Key Points
Question
What are the contributions of white matter rarefaction and cerebrovascular disease to dementia in older, deceased individuals who had played football and developed chronic traumatic encephalopathy?
Findings
In this cross-sectional study of 180 deceased individuals older than 40 years who had played football and had chronic traumatic encephalopathy, the number of years of football play (a proxy for repetitive head impacts) was associated with worse white matter rarefaction and greater dorsolateral frontal cortex neurofibrillary tangles. White matter rarefaction and neurofibrillary tangles were associated with dementia; arteriolosclerosis was not associated with the number of years of play, but it contributed to dementia.
Meaning
In chronic traumatic encephalopathy, dementia is likely a result of neuropathologic changes associated with repetitive head impacts, including white matter rarefaction and phosphorylated tau, in addition to nonhead trauma–associated pathologic changes, such as arteriolosclerosis.
This cross-sectional study investigates association of white matter rarefaction and cerebrovascular disease with dementia in deceased men older than 40 years who had played football and been found to have chronic traumatic encephalopathy.
Abstract
Importance
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease associated with repetitive head impacts, including those from US football, that presents with cognitive and neuropsychiatric disturbances that can progress to dementia. Pathways to dementia in CTE are unclear and likely involve tau and nontau pathologic conditions.
Objective
To investigate the association of white matter rarefaction and cerebrovascular disease with dementia in deceased men older than 40 years who played football and had CTE.
Design, Setting, and Participants
This cross-sectional study involves analyses of data from the ongoing Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study, which is conducted via and included brain donors from the Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank between 2008 and 2017. An original sample of 224 men who had played football and were neuropathologically diagnosed with CTE was reduced after exclusion of those younger than 40 years and those missing data.
Exposures
The number of years of football play as a proxy for repetitive head impacts.
Main Outcomes and Measures
Neuropathological assessment of white matter rarefaction and arteriolosclerosis severity (on a scale of 0-3, where 3 is severe); number of infarcts, microinfarcts, and microbleeds; and phosphorylated tau accumulation determined by CTE stage and semiquantitative rating of dorsolateral frontal cortex (DLFC) neurofibrillary tangles (NFTs) (none or mild vs moderate or severe). Informant-based retrospective clinical interviews determined dementia diagnoses via diagnostic consensus conferences.
Results
A total of 180 men were included. The mean (SD) age of the sample at death was 67.9 (12.7) years. Of 180, 120 [66.7%]) were found to have had dementia prior to death. Moderate to severe white matter rarefaction (84 of 180 [46.6%]) and arteriolosclerosis (85 of 180 [47.2%]) were common; infarcts, microinfarcts, and microbleeds were not. A simultaneous equations regression model controlling for age and race showed that more years of play was associated with more severe white matter rarefaction (β, 0.16 [95% CI, 0.02-0.29]; P = .03) and greater phosphorylated tau accumulation (DLFC NFTs: β, 0.15 [95% CI, 0.004-0.30]; P = .04; CTE stage: β, 0.27 [95% CI, 0.14-0.41]; P < .001). White matter rarefaction (β, 0.16 [95% CI, 0.02-0.29]; P = .03) and DLFC NFTs (β, 0.16 [95% CI, 0.03-0.28]; P = .01) were associated with dementia. Arteriolosclerosis and years of play were not associated, but arteriolosclerosis was independently associated with dementia (β, 0.21 [95% CI, 0.07-0.35]; P = .003).
Conclusions and Relevance
Among older men who had played football and had CTE, more years of football play were associated with more severe white matter rarefaction and greater DLFC NFT burden. White matter rarefaction, arteriolosclerosis, and DLFC NFTs were independently associated with dementia. Dementia in CTE is likely a result of neuropathologic changes, including white matter rarefaction and phosphorylated tau, associated with repetitive head impact and pathologic changes not associated with head trauma, such as arteriolosclerosis.
Introduction
Exposure to repetitive head impacts (RHI)1 is associated with chronic traumatic encephalopathy (CTE).2,3,4,5,6 This condition has been diagnosed neuropathologically in athletes who played contact sports, particularly US football players, using criteria developed by a consensus conference sponsored by National Institute of Neurological Disorders and Stroke and National Institute of Biomedical Imaging and Bioengineering.2,3,4,7 Chronic traumatic encephalopathy clinically presents with cognitive, behavioral, and mood symptoms that can progress to dementia.2,3,5,8,9,10 The contribution of phosphorylated tau pathologic changes, comorbid neurodegenerative disease, or other pathologic conditions to dementia in CTE remains unclear.11,12,13,14
The late effects of RHI might contribute to dementia in CTE via tau and nontau pathways, including cerebrovascular disease (CBVD). Cerebrovascular disease often coexists with neurodegenerative diseases, especially Alzheimer disease (AD), and contributes to cognitive decline.15,16,17,18,19,20,21,22 Arteriolosclerosis, infarcts, and microinfarcts are common with aging and cardiovascular disease (CVD), and CVD is frequent in individuals who had previously played US football.23,24 In the setting of CTE, CBVD may also encompass white matter (WM) degeneration secondary to RHI. In living men who had previously played for the National Football League, WM alterations are present on magnetic resonance imaging,25,26,27 are associated with RHI,25 and affect executive function.25 In vivo imaging studies27,28,29,30,31 in athletes who played contact sports (eg, US football players, boxers) show cerebral blood flow alterations and blood-brain barrier disruption. These brain changes may be associated with or result in chronic WM degeneration and affect cognitive outcomes.32
Neuropathological studies2,33,34,35 show that WM degeneration and CBVD are common comorbidities in CTE, but their role in the clinical manifestations of CTE is poorly understood. We investigated the association of WM pathologic changes and CBVD with dementia in older men with CTE who were deceased and had played football by examining arteriosclerosis, infarcts, microinfarcts, microbleeds, and WM rarefaction. White matter rarefaction might reflect both CBVD pathologic conditions associated with CVD36 and pathologic conditions (eg, axonal loss) that are secondary to RHI.12,13 We hypothesized that WM rarefaction would be associated with years of football (a proxy for RHI exposure), that WM rarefaction would contribute to dementia, and that other markers of CBVD pathologic changes associated with CVD (eg, arteriolosclerosis) would independently contribute to dementia.
