Key Points
Question
Is a standardized perioperative management approach safe for patients with atrial fibrillation who use a direct oral anticoagulant and require elective surgery or procedure?
Findings
In this cohort study of 3007 patients with atrial fibrillation using apixaban, dabigatran, or rivaroxaban, the direct oral anticoagulant treatment was stopped and resumed before and/or after elective surgery or procedure using standardized protocols without heparin bridging. The 30-day postoperative rates of major bleeding were less than 2%, and the rates of stroke were less than 1%.
Meaning
In this study, in patients treated with a direct oral anticoagulant, a simple standardized perioperative management approach was associated with low rates of bleeding and stroke.
Abstract
Importance
Patients with atrial fibrillation (AF) who use a direct oral anticoagulant (DOAC) and request elective surgery or procedure present a common clinical situation yet perioperative management is uncertain.
Objective
To investigate the safety of a standardized perioperative DOAC management strategy.
Design, Setting, and Participants
The Perioperative Anticoagulation Use for Surgery Evaluation (PAUSE) cohort study conducted at 23 clinical centers in Canada, the United States, and Europe enrolled and screened patients from August 1, 2014, through July 31, 2018. Participants (n = 3007) had AF; were 18 years of age or older; were long-term users of apixaban, dabigatran etexilate, or rivaroxaban; were scheduled for an elective surgery or procedure; and could adhere to the DOAC therapy interruption protocol.
Interventions
A simple standardized perioperative DOAC therapy interruption and resumption strategy based on DOAC pharmacokinetic properties, procedure-associated bleeding risk, and creatinine clearance levels. The DOAC regimens were omitted for 1 day before a low–bleeding-risk procedure and 2 days before a high–bleeding-risk procedure. The DOAC regimens were resumed 1 day after a low–bleeding-risk procedure and 2 to 3 days after a high–bleeding-risk procedure. Follow-up of patients occurred for 30 days after the operation.
Main Outcomes and Measures
Major bleeding and arterial thromboembolism (ischemic stroke, systemic embolism, and transient ischemic attack) and the proportion of patients with an undetectable or minimal residual anticoagulant level (<50 ng/mL) at the time of the procedure.
Results
The 3007 patients with AF (mean [SD] age of 72.5 [9.39] years; 1988 men [66.1%]) comprised 1257 (41.8%) in the apixaban cohort, 668 (22.2%) in the dabigatran cohort, and 1082 (36.0%) in the rivaroxaban cohort; 1007 patients (33.5%) had a high–bleeding-risk procedure. The 30-day postoperative rate of major bleeding was 1.35% (95% CI, 0%-2.00%) in the apixaban cohort, 0.90% (95% CI, 0%-1.73%) in the dabigatran cohort, and 1.85% (95% CI, 0%-2.65%) in the rivaroxaban cohort. The rate of arterial thromboembolism was 0.16% (95% CI, 0%-0.48%) in the apixaban cohort, 0.60% (95% CI, 0%-1.33%) in the dabigatran cohort, and 0.37% (95% CI, 0%-0.82%) in the rivaroxaban cohort. In patients with a high–bleeding-risk procedure, the rates of major bleeding were 2.96% (95% CI, 0%-4.68%) in the apixaban cohort and 2.95% (95% CI, 0%-4.76%) in the rivaroxaban cohort.
Conclusions and Relevance
In this study, patients with AF who had DOAC therapy interruption for elective surgery or procedure, a perioperative management strategy without heparin bridging or coagulation function testing was associated with low rates of major bleeding and arterial thromboembolism.
This cohort study examines the risk of bleeding and stroke associated with surgical procedures and the value of perioperative management among patients with atrial fibrillation who use anticoagulants.
Introduction
The perioperative management of patients who receive a direct oral anticoagulant (DOAC) for atrial fibrillation (AF) and require elective surgery or procedure is a common clinical scenario for which best practices are uncertain.1 Each year, 1 in 6 patients with AF, or an estimated 6 million patients worldwide, will require perioperative anticoagulant management.2,3 When DOAC regimens became available for clinical use in AF, starting in 2010, no studies had been conducted to inform the timing of perioperative DOAC therapy interruption and resumption, whether heparin bridging should be given, and whether preoperative coagulation function testing was needed.4 Uncertainty about the perioperative management of DOACs may be associated with unsubstantiated practices and increased harm to patients. Thus, a DOAC therapy interruption interval that is too long may increase the risk for thromboembolism, whereas an interruption interval that is too short may increase the risk for bleeding which, in turn, delays anticoagulant resumption.5 Perioperative heparin bridging has been used in DOAC-treated patients,6 but this practice does not make pharmacologic sense given the short, 8- to 14-hour DOAC elimination half-lives,4 its association with increased bleeding, and its questionable efficacy.6,7 Preoperative coagulation testing has been suggested to identify patients with an excessive residual anticoagulant level in whom a procedure can be delayed or the DOAC reversed,8 but this suggestion is problematic because DOAC-specific coagulation tests are not widely available, reference ranges are lacking, and such testing may not be advantageous for patients.9
Most clinical studies investigating perioperative DOAC regimen management are retrospective subanalyses of randomized clinical trials that assessed DOAC regimens for stroke prevention in AF or are patient registries.3,10,11,12,13 One study that assessed standardized perioperative management included only patients who were taking dabigatran etexilate.14 The perioperative management of DOAC regimens varies widely in clinical practice,15 and practice guidelines provide weak and inconsistent management recommendations.16,17,18,19,20
We designed the Perioperative Anticoagulation Use for Surgery Evaluation (PAUSE) study to assess the safety of a standardized perioperative management strategy for a DOAC regimen. We hypothesized that a simple management approach, which is based on DOAC-specific interruption and resumption intervals, forgoes perioperative heparin bridging, and does not require preoperative coagulation function testing, is safe to use for patient care. For each DOAC cohort that received DOAC-specific perioperative management, we defined safety as excluding 30-day perioperative rates of major bleeding of 2% and arterial thromboembolism of 1.5%, according to expected outcome rates (1% for major bleeding and 0.5% for arterial thromboembolism) observed with optimal perioperative management of warfarin sodium3,21 and with a proof-of-concept prospective study of standardized perioperative dabigatran management.14 We also postulated that this management would yield a high proportion of patients (>90%) with an undetectable or minimal residual anticoagulant level at the time of the procedure.
