Key Points
Question
Is high-dose vitamin D supplementation during the third trimester of pregnancy associated with long-term dental health during childhood?
Findings
In a 6-year follow-up of a double-blind randomized clinical trial that included 623 pregnant women, high-dose vitamin D supplementation during third trimester was associated with reduced odds of enamel defects in the offspring by approximately 50%. No associations with caries were observed.
Meaning
Prenatal high-dose vitamin D supplementation may be a clinically relevant preventive intervention for enamel defects.
Abstract
Importance
Enamel defects of developmental origin affect up to 38% of schoolchildren and is recognized as a global public health challenge. The impaired enamel formation results in pain owing to hypersensitivity, posteruptive breakdowns, rapid caries progression, and extractions in some cases. The etiology is unknown; therefore, prevention is currently not possible.
Objective
To assess the association of a high-dose vitamin D supplementation in pregnant women with enamel defects and caries in their offspring.
Design, Setting, and Participants
Post hoc analysis of a double-blind, single-center, randomized clinical trial, the Copenhagen Prospective Studies on Asthma in Childhood 2010 cohort (COPSAC2010). Enrollment began March 2009 and included 623 women recruited at 24 weeks of pregnancy and 588 of their children. A dental examination was completed at age 6 years in 496 of 588 children (84%). Data were analyzed in 2018.
Intervention
High-dose vitamin D3 (2400 IU/d; N = 315) or matching placebo tablets (N = 308) from pregnancy week 24 to 1 week post partum. In addition, all women received 400 IU/d of vitamin D3 as part of standard care.
Main Outcomes and Measures
Enamel defect was defined as having at least 1 molar affected by demarcated opacity, enamel breakdown, and/or atypical restoration. Caries was defined as decayed, missing, or filled surfaces in both the deciduous and permanent dentitions (World Health Organization standard).
Results
The risk of enamel defects in the permanent dentition was lower in the offspring of mothers who received high-dose vitamin D supplementation during pregnancy compared with standard dose (15.1% [n = 26 of 172] vs 27.5% [n = 44 of 160]; odds ratio, 0.47; 95% CI, 0.27-0.81). A similar association was observed for the deciduous dentition (8.6% [n = 21 of 244] vs 15.9% [n = 40 of 252]; odds ratio, 0.50; 95% CI, 0.28-0.87). There was no association between supplementation and caries.
Conclusions and Relevance
High-dose vitamin D supplementation during pregnancy was associated with approximately 50% reduced odds of enamel defects in the offspring. This suggests prenatal vitamin D supplementation as a preventive intervention for enamel defects, with a clinically important association with dental health.
Trial Registration
ClinicalTrials.gov identifier: NCT00856947
This secondary analysis of a randomized clinical trial assesses the association of a high-dose vitamin D supplementation in pregnant women with enamel defects and caries in their offspring.
Introduction
Dental health of children has significantly improved in industrialized countries1 owing to a massive focus on the identification of risk factors and the improvement of preventive strategies.2 However, while caries prevalence has declined, enamel defects have gained increasing attention and are now considered a global burden, with prevalence estimates in Western Europe reported from 6% to 38%.3,4,5,6
Enamel defects of developmental origin are present when the affected tooth erupts, and the enamel appears hypomineralized and porous. As a consequence, the affected teeth may easily break and need to be extracted early in life, especially the permanent first molars (Figure 1), and enamel defects also predispose to caries later in life.7 Even in mild cases of enamel defects, children experience pain owing to exposure of sensitive dentin, aesthetically unacceptable discoloration, and a considerable dental treatment need.8,9 Enamel defects are therefore a major public health problem, with significant effect on quality of life and health care use.
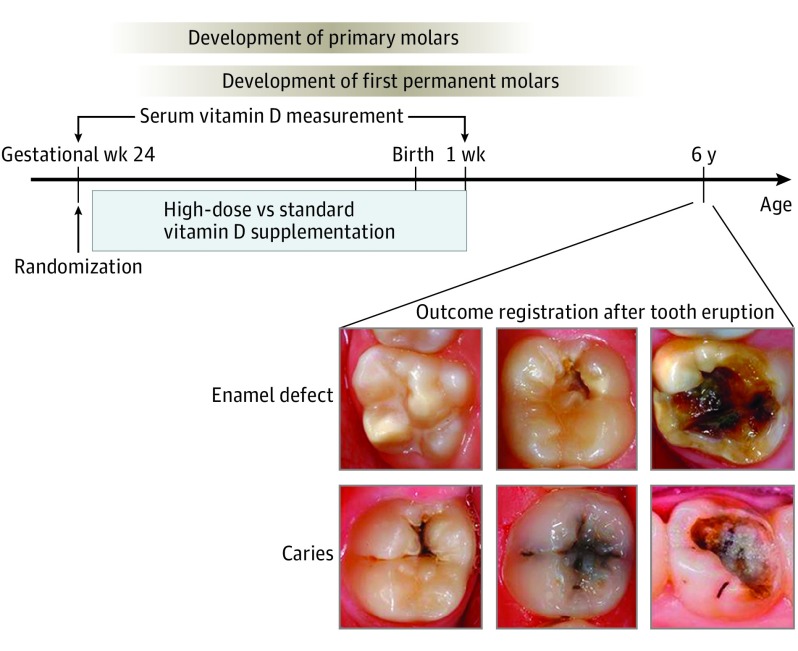
Figure 1. Timeline of High-Dose Vitamin D Supplementation.