Methods
Participants
The sample included deceased men who had been football players and were neuropathologically diagnosed with CTE, from the Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) Study and Veterans Affairs–Boston University–Concussion Legacy Foundation brain bank.8,37 The next of kin contacted the CTE Center to arrange brain donation near the time of or after the individual’s death, in some cases. Other brain donors were referred by medical examiners, recruited by the Concussion Legacy Foundation, or agreed to donation during life. To be eligible, donors needed to have a history of RHI (eg, from contact sports or military service). Brain donors were excluded for a prolonged postmortem interval or poor tissue quality. Antemortem symptomatic status was not an inclusion or exclusion criterion. Institutional review board approvals for brain donation were obtained through the Boston University Medical Campus. Approval for postmortem clinical record review, interviews with informants, and neuropathological evaluation was obtained through the Boston University Medical Campus institutional review board. The next of kin provided written informed consent.
Neuropathological Diagnoses
Neurodegenerative Diseases
Methods for pathological processing and evaluation have been published elsewhere and follow established procedures.38,39 Neuropathologists (T.D.S., B.R.H., and A.C.M.) were blinded to clinical data. Non-CTE neurodegenerative diseases were diagnosed using established neuropathological diagnostic criteria.7 The neuropathological diagnosis of CTE was made using criteria defined by the National Institutes of Neurological Disease and Stroke and National Institute of Brain Imaging and Behavior consensus panel.7 The CTE stage and severity of NFT burden in the dorsolateral frontal cortex (DLFC) served as semiquantitative scales of phosphorylated tau severity. The pathological stage of each CTE case was graded using the 4-stage classification scheme2,3,8,9,10,11,13 (eMethods 1 in the Supplement contains a description of the CTE staging scheme). Phosphorylated tau density of the dorsolateral frontal cortex (DLFC) (on a scale of 0 to 3 points; 0 indicates no neurofibrillary tangles [NFTs], while 3 indicates severe NFTs) was examined because it is an initial site of phosphorylated tau deposition in CTE that becomes severely affected with disease advancement, and phosphorylated tau severity in this region has been shown to be associated with dementia in CTE.2,3,13
White Matter Rarefaction and CBVD
The neuropathological examination included semiquantitative assessment of WM rarefaction and arteriolosclerosis, as well as quantitative assessment of infarcts, microinfarcts, and microbleeds.40 White matter rarefaction and arteriolosclerosis were assessed using slides stained with luxol fast blue and hemotoxylin-eosin. White matter rarefaction is a measure of overall WM integrity and defined by the degree of myelin loss, extent of tissue attenuation or vacuolization around small blood vessels, and density of reactive astrocytes. Arteriolosclerosis was defined as the concentric hyaline thickening of the media of arterioles. Overall ratings of WM rarefaction and arteriolosclerosis severity were made by the neuropathologists (T.D.S., B.R.H., and A.C.M.) using a scale of 0 to 3 points, with 0 indicating none and 3 indicating severe changes, and these findings were based on the evaluation of the subcortical WM of the middle frontal, inferior parietal, superior temporal, and occipital cortices, as well as deep WM of the basal ganglia. The presence or absence and number of remote (not acute) infarcts, microinfarcts, and microbleeds were evaluated in the cerebral cortex, subcortical WM, deep WM, basal ganglia, brainstem, and cerebellum.
Informant-Based Retrospective Clinical Evaluation
Clinical data were obtained through online surveys and retrospective telephone clinical interviews with informants.3,8,9,37 A neurologist and/or neuropsychologist (M.L.A., R.A.S., and J.M.) with expertise in neurodegenerative disorders conducted telephone clinical interviews with informants to obtain medical and clinical histories, including the presence, nature, and timeline of symptoms associated with cognition, behavior or mood, and daily functioning. A consensus diagnosis of dementia was adjudicated based on modified Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) criteria. eMethods 2 in the Supplement provides further details.
Statistical Analyses
The DLFC NFT burden was recoded into the categories none to mild vs moderate to severe. The CTE diagnoses were regrouped into stages I and II vs III and IV. These variables were recoded to optimize equal group sample sizes and limit ambiguity regarding the severity of CTE pathologic changes (eg, between CTE stages I and II). Arteriolosclerosis and WM rarefaction were not dichotomized because of a relatively equal distribution across scales of 0 to 3 points; the model was repeated with them as binary variables to determine potential differences in model fit. Years of US football play served as a proxy for RHI.2,9,13 The level of play (ie, youth or high school, college, semiprofessional, or professional) was not analyzed because it is highly correlated with the number of years of play (r = 0.64).
As described in eMethods 3 in the Supplement, proportional odds and logistic regression models were performed to guide selection of variables into a simultaneous equations regression model and determine model pathways among years of football play, WM rarefaction, CBVD, phosphorylated tau pathologic characteristics, and dementia. A P value of .20 or less served as the selection criterion into the model. Age and race were included as covariates. The model tested for direct (ie, from independent variable to outcome pathways) and indirect (ie, partial or complete mediation) results. The model fit was evaluated by the Akaike information criteria, standardized root mean square residual, goodness of fit index, Bentler-Bonett normed fit index, and root mean square error of approximation.41 Standardized coefficients (β’s) are presented. For model factors associated with dementia, a binary logistic regression model that controlled for age and race was used to report on their association with odds for dementia. Statistical significance was defined by P values less than .05. Data analysis was completed from February 2018 to February 2019 with SAS version 9.4 (SAS Institute Inc).
Results
Participants included 224 deceased men who had played US football; this was reduced to 180 individuals after exclusion of individuals missing data on independent variables and outcome variables (n = 14) and participants younger than 40 years at death (n = 30). Younger participants were excluded because dementia and neuropathological outcomes are associated with age. Only 2 individuals younger than 40 years had dementia; CTE, arteriolosclerosis, and WM rarefaction severity were mild or absent in participants younger than 40 years (Table 1). Spline analyses (not shown) showed that odds for dementia and severity of CTE, arteriolosclerosis, and WM rarefaction pathologic changes tended to increase starting at age 40 years.
