Abstract
The intervertebral disc (IVD) is a highly hydrated tissue, the rich proteoglycan matrix imbibes water, enabling the disc to withstand compressive loads. During aging and degeneration increased matrix degradation leads to dehydration and loss of function. Aquaporins (AQP) are a family of transmembrane channel proteins that selectively allow the passage of water in and out of cells and are responsible for maintaining water homeostasis in many tissues. Here, the expression of all 13 AQPs at gene and protein level was investigated in human and canine nondegenerate and degenerate IVDs to develop an understanding of the role of AQPs during degeneration. Furthermore, in order to explore the transition of notochordal cells (NCs) towards nucleus pulposus (NP) cells, AQP expression was investigated in canine IVDs enriched in NCs to understand the role of AQPs in IVD maturation. AQP0, 1, 2, 3, 4, 5, 6, 7, and 9 were expressed at gene and protein level in both nondegenerate and degenerate human NP tissue. AQP2 and 7 immunopositivity increased with degeneration in human NP tissue, whereas AQP4 expression decreased with degeneration in a similar way to AQP1 and 5 shown previously. All AQP proteins that were identified in human NP tissue were also expressed in canine NP tissue. AQP2, 5, 6, and 9 were found to localize to vacuole‐like membranes and cell membranes in NC cells. In conclusion, AQPs were abundantly expressed in human and canine IVDs. The expression of many AQP isotypes potentially alludes to multifaceted functions related to adaption of NP cells to the conditions they encounter within their microenvironment in health and degeneration. The presence of AQPs within the IVD may suggest an adaptive role for these water channels during the development and maintenance of the healthy, mature IVD.
Keywords: aquaporins, degeneration, intervertebral disc, maturation, notochordal cells, nucleus pulposus cells
1. INTRODUCTION
The intervertebral disc (IVD), composed of the central gelatinous nucleus pulposus (NP), surrounded by the annulus fibrosus (AF) and cartilaginous end plates, is a highly specialized tissue facilitating the biomechanical function of the spine.1 Resident cells within the NP produce vast amounts of extracellular matrix (ECM) that contain high concentrations of proteoglycans whose hydrophilic glycosaminoglycan side‐chains promote water and cation retention.2 This allows the disc to resist compressive loading and permits motion of the spine due to a high level of hydration.1, 2, 3, 4, 5, 6 Therefore, in comparison to other tissues, the healthy IVD maintains a hyperosmolar environment.2, 7, 8, 9 However, the hydration and osmolality of the disc is not constant.10 With applied loading, between 18% and 25% of fluid is recycled during a diurnal cycle.7, 11 The increased physiological osmolality within the disc has been shown to increase matrix synthesis by NP cells compared to lower osmolalities.12, 13 This highlights that NP cells can respond to osmotic changes within the disc which may be via control of water transport.14
During IVD development the NP contains populations of large, vacuolated notochordal (NC) cell clusters that are replaced by mature NP cells during aging.15 Interestingly, dogs classified as nonchondrodystrophic (NCD) retain NC cells into adulthood and suffer infrequent IVD degeneration, whereas chondrodystrophic (CD) dogs lose NC cells and suffer early‐onset IVD degeneration.16, 17 To preserve their phenotype during culture, NC cells require a hyperosmolar environment18 and they have been shown to provide a protective and regenerative effect on NP cells when cocultured together.19, 20 This indicates that the behavior of disc cells during development and maturation is, in some part, controlled by fluctuations in extracellular osmolality, and that responses may vary between NC cells and mature NP cells.
IVD degeneration can also further disrupt the osmotic flux of the disc. Around 40% of chronic low back pain cases are attributed to IVD degeneration,21 which is characterized by altered biomechanical properties of the spine caused by an imbalance between anabolic and catabolic mechanisms leading to the destruction of the ECM.22, 23, 24, 25, 26 The production of proinflammatory cytokines by NP cells is known to play a role in the degenerative cascade of IVD degeneration.27, 28, 29, 30, 31, 32, 33, 34, 35 Specifically, over‐expression of cytokines leads to increased production of matrix degrading enzymes,36, 37, 38, 39, 40, 41 and the resulting loss of hydrophilic proteoglycans causes a decrease in IVD osmolality compared to normal physiological conditions. This could further contribute to degeneration, as under low osmolality matrix metalloproteinase‐3 (MMP‐3) expression is increased and aggrecan expression is decreased in NP cells.13, 42 This further highlights the importance of regulating osmotic responses in the disc.
Aquaporins (AQPs) are a family of transmembrane channel proteins that function as selective water pores driven by osmotic gradients.43 Aquaporin 1 (AQP1) was the first member to be discovered in erythrocytes and renal tubules44 and since then 13 members have been discovered (AQP0‐12). These channels are localized to tissues with roles relating to water homeostasis, cell volume regulation and structural function,45 and therefore may contribute towards the adaptation and responses of NC and NP cells when exposed to the fluctuating osmolality of the healthy and degenerate disc.
In human and murine discs, expression of AQP1, 2, 3, and 5 have been identified.14, 46, 47, 48, 49, 50 Studies have also demonstrated that expression of AQPs in NP and NCs can be regulated by physiological conditions. Hyperosmolality upregulates AQP2 in murine NP cells,14 and AQP3 in murine NC cells.50 Whilst the expression of AQP1 and AQP5 is maintained at basal levels by hypoxia‐inducible factor 1 alpha in NP cells.49 Johnson et al49 also reported that AQP1 and AQP5 expression decreased in human IVDs with increasing degeneration.
To date only AQP1, 2, 3, and 5 have been shown to be expressed by NP cells46, 47, 48, 49 and AQP1, 2, and 3 by NC cells.14, 50 However, no studies have investigated expression of the remaining AQP family members, and few have investigated expression in NC and NP cells and determined whether expression alters during maturation or degeneration. The elucidation of all AQP family members present within the IVD may indicate how resident cells have adapted to their hyperosmotic environment with regards to water homeostasis and cell volume regulation, and how this adaptation is potentially altered as the IVD matures and degenerates, during which AQP expression may be modified. Hence, this study aimed to identify the expression of all 13 AQP family members in human and canine NP tissues. Discs from NCD and CD canines were used as a model to investigate changes in AQP expression during NC to NP cell transition in IVD maturation, and adult human discs were used to investigate changes in AQP expression in NP cells during IVD degeneration.
2. MATERIALS AND METHODS
2.1. Experimental design
As only few AQP family members have been identified in the human NP, within this study we firstly utilized quantitative real‐time polymerase chain reaction (qRT‐PCR) to identify the gene expression of all 13 AQP family members. Immunohistochemistry (IHC) was then used to determine protein expression of AQPs that were expressed at gene level. Human NP samples used were graded histologically from nondegenerate to severely degenerate which enabled this study to identify changes in AQP expression in mature human tissue as IVD degeneration progresses. This study also aimed to determine the expression of AQPs during IVD maturation which was achieved via AQP protein expression using IHC in NCD and CD canine NP samples. The differences in physiology between NCD and CD canine IVD samples enables changes in AQP expression to be identified as NC cells transition into NP cells during IVD maturation. The isolation of NP cells and NC cells from human and canine IVD samples, and subsequent ICC/IF experiments enabled the localization of AQPs within IVD cells to be investigated. The experimental design is summarized in Supplementary Figure 1, Supporting Information.
2.2. Human tissue
Human IVD tissue was obtained from patients undergoing microdiscectomy surgery for the treatment of nerve root compression as a result of IVD herniation or post‐mortem (PM) examination with informed consent of the patients or relatives (Sheffield Research Ethics Committee [09/H1308/70]). This study utilized 97 surgical samples from 97 individuals and 5 pm samples from three individuals (Supplementary Table 1).
