Abstract
This study examines industry payments made to physician directors of National Cancer Institute–designated cancer centers from 2015 through 2017.
National Cancer Institute (NCI)–designated cancer centers shape cancer care in the United States and are supported by substantial public funds (in fiscal year 2018, $330 million in core funding for 70 cancer centers).1 Cancer care is also shaped by industry, because developing new cancer therapeutics represents a major market opportunity. Industry payments to academic physicians risk blurring the line between innovation and the evaluation of new therapies. We used Open Payments data from the Centers for Medicare & Medicaid Services (CMS) to examine industry payments made to physician directors of NCI-designated cancer centers.
Methods
We obtained data on industry payments for calendar years 2015 through 2017 from the Open Payments database. Pharmaceutical and medical device companies are required to provide this information for all US physicians; nonphysician directors of NCI-designated cancer centers (16 in 2017) are not in the database. We identified physician directors using cached versions of the NCI website (https://www.cancer.gov/research/nci-role/cancer-centers) obtained from archive.org. As of July 2017, there were 53 physician directors. We then looked back to determine whether the director held the position in prior years (using cached data from July 2016 and July 2015). We sought data on all industry payments (total payments) and for their component research payments and payments unrelated to research, which includes consulting fees, travel and lodging, and honoraria. Although physicians may dispute the accuracy of their data, no payments were disputed in the data we examined.
Results
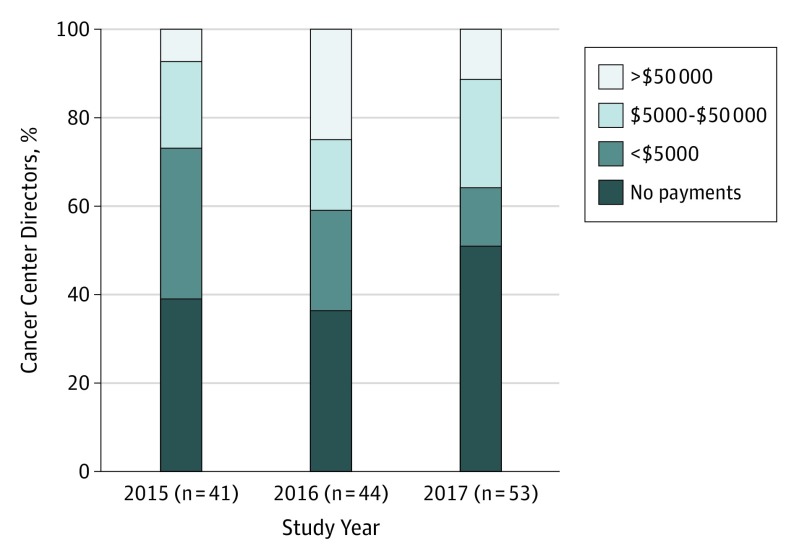
Of the 53 physician cancer center directors listed in 2017, 44 held the position in 2016 and 41 in 2015. Figure 1 shows the distribution of industry payments to these directors from 2015 to 2017. In 2017, total payments were $4.42 million, including $1.89 million in research payments to 12 directors and $2.53 million in nonresearch payments to 22 directors. Twenty-seven of the 53 directors (51%) received no payments, whereas 19 (36%) received more than $5000.
Figure 1. Industry Payments to Physician Directors of National Cancer Institute–Designated Cancer Centers.
The distribution of total payments from calendar years 2015 through 2017 is shown.