Timeline of high-dose vitamin D supplementation, blood sampling, dental examination, and enamel formation in relation to the week of gestation and the child’s age.
Developmental enamel defects results from disturbances during amelogenesis, which starts in incisors and first permanent molars in the fetus during the third trimester of pregnancy, and the mineralization process continues during the first 3 years of life. Therefore, the etiologic factors implicated in developmental enamel defects should be sought in prenatal and early postnatal life. Studies have shown associations between enamel defects and prenatal and perinatal factors, such as maternal illness and medication use in pregnancy, prematurity, birth complications, and early childhood illness10,11; however, in contrast to caries, the evidence for the potential risk factors is inconclusive, and prevention is therefore not possible.
Vitamin D plays a key role in the enamel formation,12,13 and vitamin D deficiency is now also considered a common health challenge in westernized societies.14 Although vitamin D supplementation in pregnancy is recommended by health authorities in most European countries, numerous studies demonstrate that the recommended dose is often inadequate to achieve optimal blood levels of vitamin D3 during pregnancy.15 A few observational studies have suggested an association between high vitamin D3 levels in blood and a reduced risk of enamel defect and/or caries.16,17,18,19,20
Based on these findings, we speculated that vitamin D deficiency during pregnancy and shortly after birth contributes to enamel defects and/or caries development and that vitamin D supplementation during pregnancy may lower the risk. We studied this in a post hoc analysis of a double-blind, single-center, randomized clinical trial (DB-RCT) of high-dose vitamin D supplementation during the third pregnancy trimester in the population-based Copenhagen Prospective Studies on Asthma in Childhood 2010 mother-child cohort (COPSAC2010), where the children completed a comprehensive dental examination at age 6 years.
Methods
This DB-RCT was approved by the Danish National Committee on Health Research Ethics (H-B-2009-014), and the Danish Data Protection Agency (2008-41-2599). It was registered with EudraCT number 2008-007871-26 and ClinTrial.gov ID NCT007 98226. The main COPSAC2010 cohort study was approved by the local ethics committee (COPSAC2010: H-B-2008-093) and the Danish Data Protection Agency (2015-41-3696). The dental study was approved by the local ethics committee (Oral Examination: H-15017900), and both parents gave oral and written informed consent before enrollment. The formal trial protocols can be found in Supplement 1.
The COPSAC2010 Birth Cohort
This study was embedded in the population-based mother-child cohort, COPSAC2010, where 738 pregnant Danish women were recruited between November 2008 and November 2010 as previously described.21,22,23 The first visit to the COPSAC clinic was planned for pregnancy week 24. Exclusion criteria were any endocrine, cardiovascular, or nephrologic disorders or vitamin D3 (cholecalciferol) intake greater than 600 IU/d. The children were enrolled at age 1 week and followed up closely at the COPSAC single-center research unit, with 12 scheduled clinical visits before age 6 years.21
Study Intervention
The pregnant women were randomized 1:1 to a daily dose of 2400 IU vitamin D3 supplementation or matching placebo tablets from pregnancy week 24 to 1 week post partum. In addition, all women were instructed to continue supplementation of 400 IU of vitamin D3 during pregnancy as recommended by the Danish National Board of Health. Thus, the study was a dose comparison of 2800 IU/d vs 400 IU/d of vitamin D3 supplementation. Women were randomized using a computer-generated list of random numbers. Serum vitamin D3 level was assessed at the time of randomization (week 24) and week 1 post partum. Counting returned capsules complemented assessment of compliance. The study was unblinded at a mean age of 4 years, ie, when the last study participant reached age 3 years. The primary outcome in the vitamin D study was asthma/persistent wheeze in the offspring and has been reported previously.23,24
All the women also participated in a concomitant factorial designed DB-RCT of 2.4 g per day of long-chain ω-3 polyunsaturated fatty acids (PUFAs) during pregnancy as reported previously.22 The randomization code was generated to assure balanced numbers in the 4 vitamin D/PUFA treatment/control strata according to the factorial design.