2.3. Canine tissue
Canine IVD samples (n = 35) were obtained from 19 NCD and CD dogs (Thompson grade 1‐5) euthanized for unrelated experiments with approval from the Ethics Committee of Animal Experiments of Utrecht University (Supplementary Table 2). The gross morphology of canine discs was categorized using a modified Thompson grading scheme (TG), specifically developed for canine IVDs, and each sample given a grade (1‐5) to determine the state of disc degeneration.16
2.4. Tissue processing
Human tissue was fixed in 10% (v/v) neutral buffered formalin (Leica Microsystems, Milton Keynes, UK) and embedded into paraffin wax. Following embedding, 4 μm sections were cut and human IVDs histologically graded using previously published methods.24 Briefly, sections were rehydrated in industrial methylated spirits (IMS) 3 × 5 minutes before 1 minute staining with Mayer's Hematoxylin (Leica Microsystems). Sections were left to “blue” in running tap water for 5 minutes before subsequent staining with alcoholic eosin (Leica Microsystems) for 1 minute. Sections were then dehydrated in IMS 3 × 5 minutes and cleared in sub‐X (Leica Microsystems) 3 × 5 minutes. Sections were finally mounted with pertex (Leica Microsystems). NP tissue samples were scored (0‐3) on four microscopic signs of IVD degeneration: demarcation of NP and AF, presence of fissures, cell clusters and loss of pericellular matrix staining, and separated into cohorts depending on grade: nondegenerate (grade 0‐4); moderately degenerate (grade 4.1‐6.9); and severely degenerate (grade 7‐12). Canine lumbar spinal segments, that is, the IVD and adjacent vertebral bodies, were separated into cohorts according to their modified TG, fixed in 4% (v/v) neutral buffered formaldehyde (Klinipath, Duiven, the Netherlands), decalcified as described previously,51 paraffin embedded and 5 μm sections were cut.
2.5. Human NP cell isolation and culture
Human NP cells were isolated using collagenase type I (Sigma‐Aldrich, Gillingham, UK) as described previously.49 NP cells were either used for direct RNA extraction or cell culture. Following extraction, human NP cells (patient samples n = 4, age = 34.25 ± 10.4, grade of degeneration = 4.75 ± 0.96) were expanded in monolayer in Dulbecco's Modified Eagle Media (DMEM) (Life Technologies, Paisley, UK) supplemented with 10% v/v heat inactivated fetal bovine serum (FBS), P/S, 2 mM glutamine (all Life Technologies), 50 μg/mL amphotericin B and 50 μg/mL ascorbic acid (Sigma) and maintained at 37°C in a humidified atmosphere containing 5% (v/v) CO2. Human NP cells, at passage 2, were seeded into chamber slides (Life Technologies) at a density of 1 × 104 cells/well and allowed to adhere overnight. Media aspirated, and cells washed with phosphate‐buffered saline (PBS) before fixing with 10% (v/v) neutral buffered formalin (Leica Microsystems) for 5 minutes at room temperature. These cells were utilized to investigate colocalization with cytoskeletal components which is difficult to perform in 3D culture.
2.6. Investigation of AQP gene expression in directly extracted human NP cells
RNA was extracted using Trizol (Life Technologies) and cDNA synthesized as previously published.49 Expression of target genes: AQP0‐12 together with housekeeping genes: glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) and 18S rRNA (18S), was investigated using qRT‐PCR employing predesigned primer/probe mixes (Life Technologies) (Supplementary Table 4). qRT‐PCR was conducted on 19 nondegenerate samples (grade ≤4), 13 moderately degenerate samples (grade 4.1‐6.9), 38 severely degenerate samples (grade 7‐12), and 32 samples with evidence of immune cell infiltration identified histologically (Supplementary Table 1). It has previously been identified that infiltrating cells in surgical human NP tissue are immune cells, due to positive CD11b staining, and are morphologically distinct from resident NP cells.52 Results were analyzed using the 2−ΔCt method and presented as relative gene expression normalized to the average CT of GAPDH and 18S. Both housekeeping genes used in this study have been previously shown to be stably expressed, using geNorm algorithm, in human IVD samples across all grades of degeneration.53
2.7. Isolation and culture of NCs from Canine discs
NCs were isolated from spines of six mongrel dogs, euthanized for other unrelated experiments as previously published,18 24.4 ± 0.9 × 106 (mean ± SD) live NCs were recovered per dog. Cells were encapsulated at 3 × 106/mL in alginate beads as described previously18 and maintained for 28 days in α‐MEM (Life Technologies) at 400 mOsm/L before being fixed in 10% neutral buffered formalin (Leica Microsystems) and embedded into paraffin wax. Four micrometer sections were cut and used in immunofluorescence experiments.
2.8. Immunohistochemistry
After identifying which AQPs were expressed at gene level in directly extracted human NP cells, investigations into AQP protein expression in human and canine NP tissue were performed. Thirty human tissue samples and 35 canine tissue samples were selected to represent the grades of degeneration (Supplementary Tables 1 & 2). IHC was conducted to investigate the protein expression of AQP0, 2, 3, 4, 6, 7, and 9 in human and AQP0‐7 and 9 in canine IVD tissues as described previously54, specific IHC details provided in Supplementary Table 3.
2.9. Immunofluorescence
Sectioned canine NC cells in alginate were dewaxed and rehydrated as previously described.18 Fixed human NP cells and canine alginate sections were washed in PBS for 3 × 5 minutes and human NP cells permeabilized in PBS 1% (v/v) Triton‐X100 (Sigma‐Aldrich) for 5 minutes at room temperature (RT). For both human NP cells and canine samples, nonspecific binding sites were blocked for 1 hour at RT in 1% (w/v) bovine serum albumin (BSA) (Sigma‐Aldrich) in PBS with either 25% (w/v) rabbit or goat serum (Abcam). Samples were incubated overnight at 4°C with either mouse monoclonal or rabbit polyclonal primary antibodies in blocking solution (Supplementary Table 5). Mouse or rabbit IgG controls (Abcam) were used in place of primary antibodies at an equal protein concentration. After washing in 0.1% (v/v) Tween 20 (Sigma‐Aldrich) in PBS (PBST) samples were incubated with a 1:500 dilution of fluorescent‐conjugated secondary antibody (Supplementary Table 4) for 1 hour at room temperature. After 3 × 5 minutes washes in PBST slides were mounted with diamond antifade mountant with DAPI (4',6‐diamidino‐2‐phenylindole) (Life Technologies).
2.10. Image capture and statistical analysis
Slides were visualized with an Olympus BX60 microscope and images captured using software program CellSens (Olympus, Southend, UK) and MicroCapture v5.0 RTV digital camera (Q Imaging, Buckinghamshire, UK). IHC staining was represented as percentage immunopostivity following analysis of 200 NP cells per sample. Canine analysis was restricted to morphologically distinct NP tissue and the presence of NC cells was identified by positive brachyury staining and morphological features (clusters of large, vacuolated cells) within the NP on consecutive sections. Data was shown to be nonparametric, therefore proportionality tests were performed to identify differences between the proportions of samples expressing mRNA for AQPs in directly extracted cohorts (P ≤ 0.05). Kruskall‐Wallis with Dwass‐Steel‐Critchlow‐Fligner post hoc analysis test was used to identify significant differences in immunopositivity between grades of degeneration in human and canine cohorts (P ≤ 0.05). To determine significant differences in the AQP immunopositivity of NC cells vs NP cells in pair matched canine samples, Wilcoxon's signed ranks test was performed (P ≤ 0.05).