Methods
Study Design and Oversight
The PAUSE study design and data analysis plan were developed by the steering committee and are described elsewhere.22 The study was managed by the McMaster Centre for Transfusion Research, which was responsible for the study organization as well as data collection, validation, maintenance, and analysis. Study data were collected and managed using REDCap electronic data capture tools.23 The institutional review board of each of the 23 participating clinical center in Canada, the United States, and Europe approved PAUSE, and all study participants provided written informed consent.
PAUSE is a prospective management study involving DOAC-treated patients with AF who required anticoagulant therapy interruption for elective surgery or procedure. Patients were separated into 3 cohorts on the basis of DOAC used (apixaban, dabigatran, or rivaroxaban) and received standardized perioperative management according to the DOAC. A randomized clinical trial design was considered to assess the proposed (experimental) management but was not adopted because no alternative strategy existed that would be suitable as a comparator (control) management. For example, a management approach that omits DOAC regimens for a longer (4- to 6-day) preoperative period, as suggested in other studies,16 would not make clinical sense as a comparator given the short DOAC elimination half-lives; moreover, the longer period without anticoagulation might expose patients to an increased thromboembolic risk. Similarly, adopting unspecified usual care as a comparator would also be unsuitable, as the usual care may be too heterogeneous to allow a meaningful comparison to the standardized, more uniform management used in this study.15 A cohort design is appropriate for assessing a management strategy when expected rates of clinical outcomes are low (0.5%-1% in PAUSE) and when there is sufficient statistical power to exclude clinically important higher outcome rates (1.5%-2% in PAUSE).23,24
Patients
Consecutive patients with the following characteristics were assessed for study eligibility: adults (aged ≥18 years) with AF who were long-term users of apixaban (5 mg or 2.5 mg twice daily), dabigatran etexilate (150 mg or 110 mg twice daily), or rivaroxaban (20 mg or 15 mg daily); were scheduled to have an elective surgery or procedure that required interruption of the anticoagulant regimen; and were able to adhere to the DOAC therapy interruption protocol at the time of enrollment. Patients were excluded if they fit 1 or more of the following criteria: creatinine clearance (CrCl) level less than 25 ml/min for apixaban users or CrCl level less than 30 ml/min for dabigatran or rivaroxaban users (to convert CrCl level to milliliters per second per meter squared, multiply by 0.0167),25 cognitive impairment or psychiatric illness, did not consent to participate, previous study participation, or more than 1 procedure planned within 30 days. Before the procedure, patients were categorized as having a high- or low–bleeding-risk procedure according to a prespecified classification (eAppendix 1 in the Supplement)7; this classification informed the timing of DOAC therapy interruption and resumption.22 Our aim was that at least one-third of patients enrolled into each DOAC cohort would be classified as high bleeding risk.
Procedures
The perioperative management strategy for a DOAC regimen was designed with 2 broad aims: (1) to have the shortest duration of DOAC therapy interruption before and after the procedure so as to minimize the risks for bleeding and thromboembolism, and (2) to have a simple interruption and resumption protocol for each DOAC that would be easy to use by clinicians and easily understood by patients.
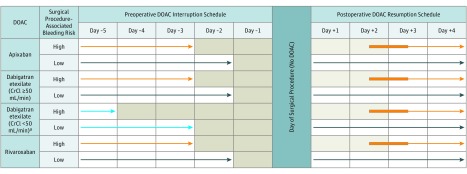
Patients were enrolled and managed using a standardized perioperative DOAC strategy based on DOAC pharmacokinetic properties (10- to 14-hour half-lives, and 1- to 3-hour peak action), the procedure–associated bleeding risk, and patient CrCl level (Figure).22 Before the procedure, DOAC regimens were omitted for 1 day before a low–bleeding-risk procedure (36- to 42-hour interval corresponding to approximately 3 DOAC half-lives) and were omitted 2 days before a high–bleeding-risk procedure (60- to 68-hour interval corresponding to approximately 5 DOAC half-lives). Patients using dabigatran with a CrCl level less than 50 mL/min had longer interruption intervals to account for renal dependence of dabigatran clearance.1 Blood samples were taken from patients just before the procedure to measure their residual anticoagulant level, but these results were not available for clinical use. Plasma samples were frozen and stored at each clinical site and later analyzed in a centralized laboratory using standardized blood processing and assay methods (eAppendix 2 in the Supplement). After the operation, DOAC regimens were resumed 1 day (approximately 24 hours) after a low–bleeding-risk procedure and 2 to 3 days (48-72 hours) after a high–bleeding-risk procedure, provided that hemostasis was achieved. Patient thromboembolic risk, based on the CHADS2 (congestive heart failure, hypertension, aged 75 years or older, diabetes, and previous stroke or transient ischemic attack) risk score, did not affect perioperative DOAC regimen management because this risk score is used in a perioperative setting to assess the need for heparin bridging, which was not performed in the present study.26,27 Patients at high risk for venous thromboembolism could receive a prophylactic dose of heparin after the operation until DOAC therapy resumption.
Figure. Perioperative Direct Oral Anticoagulant (DOAC) Management Protocol.
No DOAC was taken on certain days (shaded) and on the day of the elective surgery or procedure. The light blue arrows refer to an exception to the basic management, a subgroup of patients taking dabigatran with a creatinine clearance (CrCl) less than 50 ng/mL. The orange arrows refer to patients having a high–bleed-risk surgical procedure. Dark blue arrows refer to patients having a low–bleed-risk surgical procedure. The thickened orange part of arrows refer to flexibility in the timing of DOAC resumption after a procedure.
aCancer diagnosed within 3 months or has been treated within 6 months or metastatic.