Dental Examination
The children were invited to a comprehensive 6-year follow-up visit, including a dental examination, to collect detailed information regarding each patient’s dental health status. Each child was examined at the designated appointment by 1 dental professional, and the dental examiner was blinded to the DB-RCT allocation. The examiner was trained before the study by an experienced training dentist. The interrater and intrarater reliability of the examiner were obtained after calibration training by comparing the scorings of the trainee with the reference standard (consensus finding between the training dentist and the dental examiner). The weighted κ values were in a good (0.60-0.79) to excellent (>0.80) order of magnitude (intraexaminer reproducibility of the dental examiner for occlusal/smooth surfaces: caries, 0.92/0.97 and enamel defects, 0.91/0.90; interexaminer reproducibility of the dental examiner: caries on occlusal/smooth surfaces, 0.65/0.71; enamel defects on occlusal/smooth surfaces, 0.77/0.83 [first examination] and caries, 0.67/0.74; enamel defects, 0.86/ 0.93 [second examination]).
The participants were examined in a standard dental unit with a halogen lamp and water/airspray. Examinations were performed with a dental mirror and a probe. Teeth were not cleaned prior to the examination. In case of plaque accumulation on tooth surfaces, plaque was removed by the examiner.
Dental Outcome Measures
Enamel defects were defined as presence of hypomineralized enamel of systemic origin with demarcated opacities, posteruptive enamel breakdown, atypical restorations, and/or extractions of molars according to the European Academy of Paediatric Dentistry criteria25 (Figure 1). Demarcated opacities with a diameter less than 2 mm were not scored. Likewise, other enamel disturbances, eg, hypoplasia and dental fluorosis, were not scored. Children with at least 1 affected permanent molar were considered to have enamel defect. In addition, children with demarcated opacities in second molars in the deciduous dentition were identified.6 Caries was defined as decayed, missing, or filled surfaces in both the deciduous and permanent molars (World Health Organization 2013).
Study Power
The sample size was prespecified by recruitment to the original trial protocol focusing on asthma/persistent wheeze. The minimum detectable differences between the treatment groups given the fixed sample size, and an 80% power level (2-tailed α = .05) was 12.6% for enamel defects in the permanent teeth (expecting a prevalence of 38% in the control group).
Statistical Analysis
The association of the prenatal vitamin D supplementation with the binary variables of enamel defect in the permanent and the deciduous dentition and caries was analyzed by logistic regression. In the analysis of the permanent dentition, children with at least 1 fully erupted permanent molar were included, and the number of erupted permanent molars was included as covariate. For the deciduous dentition, all children were included because all their second primary molars were present. An additional model was analyzed with covariate adjustment for participation in the PUFA DB-RCT, sex, birth season, maternal serum vitamin D3 level at randomization, and socioeconomic status. A 2-sided P value less than .05 was considered significant in all models. The data processing was performed using R, version 3.4.0 (R Foundation for Statistical Computing).
Results
Study Group
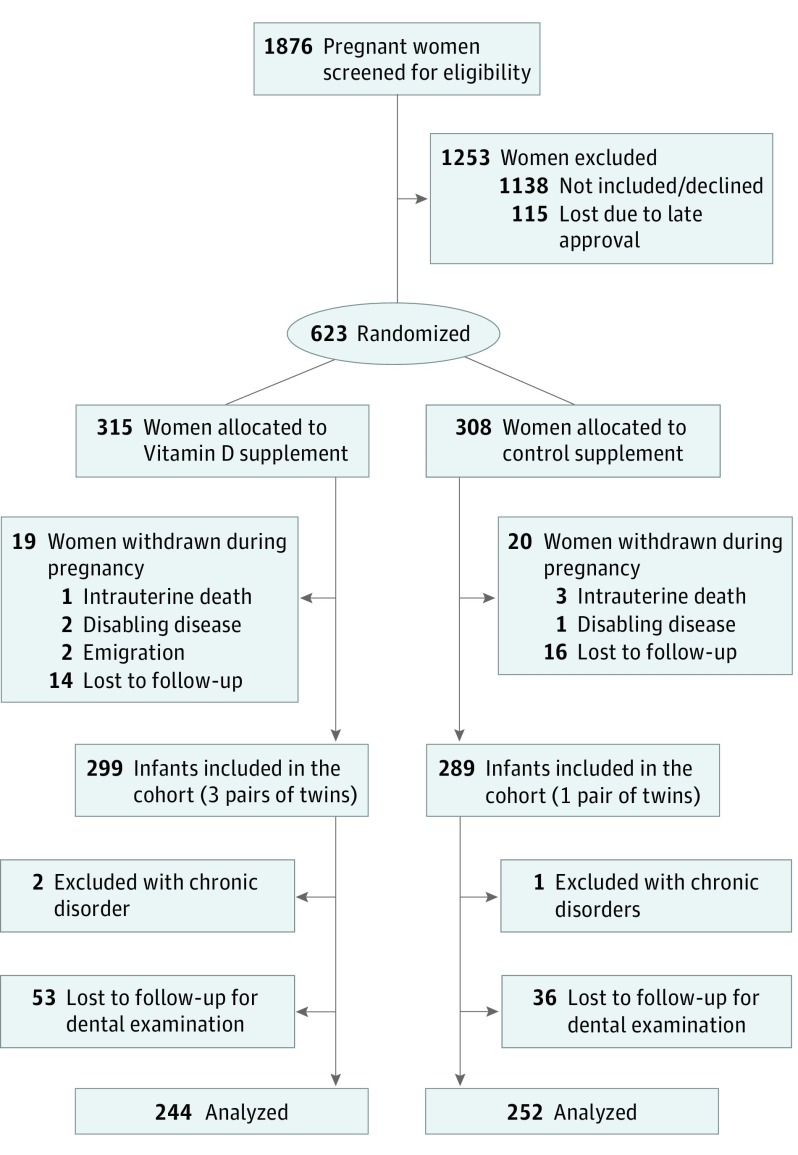
A total of 623 of 738 women from the COPSAC2010 study participated in the vitamin D trial from March 4, 2009, to November 17, 2010, because the ethical approval of the vitamin D trial was delayed during enrollment of the first 115 eligible women. A total of 588 children of these mothers were recruited to the birth cohort study. The clinical dental follow-up rate was 496 children of 588 potential participants at age 6 years (84%) (Figure 2). Baseline characteristics of the pregnant women and their children showed an unbiased randomization (Table 1).