3. RESULTS
3.1. Identification of aquaporin gene expression in human NP cells
The native gene expression of all mammalian AQP family members in directly extracted NP cells was investigated using qRT‐PCR, of which AQPs 0‐7 and 9 were identified within human IVD tissue (Figure 1). AQP8 was only expressed in 4 out of 97 samples, with no trends seen across grades of degeneration (data not shown) and AQPs 10, 11, and 12 were not identified in any sample. Whilst the levels of AQP0‐7 and 9 expression did not alter significantly between grades of degeneration, significant differences were seen in terms of the proportion of samples expressing AQPs between grades of degeneration (Figure 1). Proportions of samples expressing AQP0, 1, and 2 were increased in moderately or severely degenerate samples compared to nondegenerate samples, whilst AQP3 was decreased in moderately degenerate samples compared to severely degenerate samples (Figure 1). In contrast AQP9 was decreased in degenerate and infiltrated samples compared to nondegenerate samples and AQP2 and 7 in degenerate compared to moderately degenerate samples (P ≤ 0.05) (Figure 1). The proportions of samples expressing AQP4, 5, and 6 did not change significantly across grades of degeneration (Figure 1).
Figure 1.
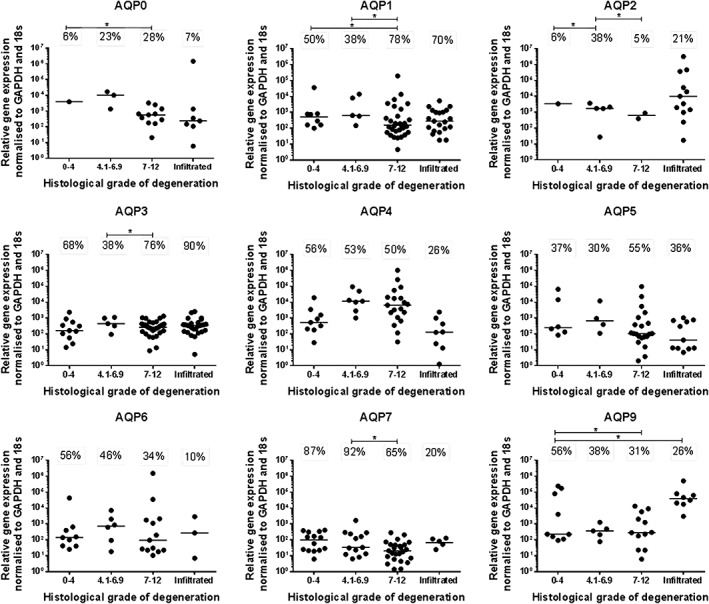
Gene expression of aquaporin family members within directly extracted human nucleus pulposus (NP) cells. The number of disc samples in each cohort expressing target genes is represented as percentage expression; the median value is shown by the bars. Target gene expression is separated into cohorts determined by histological grade of degeneration and evidence of infiltrating cells—grade 0‐4 (nondegenerate n = 16), grade 4.1‐6.9 (moderately degenerate n = 13), grade 7‐12 (severely degenerate n = 38), infiltrated (n = 30), total number of samples n = 97. Statistical analysis was performed on proportions expressed P ≤ 0.05
3.2. Immunodetection of aquaporin proteins in human IVD tissue
We have previously identified AQP1 and 5 in human IVD tissues at protein levels,49 therefore AQPs 0, 2, 3, 4, 6, 7, and 9 were investigated here for their presence in human NP tissue in different histological grades of degeneration. Protein expression of all AQPs investigated was identified in the NP cells of human IVD tissues. However, the percentage immunopositivity for AQPs 0, 3, 6, and 9 was not significantly altered between grades of degeneration (Supplementary Figure 2). The percentage immunopositivity for AQP2 in NP tissue significantly increased in severely degenerate discs compared to moderately degenerate discs (Figure 2) (P ≤ 0.05), and the percentage immunopositivity for AQP7 was increased in severely degenerate samples compared to nondegenerate and moderately degenerate samples (Figure 2) (P ≤ 0.05). In contrast, the percentage immunopositivity for AQP4 was significantly reduced in the moderately and severely degenerate cohorts compared to nondegenerate samples (Figure 2) (P ≤ 0.05). Correlation analysis was determined for percentage of immunopositve cells with grade of degeneration and age, AQP4 was negatively correlated with grade of degeneration (P < 0.0001) but not age (P = 0.1614) (Supplementary Figure 3). Whilst AQP7 was positively correlated with grade of degeneration (P = 0.0012), but not age (P = 0.1116) (Supplementary Figure 3). Other AQPs were not seen to correlate with grade of degeneration or age (Data not shown).
Figure 2.
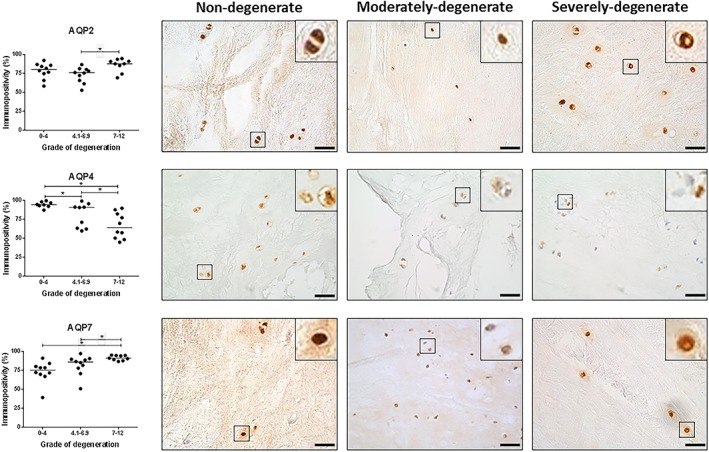
Immunopositivity of aquaporin 2, 4, and 7 within human intervertebral disc (IVD). Three cohorts were investigated for the expression of aquaporin (AQP) family members in human IVD; nondegenerate (grade 0‐4) (n = 10), moderately degenerate (grade 4.1‐6.9) (n = 10), severely degenerate (grade 7‐12) (n = 10). Immunopositive cells were expressed as a percentage of total count; the median value is represented by the bars. Statistical analysis was performed on changes of percentage immunopositivity P ≤ 0.05. Scale bar 50 μm
3.3. Aquaporin expression and localization in cultured human NP cells
All aquaporins, which were expressed in native human NP tissue (AQPs 0‐7 and 9) were also identified in monolayer cultured human NP cells. To identify potential localization of AQPs, expression was compared to cytoskeletal components F‐actin and β‐tubulin. Differential localization was seen for AQPs in human NP cells (Figure 3). AQP1 and 5 showed punctate staining pattern, whereas AQP0, 3, 6, 7, and 9 staining was dispersed throughout NP cells. Interestingly the expression of AQP2 appeared to co‐localize with actin at cell pseudopodia and AQP4 appeared to colocalize with β‐tubulin (Supplementary Figure 4), potentially indicating a means of transporting AQP4 throughout the cell in response to certain stimuli.
Figure 3.