Table 1. Dementia and Pathologic Characteristics in Those Younger Than 40 Years Compared With Those 40 Years and Older.
| Characteristic | Individuals, No. (%) | P Value | |
|---|---|---|---|
| <40 y (n = 30)a | ≥40 y (n = 180)a | ||
| Dementia | 2 (6.7) | 120 (66.7) | <.001 |
| Chronic traumatic encephalopathy stage | |||
| I/II | 30 (100.0) | 35 (19.4) | <.001 |
| III/IV | 0 | 145 (80.6) | |
| Dorsolateral frontal cortex neurofibrillary tangle density rating | |||
| None or mild | 20 (66.7) | 50 (27.8) | <.001 |
| Moderate or severe | 10 (33.3) | 130 (72.2) | |
| Arteriolosclerosisb | |||
| None | 26 (86.7) | 54 (30.0) | <.001 |
| Mild | 4 (13.3) | 41 (22.8) | |
| Moderate | 0 | 67 (37.2) | |
| Severe | 0 | 18 (10.0) | |
| White matter rarefaction, mean (SD)b | |||
| None | 16 (53.3) | 22 (12.2) | <.001 |
| Mild | 7 (23.3) | 74 (41.1) | |
| Moderate | 4 (13.3) | 58 (32.2) | |
| Severe | 3 (10.0) | 26 (14.4) | |
| Remote microinfarcts | 1 (3.3) | 49 (27.2) | .002 |
Individuals who were younger than 40 years were excluded from analyses because of low pathologic burden and minimal presence of dementia (remote infarcts and microbleeds are not shown because of low burden in the overall sample). The final sample size of 180 individuals was finalized after exclusion of individuals for missing data and those younger than 40 years. Analyses were completed after exclusion of the 14 individuals who had missing data.
Ordinal regression was conducted for arteriolosclerosis and white matter rarefaction. Fisher exact tests were used to examine differences between the 2 groups for all other variables because of small cell sizes.
Sample Characteristics
Table 2 and Table 3 present sample characteristics. When the DLFC NFT burden was recoded into 2 categories, 50 individuals were in the none-to-mild group vs 130 in the moderate-to-severe group. Thirty-five individuals had CTE at stages I and II, and 145 had CTE at stages III and IV.
Table 2. Sample Characteristics of the Deceased US Football Players.
| Characteristic | Patients, No. (%) |
|---|---|
| Total, No. | 180a |
| Demographic Factors | |
| Age at death, mean (SD), y | 67.9 (12.7) |
| African American | 34 (18.9) |
| Education level | |
| Less than high school or some high school | 1 (0.6) |
| High school or graduate equivalency diploma | 2 (1.1) |
| Some college | 34 (18.9) |
| College degree | 94 (52.2) |
| More than college | 10 (5.6) |
| Graduate degree | 39 (21.7) |
| Athletic participation time variables, mean (SD), y | |
| Football play | 14.9 (5.7) |
| Since retirement | 40.9 (12.7) |
| Age at first exposure to football | 12.1 (2.8) |
| Highest level played | |
| Youth or high school | 7 (3.9) |
| College | 45 (25.0) |
| Semiprofessional | 7 (3.9) |
| Professional | 121 (67.2) |
| Football primary position | |
| Lineman (offensive or defensive) | 83 (46.1) |
| Linebacker | 22 (12.2) |
| Defensive back or safety | 18 (10.0) |
| Running back | 27 (15.0) |
| Quarterback | 13 (7.2) |
| Special teams | 1 (0.6) |
| Other or multiple | 9 (5.0) |
| Wide receiver | 2 (1.1) |
| Unknown | 5 (2.8) |
| Any other contact sport history | 28 (15.6) |
| Military historyb | 53 (29.4) |
| Combat exposure | 7 (3.9) |
| Neuropathologic Factors | |
| Chronic traumatic encephalopathy | |
| I | 12 (6.7) |
| II | 23 (12.8) |
| III | 86 (47.8) |
| IV | 59 (32.8) |
| Dorsolateral frontal cortex neurofibrillary tangles density rating | |
| None | 5 (2.8) |
| Mild | 45 (25.0) |
| Moderate | 40 (22.2) |
| Severe | 90 (50.0) |
| Lewy body disease | |
| Brainstem predominant | 9 (5.0) |
| Limbic or neocortical | 29 (16.1) |
| Frontotemporal lobar degeneration | |
| Tau | 8 (4.4) |
| TDP-43 | 8 (4.4) |
| Motor neuron disease | 10 (5.6) |
| Prion disease | 2 (1.1) |
| Alzheimer disease neuropathologic changes | |
| Not Alzheimer disease | 90 (50.0) |
| Alzheimer disease neuropathologic change severity | |
| Low | 25 (13.9) |
| Intermediate | 41 (22.8) |
| High | 24 (13.3) |
| Consortium to Establish a Registry for Alzheimer Disease neuritic plaque score | |
| None | 101 (56.1) |
| Sparse | 50 (27.8) |
| Moderate | 17 (9.4) |
| Frequent | 12 (6.7) |
| Braak stage | |
| 0-III | 142 (80.2) |
| IV-VI | 35 (19.8) |
Abbreviation: TDP-43, transactive response DNA-binding protein 43 kDa.
Of the 180 participants, samples sizes were reduced to 179 individuals for combat history and 177 individuals for Braak stage because of missing data.
Military history refers to any history of service in the military, regardless of combat exposure.
Table 3. Cerebrovascular Disease Characteristics.