Clinical Outcomes and Residual Anticoagulant Level
Study clinical outcomes were assessed from the time the first DOAC dose was interrupted until 30 days after the operation. Patients had scheduled weekly telephone follow-up and additional clinic visits as needed to document clinical outcomes. The primary clinical outcomes were major bleeding and arterial thromboembolism (ischemic stroke, transient ischemic attack, and systemic embolism). The secondary clinical outcomes were clinically relevant nonmajor bleeding, minor bleeding, death, myocardial infarction, deep vein thrombosis, pulmonary embolism, and catheter-associated venous or arterial thrombosis. Study outcomes were defined according to standardized criteria (eAppendix 3 in the Supplement)28,29 and were independently adjudicated by a committee that was blinded to the DOAC cohort, procedure bleeding risk, and preoperative DOAC treatment levels.
The residual anticoagulant level just before the procedure was measured by DOAC-specific anti–factor Xa assays for apixaban and rivaroxaban as well as by the dilute thrombin time for dabigatran.30 The residual anticoagulant level was also measured with nonspecific coagulation tests: prothrombin time, international normalized ratio, activated partial thromboplastin time, and thrombin time.31
Study Hypothesis and Sample Size Determination
We hypothesized that, for each DOAC cohort, the PAUSE management would be associated with a 1% rate of major bleeding (with the upper limit of the 1-sided 95% CI to exclude a 2% rate) and a 0.5% rate of arterial thromboembolism (with the upper limit of the 1-sided 95% CI to exclude a 1.5% rate). Thus, the null hypothesis was that the proposed protocol was unsafe; that is, the proportion of the major bleeding (or arterial thromboembolism) was 2% or higher (or ≥1.5%); an alternative hypothesis was that the protocol was safe; that is, the proportion was lower than 2% (or <1.5%). A 1-sided P < .05 was considered statistically significant, and a statistically significant result would mean that, with the 1-sided 95% CI, the true incidence of major bleeding was lower than 2% and arterial thromboembolism was lower than 1.5% for each DOAC cohort, rejecting the null hypothesis.
When PAUSE was designed in 2013, we were more confident about estimates, based on findings from available studies,3,21,32 of perioperative rates of major bleeding than arterial thromboembolism. Therefore, major bleeding was the primary determinant of sample size, and the sample size calculation was based on an expected rate of 1%. The required sample size was 987 patients per DOAC cohort, which provided 80% power at the .05 significance level (1-sided) to detect a proportion that was lower than 2% for major bleeding. With this sample size, there was also 80% power at the 5% significance level (1-sided) to detect a proportion that was lower than 1.5% for arterial thromboembolism, based on an expected rate of 0.5%.
The number of patients per DOAC cohort was increased by 10% (to 1097) to anticipate cancelled operations and patients lost to follow-up. We also postulated that the DOAC therapy interruption protocol would yield more than 90% of patients with a preoperative residual anticoagulant level less than 50 ng/mL, which was considered empirically as a level that would allow a procedure to proceed safely.
Statistical Analysis
For the primary clinical outcomes, a 1-sided test for 1 proportion with continuity correction was used to determine within each DOAC cohort at the patient level if the proportion of major bleeding was lower than 2% and if the proportion of arterial thromboembolism was lower than 1.5%.33 The primary analysis was conducted in the main study population of patients who had at least 1 DOAC dose interrupted. For each primary outcome within each DOAC cohort, we reported the proportion and associated 1-sided 95% CI as well as the P value from the 1-sided test for 1 proportion to check that the outcome rate was lower than the expected rate of 2% for major bleeding and 1.5% for arterial thromboembolism. For the secondary clinical outcomes, we assessed rates of mortality and other adverse events for patients within each DOAC cohort. We reported the proportions with 2-sided 95% CIs of the secondary outcomes for each cohort.
For the preoperative residual anticoagulant level outcome, we identified the proportion of patients with an anti–factor Xa level (for apixaban or rivaroxaban) or dilute thrombin time (for dabigatran) of less than 50 ng/mL (30-49.9 ng/mL and <30 ng/mL) or 50 ng/mL or greater; this calculation was done separately for patients with a low–bleeding risk and patients with a high–bleeding-risk procedure because the bleeding risk determined the DOAC therapy interruption interval, which would affect the residual anticoagulant level. We also identified the median (interquartile range [IQR]) prothrombin time, international normalized ratio, activated partial thromboplastin time, and thrombin time as well as the proportion of patients with an elevated prothrombin time, international normalized ratio, activated partial thromboplastin time, and thrombin time.
Because the analyses of secondary clinical outcomes and coagulation test outcomes were descriptive, no statistical hypothesis testing was considered. In this analysis, we assessed rates of major bleeding in patients according to procedure-associated bleeding risk as perioperative management differed between patients considered at high and low risk for bleeding. We also assessed rates of primary outcomes in a population of patients within each cohort who adhered to the DOAC therapy interruption and resumption protocols.
Results
Patients
We screened 3640 patients from August 1, 2014, through July 31, 2018, from 23 clinical sites in Canada, the United States, and Europe (eAppendix 4 in the Supplement). Of these patients, 3007 (82.6%) were enrolled and were included in the primary analysis: 1257 (41.8%) in the apixaban cohort, 668 (22.2%) in the dabigatran cohort, and 1082 (36.0%) in the rivaroxaban cohort (eFigure in the Supplement). The baseline characteristics of the patients in each DOAC cohort are shown in Table 1. Overall, patients had a mean (SD) age of 72.5 (9.39) years and were predominantly male (1988 [66.1%]). The types of procedures that patients underwent in each DOAC cohort are shown in eAppendix 5 in the Supplement.
Table 1. Baseline Patients Characteristics .