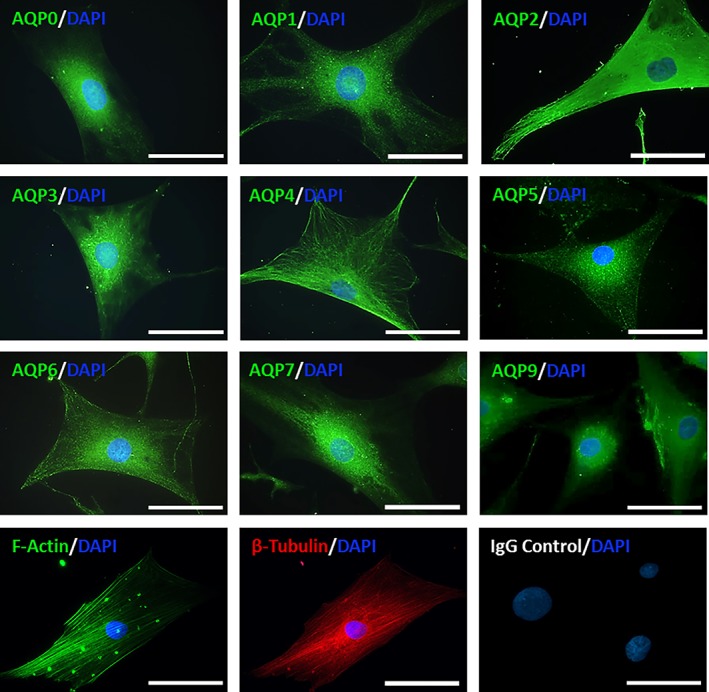
Localization of aquaporins within human nucleus pulposus (NP) cells. All aquaporins expressed in human NP tissue are also expressed in extracted human NP cells in monolayer culture at passage 2. The localization of aquaporins (AQPs) is compared to the expression of cytoskeletal components F‐actin and β‐tubulin within cells. Scale bar 20 μm
3.4. Aquaporin expression in canine IVD tissue
Expression of aquaporins, identified in human NP tissue were also investigated in native NC and NP cells within canine NP tissue. All AQPs investigated were expressed within both NC and NP cells in canine discs (Figure 4). Brachyury staining and the presence of distinct morphological features, such as clusters of large, vacuolated cells, enabled the distinction between NC and NP cells. NC cells were only observed in TG 1‐3 (Figure 4). The number of NP cells expressing AQP2 decreased between TG 1‐3 when compared with grade 4 (P ≤ 0.05) but the number of NC cells expressing AQP2 was not altered by grade (Figure 4). AQP5 immunopositivity of NP cells significantly decreased from grade 1 to 4 (P ≤ 0.05) whilst NC cell expression remained unaffected by degeneration (Figure 4). As TG increased from 1 and 2 up to 4 and 5 AQP6 expression in NP cells significantly increased (P ≤ 0.05), which was also seen to correlate with TG and age (P < 0.0001) (Supplementary Figure 5), however, AQP6 expression in NC cell populations decreased with increasing TG from 1 to 3 (P ≤ 0.05). NP cell expression of AQP7 also increased from grade 1 and 2 to grade 5 (P ≤ 0.05), which was also seen to correlate with TG and age (P < 0.0001) (Supplementary Figure 5), whereas NC expression remained constant across all TG. AQP9 immunopositivity significantly decreased in NP cells between grade 1 and 2 to grade 5 (P ≤ 0.05) and in NC cells between grade 1 and 3 (P ≤ 0.05). AQPs 0, 1, 3, and 4 were also expressed by both native canine NC and NP cells irrespective of TG. The percentage of AQP2, 3, 6, 9, and brachyury expressed by NC cells was significantly increased compared to NP cells in pair matched canine IVD samples (P ≤ 0.05). In contrast, the percentage of AQP1 immunopositivity in NC cells was significantly reduced compared to NP cells in pair matched canine samples (P ≤ 0.05). When AQP expression was compared to histological grade of degeneration similar trends were observed (Supplementary Figure 6), furthermore the histological grade of degeneration was seen to correlate with the Thompson grading for canine discs (P < 0.0001)(Supplementary Figure 7). AQP expression was maintained in NC cell clusters cultured in alginate beads for 28 days in αMEM at 400 mOsm/L (Figures 5 and 6). Interestingly strong immunopositivity was observed localized to the membranes of vacuoles within NC cell clusters, particularly for AQP2, 5, 6, and 9 (Figures 5 and 6).
Figure 4.
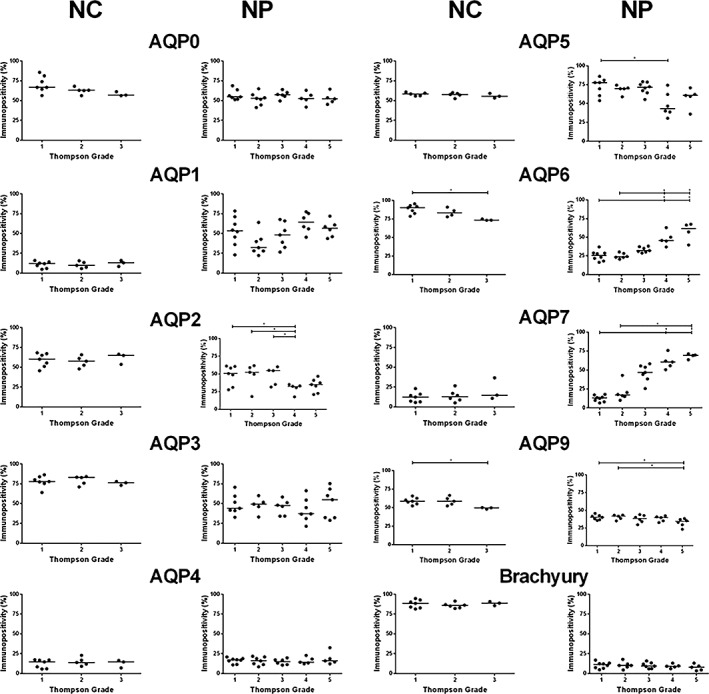
Immunopositivity of aquaporins (AQPs) in notochordal cell (NC) and nucleus pulposus (NP) cells within canine intervertebral disc (IVD). Three cohorts were investigated for the expression of AQP family members in NC cells; Thompson grade 1‐3, and NP cells; Thompson grade 1‐5. Immunopositive cells were expressed as a percentage of total count; the median value is represented by the bars. Statistical analysis was performed on changes of percentage immunopositivity P ≤ 0.05
Figure 5.

Expression and localization of aquaporins (AQPs) in native canine intervertebral disc (IVD) tissue cells. Immunocytochemistry/Immunofluorescence (ICC/IF) images show expression of AQPs in NC cell clusters after 3D culture and localization at vacuolar‐like membranes. Scale bar 20 μm. immunohistochemistry (IHC) staining represents AQP expression in canine NC cells and NP cells across Thompson grade 2 and 5. Scale bar 50 μm. Brachyury is used as a positive marker of NC cells
Figure 6.