Figure 2. Flow of Participants Through the Copenhagen Prospective Studies on Asthma in Childhood 2010 (COPSAC2010) Vitamin D Randomized Clinical Trial.
The diagram shows the flow of participants from enrollment in the randomized clinical trial to the dental examination at age 6 years. Exclusion criteria were gestational age greater than week 26; any endocrine, cardiovascular, or nephrological disorders or vitamin D intake greater than 600 IU/d.
Table 1. Baseline Characteristics of the Interventional and Control Groupa.
| Characteristics of the Study Population | Randomization, No. (%) | |
|---|---|---|
| High-Dose Vitamin D Supplementation | Standard-Dose Vitamin D Supplementation | |
| Mothers | ||
| Patient allocation | 244 (49.2) | 252 (50.8) |
| Age at birth, mean (SD), y | 32.8 (4.3) | 32.1 (4.2) |
| Household income during last 3 mo before birth, % | ||
| <$24 000 | 70 (28.7) | 80 (31.7) |
| $24 000-$40 000 | 133 (54.5) | 137 (54.4) |
| >$40 000 | 41 (16.8) | 35 (13.9) |
| Smoking in pregnancy | 15 (6.1) | 21 (8.3) |
| Serum vitamin D3 level, mean (SD), ng/mL | ||
| Before intervention | 30.77 (10.34) | 30.61 (10.22) |
| 1 wk Post partum | 43.35 (14.14)b | 28.97 (12.82)b |
| Children | ||
| Male children | 131 (53.7) | 118 (46.8) |
| Nonwhite children | 10 (4.1) | 14 (5.6) |
| Day in y of birth, day 1 = January 1, mean (SD) | 167.1 (114.9) | 176.8 (117.5) |
| Birth | ||
| Term birth >37 wk | 233 (95.5) | 243 (96.4) |
| Gestational age, mean (SD) | 279.0 (12.6) | 279.2 (10.9) |
SI conversion factor: To convert vitamin D3 to nanomoles per liter, multiply by 2.496.
Comparisons between the high-dose vitamin D supplementation and the standard-dose supplementation groups were calculated by χ2 test for categorical variables and by t test for continuous variables.
Statistically significant difference (P < .05).
The proportion of children receiving fish oil supplementation as part of the concomitant PUFA trial was 49.6% in the vitamin D group (n = 121 of 244) and 48.4% in the placebo group (n = 121 of 250). Dropout analyses illustrated that mothers of children aged 6 years who were examined were on average older, had a higher gestational age, and gave birth earlier in the year (eTable in Supplement 2).
Adherence to the intervention, defined as mothers taking more than 80% of the prescribed tablets, was 74% (n = 462 of 623). The vitamin D intervention resulted in a significant increase in maternal serum vitamin D3 level in the treatment group from randomization to the postpartum assessment (mean [SD], 30.77 [10.34] ng/mL vs 43.35 [14.14] ng/mL; to convert to nanomoles per liter, multiply by 2.496) but not in the control group (30.61 [10.22] ng/mL vs 28.97 [12.82] ng/mL).
Enamel Defects
Of the 496 examined children, 332 (66.9%) had at least 1 fully erupted first permanent molar and 234 (47.2%) had all 4. Enamel defects in the permanent dentition and the deciduous dentition were diagnosed in 70 children (21.1%) and 61 children (12.3%), respectively, and in either the permanent or the deciduous dentition in 118 children (23.8%). The odds of having enamel defects in the permanent dentition were more than doubled if it was also present in the deciduous dentition (odds ratio [OR], 2.5; 95% CI, 1.26-4.88). There was no difference in the number of erupted permanent molars between the intervention and the control group (in the intervention group, 7.6% had 1 erupted molar, 16.3% had 2, 7.0% had 3, and 69.2% had 4 vs 9.4%, 12.5%, 6.3%, and 71.9%, respectively, in the control group; P = .73).