| Variable | No. (%) | ||
|---|---|---|---|
| Apixaban Cohort (n = 1257) | Dabigatran Cohort (n = 668) | Rivaroxaban Cohort (n = 1082) | |
| Age, mean (SD), y | 73.1 (9.15) | 72.4 (9.9) | 72.0 (9.3) |
| Male | 805 (64.0) | 458 (68.6) | 725 (67.0) |
| BMI, mean (SD) | 29.49 (6.2) | 30.24 (6.8) | 29.8 (6.5) |
| Race/ethnicity | |||
| White | 1204 (95.8) | 654 (97.9) | 1045 (96.6) |
| Non-white | 43 (3.4) | 12 (1.8) | 25 (2.3) |
| Unknown | 10 (0.8) | 2 (0.3) | 12 (1.1) |
| Risk stratification scores, mean (SD) | |||
| CHADS2a | 2.1 (1.3) | 2.2 (1.3) | 2.0 (1.3) |
| CHADS2–VA2Scb | 3.5 (1.7) | 3.5 (1.6) | 3.3 (1.6) |
| Modified HAS-BLEDc | 2.0 (0.9) | 1.9 (0.9) | 1.8 (0.9) |
| Medical condition | |||
| Congestive heart failure | 243 (19.3) | 111 (16.6) | 140 (12.9) |
| Hypertension | 933 (74.2) | 504 (75.4) | 784 (72.5) |
| Diabetes | 337 (26.8) | 185 (27.7) | 273 (25.2) |
| Stroke | 98 (7.8) | 64 (9.6) | 77 (7.1) |
| Transient ischemic attack | 117 (9.3) | 93 (13.9) | 99 (9.1) |
| Coronary artery disease | 232 (18.5) | 113 (16.9) | 177 (16.4) |
| Peripheral arterial disease | 8 (0.6) | 6 (0.9) | 13 (1.2) |
| Bioprosthetic heart valve | 35 (2.8) | 10 (1.5) | 20 (1.8) |
| Mitral valve disease | 125 (9.9) | 51 (7.6) | 86 (7.9) |
| Venous thromboembolism | 77 (6.1) | 40 (6.0) | 85 (7.9) |
| Active cancerd | 105 (8.3) | 57 (8.5) | 107 (9.9) |
| Laboratory values, mean (SD) | |||
| Hemoglobin, g/L | 134.4 (17.8) | 140.1 (50.0) | 136.8 (31.6) |
| Platelets <100 × 106/L | 8 (0.6) | 2 (0.3) | 3 (0.3) |
| Serum creatinine, μmol/L | 94.1 (28.8) | 87.7 (21.6) | 90.3 (22.5) |
| Creatinine clearance, ml/mine | 77.9 (32.0) | 85.9 (35.7) | 82.2 (32.8) |
| Medication use | |||
| Lower-dose DOAC regimenf | 252 (20.0) | 248 (37.1) | 181 (16.7) |
| Aspirin | 156 (12.4) | 98 (14.7) | 99 (9.1) |
| P2Y12 inhibitorg | 12 (0.9) | 7 (1.0) | 11 (1.0) |
| P-glycoprotein or cytochrome P450 3A4 inhibitor or inducerh | 76 (6.0) | 53 (7.9) | 55 (5.1) |
| Elective surgery or procedure type | |||
| High bleeding risk | 406 (32.3) | 228 (34.1) | 373 (34.5) |
| Low bleeding risk | 851 (67.7) | 440 (65.9) | 709 (65.5) |
| Anesthesia type | |||
| General | 410 (32.6) | 193 (28.9) | 384 (35.5) |
| Neuraxial | 103 (8.2) | 57 (8.5) | 70 (6.5) |
| Other | 689 (54.8) | 369 (55.2) | 584 (54.0) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DOAC, direct oral anticoagulant.
SI conversion factor: To convert creatinine clearance to milliliters per second per square meter, multiply by 0.0167.
CHADS2 risk score range: 1-6; risks include congestive heart failure, hypertension, age 75 years or older, diabetes, and previous stroke or transient ischemic attack.
CHADS2–VA2Sc risk score range: 1-9; risks include congestive heart failure, hypertension, age 75 years or older or 65 years or older, diabetes, previous stroke or transient ischemic attack, female sex, and vascular disease.
HAS-BLED bleeding risk score range: 1-7; risks include hypertension, abnormal renal or liver function, previous stroke, previous bleed or bleed predisposition, labile international normalized ratio (omitted), age 65 years or older, and drug use that affects hemostasis or alcohol use (omitted).
Cancer diagnosed within 3 months or treated within 6 months or metastatic.
Based on Cockroft-Gault formula.
Apixaban 2.5 mg twice daily, or dabigatran etexilate 110 mg twice daily, or rivaroxaban 15 mg daily.
Clopidogrel bisulfate, ticagrelor, prasugrel hydrochloride, or ticlopidine hydrochloride.
Drugs that can inhibit or induce DOAC activity (eAppendix 9 in the Supplement).
Perioperative Anticoagulant Management
Table 2 shows the DOAC therapy interruption intervals for the apixaban, dabigatran (≥50 mL/min and <50 mL/min subgroups), and rivaroxaban cohorts as well as the DOAC therapy resumption intervals for the apixaban, dabigatran, and rivaroxaban cohorts. Of the 3007 patients in the primary analysis cohort (≥1 dose interrupted), 159 (5.3%) deviated from the DOAC therapy interruption protocol, 202 (6.7%) deviated from the DOAC therapy resumption protocol, and 22 (0.7%) were lost to follow-up, leaving 2624 patients (87.3%) to be included in the per protocol analysis.
Table 2. Preoperative and Postoperative Direct Oral Anticoagulant Interruption and Resumption.