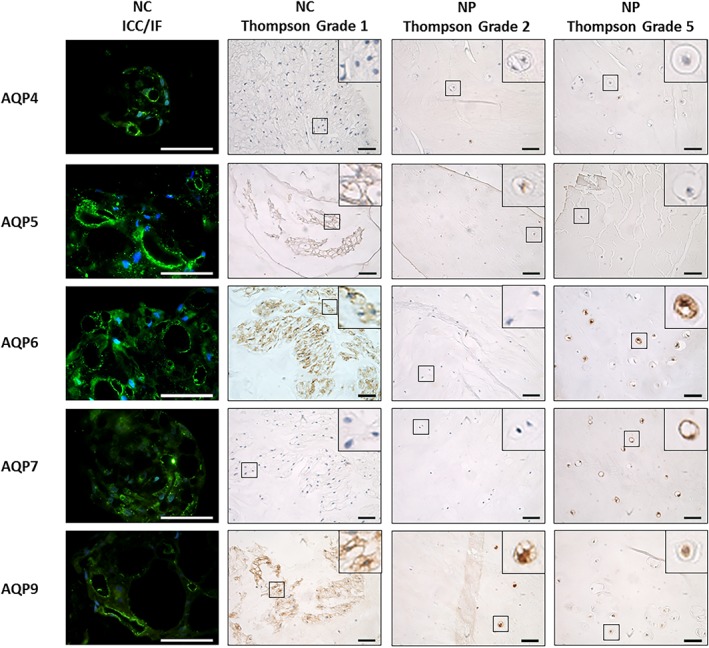
Expression and localization of aquaporins (AQPs) in native canine intervertebral disc (IVD) tissue cells. Immunocytochemistry/Immunofluorescence (ICC/IF) images show expression of AQPs in notochordal cell (NC) cell clusters after 3D culture and localization at vacuolar‐like membranes. Scale bar 20 μm. immunohistochemistry (IHC) staining represents AQP expression in canine NC cells and nucleus pulposus (NP) cells across Thompson grade 2 and 5. Scale bar 50 μm
4. DISCUSSION
NP cells reside within an osmotically challenging environment,6, 7, 8, 9 where cells must be able to adapt to their environment to allow correct function. Previously AQPs 1, 2, 3, and 5 have been shown to be expressed by NP cells,14, 46, 47, 48, 49 expression of AQP1, 2, and 5 is regulated by physiological conditions found in the disc such as hyperosmolality14 and AQP1 and 5 expression levels decrease with IVD degeneration.49 This highlights that water transport via AQPs in the disc must be tightly controlled to allow the survival and function of NP cells, and such regulation is diminished in degeneration, possibly aggravating the catabolic cascade. However, to date, no studies have investigated the expression of all AQP family members in the IVD or determined how their expression is altered with degeneration, thus it is difficult to gain a complete understanding of the roles for AQPs in the IVD. For the first time we show the expression of 10 AQP family members, AQPs 0‐9, in human and canine NP tissue. We determined that the expression of certain AQPs is altered during IVD degeneration of humans and canines (Supplementary Figure 4). Utilizing canine discs we were also able to investigate expression and localization during cellular maturation and demonstrated nine AQPs were expressed in canine NC cells, which were particularly associated with vacuolar membranes, suggesting a role in water transport into vacuoles.
4.1. AQP expression in IVD tissues
Our results agree with studies that have shown AQP1, 2, 3, and 5 expression in NP cells.14, 46, 47, 48, 49 However whilst Richardson et al46 identified that AQP1 and AQP3 were expressed within human IVD in agreement with our study, they failed to demonstrate expression of AQP2. However, in the current study both gene and protein expression of AQP2 was identified in human discs and protein in canine discs. In the earlier study46 only 10 pm samples from human discs were used for the identification of AQP protein expression, in contrast, here we have used a larger sample size with a total of 102 human samples to assess gene expression, and 30 surgical and PM human and 35 pm canine samples for IHC analysis. Our data is in agreement with a study by Gajghate et al14 which demonstrated AQP2 gene and protein expression in rat and human IVD tissues. Limited AQP family members have been identified in other musculoskeletal tissues, including AQP1, 3, 4, and 9 in articular cartilage,55, 56, 57, 58, 59 AQP1 and AQP9 in synovial tissues,55, 60 AQP1 and three in osteoblasts,61, 62 and AQP9 expression in osteoclasts.63, 64 Here, for the first time gene expression of AQPs 0, 4, 6‐9 was shown in human NP tissue, although AQP8 was only expressed in a small number of samples at gene level (n = 4). Furthermore we demonstrate for the first time protein expression of AQPs 0, 4, 6, 7, and 9. Together this data demonstrates NP cells express many AQPs which may play important roles in the disc such as contributing towards water homeostasis, solute transport, cell volume regulation, cell adhesion, and protein localization.45, 65
All AQPs expressed within human discs were also identified in canine discs from NCD and CD dog breeds, which were used to study AQP expression in the transition from NCs to NPs. Both NC and NP cells from canine discs expressed all AQPs investigated suggesting AQP family members are important during the development and maturation of the IVD. In canines and humans, the proportion of cells expressing AQPs and changes in their expression during degeneration differed, suggesting functions of AQPs during different stages of disc biology and within different species may vary. On the other hand, differences in sample preparation methods between human and canine samples may have resulted in the differences in immunopositivity results between species, as decalcification which was utilized for canine but not human samples, has been shown to effect IHC staining on IVD tissue previously.54
4.2. AQP expression during maturation of the IVD
Whilst NC and NP cells were seen to express all AQPs investigated, AQP expression was observed on higher numbers of NC cells vs NP cells for AQPs 2, 3, 6, 9, whilst AQP1 was seen on higher numbers of NP cells than NCs in pair matched canine discs. This may be due to differences in the disc environment, such as osmolality, nutrition, pH and mechanical loading that both NC and NP cells withstand during development, suggesting a role for AQPs in NC physiology. Of particular note IHC on native canine tissue and immunocytochemistry on cultured canine NC cells revealed AQP expression along vacuolar membranes, particularly for AQP2, 5, 6, and 9. This expression may suggest a physiological role for AQPs on vacuole membranes, indicating potential water and solute transport regulation in NC cells. As AQP5, along with AQP1 and 4, contributes to regulatory volume decrease and the control of cell volume in response to osmotic flux, there is also evidence to suggest AQP2 is also involved.66 Therefore AQP2 and 5 expression may indicate that vacuoles regulate NC cell responses to their hyperosmolar environment, and their decreased expression during NC to NP transition could regulate the decreased cell size seen during maturation. AQP6 expression potentially indicates vacuoles play a role in regulating acid‐base balance in NC cells. In the kidney, where AQP6 is expressed intracellularly, low pH activates the anion permeability of AQP667 and increases AQP6 expression.68 An increase in AQP6 expression in response to low pH also potentially explains the increase in AQP6 observed in canine NP cells during degeneration, where extracellular pH decreases.
4.3. AQP expression during degeneration of the IVD
Expression of AQP1 and 5 in the disc has been shown to be sensitive to degeneration within human IVD tissue previously,49 with levels decreasing with degeneration. Here, AQP4 protein expression was also shown to decrease during human IVD degeneration. This potentially indicates shared functions of these AQPs which, during degeneration when expression is decreased, results in a diminished ability of NP cells to control water transport and cell volume regulation as the surrounding environment becomes increasingly osmotically challenging. This decrease in AQP4 expression is of particular importance as within the AQP family, AQP4 has the fastest rate of water permeability followed by AQP1 and 5,65, 69 and these AQPs have all been implicated in the control of regulatory volume decrease when cells are exposed to hypo‐osmotic stimuli.70, 71 Thus the decreased expression of AQPs 1, 4, and 5 seen during human IVD degeneration could be a result of hypo‐osmotic stimuli observed during IVD degeneration.2
AQP gene expression investigated in patient‐derived NP tissue identified that no AQPs were expressed in 100% of patient samples, this indicates there is biological variability across patients but AQP gene expression was not altered due to IVD degeneration. In contrast, AQP protein expression was observed in all patient‐derived NP tissue used for IHC and percentage immunopositivity was altered during degeneration, for AQP2, 4, and 7. This suggests that AQP gene expression is more transient than stably expressed AQP proteins, which may be more reliable when determining AQP expression and regulation in IVD tissue and cells in the future.