The prevalence of enamel defects in the permanent dentition was lower in the children whose mothers received high-dose vitamin D supplementation in pregnancy compared with standard dose, and a similar association was observed for enamel defects in the deciduous dentition and in the combined analysis of both the deciduous and permanent dentition (Table 2). Covariate adjustment did not change the results (Table 2).
Table 2. Association of Vitamin D Supplementation During Pregnancy With Dental Health of the Child at Age 6 Years.
| Dental Health Outcomes at the 6-y Follow-up | Vitamin D Supplementation, No./Total No. (%) | Logistic Regression Analysis, OR (95% CI) | ||
|---|---|---|---|---|
| High Dose | Standard Dose | Crude | Adjusteda | |
| Enamel defects | ||||
| Permanent dentition | 26/172 (15.1) | 44/160 (27.5) | 0.47 (0.27-0.81)b,c | 0.42 (0.23-0.73)b,c |
| Deciduous dentition | 21/244 (8.6) | 40/252 (15.9) | 0.50 (0.28-0.87)c | 0.54 (0.30-0.94)c |
| Permanent and/or deciduous dentition | 44/244 (18.0) | 74/252 (29.4) | 0.50 (0.32-0.77)b,c | 0.52 (0.33-0.79)b,c |
| Caries | ||||
| Permanent dentition | 8/172 (4.7) | 6/159 (3.8) | 1.30 (0.44-4.37)b | 1.32 (0.41-4.48)b |
| Deciduous dentition | 49/244 (20.1) | 51/252 (20.2) | 0.99 (0.64-1.54) | 1.01 (0.65-1.59) |
| Permanent and/or deciduous dentition | 54/244 (22.1) | 53/252 (21.0) | 1.05 (0.68-1.60)b | 1.08 (0.70-1.67)b |
Abbreviation: OR, odds ratio.
The model was adjusted for participation in the long-chain ωn3 polyunsaturated fatty acids, sex, birth season, maternal serum vitamin D3 level at randomization, and socioeconomic status.
The model was adjusted for number of erupted first permanent molars.
Statistically significant association (P < .05).
There were no associations between maternal levels of vitamin D3 before intervention or season of birth and enamel defects in the permanent dentition nor any interaction between vitamin D supplementation and maternal levels of vitamin D3 before intervention or season of birth in association with the odds of enamel defects in the permanent dentition or the deciduous dentition. Similarly, there was no association between PUFA supplementation and enamel defects and no evidence of interaction between vitamin D and PUFA supplementation in relation to enamel defects (data not shown).
Caries
Caries was present in 107 of 496 children (23%) with a dental examination. The prevalence was 4.2% in the permanent dentition (n = 14 of 331) and 20.2% in the deciduous dentition (n = 100 of 496). There was no association between high-dose vitamin D3 supplementation and caries in both dentitions (Table 2). Furthermore, there was no association between enamel defects and caries in the deciduous dentition (26% of children [n = 16 of 61] with enamel defects had caries and 19% of the children [n = 82 of 435] without enamel defects had caries; P = .28).
Discussion
High-dose vitamin D supplementation during pregnancy was associated with an approximately 50% reduced odds of enamel defects in the offspring at age 6 years. This suggests vitamin D supplementation as a primary preventive measure for enamel defects with a potentially large effect on dental health.
Strengths and Limitations
The major strength of this study is the randomized, double-blind, placebo-controlled supplementation of vitamin D. This design avoids confounding from lifestyle factors associated with vitamin D levels and dental problems, which are difficult to adjust for in observational studies. In addition, the mothers had good adherence to the intervention protocol. The study is furthermore strengthened by a high follow-up rate, with 84% of the included children attending the examination at age 6 years. All children were examined by a single, trained and well-calibrated dental professional, which improves consistency in the procedures and registration of the conditions.
The observed proportion of enamel defects were within the expected range based on epidemiologic reports from Western Europe.3,4,5,6 The proportion of caries is also in line with the 2018 report from the National Health Authorities in Denmark.26
It may be considered a limitation of this study that the DB-RCT was not primarily designed for dental end points and that the dental examination was performed after unblinding of the study. Nonetheless, the dental examiner was not aware of the medical information, laboratory data, or treatment assignment, and the events during tooth development leading to enamel defects are expected to take place before unblinding of the study at age 3 years.
Not all children had eruption of all their permanent molars at the dental examination, but because there was no association between supplementation with high-dose vitamin D and eruption of permanent molars, this did not bias our results. As a likely consequence of the relatively short exposure time of the first permanent molar in the oral cavity by age 6 years, the proportion of caries in these molars was low, and we therefore did not have statistical power to detect a potential effect of the supplementation on this outcome. Furthermore, owing to low statistical power, we were not able to test the effect of the intervention on the severity and extension of enamel defects, and it is therefore uncertain whether vitamin D supplementation can prevent more severe conditions, eg, enamel breakdown.
