptak AT, Bender KJ, Sohal VS. 2017. Tonic or phasic stimulation of dopaminergic projections to prefrontal cortex causes mice to maintain or deviate from previously learned behavioral strategies. J Neurosci. 37:8315–8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Manitt C, Rodaros D, Thompson KM, Rajabi H, Luk KC, Tritsch NX, Sadikot AF, Stewart J, Kennedy TE. 2005. Netrin receptor deficient mice exhibit functional reorganization of dopaminergic systems and do not sensitize to amphetamine. Mol Psychiatry. 10:606–612. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. 2006. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl). 188:567–585. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. 1996. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 379:606–612. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. 1998. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proceedings of the National Academy of Sciences. 95:9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, et al. . 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. 2003. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 10:163–173. [DOI] [PubMed] [Google Scholar]
- Grégoire S, Rivalan M, Le Moine C, Dellu-Hagedorn F. 2012. The synergy of working memory and inhibitory control: behavioral, pharmacological and neural functional evidences. Neurobiol Learn Mem. 97:202–212. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Juraska JM. 2013. The effects of abused drugs on adolescent development of corticolimbic circuitry and behavior. Neuroscience. 249:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsen I, Mørland J, Bramness JG. 2006. Impairment related to blood amphetamine and/or methamphetamine concentrations in suspected drugged drivers. Accid Anal Prev. 38:490–495. [DOI] [PubMed] [Google Scholar]
- Hoops D, Flores C. 2017. Making dopamine connections in adolescence. Trends Neurosci. 40:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops D, Reynolds LM, Restrepo-Lozano JM, Flores C. 2018. Dopamine development in the mouse orbital prefrontal cortex is protracted and sensitive to amphetamine in adolescence. eNeuro. 5:ENEURO.0372–17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE, Elsworth JD, Taylor JR, Youngren KD, Roth RH. 1997. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 277:953–955. [DOI] [PubMed] [Google Scholar]
- Jordan CJ, Andersen SL. 2017. Sensitive periods of substance abuse: early risk for the transition to dependence. Dev Cogn Neurosci. 25:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käenmäki M, Tammimäki A, Myöhänen T, Pakarinen K, Amberg C, Karayiorgou M, Gogos JA, Männistö PT. 2010. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem. 114:1745–1755. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. 1988. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 269:58–72. [DOI] [PubMed] [Google Scholar]
- Kang S, Wu MM, Galvez R, Gulley JM. 2016. Timing of amphetamine exposure in relation to puberty onset determines its effects on anhedonia, exploratory behavior, and dopamine D1 receptor expression in young adulthood. Neuroscience. 339:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karper PE, De la Rosa H, Newman ER, Krall CM, Nazarian A, McDougall SA, Crawford CA. 2002. Role of D1-like receptors in amphetamine-induced behavioral sensitization: a study using D1A receptor knockout mice. Psychopharmacology (Berl). 159:407–414. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. 2006. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 49:603–615. [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. 2014. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 68:61–72. [DOI] [PubMed] [Google Scholar]
- Kramer JC, Fischman VS, Littlefield DC. 1967. Amphetamine abuse. Pattern and effects of high doses taken intravenously. JAMA. 201:305–309. [DOI] [PubMed] [Google Scholar]
- Laatikainen LM, Sharp T, Harrison PJ, Tunbridge EM. 2013. Sexually dimorphic effects of catechol-O-methyltransferase (COMT) inhibition on dopamine metabolism in multiple brain regions. PLoS One. 8:e61839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Krimer LS, Goldman-Rakic PS. 2000. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 20:8780–8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B. 2009. Developmental changes in cognitive control through adolescence. Adv Child Dev Behav. 37:233–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. 2010. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 72:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. 2012. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 337:1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduca A, Bara A, Larrieu T, Lassalle O, Joffre C, Layé S, Manzoni OJ. 2017. Amplification of mGlu5-endocannabinoid signaling rescues behavioral and synaptic deficits in a mouse model of adolescent and adult dietary polyunsaturated fatty acid imbalance. J Neurosci. 37:6851–6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitt C, Eng C, Pokinko M, Ryan RT, Torres-Berrío A, Lopez JP, Yogendran SV, Daubaras MJJ, Grant A, Schmidt ERE, et al. . 2013. Dcc orchestrates the development of the prefrontal cortex during adolescence and is altered in psychiatric patients. Transl Psychiatry. 3:e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manitt C, Mimee A, Eng C, Pokinko M, Stroh T, Cooper HM, Kolb B, Flores C. 2011. The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry. J Neurosci. 31:8381–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak S, Nikolakopoulou AM, Dirks R, Martens GJ, Cohen-Cory S. 2007. Cell-autonomous TrkB signaling in presynaptic retinal ganglion cells mediates axon arbor growth and synapse maturation during the establishment of retinotectal synaptic connectivity. J Neurosci. 27:2444–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, West BT, Morales M, Cranford JA, Boyd CJ. 2007. Does early onset of non-medical use of prescription drugs predict subsequent prescription drug abuse and dependence? Results from a national study. Addiction. 102:1920–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Goodkind MS, Etkin A. 2016. Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res. 83:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Berridge CW, Goldman-Rakic PS, Bunney BS, Roth RH. 1993. In vivo assessment of basal and drug‐induced dopamine release in cortical and subcortical regions of the anesthetized primate. Synapse. 13:215–222. [DOI] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Di Scala G, Pape J-R, Coutureau E. 2012. Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J Neurosci. 32:16223–16232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Pichon A, Pape J-R, Coutureau E. 2013. Adolescent stimulation of D2 receptors alters the maturation of dopamine-dependent goal-directed behavior. Neuropsychopharmacology. 38:1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz SJ, Foran W, Velanova K, Luna B. 2013. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci. 33:18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. 2008. The mouse brain in stereotaxic coordinates. San Diego: Academic Press. [Google Scholar]
- Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HBM, Rakic P, Kostovic I. 2011. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Floresco SB. 2004. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J Neurosci. 24:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokinko M, Grant A, Shahabi F, Dumont Y, Manitt C, Flores C. 2017. Dcc haploinsufficiency regulates dopamine receptor expression across postnatal lifespan. Neuroscience. 346:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokinko M, Moquin L, Torres-Berrío A, Gratton A, Flores C. 2015. Resilience to amphetamine in mouse models of netrin-1 haploinsufficiency: role of mesocortical dopamine. Psychopharmacology (Berl). 232:3719–29. [DOI] [PubMed] [Google Scholar]
- Reynolds LM, Makowski CS, Yogendran SV, Kiessling S, Cermakian N, Flores C. 2015. Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a dcc-dependent manner. Neuropsychopharmacology. 40:1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LM, Pokinko M, Torres-Berrío A, Cuesta S, Lambert LC, Del Cid-Pellitero E, Wodzinski M, Manitt C, Krimpenfort P, Kolb B, et al. . 2018. DCC receptors drive prefrontal cortex maturation by determining dopamine axon targeting in adolescence. Biol Psychiatry. 83:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffee WH, Ludden TM, Wilcox RE, Gerald MC. 1978. Brain and plasma concentrations of amphetamine isomers in mice. J Pharmacol Exp Ther. 206:586–594. [PubMed] [Google Scholar]
- Robbins TW, Roberts AC, Owen AM, Sahakian BJ, Everitt BJ, Wilkinson L, Muir J, De Salvia M, Tovee M. 1994. Monoaminergic-dependent cognitive functions of the prefrontal cortex in monkey and man. In: Thierry A-M, Glowinski J, Goldman-Rakic PS, editors. Motor and cognitive functions of the prefrontal cortex. Berlin, Heidelberg: Springer-Verlag. p. 93–111.
- Séguéla P, Watkins KC, Descarries L. 1988. Ultrastructural features of dopamine axon terminals in the anteromedial and the suprarhinal cortex of adult rat. Brain Res. 442:11–22. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. 1998. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 18:2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Moghaddam B. 2017. Methylphenidate has nonlinear dose effects on cued response inhibition in adults but not adolescents. Brain Res. 1654:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Salamone JD. 1994. Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Res. 642:20–28. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. 2003. Mapping cortical change across the human life span. Nat Neurosci. 6:309–315. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. 2006. Rule learning and reward contingency are associated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucleus accumbens, and dorsal striatum. J Neurosci. 26:8810–8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. 2005. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 15:49–57. [DOI] [PubMed] [Google Scholar]
- Tseng K-Y, O’Donnell P. 2007. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 17:1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Swearingen AED, Walker QD, Kuhn CM. 2013. Sex differences in novelty- and psychostimulant-induced behaviors of C57BL/6 mice. Psychopharmacology (Berl). 225:707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosberg DE, Zhang Y, Menegaux A, Chalupa A, Manitt C, Zehntner S, Eng C, DeDuck K, Allard D, Durand F, et al. . 2018. Mesocorticolimbic connectivity and volumetric alterations in DCC mutation carriers. J Neurosci. 38:4655–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Bell MR, Flores C, Gulley JM, Willing J, Paul MJ. 2017. Adolescence and reward: making sense of neural and behavioral changes amid the chaos. J Neurosci. 37:10855–10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Cortes LR, Brodsky JM, Kim T, Juraska JM. 2017. Innervation of the medial prefrontal cortex by tyrosine hydroxylase immunoreactive fibers during adolescence in male and female rats. Dev Psychobiol. 59:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Wagner CK. 2015. a. Exposure to the synthetic progestin, 17α-hydroxyprogesterone caproate during development impairs cognitive flexibility in adulthood. Endocrinology. 157:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Wagner CK. 2015. b. Progesterone receptor expression in the developing mesocortical dopamine pathway: importance for complex cognitive behavior in adulthood. Neuroendocrinology. 103:207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamuro K, Yoshino H, Ogawa Y, Makinodan M, Toritsuka M, Yamashita M, Corfas G, Kishimoto T. 2017. Social isolation during the critical period reduces synaptic and intrinsic excitability of a subtype of pyramidal cell in mouse prefrontal cortex. Cereb Cortex. 28:998–1010. [DOI] [PubMed] [Google Scholar]
- Yetnikoff L, Almey A, Arvanitogiannis A, Flores C. 2011. Abolition of the behavioral phenotype of adult netrin-1 receptor deficient mice by exposure to amphetamine during the juvenile period. Psychopharmacology (Berl). 217:505–514. [DOI] [PubMed] [Google Scholar]
- Yetnikoff L, Labelle-Dumais C, Flores C. 2007. Regulation of netrin-1 receptors by amphetamine in the adult brain. Neuroscience. 150:764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetnikoff L, Pokinko M, Arvanitogiannis A, Flores C. 2013. Adolescence: a time of transition for the phenotype of dcc heterozygous mice. Psychopharmacology (Berl). 231:1705–14. [DOI] [PubMed] [Google Scholar]
- Yetnikoff L, Reichard RA, Schwartz ZM, Parsely KP, Zahm DS. 2014. Protracted maturation of forebrain afferent connections of the ventral tegmental area in the rat. J Comp Neurol. 522:1031–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.