An important response to osmotic stress is translocation of AQPs to the plasma membrane, in primary rat cortical astrocytes, HEK293 and MDCK cells AQP4 is rapidly translocated to the plasma membrane in response to osmotic changes.72, 73 This translocation is governed by many mechanisms including vesicle transport along microtubules.72, 73 In human NP cells we observed that AQP4 co‐localized with β‐tubulin, a major component of microtubules, indicating AQP4 is possibly translocated between the cytosol and plasma membrane in order to respond and adapt to changes in the extracellular osmolality. However, this colocalization was observed in monolayer NP cells which may not represent the localization seen in vivo as monolayer culture is known to induce dedifferentiation. It would be pertinent to investigate this localization within 3D culture however visualization is complicated in 3D culture. The investigation of localization in canine NP and NC would also be interesting to enable comparison between human and canine cells and determine if this colocalization with cytoskeletal proteins is species specific.
In contrast to the decrease seen in AQPs 1, 4, and 5 with degeneration, the number of NP cells expressing AQP2 and AQP7 increased in moderate or severely degenerate human IVD tissues respectively. This may indicate that AQP2 and 7 are regulated by different underlying mechanisms and have different functions compared to AQP1, 4, and 5. The increase in the number of cells expressing these proteins may be a consequence of degeneration or a potential repair mechanism employed by NP cells to re‐establish homeostasis dysregulated during degeneration. As the disc degenerates, the micro‐environment in which cells reside, irreversibly changes, such as a decrease in disc pH.7 This potentially explains the increase in AQP2 protein expression, as AQP2 expression is increased in the rat kidney when exposed to lowered pH.74 Immunopositivity of AQP3, 6, and 9 in human IVD tissue was not altered during degeneration, yet the majority of NP cells in native IVD tissue expressed all three AQPs, potentially indicating roles which may not be sensitive to IVD degeneration.
Within canine discs some effects mirrored the effects seen in human discs; however some AQPs showed differential responses between species that remain to be explored. AQP5 immunopositivity decreased49 and AQP7 immunopositivity increased in human and canine NP cells with IVD degeneration. It is also important to note that only the percentage of cells expressing AQP proteins during IVD maturation and degeneration was investigated in this study, other more quantitative methods must be used in future investigations to determine how the relative expression levels of AQPs may be affected during IVD biology, which may not be represented by percentage immunopositivity.
5. CONCLUSION
As the disc is an osmotically challenged tissue which is further altered during degeneration, NP cells must employ several tightly regulated mechanisms to survive and function within their unique environment. We postulate that the expression of numerous AQP family members in the disc highlights the importance of maintaining intricate control over regulation of water transport and cell volume in NP cells. The increased proportion of NP cells expressing AQP2 and AQP7 during degeneration may reflect consequences or potential repair mechanisms employed by the disc as NP cells lose their ability to adapt to the degenerate hypo‐osmolar environment, which is identified by the loss of AQP1, AQP4, and AQP5 expression during degeneration.
The expression of numerous AQPs within the disc may highlight the importance of controlling water and solute transport in relation to maintaining NC and NP cell function. As yet the actual roles of AQPs within disc maturation and degeneration are unknown; further investigation is warranted to identify mechanisms of regulation and functions of AQPs to elucidate the true action of these transmembrane channels proteins on the overall behavior and health of the disc.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Author contributions
J.S., M.C., R.B., M.T. and C.L.M. contributed to study design. R.D. performed some of the RT‐qPCR experiments, F.B. isolated, cultured, and processed canine alginate beads, and prepared canine sections. J.S. performed all other experiments, completed data and statistical analysis, and drafted the manuscript. C.L.M. conceived the study, participated in the design and coordination, aided data analysis, secured funding, and critically revised the manuscript. All authors read and approved the final manuscript.
Supporting information
Supplementary Figure 1 Experimental design of methods used in this study.
Supplementary Figure 2 Immunopositivity of aquaporin 0, 3, 6 and within human IVD.
Supplementary Figure 3 Human IHC correlation. In human NP tissue AQP4 and 7% immunopostivity significantly correlated with grade of degeneration but not with age. Correlations determined using Spearman's rank correlation *P < 0.05.
Supplementary Figure 4 ICC/IF identifying AQP4 (green) and β‐tubulin (red) localization in human NP cells. Merged image shows colocalization of AQP4 and β‐tubulin (orange). Nuclei counterstained with DAPI(4',6‐diamidino‐2‐phenylindole) (blue). Scale bar 20 μm.
Supplementary Figure 5.Canine IHC correlation. In canine NP tissue AQP6 and 7 % immunopositivity significantly correlated with Thompson grade and age. Correlations determined using Spearman's rank correlation *P ≤ 0.05.
Supplementary Figure 6.Canine IVD tissue was analysed with the H & E grading system for the expression of AQP family members in canine NC and NP cells. Non‐degenerate (grade 0‐4), moderately degenerate (grade 4.1‐6.9), severely degenerate (grade 7‐12). Immunopositive cells were expressed as a percentage of total count; the median value is represented by the bars. Statistical analysis was performed on changes of percentage immunopositivity *P ≤ 0.05.
Supplementary Figure 7.H & E and Thompson grading systems for canine IVD tissue significantly correlate. Correlations determined using Spearman's rank correlation *P ≤ 0.05.
Supplementary Table 1 Human intervertebral disc sample information. Samples were separated into different grades, 0‐4 (Nondegenerate, ND), 4.1‐6.9 (Moderately‐degenerate, MD), and 7‐12 (severely‐degenerate, SD). X indicates samples used for direct extraction (DE) of RNA and RT‐qPCR and immunohistochemistry (IHC).
Supplementary Table 2 Canine sample information. Nonchondrodystrophic (NC) = 0, chondrodystrophic (C) = 1, not determined (ND).
Supplementary Table 3 (A) Antibody information used for IHC on human IVD tissue, (B) antibody information used for IHC on canine IVD tissue. All antibodies purchased from Abcam, Cambridge, UK. ab6720 (goat anti Rabbit) and ab7074 (rabbit anti mouse) secondary antibodies at 1:500 were used. After endogenous peroxidase blocking antigen retrieval methods were used. Enzyme: slides were added to 0.1% (w/v) α‐chymotrypsin (Sigma‐Aldrich) in 1×tris‐buffered saline (TBS) containing 1% (w/v) CaCl2 for 30 minutes at 37°C. Heat: slides were added to 50 mM tris, pH 9.5 (pre‐heated to 60°C) and irradiated at 40% power in a microwave oven (Sanyo) for 5 minutes, left to stand at room temperature for 1 minute and further irradiated for 5 minutes at 20% power. Slides were then cooled at room temperature for 15 minutes.
Supplementary Table 4 Probe IDs of TaqMan gene expression assays used in this study. All primers were purchased from Thermo Fisher Scientific (Paisley, UK).
Supplementary Table 5 (A) Antibody information used for ICC/IF on human NP cells and 3D‐cultured canine IVD cells.
ACKNOWLEDGEMENTS
The Authors would like to thank the surgeons: Mr Ashley Cole, Mr Neil Chiverton, Mr Antony Michael, Mr Lee Breakwell, Mr Michael Athanassacopoulos, Mr Marcel Ivanov, and Mr James Tomlinson from Northern General Hospital, Sheffield Teaching Hospitals NHS Trust for supply of human disc samples. The funding contribution of the Dutch Arthritis Society (LLP22) is acknowledged.