| Cohort | Preoperative Management | Postoperative Management | ||||||
|---|---|---|---|---|---|---|---|---|
| DOAC Preoperative Omission, No. (IQR), d | Interruption Interval (IQR), h | Patient Adherence to Interruption Protocol, No. (%) | DOAC Postoperative Resumption, No. (IQR), d | Resumption Interval (IQR), h | Patient Adherence to Resumption Protocol, No. (%) | Patient Receipt of Prophylactic-Dose LMWH, No. (%) | ||
| Apixaban | ||||||||
| Low bleeding risk (n = 851) | 1 (1-1) | 39.3 (37.4-41.5) | 819 (96.24) | 1 (1-1) | 22.2 (19.3-31.9) | 745 (87.5) | 16 (1.9) | |
| High bleeding risk (n = 406) | 2 (2-2) | 63.8 (61-67 | 378 (93.1) | 3 (2-4) | 67.8 (45.1-91.4) | 399 (98.3) | 133 (32.8) | |
| Dabigatran etexilate, CrCl ≥50 mL/min | ||||||||
| Low bleeding risk (n = 386) | 1 (1-1) | 39.7 (38-41.9) | 368 (95.34) | NA | NA | NA | NA | |
| High bleeding risk (n = 202) | 2 (2-2) | 63.2 (61.5-67.2) | 187 (92.57) | NA | NA | NA | NA | |
| Dabigatran, CrCl <50 mL/min | ||||||||
| Low bleeding risk (n = 54) | 2 (2-2) | 64.4 (62-66) | 50 (92.59) | NA | NA | NA | NA | |
| High bleeding risk (n = 26) | 4 (4-4) | 110.2 (108.3-112.7) | 22 (84.62) | NA | NA | NA | NA | |
| Dabigatran (all patients)a | ||||||||
| Low bleeding risk (n = 440) | NA | NA | NA | 1 (1-1) | 23 (20.5-33.6) | 425 (96.6) | 7 (1.6) | |
| High bleeding risk (n = 228) | NA | NA | NA | 3 (2-3) | 66.4 (45.1-81.4) | 227 (99.6) | 85 (37.3) | |
| Rivaroxiban | ||||||||
| Low bleeding risk (n = 709) | 1 (1-1) | 48 (40.7-51) | 674 (95.06) | 1 (1-1) | 25 (20.8-33.5) | 641 (90.41) | 8 (1.13) | |
| High bleeding risk (n = 373) | 2 (2-2) | 72 (65.6-75) | 350 (93.83) | 3 (2-4) | 69.4 (46.4-94) | 370 (99.2) | 131 (35.1) | |
Abbreviations: CrCl, creatinine clearance; DOAC, direct oral anticoagulant; IQR, interquartile range; LMWH, low-molecular-weight heparin; NA, not applicable.
SI conversion factor: To convert creatinine clearance to milliliters per second per square meter, multiply by 0.0167.
Postoperative resumption of dabigatran was the same for patients with a CrCl 50 milliliters per minute or more and less than 50 milliliters per minute.
Study Outcomes
In the primary analysis cohort (Table 3), the 30-day postoperative rate of major bleeding was 1.35% (95% CI, 0%-2.00%) in the apixaban cohort, 0.90% (95% CI, 0%-1.73%) in the dabigatran cohort, and 1.85% (95% CI, 0%-2.65%) in the rivaroxaban cohort. The rate of arterial thromboembolism was 0.16% (95% CI, 0%-0.48%) in the apixaban cohort, 0.60% (95% CI, 0%-1.33%) in the dabigatran cohort, and 0.37% (95% CI, 0%-0.82%) in the rivaroxaban cohort. All 43 major bleeding events occurred postoperatively at a median (IQR) of 2 (0-6) days; 9 of 10 arterial thromboembolic events occurred postoperatively at a median (IQR) of 2 (0-6) days. Rates of major bleeding according to procedure-associated bleeding risk are shown in Table 4; in the high–bleed-risk subgroups, the rate of major bleeding was 2.96% (95% CI, 0%-4.68%) in the apixaban cohort, 0.88 (95% CI, 0%-2.62%) in the dabigatran cohort, and 2.95% (95% CI, 0%-4.76%) in the rivaroxaban cohort.
Table 3. Primary Study Outcomes.
| Outcome | DOAC Cohort | ||
|---|---|---|---|
| Apixaban (n = 1257) | Dabigatran Etexilate (n = 668) | Rivaroxaban (n = 1082) | |
| Primary | |||
| Major bleedinga | |||
| No. (%) | 17 (1.35) | 6 (0.90) | 20 (1.85) |
| 1-Sided 95% CI | 0-2.00 | 0-1.73 | 0-2.65 |
| P value | .051 | .02 | .36 |
| Arterial thromboembolismb,c | |||
| No. (%) | 2 (0.16) | 4 (0.60) | 4 (0.37) |
| 1-Sided 95% CI | 0-0.48 | 0-1.33 | 0-0.82 |
| P value | <.001 | .03 | .001 |
| Secondary | |||
| Death | |||
| No. (%) | 3 (0.24) | 3 (0.45) | 3 (0.28) |
| 2-Sided 95% CI | 0.08-0.70 | 0.15-1.31 | 0.09-0.81 |
| Myocardial infarction | |||
| No. (%) | 1 (0.08) | 0 (0) | 0 (0) |
| 2-Sided 95% CI | 0.01-0.45 | 0-0.57 | 0-0.35 |
| Deep vein thrombosis | |||
| No. (%) | 2 (0.16) | 1 (0.15) | 0 (0) |
| 2-Sided 95% CI | 0.04-0.58 | 0.03-0.84 | 0-0.35 |
| Pulmonary embolism | |||
| No. (%) | 4 (0.32) | 1 (0.15) | 1 (0.09) |
| 2-Sided 95% CI | 0.12-0.82 | 0.03-0.84 | 0.02-0.52 |
| Arterial catheter thrombosisd | |||
| No. (%) | 1 (0.08) | 1 (0.15) | 0 (0) |
| 2-Sided 95% CI | 0.01-0.45 | 0.03-0.84 | 0-0.35 |
| Clinically relevant nonmajor bleeding | |||
| No. (%) | 21 (1.67) | 13 (1.95) | 26 (2.4) |
| 2-Sided 95% CI | 1.10-2.54 | 1.14-3.30 | 1.65-3.50 |
| Minor bleeding | |||
| No. (%) | 54 (4.3) | 38 (5.69) | 62 (5.73) |
| 2-Sided 95% CI | 3.31-5.56 | 4.17-7.71 | 4.5-7.28 |
Abbreviation: DOAC, direct oral anticoagulant.
P value of the 1-sided test for 1 proportion to check that the proportion of major bleeding per DOAC was less than 2%.
P value of the 1-sided test for 1 proportion to check that the proportion of arterial thromboembolism per DOAC was less than 1.5%.
All episodes of arterial thromboembolism were ischemic stroke.
No episodes of catheter-related venous thrombosis were reported.