| Characteristic | Patients, No. (%) | P Valueb | ||
|---|---|---|---|---|
| Total Sample (N = 180) | CTE Only (n = 101) | CTE and Comorbidities (n = 79)a | ||
| White matter rarefaction | ||||
| None | 22 (12.2) | 16 (15.8) | 6 (7.6) | .91 |
| Mild | 74 (41.1) | 39 (38.6) | 35 (44.3) | |
| Moderate | 58 (32.2) | 32 (32.7) | 25 (31.6) | |
| Severe | 26 (14.4) | 13 (12.9) | 13 (16.5) | |
| Atherosclerosis | ||||
| None | 102 (60.0) | 58 (59.2) | 44 (58.7) | .30 |
| Mild | 34 (20.0) | 19 (20.0) | 15 (20.0) | |
| Moderate | 25 (14.7) | 11 (11.6) | 14 (18.7) | |
| Severe | 9 (5.3) | 7 (7.4) | 2 (2.7) | |
| Arteriolosclerosis | ||||
| None | 54 (30.0) | 29 (28.7) | 25 (31.6) | .04 |
| Mild | 41 (22.8) | 20 (19.8) | 21 (26.6) | |
| Moderate | 67 (37.2) | 43 (42.6) | 24 (30.4) | |
| Severe | 18 (10.0) | 9 (8.9) | 9 (11.4) | |
| Remote microinfarcts | 49 (27.2) | 29 (28.7) | 20 (25.3) | .09c |
| Cerebral cortex, No. | ||||
| 1 | 8 | 4 | 4 | |
| 2 | 10 | 5 | 5 | |
| ≥3 | 10 | 9 | 1 | |
| Subcortical and periventricular white matter, No. | ||||
| 1 | 7 | 4 | 3 | |
| 2 | 4 | 3 | 1 | |
| ≥3 | 8 | 3 | 5 | |
| Subcortical gray matter, No. | ||||
| 1 | 5 | 1 | 4 | |
| 2 | 5 | 4 | 1 | |
| ≥3 | 5 | 3 | 2 | |
| Brainstem and cerebellum, No. | ||||
| 1 | 7 | 7 | 0 | |
| 2 | 4 | 2 | 2 | |
| ≥3 | 4 | 3 | 1 | |
| Remote infarcts | 30 (16.9) | 18 (18.0) | 12 (15.6) | .39c |
| Cerebral cortex, No. | ||||
| 1 | 11 | 9 | 2 | |
| 2 | 4 | 2 | 2 | |
| ≥3 | 2 | 1 | 1 | |
| Deep cerebral gray matter and internal capsule, No. | ||||
| 1 | 6 | 3 | 3 | |
| 2 | 1 | 1 | 0 | |
| ≥3 | 0 | 0 | 0 | |
| Subcortical and periventricular white matter, No. | ||||
| 1 | 7 | 3 | 4 | |
| 2 | 0 | 0 | 0 | |
| ≥3 | 1 | 1 | 0 | |
| Brainstem and cerebellum, No. | ||||
| 1 | 4 | 2 | 2 | |
| 2 | 1 | 1 | 0 | |
| ≥3 | 0 | 0 | 0 | |
| Remote microbleeds | 5 (2.8) | 2 (2.0) | 3 (3.8) | .62c |
| Cerebral cortex, No. | ||||
| 1 | 2 | 1 | 1 | |
| 2 | 1 | 0 | 1 | |
| ≥3 | 2 | 1 | 1 | |
| Subcortical and periventricular white matter, No. | ||||
| 1 | 0 | 0 | 0 | |
| 2 | 0 | 0 | 0 | |
| ≥3 | 2 | 1 | 1 | |
| Subcortical gray matter, No. | ||||
| 1 | 0 | 0 | 0 | |
| 2 | 1 | 0 | 1 | |
| ≥3 | 1 | 1 | 0 | |
| Brainstem and cerebellum, No. | ||||
| 1 | 0 | 0 | 0 | |
| 2 | 0 | 0 | 0 | |
| ≥3 | 2 | 1 | 1 | |
Abbreviation: CTE, chronic traumatic encephalopathy.
Participants with CTE and other comorbid neurodegenerative disease diagnoses, whereas CTE only includes those with CTE and who did not have comorbid neurodegenerative disease diagnoses.
Ordinal regression controlling for age compared differences between participants with CTE only and those with CTE and comorbid diagnoses for white matter rarefaction, atherosclerosis, and arteriolosclerosis. Logistic regression controlling for age was used to examine differences between the participant groups on all remaining variables.
Compared absence vs presence. Of the 180 participants, sample sizes were reduced because of missing data to 170 participants for atherosclerosis and 177 participants for remote infarcts. There are variable sample sizes across the regional assessments because of missing data.
Of the 180 men who donated their brains, 120 were determined to have had antemortem dementia. Microinfarcts were examined globally (ie, as an overall absence or presence) because of the insufficient sample size at the level of brain region. Because of the small number of participants who had infarcts and microbleeds, these variables were not examined.
Simultaneous Equations Regression Model Results
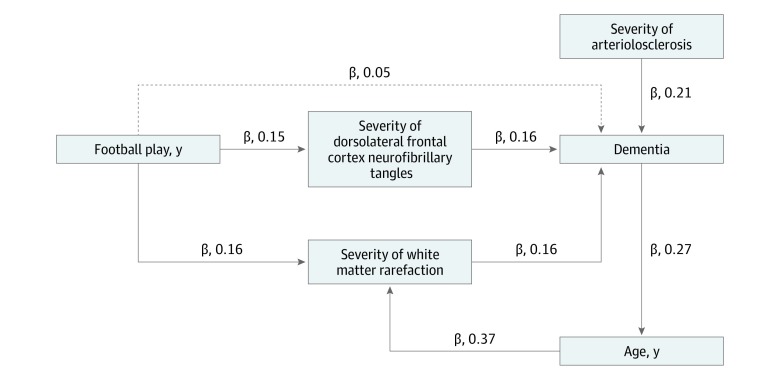
Results of the proportional odds and logistic regression analyses are presented in eMethods 4 in the Supplement. Results from these models remain unchanged when controlling for the football position(s) played and age at first exposure to US football. These were not included the final model. Figure 1 depicts a summary of the primary findings from the simultaneous equations regression model. The model fit was excellent (Akaike information criteria, 77.56; standardized root mean square residual, 0.02; goodness of fit index, 0.99; root mean square error of approximation <0.01; Bentler-Bonett normed fit index, 0.98; χ2 = 3.56; P = .83), and findings were similar when WM rarefaction and arteriolosclerosis were recoded as none to mild vs moderate to severe (Akaike information criteria, 76.38; standardized root mean square residual, 0.02; goodness of fit index, 0.99; Bentler-Bonett normed fit index, 0.99; root mean square error of approximation <0.01; χ2 = 2.38; P = .94). Increasing age was associated with more severe WM rarefaction (β, 0.37 [95% CI, 025-0.50]; P < .001), high CTE stage (β, 0.32 [95% CI, 0.19-0.45]; P < .001), and increased likelihood for dementia (β, 0.27 [95% CI, 0.13-0.42]; P < .001).
Figure 1. Cross-Sectional Model on the Contributions of White Matter Rarefaction, Arteriolosclerosis, and Phosphorylated Tau to Dementia in Chronic Traumatic Encephalopathy (CTE).
Simultaneous equations regression models tested the association of pathological markers of cerebrovascular disease with dementia in older deceased individuals who had played football and had CTE. The Figure provides an illustrative conceptual summary of the primary significant findings. Not all variables or pathways are displayed for ease of presentation. See eMethods 3 and 4 in the Supplement for a summary of all variables included in the model and associated pathways estimated. The β values shown are standardized estimates, and each path shown is significant at an α less than .05. Standardized estimates are interpreted as changes in SD units. For instance, there was a 0.16-SD change in dementia per unit SD increase in white matter rarefaction and dorsolateral frontal cortex neurofibrillary tangles of hyperphosphorylated tau. Standardized estimates permit direct comparison of the effect sizes across pathways. The dashed line between years of football play and dementia denotes a significant indirect effect in which the association of years of football play with dementia was mediated by dorsolateral frontal cortex neurofibrillary tangles and severity of white matter rarefaction. Arteriolosclerosis and white matter rarefaction were rated on a scale of 0 to 3 points, with 3 indicating severe pathologic changes. Dorsolateral frontal cortex neurofibrillary tangles was a binary variable, with 0 indicating a low burden of neurofibrillary tangles and 1 a high burden.