Snuggs JW, Day RE, Bach FC, et al. Aquaporin expression in the human and canine intervertebral disc during maturation and degeneration. JOR Spine. 2019;2:e1049. 10.1002/jsp2.1049
Funding information Dutch Arthritis Society, Grant/Award Number: LLP22; Sheffield Hallam University Vice Chancellors PhD Fellowship
REFERENCES
- 1. Urban JP, Holm S, Maroudas A, Nachemson A. Nutrition of the intervertebral disk. An in vivo study of solute transport.Clin Orthop Relat Res. 1977;129:101‐114. [PubMed] [Google Scholar]
- 2. Ishihara H, Warensjo K, Roberts S, Urban JP. Proteoglycan synthesis in the intervertebral disk nucleus: the role of extracellular osmolality.Am J Physiol. 1997;272:C1499‐C1506. [DOI] [PubMed] [Google Scholar]
- 3. Maroudas A, Muir H, Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage.Biochim Biophys Acta. 1969;177:492‐500. [DOI] [PubMed] [Google Scholar]
- 4. Maroudas A. Distribution and diffusion of solutes in articular cartilage.Biophys J. 1970;10:365‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Urban JP, Holm S, Maroudas A. Diffusion of small solutes into the intervertebral disc: as in vivo study.Biorheology. 1978;15:203‐221. [DOI] [PubMed] [Google Scholar]
- 6. Kraemer J, Kolditz D, Gowin R. Water and electrolyte content of human intervertebral discs under variable load.Spine (Phila Pa 1976). 1985;10:69‐71. [DOI] [PubMed] [Google Scholar]
- 7. Urban JPG. The role of the physicochemical environment in determining disc cell behaviour.Biochem Soc Trans. 2002;30:858‐864. [DOI] [PubMed] [Google Scholar]
- 8. van Dijk B, Potier E, Ito K. Culturing bovine nucleus pulposus explants by balancing medium osmolarity.Tissue Eng Part C Methods. 2011;17:1089‐1096. [DOI] [PubMed] [Google Scholar]
- 9. Johnson ZI, Shapiro IM, Risbud MV. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: evolving role of TonEBP.Matrix Biol. 2014;40:10‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Urban JP, McMullin JF. Swelling pressure of the inervertebral disc: influence of proteoglycan and collagen contents.Biorheology. 1985;22:145‐157. [DOI] [PubMed] [Google Scholar]
- 11. McMillan DW, Garbutt G, Adams MA. Effect of sustained loading on the water content of intervertebral discs: implications for disc metabolism.Ann Rheum Dis. 1996;55:880‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Connell GD, Newman IB, Carapezza MA. Effect of long‐term osmotic loading culture on matrix synthesis from intervertebral disc cells.Biores Open Access. 2014;3:242‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neidlinger‐Wilke C, Mietsch A, Rinkler C, Wilke HJ, Ignatius A, Urban J. Interactions of environmental conditions and mechanical loads have influence on matrix turnover by nucleus pulposus cells.J Orthop Res. 2012;30:112‐121. [DOI] [PubMed] [Google Scholar]
- 14. Gajghate S, Hiyama A, Shah M, et al. Osmolarity and intracellular calcium regulate aquaporin2 expression through TonEBP in nucleus pulposus cells of the intervertebral disc.J Bone Miner Res. 2009;24:992‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord.Dev Dyn. 2010;239:2141‐2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bergknut N, Smolders LA, Grinwis GCM, et al. Intervertebral disc degeneration in the dog. Part 1: Anatomy and physiology of the intervertebral disc and characteristics of intervertebral disc degeneration.Vet J. 2013;195:282‐291. [DOI] [PubMed] [Google Scholar]
- 17. Smolders LA, Bergknut N, Grinwis GCM, et al. Intervertebral disc degeneration in the dog. Part 2: Chondrodystrophic and non‐chondrodystrophic breeds.Vet J. 2013;195:292‐299. [DOI] [PubMed] [Google Scholar]
- 18. Spillekom S, Smolders LA, Grinwis GCM, et al. Increased osmolarity and cell clustering preserve canine notochordal cell phenotype in culture.Tissue Eng Part C Methods. 2014;20:652‐662. [DOI] [PubMed] [Google Scholar]
- 19. de Vries SAH, van Doeselaar M, Meij BP, Tryfonidou MA, Ito K. The stimulatory effect of notochordal cell‐conditioned medium in a nucleus pulposus explant culture.Tissue Eng Part A. 2016;22:103‐110. [DOI] [PubMed] [Google Scholar]
- 20. Mehrkens A, Matta A, Karim MZ, et al. Notochordal cell‐derived conditioned medium protects human nucleus pulposus cells from stress‐induced apoptosis.Spine J. 2017;17:579‐588. [DOI] [PubMed] [Google Scholar]
- 21. Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari‐Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration.Spine (Phila Pa 1976). 2000;25:487‐492. [DOI] [PubMed] [Google Scholar]
- 22. Haefeli M, Kalberer F, Saegesser D, Nerlich AG, Boos N, Paesold G. The course of macroscopic degeneration in the human lumbar intervertebral disc.Spine (Phila Pa 1976). 2006;31:1522‐1531. [DOI] [PubMed] [Google Scholar]
- 23. Freemont AJ, Watkins A, Le Maitre C, Jeziorska M, Hoyland JA. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy.J Pathol. 2002;196:374‐379. [DOI] [PubMed] [Google Scholar]
- 24. Maitre L, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration.Arthritis Res Ther. 2007;9:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maitre L, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration.Biochem Soc Trans. 2007;35:652‐655. [DOI] [PubMed] [Google Scholar]
- 26. Le Maitre CL, Binch AL, Thorpe AA, Hughes SP. Degeneration of the intervertebral disc with new approaches for treating low back pain.J Neurosurg Sci. 2015;59:47‐61. [PubMed] [Google Scholar]
- 27. Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine.Spine (Phila Pa 1976). 1996;21:218‐224. [DOI] [PubMed] [Google Scholar]
- 28. Burke JG, Watson RWG, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators.J Bone Joint Surg Br. 2002;84:196‐201. [DOI] [PubMed] [Google Scholar]
- 29. Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls.Spine (Phila Pa 1976). 2005;30:44‐53; discussion 54. [DOI] [PubMed] [Google Scholar]
- 30. Le Maitre C, Freemont AJ, Hoyland J. The role of interleukin‐1 in the pathogenesis of human intervertebral disc degeneration.Arthritis Res Ther. 2005;7:R732‐R745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maitre L, Hoyland F. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL‐1beta and TNFalpha expression profile.Arthritis Res Ther. 2007;9:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Studer RK, Vo N, Sowa G, Ondeck C, Kang J. Human nucleus pulposus cells react to IL‐6: independent actions and amplification of response to IL‐1 and TNF‐α.Spine (Phila Pa 1976). 2011;36:593‐599. [DOI] [PubMed] [Google Scholar]
- 33. Andrade P, Hoogland G, Garcia MA, Steinbusch HW, Daemen MA, Visser‐Vandewalle V. Elevated IL‐1β and IL‐6 levels in lumbar herniated discs in patients with sciatic pain.Eur Spine J. 2013;22:714‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, Iatridis J. A role for TNFα in intervertebral disc degeneration: a non‐recoverable catabolic shift.Biochem Biophys Res Commun. 2013;433:151‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content.Nat Rev Rheumatol. 2014;10:44‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc.Spine (Phila Pa 1976). 2000;25:3005‐3013. [DOI] [PubMed] [Google Scholar]