Interpretation
Very little is known about the etiology of developmental enamel defects, and therefore, no causal preventive strategies are currently available. These results from a DB-RCT, suggesting a protective role of vitamin D supplementation during pregnancy against enamel defects, indicate a breakthrough in understanding and prevention of the disease. Interestingly, there are also potential health benefits from prenatal vitamin D supplementation in relation to development of persistent wheeze before age 3 years23,27 and other childhood outcomes, and to our knowledge, no adverse effects have been observed in the current or previous trials.23,28 Therefore, our finding suggests a simple and safe prevention strategy against enamel defects with a considerable effect size, which could have an important effect on long-term dental health.
Considering the key role of vitamin D in enamel mineralization,12,13,29,30 the association between vitamin D supplementation and enamel defects seems biologically plausible. It can be speculated that ameloblast function and mineralization processes under early tooth formation are affected by agents causing enamel defects and that supplementation with high-dose vitamin D has a protective association with the development and strength of the enamel.
High-dose vitamin D supplementation was provided from week 24 of gestation to 1 week after birth, and considering the half-life of vitamin D3 in blood of approximately 2-3 weeks,31 the period of increased vitamin D3 levels owing to supplementation will be expected to last from week 24 of gestation until approximately 3 months after birth. This period is relevant in relation to the age of tooth and enamel formation (Figure 1). Our study showed similar association of the intervention with enamel defects in both the deciduous and permanent dentition, indicating that the third trimester of pregnancy and early prenatal life is a critical period for the development of enamel defects in both dentitions.
Two previous observational studies have investigated the association between vitamin D and enamel defects. One cohort study16 found that an increased level of serum vitamin D3 concentration at age 10 years was associated with a lower risk of having enamel defects, while another cohort study32 found no association between vitamin D3 levels in maternal blood during pregnancy, in cord blood, or blood in the offspring at 6 years and enamel defects. However, it should be noted that vitamin D3 levels are strongly associated with various socioeconomic and lifestyle factors,33,34,35,36 and residual confounding can never be excluded in observational studies, which is mitigated in a DB-RCT design. The maternal level of vitamin D3 before intervention did not have an association with the risk of enamel defects. This suggests that a high-dose supplementation is imperative to prevent enamel defects because standard supplementation of vitamin D in pregnancy is not adequate.
We found no association of high-dose vitamin D supplementation with risk of caries. This is in contrast to previous observational studies that suggested an association between risk of caries and maternal gestational vitamin D3 levels,17 vitamin D supplementation in the first year of life,18 and low blood levels of vitamin D3 in childhood.19,20 This discrepancy might be owing to the previous observations being confounded from lifestyle factors. Another potential factor is the relatively short follow-up in this study. In contrast to previous studies,7,37,38 we did not see any statistically significant association between enamel defects and caries, which presumably is a result of the short period that molars, particularly the permanent molars, have been exposed in the oral cavity. Thus, given the association of prenatal vitamin D supplementation with enamel defects, it will be expected to reduce the risk of developing caries later in life.
Enamel defects are a challenging dental health problem for many children and adults.39,40 Children with severe defects experience pain and need for dental treatments, have increased risk of caries, and may develop dental anxiety over time.7 In some cases, enamel defects lead to extensive and expensive treatment types, ie, artificial crowns or orthodontic treatment as a consequence of extraction of permanent first molars, which pose a significant economic burden to society and negatively affect quality of life for the affected children. The implications of our findings of prenatal high-dose vitamin D supplementation as a new and nontoxic primary preventive agent of enamel defects, with a clinically significant odds reduction of approximately 50%, may therefore have substantial effects on dental health and health care use.
A follow-up of this study at an older age will be important to analyze potential effects on caries. Furthermore, future studies should consider earlier start of high-dose vitamin D supplementation and higher supplementation doses in the infant.
Conclusions
High-dose vitamin D supplementation during pregnancy was associated with reduced odds of enamel defects by approximately 50% in children aged 6 years. This suggests prenatal high-dose vitamin D supplementation as a preventive intervention to reduce the prevalence of enamel defects with a significant potential effect on dental health.