The association between age and dementia was partially mediated by WM rarefaction (indirect effect: β, 0.05 [95% CI, −0.005 to 0.11]; P = .07). There were no significant associations of race with WM rarefaction, CTE stage, DLFC NFT burden, or dementia.
More years of play was associated with increased likelihood for more severe WM rarefaction (β, 0.16 [95% CI, 0.02-0.29]; P = .02), as well as moderate to severe DLFC NFT burden (β, 0.15 [95% CI, 0.004-0.30]; P = .04) and high CTE stage (β, 0.27 [95% CI, 0.14-0.41]; P < .001). More severe WM rarefaction (β, 0.16 [95% CI, 0.02-0.29]; P = .03) and greater DLFC NFT burden (β, 0.16 [95% CI, 0.03-0.28]; P = .01) subsequently corresponded to increased likelihood for having dementia. As shown by the standardized β values, WM rarefaction and DLFC NFT burden were equally associated with dementia. The association of years of play with dementia was mediated by WM rarefaction and DLFC NFT burden (indirect effect: β, 0.05 [95% CI, 0.006-0.09]; P = .03). Although arteriolosclerosis was not associated with years of football play, it was independently associated with dementia (β, 0.21 [95% CI, 0.07-0.35]; P = .003). The simultaneous equations regression model accounted for all variance in the model; therefore, the association of WM rarefaction and arteriolosclerosis with dementia were independent of all variables, including phosphorylated tau severity. Figure 2 shows exemplar images of arteriolosclerosis, WM rarefaction, and phosphorylated tau pathologic changes.
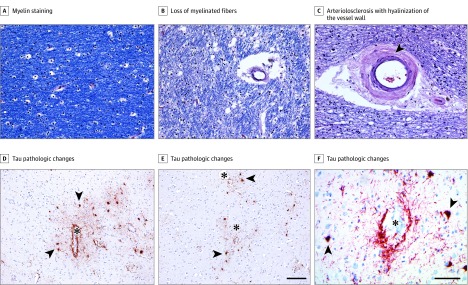
Figure 2. White Matter Rarefaction, Arteriolosclerosis, and Dorsolateral Frontal Cortex Tau Pathologic Changes in Participants With Chronic Traumatic Encephalopathy.
A, Luxol fast blue with hemotoxylin-eosin histochemical staining shows robust myelin staining (blue) in a former US football college player in his early 40s who was neuropathologically diagnosed with chronic traumatic encephalopathy (CTE) (stage I/II) who was not determined to have had antemortem dementia. B and C, In a man who had played professional US football, was in his mid-80s, had been neuropathologically diagnosed with CTE (stage III/IV), and was determined by consensus to have dementia, there was severe loss (3+) of myelinated fibers (B), as well as marked arteriolosclerosis with hyalinization of the vessel wall (C, arrowhead). D-F, Immunohistochemical staining for tau pathologic changes (antibody AT8) in the dorsolateral frontal cortex shows perivascular accumulations of abnormal tau at the sulcal depths within neurons and cell processes (asterisks indicate the lumen of cortical vessels; arrowheads, examples of pretangles and tangles within neurons). The scale bar for A-C and F is 100 μm; for D and E, 150 μm.
Sensitivity analyses were conducted to clarify whether the associations of WM rarefaction and arteriolosclerosis with dementia were independent of comorbid neurodegeneration. Overall model fit and significant associations of WM rarefaction and arteriolosclerosis with dementia remained when AD Neuropathologic Change was added as a covariate, as well as when individuals diagnosed with prion disease and motor neuron disease were excluded from the sample. The model was repeated in participants who had CTE alone (n = 101). In this reduced sample, the effect sizes of WM rarefaction (β, 0.13 [95% CI, −0.07 to 0.32]) and arteriolosclerosis (β, 0.25 [95% CI, 0.06-0.43]) on dementia were similar to those in the entire sample.
White Matter Rarefaction and Arteriolosclerosis and Dementia: Logistic Regression
A logistic regression controlling for age and race showed that participants who had more severe WM rarefaction were at 1.69 times greater odds for dementia (odds ratio [OR], 1.69 [95% CI, 1.08-2.62]; P = .03). Similar outcomes were observed for arteriolosclerosis (OR, 1.81 [95% CI, 1.23-2.67]; P = .003). The ORs for WM rarefaction (OR, 1.68 [95% CI, 1.08-2.62]; P = .02) and arteriolosclerosis (OR, 1.84 [95% CI, 1.23-2.75]; P = .003) remained unchanged when DLFC NFTs were included as a covariate. A logistic regression showed the dementia outcomes associated with WM rarefaction (OR, 1.59 [95% CI, 1.02-2.47]; P = .04) and arteriolosclerosis (OR, 1.72 [95% CI, 1.16-2.55]; P = .007) remained stable after controlling for AD Neuropathologic Change (and age and race). Compared with participants with a low DLFC NFT burden, those with a high DLFC NFT burden had 2.65 times the odds of having dementia (OR, 2.65 [95% CI, 1.24-5.70]; P = .01), after controlling for age and race.
Sensitivity Analyses: Associations With Atherosclerosis and Cardiovascular Disease
Cumulative logit proportional odds (ordinal outcomes) and logistic (binary outcomes) regression models that controlled for age and race examined whether WM rarefaction and arteriolosclerosis reflect downstream sequelae from large-vessel disease (eg, atherosclerosis). The sample size for these analyses was 170 individuals because of missing atherosclerosis data. Large-vessel atherosclerosis increased the odds for more severe small-vessel arteriolosclerosis (OR, 1.62 [95% CI, 1.16-2.27]; P = .005) and microinfarcts (OR, 2.27 [95% CI, 1.48-3.48]; P < .001). Atherosclerosis was not associated with WM rarefaction (data not shown). Statistically significant associations were not found between WM rarefaction with arteriolosclerosis and microinfarcts.