Table 4. Incidence of Major Bleeding by Elective Surgery or Procedure–Associated Bleeding Risk.
| Procedure-Associated Bleeding Risk | Apixaban Cohort (n = 1257) | Dabigatran Etexilate Cohort (n = 668) | Rivaroxaban Cohort (n = 1082) |
|---|---|---|---|
| Low bleeding risk | |||
| No. (%) | 851 (67.7) | 440 (65.9) | 709 (65.5) |
| 30-d Postoperative rate of major bleeding, % (95% CI) | 0.59 (0-1.20) | 0.91 (0-2.01) | 1.27 (0-2.17) |
| High bleeding risk | |||
| No. (%) | 406 (32.3) | 228 (34.1) | 373 (34.5) |
| 30-d Postoperative rate of major bleeding, % (95% CI) | 2.96 (0-4.68 | 0.88 (0-2.62) | 2.95 (0-4.76) |
In the secondary analysis of patients who adhered to the DOAC therapy interruption and resumption protocols, the 30-day postoperative rate of major bleeding was 1.2% (95% CI, 0%-1.89%) in the apixaban cohort, 1.0% (95% CI, 0%-1.93%) in the dabigatran cohort, and 1.69% (95% CI, 0%-2.53%) in the rivaroxaban cohort. The rate of arterial thromboembolism was 0.19% (95% CI, 0%-0.56%) in the apixaban cohort, 0.50% (95% CI, 0%-1.25%) in the dabigatran cohort, and 0.42% (95% CI, 0%-0.94%) in the rivaroxaban cohort (eAppendix 6 in the Supplement). Results according to clinical site are shown in eAppendix 7 in the Supplement.
Preoperative DOAC treatment levels were measured for 2541 patients (84.5%) (eAppendix 10 in the Supplement). The proportion of patients with a level less than 50 ng/mL was 90.5% in the apixaban cohort, 95.1% in the dabigatran cohort, and 96.8% in the rivaroxaban cohort. Among 1007 patients who had a high–bleeding-risk procedure, 832 (82.6%) had anticoagulant measurements, of whom the proportion with a residual anticoagulant level less than 50 ng/mL was 98.8%. The proportion of patients with a residual anticoagulant level of 30 to 49.9 ng/mL in the high–bleeding-risk procedure group was 4.8% in the apixaban cohort, 0.55% in the dabigatran cohort, and 14.0% in the rivaroxaban cohort. Results for the nonspecific coagulation tests are shown in eAppendix 8 in the Supplement.
Discussion
We found that in patients with AF who were receiving a DOAC (apixaban, dabigatran, or rivaroxaban) and required interruption of the anticoagulant regimen for elective surgery or procedure, a simple standardized perioperative management strategy that did not require the use of heparin bridging or preoperative coagulation function testing was associated with low rates of perioperative major bleeding (<2%) and arterial thromboembolism (<1%). Furthermore, a high proportion of patients (>90% overall; 98.8% of those at high bleeding risk) had a minimal or no residual anticoagulant level at the time of the procedure.
Based on the primary analysis cohort, our hypothesis that the PAUSE perioperative management strategy would exclude a 2% rate of major bleeding was supported in the dabigatran cohort (0.90%; 95% CI, 0%-1.73%) but not in the apixaban cohort (1.35%; 95% CI, 0%-2.0%) or rivaroxaban cohort (1.85%; 95% CI, 0%-2.65%), whereas our hypothesis that this management strategy would exclude a 1.5% rate of arterial thromboembolism was supported in all 3 cohorts. In the per protocol analysis, excluding a 2% rate of major bleeding was supported in the dabigatran cohort (1.0%; 95% CI, 0%-1.93%) and the apixaban cohort (1.2%; 95% CI, 0%-1.89%) but not in the rivaroxaban cohort (1.69%; 95% CI, 0%-2.53%); excluding a rate of arterial thromboembolism of 1.5% was supported in all 3 cohorts.
Our exploratory postulation that a high proportion of patients (>90%) would have a residual anticoagulant level less than 50 ng/mL at the time of the operation was supported in all 3 DOAC cohorts. In addition, we found that, among patients with a high–bleeding-risk procedure (which included any patient with neuraxial anesthesia) in whom there was concern of bleeding complications associated with an excessive residual anticoagulant level,16,18,19 almost all patients (98.8%) had a residual anticoagulant level less than 50 ng/mL. Moreover, the proportion of such patients with a residual anticoagulant level less than 30 mg/mL, which some experts consider an optimal preoperative anticoagulant level,13 was high in the apixaban cohort (93.1%) and dabigatran cohort (98.9%). Among patients in the rivaroxaban cohort with a high–bleeding-risk procedure, a lower proportion (85.4%) had a residual anticoagulant level less than 30 ng/mL, an observation that requires further study. When the findings were assessed according to procedure-associated high– and low–bleeding risk, rates of major bleeding appeared to be higher among patients with a high–bleeding-risk procedure in the apixaban and rivaroxaban cohorts. This finding may reflect an intrinsically higher rate of bleeding expected with major procedures. Further study is needed to assess the PAUSE perioperative DOAC regimen management in patients with high–bleeding-risk procedures.
Most other studies that assessed perioperative DOAC regimen management are not comparable to this study because their management was not standardized, perioperative heparin bridging was allowed, and fewer patients (10%-20%) with high bleeding risk were studied.3,10,11,12,13,14 In these studies, perioperative rates of major bleeding, for example, varied and were as high as 6%.3 Two pertinent studies that assessed standardized perioperative anticoagulant management without heparin bridging had similar adverse outcome rates to those in this study. In a cohort study of 541 patients receiving dabigatran who had standardized perioperative interruption and resumption of their treatment, the 30-day postoperative rate of major bleeding was 1.8% and the arterial thromboembolism rate was 0.2%.14 In the BRIDGE trial, which evaluated a bridging strategy in patients with AF who had perioperative warfarin treatment interruption, patients who were not bridged had a 30-day postoperative rate of major bleeding of 1.3% and arterial thromboembolism rate of 0.4%,7 and those who underwent a high–bleeding-risk procedure had a rate of major bleeding of 3.2%.34
Limitations and Strengths
This study has limitations. First, although a cohort study design may introduce patient selection bias, this was unlikely because a high proportion (83%) of screened patients participated in this study, and their risk factor profile, as measured by the CHA2DS2VASc risk score, was comparable to that of patients with AF included in population-based studies.35 Second, although few patients (n = 230) received neuraxial anesthesia, in whom there was a concern about bleeding risk during the operation associated with an excessive residual anticoagulant level, the management of such patients was the same as all patients undergoing a high–bleeding-risk procedure (n = 1007). Accordingly, the high proportion (98.8%) of patients with minimal to no residual anticoagulant level in this group would be applicable to those with neuraxial anesthesia. Third, the dabigatran cohort did not reach the expected sample size, owing to the decrease in dabigatran use compared with other DOAC regimens during the study, but the number of patients accrued was sufficient to address the study hypotheses in this cohort. Fourth, patients using edoxaban tosylate were not included as the drug was not available for clinical use when the PAUSE study started, and the results are not generalizable to this DOAC. Fifth, the 50 ng/mL cut point used in this study to define a clinically important residual preoperative DOAC level was not established, and further study is needed to assess a correlation between preoperative DOAC treatment levels and bleeding. Sixth, most patients included were white, and additional studies are needed in nonwhite populations. Seventh, patients with venous thromboembolism, who represent a different study population,36 were not included.