Trial Protocol
eTable. Dropout Analyses in Relation to Baseline Characteristics of the Study Population
References
- 1.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJL, Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J Dent Res. 2015;94(5):650-658. doi: 10.1177/0022034515573272 [DOI] [PubMed] [Google Scholar]
- 2.Pitts NB, Zero DT, Marsh PD, et al. Dental caries. Nat Rev Dis Primers. 2017;3:17030. doi: 10.1038/nrdp.2017.30 [DOI] [PubMed] [Google Scholar]
- 3.Jälevik B. Prevalence and diagnosis of molar-incisor-hypomineralisation (MIH): a systematic review. Eur Arch Paediatr Dent. 2010;11(2):59-64. doi: 10.1007/BF03262714 [DOI] [PubMed] [Google Scholar]
- 4.Kühnisch J, Heitmüller D, Thiering E, et al. Proportion and extent of manifestation of molar-incisor-hypomineralizations according to different phenotypes. J Public Health Dent. 2014;74(1):42-49. doi: 10.1111/j.1752-7325.2012.00365.x [DOI] [PubMed] [Google Scholar]
- 5.Wogelius P, Haubek D, Poulsen S. Prevalence and distribution of demarcated opacities in permanent 1st molars and incisors in 6 to 8-year-old Danish children. Acta Odontol Scand. 2008;66(1):58-64. doi: 10.1080/00016350801926941 [DOI] [PubMed] [Google Scholar]
- 6.Elfrink MEC, Ghanim A, Manton DJ, Weerheijm KL. Standardised studies on molar incisor hypomineralisation (MIH) and hypomineralised second primary molars (HSPM): a need. Eur Arch Paediatr Dent. 2015;16(3):247-255. doi: 10.1007/s40368-015-0179-7 [DOI] [PubMed] [Google Scholar]
- 7.Kühnisch J, Kabary L, Malyk Y, et al. Relationship between caries experience and demarcated hypomineralised lesions (including MIH) in the permanent dentition of 15-year-olds. Clin Oral Investig. 2018;22(5):2013-2019. doi: 10.1007/s00784-017-2299-4 [DOI] [PubMed] [Google Scholar]
- 8.Schwendicke F, Elhennawy K, Reda S, Bekes K, Manton DJ, Krois J. Global burden of molar incisor hypomineralization. J Dent. 2018;68:10-18. doi: 10.1016/j.jdent.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 9.Elhennawy K, Jost-Brinkmann P-G, Manton DJ, Paris S, Schwendicke F. Managing molars with severe molar-incisor hypomineralization: a cost-effectiveness analysis within German healthcare. J Dent. 2017;63:65-71. doi: 10.1016/j.jdent.2017.05.020 [DOI] [PubMed] [Google Scholar]
- 10.Alaluusua S. Aetiology of molar-incisor hypomineralisation: a systematic review. Eur Arch Paediatr Dent. 2010;11(2):53-58. doi: 10.1007/BF03262713 [DOI] [PubMed] [Google Scholar]
- 11.Silva MJ, Scurrah KJ, Craig JM, Manton DJ, Kilpatrick N. Etiology of molar incisor hypomineralization: a systematic review. Community Dent Oral Epidemiol. 2016;44(4):342-353. doi: 10.1111/cdoe.12229 [DOI] [PubMed] [Google Scholar]
- 12.Berdal A, Papagerakis P, Hotton D, Bailleul-Forestier I, Davideau JL. Ameloblasts and odontoblasts, target-cells for 1,25-dihydroxyvitamin D3: a review. Int J Dev Biol. 1995;39(1):257-262. [PubMed] [Google Scholar]
- 13.Mesbah M, Nemere I, Papagerakis P, et al. Expression of a 1,25-dihydroxyvitamin D3 membrane-associated rapid-response steroid binding protein during human tooth and bone development and biomineralization. J Bone Miner Res. 2002;17(9):1588-1596. doi: 10.1359/jbmr.2002.17.9.1588 [DOI] [PubMed] [Google Scholar]
- 14.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144(pt A):138-145. doi: 10.1016/j.jsbmb.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79(5):717-726. doi: 10.1093/ajcn/79.5.717 [DOI] [PubMed] [Google Scholar]
- 16.Kühnisch J, Thiering E, Kratzsch J, Heinrich-Weltzien R, Hickel R, Heinrich J; GINIplus study group; LISAplus study group . Elevated serum 25(OH)-vitamin D levels are negatively correlated with molar-incisor hypomineralization. J Dent Res. 2015;94(2):381-387. doi: 10.1177/0022034514561657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroth RJ, Lavelle C, Tate R, Bruce S, Billings RJ, Moffatt MEK. Prenatal vitamin D and dental caries in infants. Pediatrics. 2014;133(5):e1277-e1284. doi: 10.1542/peds.2013-2215 [DOI] [PubMed] [Google Scholar]
- 18.Kühnisch J, Thiering E, Heinrich-Weltzien R, Hellwig E, Hickel R, Heinrich J. Fluoride/vitamin D tablet supplementation in infants-effects on dental health after 10 years. Clin Oral Investig. 2017;21(7):2283-2290. doi: 10.1007/s00784-016-2021-y [DOI] [PubMed] [Google Scholar]
- 19.Schroth RJ, Rabbani R, Loewen G, Moffatt ME. Vitamin D and dental caries in children. J Dent Res. 2016;95(2):173-179. doi: 10.1177/0022034515616335 [DOI] [PubMed] [Google Scholar]