In the full sample minus the individuals excluded because of missing data, 81 of 133 men (60.9%) had a reported history of hypertension, and 29 of 134 men (21.6%) had a reported history of diabetes. Logistic regression showed years of football play was not associated with hypertension or diabetes (data not shown). Simple proportional odds regression revealed that hypertension was associated with more severe arteriolosclerosis (OR, 1.26 [95% CI, 1.03-2.38]; P < .001) and atherosclerosis (OR, 2.51 [95% CI, 1.20-3.25]; P = .004), but not WM rarefaction. A history of diabetes was not associated with severity of atherosclerosis, arteriolosclerosis, or WM rarefaction (data not shown). Binary logistic regressions showed no significant associations between a history of hypertension and diabetes with dementia (data not shown). All sensitivity analyses controlled for age and race.
Discussion
In this convenience sample of deceased men older than 40 years who had played US football and were neuropathologically diagnosed with CTE, longer duration of football play was associated with more severe WM rarefaction and greater DLFC NFT burden. Worse WM rarefaction severity and greater DLFC NFT burden subsequently corresponded to an increased likelihood of dementia. Arteriolosclerosis was also independently associated with increased odds for having dementia. Dementia in older individuals with CTE may be a result of neuropathologic conditions associated with RHI, including WM rarefaction and DLFC NFTs, as well as non-RHI–associated pathologic conditions, such as arteriolosclerosis.
Arteriolosclerosis is a marker of cerebral small-vessel disease associated with aging and CVD. As expected, it was associated with hypertension and atherosclerosis in this sample, but not years of play. The causative mechanisms of WM rarefaction are multifaceted and might reflect CBVD associated with CVD.36 White matter rarefaction can also represent demyelination and axonal loss.42 White matter rarefaction was not associated with atherosclerosis, arteriolosclerosis, or CVD risk factors. Instead, more years of football, a surrogate metric of RHI exposure,2,9,13 was a corollary of more severe WM rarefaction. In CTE, WM rarefaction may encompass diffuse, long-term degeneration (eg, axonal and myelin loss, astrocytosis, neuroinflammation) secondary to RHI from football.12,13 Acute WM injuries6,43,44,45,46 from RHI might persist over time2,3,25,47,48,49 and worsen with age. Studies that include direct CVD and RHI metrics and refined measures of WM integrity are needed to improve understanding of the pathogenesis of WM rarefaction and cerebral small vessel changes in CTE.
White matter rarefaction and arteriolosclerosis were associated with increased odds for dementia independent of phosphorylated tau; they were not associated with phosphorylated tau severity. White matter rarefaction and arteriolosclerosis thus had independent or additive associations with dementia. These pathologic conditions also independently affect the clinical expression of AD.19,50,51,52,53,54 Cases of CTE often present with various cognitive and neuropsychiatric changes that can progress to dementia.3,8,55 The extent to which these symptoms are accounted for by phosphorylated tau is unclear, especially behavioral and mood changes that occur early in life.3,8,9,56 Nontau pathologic changes, including WM degeneration associated with RHI and arteriolosclerosis associated with aging or CVD, may affect the clinical expression of CTE and contribute to clinical hetereogeneity.46
The DLFC is an initial region of phosphorylated tau accumulation in CTE, and DLFC NFTs burden contributed to dementia in this sample of participants with CTE and other comorbid neurodegenerative pathologic characteristics. These findings are similar to data linking plaques and NFTs from AD with clinical decline.19,50,57 Dementia in AD is a result of mixed neuropathologic changes (not only phosphorylated tau),11,58,59,60,61,62 and the current findings support a similar phenomenon in CTE. In 2013, Stern et al8 reported a dementia rate of approximately 30% (compared with 67% in this sample) among a small sample of men (n = 36) who had played US football, were neuropathologically diagnosed with CTE, and were without comorbid neurodegenerative disease. That smaller sample was younger (mean age, 57 years; compared with 68 years in the current sample). The lower rate of dementia might reflect the lack of comorbid neurodegenerative disease in the Stern et al study.8
Limitations
The current findings are limited by ascertainment bias that reduces the generalizability of the findings to the broader population of individuals who play US football. Population-based studies are needed to overcome potential biases from sample selection. Ascertainment bias may have had minimal influence on the estimated associations. The accuracy of the estimated associations would only be influenced by ascertainment bias if both the independent variables and outcomes were differentially associated with brain bank selection. It is theoretically unlikely that CBVD had an independent differential association with selection into the brain bank. Recent research showed that the associations of age at first exposure to football on clinical outcomes remained unchanged after adjusting (through inverse-probability weighting) for some factors shown to affect brain bank selection among older adults (eg, dementia, depression, age, race).9,63 Continued studies that elucidate potential biases associated with sample selection into the Understanding Neurologic Injury and Traumatic Encephalopathy study will be important to place the current and future findings in context.
The DLFC NFTs were examined based on a priori hypotheses and the extant data.2,3,13 Regional evaluation of phosphorylated tau was beyond the scope of this study and not examined to avoid an overidentified model, decreased statistical power attributable to the number of pathways to be estimated, violation of statistical assumptions associated with multicollinearity, and decreased sample size attributable to missing data across the regions. A separate study that uses advanced statistical modeling and refined quantitative measures of regional phosphorylated tau will clarify the associations between regional phosphorylated tau severity, RHI, and dementia. Granular examinations of microvasculopathy were not conducted, which may be more sensitive to the associations of head trauma with CTE.6,34 Future research should examine other markers of vascular injury, such as C-reactive protein, vascular cell adhesion molecule 1, intercellular adhesion molecule 1, and measures of vessel tortuosity. Inclusion of a neurodegenerative disease comparison group, such as individuals with AD, would provide insight into distinctive patterns of CBVD severity in CTE, as well as comparative contributions of CBVD to dementia. Dementia was determined by consensus diagnostic conferences based on information obtained through retrospective telephone interviews with informants. This approach might have affected the accuracy of the estimated outcomes because of subjectivity and recall biases. Corroboration of in-life dementia diagnoses through medical records could be an added source of supporting evidence. Data from medical records collected as part of the larger study of this sample are not yet ready for analysis. In addition, APOE genotyping is ongoing and not included here. The exact role of apolipoprotein E in CTE is unclear,2,8 but apolipoprotein E status might have influenced many of the associations examined.
Conclusions
In this convenience sample of older football players with neuropathologically confirmed CTE, more years of football play were associated with more severe WM rarefaction and greater phosphorylated tau accumulation. White matter rarefaction, DLFC NFTs, and arteriosclerosis were independently associated with dementia. Increased understanding of comorbid contributors to the pathogenesis and symptom profile of CTE is imperative to fully diagnose, treat, and prevent this progressive brain disease.
eMethods 1. CTE stage classification scheme
eMethods 2. Dementia diagnostic procedures
eMethods 3. Statistical analyses
eMethods 4. Results of proportional odds and logistic regression models
eReferences.