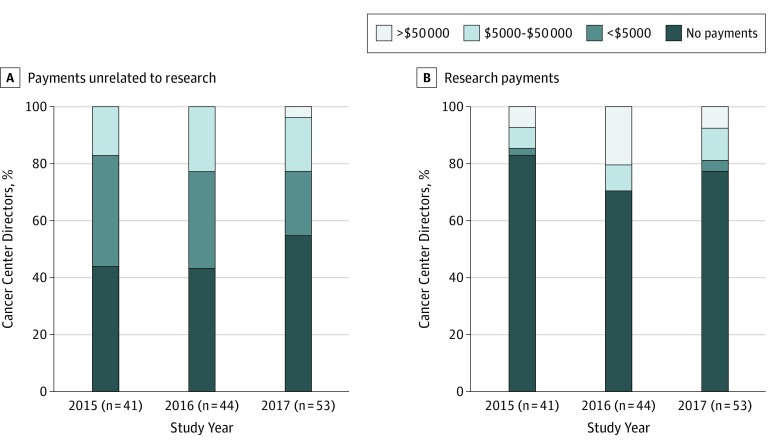
Physician directors were more likely to receive payments unrelated to research than research payments (Figure 2). In 2017, 12 of 53 (23%) had payments unrelated to research exceeding $5000, the threshold defined by the NCI for payments unrelated to research as a significant financial interest.2 Two directors received payments of more than $50 000. The larger of these payments was for $2.27 million, almost all of which was categorized in Open Payments as “compensation for services other than consulting, including serving as faculty or a speaker at a venue other than a continuing education program.”
Figure 2. Components of Industry Payments to Physician Directors of National Cancer Institute–Designated Cancer Centers.
The distribution of payments unrelated to research and research payments from calendar years 2015 through 2017 is shown.
The largest research payment in 2017 was $863 000; the median value of research payments was larger than the median value for payments unrelated to research ($37 036 [range, $136-$863 000] vs $5828 [range, $15-$2.3 million]). Of the 12 directors receiving research payments, 10 received more than $5000 and 4 received more than $50 000.
Discussion
In 2017, about half of physician directors of NCI cancer centers received industry payments, and about half did not. Industry payments were thus less frequent than has been reported for authors of National Comprehensive Cancer Network Guidelines in 20143 and leaders of academic oncology units in 2015 (ie, department chairs and section chiefs).4 Almost one-quarter of physician directors received more than $5000 in payments unrelated to research, constituting a significant financial interest as defined by the NCI (the NCI conflict of interest policy is silent on research payments2).
Some argue that although physicians have few reasons to receive payments unrelated to research, payments for bona fide research are less problematic.5 Others have raised concerns about the far-reaching effects of industry funding of any type, because this funding can affect all stages of clinical research, from formulation of research questions and trial design to dissemination of research findings.6 Our findings raise the question of whether industry payments to the directors of publicly supported institutions, such as NCI-designated cancer centers, serve the public interest. Policy makers—and the public—should consider whether such payments should be allowed, limited (eg, to <$5000 a year), or eliminated.
Open payments data are limited because they lack detail about the specific service provided. Furthermore, they provide no information on whether the payments influenced the cancer center director.
References
- 1.National Cancer Institute. Grants to NCI-designated cancer centers: NCI-designated cancer center totals, FY 2018. https://www.cancer.gov/about-nci/budget/fact-book/extramural-programs/cancer-centers. Posted December 20, 2018. Accessed April 3, 2019.
- 2.National Cancer Institute Division of Cancer Treatment and Diagnosis. Conflict of interest policy for NCI/DCTD-supported Cooperative Group or National Clinical Trials Network randomized phase 2 and phase 3 clinical trials. https://ctep.cancer.gov/investigatorResources/docs/Conflict_Of_Interest_Policy.pdf. Published August 2012. Accessed December 6, 2018.
- 3.Mitchell AP, Basch EM, Dusetzina SB. Financial relationships with industry among national comprehensive cancer network guideline authors. JAMA Oncol. 2016;2(12):1628-1631. doi: 10.1001/jamaoncol.2016.2710 [DOI] [PubMed] [Google Scholar]
- 4.Yoo SK, Ahmed AA, Ileto J, et al. Industry funding among leadership in medical oncology and radiation oncology in 2015. Int J Radiat Oncol Biol Phys. 2017;99(2):280-285. doi: 10.1016/j.ijrobp.2017.01.202 [DOI] [PubMed] [Google Scholar]
- 5.Steinbrook R. Industry payments to physicians and prescribing of brand-name drugs. JAMA Intern Med. 2016;176(8):1123. doi: 10.1001/jamainternmed.2016.2959 [DOI] [PubMed] [Google Scholar]
- 6.Stamatakis E, Weiler R, Ioannidis JPA. Undue industry influences that distort healthcare research, strategy, expenditure and practice: a review. Eur J Clin Invest. 2013;43(5):469-475. doi: 10.1111/eci.12074 [DOI] [PubMed] [Google Scholar]