A strength of this study is the generalizability of the results to patients assessed in clinical practice, as a high proportion of screened patients were enrolled (83%) and few were lost to follow-up (<1%). Another strength is the clinical applicability of the DOAC regimen management we assessed, as most patients adhered to the perioperative DOAC therapy interruption (95%) and resumption (93%) management protocol. The simple strategy of omitting DOAC regimens for 1 day before and after a low–bleeding-risk procedure and 2 days before and after a high–bleeding-risk procedure (except for patients using dabigatran with a CrCl <50 mL/min) is, therefore, likely to be easily adoptable in clinical practice.
Conclusions
In this study, patients with AF who had DOAC therapy interruption for elective surgery or procedure, a simple standardized perioperative management strategy without heparin bridging or measurement of coagulation function was associated with low rates of major bleeding and arterial thromboembolism.
eFigure. Screening of Patients, Enrollment into DOAC Cohort, and Study Completion
eAppendix 1. Classification of Surgery/Procedure as High or Low Bleeding Risk
eAppendix 2. Blood Processing Methods and Coagulation Assays Used
eAppendix 3. Clinical Outcome Definitions
eAppendix 4. List of Clinical Sites, Site Investigators, and Patients Recruited per Site
eAppendix 5. Types of Surgery/Procedures Patients Underwent
eAppendix 6. Study Outcomes in Patients Adhering to DOAC Interruption and Resumption Protocols
eAppendix 7. Primary Outcome Rates According to Clinical Site
eAppendix 8. Anticoagulant Level at Time of Surgery/Procedure based on Non-specific Coagulation Tests
eAppendix 9. Drugs That Can Inhibit or Induce DOAC Activity
eAppendix 10. Anticoagulant Level at Time of Surgical Procedure Based on Direct Oral Anticoagulant–Specific Coagulation Tests
References
- 1.Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885. doi: 10.1111/jth.13305 [DOI] [PubMed] [Google Scholar]
- 2.Zulkifly H, Lip GYH, Lane DA. Epidemiology of atrial fibrillation. Int J Clin Pract. 2018;72(3):e13070. doi: 10.1111/ijcp.13070 [DOI] [PubMed] [Google Scholar]
- 3.Healey JS, Eikelboom J, Douketis J, et al. ; RE-LY Investigators . Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial[published correction appears in Circulation. 2012;126(10):e160]. Circulation. 2012;126(3):343-348. doi: 10.1161/CIRCULATIONAHA.111.090464 [DOI] [PubMed] [Google Scholar]
- 4.Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120(15):2954-2962. doi: 10.1182/blood-2012-06-415943 [DOI] [PubMed] [Google Scholar]
- 5.Dunn AS, Spyropoulos AC, Turpie AG. Bridging therapy in patients on long-term oral anticoagulants who require surgery: the Prospective Peri-operative Enoxaparin Cohort Trial (PROSPECT). J Thromb Haemost. 2007;5(11):2211-2218. doi: 10.1111/j.1538-7836.2007.02729.x [DOI] [PubMed] [Google Scholar]
- 6.Douketis JD, Healey JS, Brueckmann M, et al. Perioperative bridging anticoagulation during dabigatran or warfarin interruption among patients who had an elective surgery or procedure. Substudy of the RE-LY trial. Thromb Haemost. 2015;113(3):625-632. doi: 10.1160/TH14-04-0305 [DOI] [PubMed] [Google Scholar]
- 7.Douketis JD, Spyropoulos AC, Kaatz S, et al. ; BRIDGE Investigators . Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-833. doi: 10.1056/NEJMoa1501035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tripodi A. To measure or not to measure direct oral anticoagulants before surgery or invasive procedures: reply. J Thromb Haemost. 2016;14(12):2559-2561. doi: 10.1111/jth.13513 [DOI] [PubMed] [Google Scholar]
- 9.Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. To measure or not to measure direct oral anticoagulants before surgery or invasive procedures: comment. J Thromb Haemost. 2016;14(12):2556-2559. doi: 10.1111/jth.13505 [DOI] [PubMed] [Google Scholar]
- 10.Garcia D, Alexander JH, Wallentin L, et al. Management and clinical outcomes in patients treated with apixaban vs warfarin undergoing procedures. Blood. 2014;124(25):3692-3698. doi: 10.1182/blood-2014-08-595496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherwood MW, Douketis JD, Patel MR, et al. ; ROCKET AF Investigators . Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;129(18):1850-1859. doi: 10.1161/CIRCULATIONAHA.113.005754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyer-Westendorf J, Gelbricht V, Förster K, et al. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J. 2014;35(28):1888-1896. doi: 10.1093/eurheartj/eht557 [DOI] [PubMed] [Google Scholar]
- 13.Godier A, Dincq AS, Martin AC, et al. Predictors of pre-procedural concentrations of direct oral anticoagulants: a prospective multicentre study. Eur Heart J. 2017;38(31):2431-2439. doi: 10.1093/eurheartj/ehx403 [DOI] [PubMed] [Google Scholar]
- 14.Schulman S, Carrier M, Lee AY, et al. ; Periop Dabigatran Study Group . Perioperative management of dabigatran: a prospective cohort study. Circulation. 2015;132(3):167-173. doi: 10.1161/CIRCULATIONAHA.115.015688 [DOI] [PubMed] [Google Scholar]
- 15.Faraoni D, Samama CM, Ranucci M, Dietrich W, Levy JH. Perioperative management of patients receiving new oral anticoagulants: an international survey. Clin Lab Med. 2014;34(3):637-654. doi: 10.1016/j.cll.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 16.Narouze S, Benzon HT, Provenzano DA, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications: guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2015;40(3):182-212. doi: 10.1097/AAP.0000000000000223 [DOI] [PubMed] [Google Scholar]