References
- 1.Montenigro PH, Alosco ML, Martin BM, et al. . Cumulative head impact exposure predicts later-life depression, apathy, executive dysfunction, and cognitive impairment in former high school and college football players. J Neurotrauma. 2017;34(2):328-340. doi: 10.1089/neu.2016.4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKee AC, Stern RA, Nowinski CJ, et al. . The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):43-64. doi: 10.1093/brain/aws307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mez J, Daneshvar DH, Kiernan PT, et al. . Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA. 2017;318(4):360-370. doi: 10.1001/jama.2017.8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bieniek KF, Ross OA, Cormier KA, et al. . Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015;130(6):877-889. doi: 10.1007/s00401-015-1502-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling H, Morris HR, Neal JW, et al. . Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol. 2017;133(3):337-352. doi: 10.1007/s00401-017-1680-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tagge CA, Fisher AM, Minaeva OV, et al. . Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain. 2018;141(2):422-458. doi: 10.1093/brain/awx350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKee AC, Cairns NJ, Dickson DW, et al. ; TBI/CTE group . The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131(1):75-86. doi: 10.1007/s00401-015-1515-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stern RA, Daneshvar DH, Baugh CM, et al. . Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81(13):1122-1129. doi: 10.1212/WNL.0b013e3182a55f7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alosco ML, Mez J, Tripodis Y, et al. . Age of first exposure to tackle football and chronic traumatic encephalopathy. Ann Neurol. 2018;83(5):886-901. doi: 10.1002/ana.25245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alosco ML, Mez J, Kowall NW, et al. . Cognitive reserve as a modifier of clinical expression in chronic traumatic encephalopathy: a preliminary examination. J Neuropsychiatry Clin Neurosci. 2017;29(1):6-12. doi: 10.1176/appi.neuropsych.16030043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein TD, Montenigro PH, Alvarez VE, et al. . Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015;130(1):21-34. doi: 10.1007/s00401-015-1435-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherry JD, Stein TD, Tripodis Y, et al. . CCL11 is increased in the CNS in chronic traumatic encephalopathy but not in Alzheimer’s disease. PLoS One. 2017;12(9):e0185541. doi: 10.1371/journal.pone.0185541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherry JD, Tripodis Y, Alvarez VE, et al. . Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun. 2016;4(1):112. doi: 10.1186/s40478-016-0382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holleran L, Kim JH, Gangolli M, et al. . Axonal disruption in white matter underlying cortical sulcus tau pathology in chronic traumatic encephalopathy. Acta Neuropathol. 2017;133(3):367-380. doi: 10.1007/s00401-017-1686-x [DOI] [PubMed] [Google Scholar]
- 15.Alosco ML, Duskin J, Besser LM, et al. . Modeling the relationships among late-life body mass index, cerebrovascular disease, and Alzheimer’s disease neuropathology in an autopsy sample of 1,421 subjects from the National Alzheimer’s Coordinating Center Data Set. J Alzheimers Dis. 2017;57(3):953-968. doi: 10.3233/JAD-161205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deramecourt V, Slade JY, Oakley AE, et al. . Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78(14):1043-1050. doi: 10.1212/WNL.0b013e31824e8e7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13(suppl 3):S115-S123. doi: 10.1097/00002093-199912003-00017 [DOI] [PubMed] [Google Scholar]
- 18.Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta. 2016;1862(5):887-900. doi: 10.1016/j.bbadis.2015.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toledo JB, Arnold SE, Raible K, et al. . Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136(pt 9):2697-2706. doi: 10.1093/brain/awt188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723-738. doi: 10.1038/nrn3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Viqar F, Zimmerman ME, et al. ; Dominantly Inherited Alzheimer Network . White matter hyperintensities are a core feature of Alzheimer’s disease: evidence from the dominantly inherited Alzheimer network. Ann Neurol. 2016;79(6):929-939. doi: 10.1002/ana.24647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 2016;15(9):934-943. doi: 10.1016/S1474-4422(16)30029-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron SL, Hein MJ, Lehman E, Gersic CM. Body mass index, playing position, race, and the cardiovascular mortality of retired professional football players. Am J Cardiol. 2012;109(6):889-896. doi: 10.1016/j.amjcard.2011.10.050 [DOI] [PubMed] [Google Scholar]
- 24.Tucker AM, Vogel RA, Lincoln AE, et al. . Prevalence of cardiovascular disease risk factors among National Football League players. JAMA. 2009;301(20):2111-2119. doi: 10.1001/jama.2009.716 [DOI] [PubMed] [Google Scholar]
- 25.Alosco ML, Koerte IK, Tripodis Y, et al. . White matter signal abnormalities in former National Football League players. Alzheimers Dement (Amst). 2017;10:56-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner RC, Possin KL, Hess CP, et al. . Evaluating and treating neurobehavioral symptoms in professional American football players: Lessons from a case series. Neurol Clin Pract. 2015;5(4):285-295. doi: 10.1212/CPJ.0000000000000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart J Jr, Kraut MA, Womack KB, et al. . Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross-sectional study. JAMA Neurol. 2013;70(3):326-335. doi: 10.1001/2013.jamaneurol.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amen DG, Willeumier K, Omalu B, Newberg A, Raghavendra C, Raji CA. Perfusion neuroimaging abnormalities alone distinguish National Football League players from a healthy population. J Alzheimers Dis. 2016;53(1):237-241. doi: 10.3233/JAD-160207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey DM, Jones DW, Sinnott A, et al. . Impaired cerebral haemodynamic function associated with chronic traumatic brain injury in professional boxers. Clin Sci (Lond). 2013;124(3):177-189. doi: 10.1042/CS20120259 [DOI] [PubMed] [Google Scholar]
- 30.Marchi N, Bazarian JJ, Puvenna V, et al. . Consequences of repeated blood-brain barrier disruption in football players. PLoS One. 2013;8(3):e56805. doi: 10.1371/journal.pone.0056805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissberg I, Veksler R, Kamintsky L, et al. . Imaging blood-brain barrier dysfunction in football players. JAMA Neurol. 2014;71(11):1453-1455. doi: 10.1001/jamaneurol.2014.2682 [DOI] [PubMed] [Google Scholar]
- 32.Filley CM, Kelly JP. White matter and cognition in traumatic brain injury. J Alzheimers Dis. 2018;65(2):345-362. doi: 10.3233/JAD-180287 [DOI] [PubMed] [Google Scholar]