- 20.Schroth RJ, Levi JA, Sellers EA, Friel J, Kliewer E, Moffatt MEK. Vitamin D status of children with severe early childhood caries: a case-control study. BMC Pediatr. 2013;13:174. doi: 10.1186/1471-2431-13-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bisgaard H, Vissing NH, Carson CG, et al. Deep phenotyping of the unselected COPSAC2010 birth cohort study. Clin Exp Allergy. 2013;43(12):1384-1394. doi: 10.1111/cea.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530-2539. doi: 10.1056/NEJMoa1503734 [DOI] [PubMed] [Google Scholar]
- 23.Chawes BL, Bønnelykke K, Stokholm J, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA. 2016;315(4):353-361. doi: 10.1001/jama.2015.18318 [DOI] [PubMed] [Google Scholar]
- 24.Wolsk HM, Chawes BL, Litonjua AA, et al. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: a combined analysis of two randomized controlled trials. PLoS One. 2017;12(10):e0186657. doi: 10.1371/journal.pone.0186657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lygidakis NA, Wong F, Jälevik B, Vierrou A-M, Alaluusua S, Espelid I. Best clinical practice guidance for clinicians dealing with children presenting with molar-incisor-hypomineralisation (MIH): an EAPD policy document. Eur Arch Paediatr Dent. 2010;11(2):75-81. doi: 10.1007/BF03262716 [DOI] [PubMed] [Google Scholar]
- 26.Sundhedsstyrelsen. Danish Health Authority; Central Odontologic Register (SCOR). https://www.sst.dk/da/Udgivelser/1999/Indberetning-paa-boerne--og-ungdomstandplejeomraadet---redegoerelse-vedroerende-revision-af-indberet. Accessed July 1, 2019.
- 27.Litonjua AA, Carey VJ, Laranjo N, et al. Effect of prenatal supplementation with vitamin d on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. 2016;315(4):362-370. doi: 10.1001/jama.2015.18589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bi WG, Nuyt AM, Weiler H, Leduc L, Santamaria C, Wei SQ. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172(7):635-645. doi: 10.1001/jamapediatrics.2018.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berdal A, Hotton D, Pike JW, Mathieu H, Dupret JM. Cell- and stage-specific expression of vitamin D receptor and calbindin genes in rat incisor: regulation by 1,25-dihydroxyvitamin D3. Dev Biol. 1993;155(1):172-179. doi: 10.1006/dbio.1993.1016 [DOI] [PubMed] [Google Scholar]
- 30.Celio MR, Norman AW, Heizmann CW. Vitamin-D-dependent calcium-binding-protein and parvalbumin occur in bones and teeth. Calcif Tissue Int. 1984;36(1):129-130. doi: 10.1007/BF02405306 [DOI] [PubMed] [Google Scholar]
- 31.Jones KS, Assar S, Harnpanich D, et al. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99(9):3373-3381. doi: 10.1210/jc.2014-1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Tas JT, Elfrink MEC, Heijboer AC, et al. Foetal, neonatal and child vitamin D status and enamel hypomineralization. Community Dent Oral Epidemiol. 2018;46(4):343-351. doi: 10.1111/cdoe.12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281. doi: 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 34.Jones AP, Palmer D, Zhang G, Prescott SL. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics. 2012;130(5):e1128-e1135. doi: 10.1542/peds.2012-1172 [DOI] [PubMed] [Google Scholar]
- 35.Camargo CA Jr, Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788-795. doi: 10.1093/ajcn/85.3.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85(3):853-859. doi: 10.1093/ajcn/85.3.853 [DOI] [PubMed] [Google Scholar]
- 37.Grossi JA, Cabral RN, Leal SC. Caries experience in children with and without molar-incisor hypomineralisation: a case-control study. Caries Res. 2017;51(4):419-424. doi: 10.1159/000477099 [DOI] [PubMed] [Google Scholar]
- 38.Americano GCA, Jacobsen PE, Soviero VM, Haubek D. A systematic review on the association between molar incisor hypomineralization and dental caries. Int J Paediatr Dent. 2017;27(1):11-21. doi: 10.1111/ipd.12233 [DOI] [PubMed] [Google Scholar]
- 39.Crombie FA, Manton DJ, Weerheijm KL, Kilpatrick NM. Molar incisor hypomineralization: a survey of members of the Australian and New Zealand Society of Paediatric Dentistry. Aust Dent J. 2008;53(2):160-166. doi: 10.1111/j.1834-7819.2008.00026.x [DOI] [PubMed] [Google Scholar]
- 40.Weerheijm KL, Mejàre I. Molar incisor hypomineralization: a questionnaire inventory of its occurrence in member countries of the European Academy of Paediatric Dentistry (EAPD). Int J Paediatr Dent. 2003;13(6):411-416. doi: 10.1046/j.1365-263X.2003.00498.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Dropout Analyses in Relation to Baseline Characteristics of the Study Population