- 17.Raval AN, Cigarroa JE, Chung MK, et al. ; American Heart Association Clinical Pharmacology Subcommittee of the Acute Cardiac Care and General Cardiology Committee of the Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; and Council on Quality of Care and Outcomes Research . Management of patients on non-vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting: a scientific statement from the American Heart Association. Circulation. 2017;135(10):e604-e633. doi: 10.1161/CIR.0000000000000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albaladejo P, Bonhomme F, Blais N, et al. ; French Working Group on Perioperative Hemostasis (GIHP) . Management of direct oral anticoagulants in patients undergoing elective surgeries and invasive procedures: updated guidelines from the French Working Group on Perioperative Hemostasis (GIHP) - September 2015. Anaesth Crit Care Pain Med. 2017;36(1):73-76. doi: 10.1016/j.accpm.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 19.Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional Anesthesia in the Patient Receiving Antithrombotic or Thrombolytic Therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med. 2018;43(3):263-309. doi: 10.1097/AAP.0000000000000763 [DOI] [PubMed] [Google Scholar]
- 20.Steffel J, Verhamme P, Potpara TS, et al. ; ESC Scientific Document Group . The 2018 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330-1393. doi: 10.1093/eurheartj/ehy136 [DOI] [PubMed] [Google Scholar]
- 21.Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126(13):1630-1639. doi: 10.1161/CIRCULATIONAHA.112.105221 [DOI] [PubMed] [Google Scholar]
- 22.Douketis JD, Spyropoulos AC, Anderson JM, et al. The perioperative anticoagulant use for surgery evaluation (PAUSE) study for patients on a direct oral anticoagulant who need an elective surgery or procedure: design and rationale. Thromb Haemost. 2017;117(12):2415-2424. doi: 10.1160/TH17-08-0553 [DOI] [PubMed] [Google Scholar]
- 23.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18(6):805-835. doi: 10.1097/EDE.0b013e3181577511 [DOI] [PubMed] [Google Scholar]
- 24.Euser AM, Zoccali C, Jager KJ, Dekker FW. Cohort studies: prospective versus retrospective. Nephron Clin Pract. 2009;113(3):c214-c217. doi: 10.1159/000235241 [DOI] [PubMed] [Google Scholar]
- 25.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41. doi: 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 26.Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898. doi: 10.1016/j.jacc.2016.11.024 [DOI] [PubMed] [Google Scholar]
- 27.Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. [published correction appears in Chest. 2012;141(4):1129]. Chest. 2012;141(2)(suppl):e326S-e350S. doi: 10.1378/chest.11-2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8(1):202-204. doi: 10.1111/j.1538-7836.2009.03678.x [DOI] [PubMed] [Google Scholar]
- 29.Spyropoulos AC, Douketis JD, Gerotziafas G, Kaatz S, Ortel TL, Schulman S; Subcommittee on Control of Anticoagulation of the SSC of the ISTH . Periprocedural antithrombotic and bridging therapy: recommendations for standardized reporting in patients with arterial indications for chronic oral anticoagulant therapy. J Thromb Haemost. 2012;10(4):692-694. doi: 10.1111/j.1538-7836.2012.04630.x [DOI] [PubMed] [Google Scholar]
- 30.Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest. 2017;151(1):127-138. doi: 10.1016/j.chest.2016.08.1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripodi A, Padovan L, Veena C, Scalambrino E, Testa S, Peyvandi F. How the direct oral anticoagulant apixaban affects thrombin generation parameters. Thromb Res. 2015;135(6):1186-1190. doi: 10.1016/j.thromres.2015.03.032 [DOI] [PubMed] [Google Scholar]
- 32.Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: a systematic review. Arch Intern Med. 2003;163(8):901-908. doi: 10.1001/archinte.163.8.901 [DOI] [PubMed] [Google Scholar]
- 33.Wallis S. Binomial confidence intervals and contingency tests: mathematical fundamentals and the evaluation of alternative methods. J Quant Linguist. 2013;20(3):178-208. doi: 10.1080/09296174.2013.799918 [DOI] [Google Scholar]
- 34.Clark NP, Douketis JD, Hasselblad V, Schulman S, Kindzelski AL, Ortel TL; BRIDGE Investigators . Predictors of perioperative major bleeding in patients who interrupt warfarin for an elective surgery or procedure: analysis of the BRIDGE trial. Am Heart J. 2018;195:108-114. doi: 10.1016/j.ahj.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forslund T, Komen JJ, Andersen M, et al. Improved stroke prevention in atrial fibrillation after the introduction of non-vitamin K antagonist oral anticoagulants. Stroke. 2018;49(9):2122-2128. doi: 10.1161/STROKEAHA.118.021990 [DOI] [PubMed] [Google Scholar]
- 36.Clark NP, Witt DM, Davies LE, et al. Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. JAMA Intern Med. 2015;175(7):1163-1168. doi: 10.1001/jamainternmed.2015.1843 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Screening of Patients, Enrollment into DOAC Cohort, and Study Completion
eAppendix 1. Classification of Surgery/Procedure as High or Low Bleeding Risk
eAppendix 2. Blood Processing Methods and Coagulation Assays Used
eAppendix 3. Clinical Outcome Definitions
eAppendix 4. List of Clinical Sites, Site Investigators, and Patients Recruited per Site
eAppendix 5. Types of Surgery/Procedures Patients Underwent
eAppendix 6. Study Outcomes in Patients Adhering to DOAC Interruption and Resumption Protocols
eAppendix 7. Primary Outcome Rates According to Clinical Site
eAppendix 8. Anticoagulant Level at Time of Surgery/Procedure based on Non-specific Coagulation Tests
eAppendix 9. Drugs That Can Inhibit or Induce DOAC Activity
eAppendix 10. Anticoagulant Level at Time of Surgical Procedure Based on Direct Oral Anticoagulant–Specific Coagulation Tests