- 33.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3(3):270-303. doi: 10.1017/S0033291700049588 [DOI] [PubMed] [Google Scholar]
- 34.Buée L, Hof PR, Bouras C, et al. . Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol. 1994;87(5):469-480. doi: 10.1007/BF00294173 [DOI] [PubMed] [Google Scholar]
- 35.Doherty CP, O’Keefe E, Wallace E, et al. . Blood-brain barrier dysfunction as a hallmark pathology in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2016;75(7):656-662. doi: 10.1093/jnen/nlw036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Schweizer TA, Fischer CE, Munoz DG. The role of cerebrovascular disease on cognitive and functional status and psychosis in severe Alzheimer’s disease. J Alzheimers Dis. 2017;55(1):381-389. doi: 10.3233/JAD-160506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mez J, Solomon TM, Daneshvar DH, et al. . Assessing clinicopathological correlation in chronic traumatic encephalopathy: rationale and methods for the UNITE study. Alzheimers Res Ther. 2015;7(1):62. doi: 10.1186/s13195-015-0148-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vonsattel JP, Amaya MdelP, Cortes EP, Mancevska K, Keller CE. Twenty-first century brain banking: practical prerequisites and lessons from the past: the experience of New York Brain Bank, Taub Institute, Columbia University. Cell Tissue Bank. 2008;9(3):247-258. doi: 10.1007/s10561-008-9079-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vonsattel JP, Del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol. 2008;115(5):509-532. doi: 10.1007/s00401-007-0311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beekly DL, Ramos EM, van Belle G, et al. ; NIA-Alzheimer’s Disease Centers . The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18(4):270-277. [PubMed] [Google Scholar]
- 41.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1-55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- 42.McAleese KE, Walker L, Graham S, et al. . Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 2017;134(3):459-473. doi: 10.1007/s00401-017-1738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahrami N, Sharma D, Rosenthal S, et al. . Subconcussive head impact exposure and white matter tract changes over a single season of youth football. Radiology. 2016;281(3):919-926. doi: 10.1148/radiol.2016160564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuzminski SJ, Clark MD, Fraser MA, et al. . White matter changes related to subconcussive impact frequency during a single season of high school football. AJNR Am J Neuroradiol. 2018;39(2):245-251. doi: 10.3174/ajnr.A5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayinger MC, Merchant-Borna K, Hufschmidt J, et al. . White matter alterations in college football players: a longitudinal diffusion tensor imaging study. Brain Imaging Behav. 2018;12(1):44-53. doi: 10.1007/s11682-017-9672-4 [DOI] [PubMed] [Google Scholar]
- 46.Gangolli M, Benetatos J, Esparza TJ, Fountain EM, Seneviratne S, Brody DL. Repetitive concussive and subconcussive injury in a human tau mouse model results in chronic cognitive dysfunction and disruption of white matter tracts, but not tau pathology. J Neurotrauma. 2019;36(5):735-755. doi: 10.1089/neu.2018.5700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKee AC, Cantu RC, Nowinski CJ, et al. . Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709-735. doi: 10.1097/NEN.0b013e3181a9d503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark MD, Varangis EML, Champagne AA, et al. . Effects of career duration, concussion history, and playing position on white matter microstructure and functional neural recruitment in former college and professional football athletes. Radiology. 2018;286(3):967-977. doi: 10.1148/radiol.2017170539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coughlin JM, Wang Y, Minn I, et al. . Imaging of glial cell activation and white matter integrity in brains of active and recently retired National Football League players. JAMA Neurol. 2017;74(1):67-74. doi: 10.1001/jamaneurol.2016.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett DA, Wilson RS, Arvanitakis Z, Boyle PA, de Toledo-Morrell L, Schneider JA. Selected findings from the Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2013;33(suppl 1):S397-S403. doi: 10.3233/JAD-2012-129007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chui HC, Zarow C, Mack WJ, et al. . Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann Neurol. 2006;60(6):677-687. doi: 10.1002/ana.21009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the nun study. JAMA. 1997;277(10):813-817. doi: 10.1001/jama.1997.03540340047031 [DOI] [PubMed] [Google Scholar]
- 53.Bos D, Wolters FJ, Darweesh SKL, et al. . Cerebral small vessel disease and the risk of dementia: a systematic review and meta-analysis of population-based evidence. Alzheimers Dement. 2018;14(11):1482-1492. doi: 10.1016/j.jalz.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 54.Bouhrara M, Reiter DA, Bergeron CM, et al. . Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimers Dement. 2018;14(8):998-1004. doi: 10.1016/j.jalz.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montenigro PH, Baugh CM, Daneshvar DH, et al. . Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6(5):68. doi: 10.1186/s13195-014-0068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahar I, Alosco ML, McKee AC. Psychiatric phenotypes in chronic traumatic encephalopathy. Neurosci Biobehav Rev. 2017;83:622-630. doi: 10.1016/j.neubiorev.2017.08.023 [DOI] [PubMed] [Google Scholar]
- 57.Nelson PT, Jicha GA, Schmitt FA, et al. . Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66(12):1136-1146. doi: 10.1097/nen.0b013e31815c5efb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol. 2018;83(1):74-83. doi: 10.1002/ana.25123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brenowitz WD, Hubbard RA, Keene CD, et al. . Mixed neuropathologies and associations with domain-specific cognitive decline. Neurology. 2017;89(17):1773-1781. doi: 10.1212/WNL.0000000000004567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brenowitz WD, Hubbard RA, Keene CD, et al. . Mixed neuropathologies and estimated rates of clinical progression in a large autopsy sample. Alzheimers Dement. 2017;13(6):654-662. doi: 10.1016/j.jalz.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134(2):171-186. doi: 10.1007/s00401-017-1717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016;139(11):2983-2993. doi: 10.1093/brain/aww224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haneuse S, Schildcrout J, Crane P, Sonnen J, Breitner J, Larson E. Adjustment for selection bias in observational studies with application to the analysis of autopsy data. Neuroepidemiology. 2009;32(3):229-239. doi: 10.1159/000197389 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. CTE stage classification scheme
eMethods 2. Dementia diagnostic procedures
eMethods 3. Statistical analyses
eMethods 4. Results of proportional odds and logistic regression models
eReferences.