- 37. Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc.J Pathol. 2004;204:47‐54. [DOI] [PubMed] [Google Scholar]
- 38. Patel KP, Sandy JD, Akeda K, et al. Aggrecanases and aggrecanase‐generated fragments in the human intervertebral disc at early and advanced stages of disc degeneration.Spine (Phila Pa 1976). 2007;32:2596‐2603. [DOI] [PubMed] [Google Scholar]
- 39. Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL‐1 and TNF in matrix degradation in the intervertebral disc.Rheumatology. 2008;47:809‐814. [DOI] [PubMed] [Google Scholar]
- 40. Pockert AJ, Richardson SM, Le Maitre CL, et al. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration.Arthritis Rheum. 2009;60:482‐491. [DOI] [PubMed] [Google Scholar]
- 41. Zhao C‐Q, Zhang Y‐H, Jiang S‐D, Li H, Jiang LS, Dai LY. ADAMTS‐5 and intervertebral disc degeneration: the results of tissue immunohistochemistry and in vitro cell culture.J Orthop Res. 2011;29:718‐725. [DOI] [PubMed] [Google Scholar]
- 42. Wuertz K, Urban JPG, Klasen J, et al. Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells.J Orthop Res. 2007;25:1513‐1522. [DOI] [PubMed] [Google Scholar]
- 43. Agre P, King LS, Yasui M, et al. Aquaporin water channels—from atomic structure to clinical medicine.J Physiol. 2002;542:3‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Denker BM, Smith BL, Kuhajda FP, Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules.J Biol Chem. 1988;263:15634‐15642. [PubMed] [Google Scholar]
- 45. Day RE, Kitchen P, Owen DS, et al. Human aquaporins: regulators of transcellular water flow.Biochim Biophys Acta. 2014;1840:1492‐1506. [DOI] [PubMed] [Google Scholar]
- 46. Richardson SM, Knowles R, Marples D, Hoyland JA, Mobasheri A. Aquaporin expression in the human intervertebral disc.J Mol Histol. 2008;39:303‐309. [DOI] [PubMed] [Google Scholar]
- 47. Wang F, Zhu Y. Aquaporin‐1: a potential membrane channel for facilitating the adaptability of rabbit nucleus pulposus cells to an extracellular matrix environment.J Orthop Sci. 2011;16:304‐312. [DOI] [PubMed] [Google Scholar]
- 48. Taş U, Caylı S, Inanır A, et al. Aquaporin‐1 and aquaporin‐3 expressions in the intervertebral disc of rats with aging.Balkan Med J. 2012;29:349‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnson ZI, Gogate SS, Day R, et al. Aquaporin 1 and 5 expression decreases during human intervertebral disc degeneration: novel HIF‐1‐mediated regulation of aquaporins in NP cells.Oncotarget. 2015;6:11945‐11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Palacio‐Mancheno PE, Evashwick‐Rogler TW, Laudier DM, Purmessur D, Iatridis JC. Hyperosmolarity induces notochordal cell differentiation with aquaporin3 upregulation and reduced N‐cadherin expression.J Orthop Res. 2018;36:788‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bach FC, Tellegen AR, Beukers M, et al. Biologic canine and human intervertebral disc repair by notochordal cell‐derived matrix: from bench towards bedside.Oncotarget. 2018;9:26507‐26526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang J, Tian Y, Phillips KLE, et al. Tumor necrosis factor α‐ and interleukin‐1β‐dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1.Arthritis Rheum. 2013;65:832‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Phillips KLE, Cullen K, Chiverton N, et al. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: interleukin‐1 is a master regulator of catabolic processes.Osteoarthr Cartil. 2015;23:1165‐1177. [DOI] [PubMed] [Google Scholar]
- 54. Binch ALA, Cole AA, Breakwell LM, et al. Class 3 semaphorins expression and association with innervation and angiogenesis within the degenerate human intervertebral disc.Oncotarget. 2015;6:18338‐18354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Trujillo E, González T, Marín R, Martín‐Vasallo P, Marples D, Mobasheri A. Human articular chondrocytes, synoviocytes and synovial microvessels express aquaporin water channels; upregulation of AQP1 in rheumatoid arthritis.Histol Histopathol. 2004;19:435‐444. [DOI] [PubMed] [Google Scholar]
- 56. Steinberg J, Ritchie GRS, Roumeliotis TI, et al. Integrative epigenomics, transcriptomics and proteomics of patient chondrocytes reveal genes and pathways involved in osteoarthritis.Sci Rep. 2017;7:8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mobasheri A, Trujillo E, Bell S, et al. Aquaporin water channels AQP1 and AQP3, are expressed in equine articular chondrocytes.Vet J. 2004;168:143‐150. [DOI] [PubMed] [Google Scholar]
- 58. Cai L, Lei C, Li R, Chen WN, Li CM. Aquaporin‐4 blockage by siRNA protects rat articular chondrocytes from IL‐1β‐induced apoptosis by inhibiting p38 MAPK signal pathway.Ann Clin Lab Sci. 2017;47:563‐571. [PubMed] [Google Scholar]
- 59. Takeuchi K, Hayashi S, Matumoto T, et al. Downregulation of aquaporin 9 decreases catabolic factor expression through nuclear factor‐κB signaling ins chondrocytes.Int J Mol Med. 2018;42:1548‐1558. [DOI] [PubMed] [Google Scholar]
- 60. Nagahara M, Waguri‐Nagaya Y, Yamagami T, et al. TNF‐alpha‐induced aquaporin 9 in synoviocytes from patients with OA and RA.Rheumatology (Oxford). 2010;49:898‐906. [DOI] [PubMed] [Google Scholar]
- 61. Barron ML, Rybchyn MS, Ramesh S, et al. Clinical, cellular, microscopic, and ultrastructural studies of a case of fibrogenesis imperfecta ossium.Bone Res. 2017;5:16057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mobasheri A, Wray S, Marples D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays.J Mol Histol. 2005;36:1‐14. [DOI] [PubMed] [Google Scholar]
- 63. Aharon R, Bar‐Shavit Z. Involvement of aquaporin 9 in osteoclast differentiation.J Biol Chem. 2006;281:19305‐19309. [DOI] [PubMed] [Google Scholar]
- 64. Liu Y, Song L, Wang Y, et al. Osteoclast differentiation and function in aquaglyceroporin AQP9‐null mice.Biol Cell. 2009;101:133‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kitchen P, Day RE, Salman MM, Conner MT, Bill RM, Conner AC. Beyond water homeostasis: diverse functional roles of mammalian aquaporins.Biochim Biophys Acta. 2015;1850:2410‐2421. [DOI] [PubMed] [Google Scholar]
- 66. Galizia L, Pizzoni A, Fernandez J, Rivarola V, Capurro C, Ford P. Functional interaction between AQP2 and TRPV4 in renal cells.J Cell Biochem. 2012;113:580‐589. [DOI] [PubMed] [Google Scholar]
- 67. Yasui M, Hazama A, Kwon T‐H, Nielsen S, Guggino WB, Agre P. Rapid gating and anion permeability of an intracellular aquaporin.Nature. 1999;402:184‐187. [DOI] [PubMed] [Google Scholar]
- 68. Promeneur D, Kwon T‐H, Yasui M, et al. Regulation of AQP6 mRNA and protein expression in rats in response to altered acid‐base or water balance.Am J Physiol. 2000;279:F1014‐F1026. [DOI] [PubMed] [Google Scholar]
- 69. Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1‐5 and MIP determined quantitatively by expression of epitope‐tagged constructs in Xenopus oocytes.J Biol Chem. 1997;272:16140‐16146. [DOI] [PubMed] [Google Scholar]
- 70. Benfenati V, Caprini M, Dovizio M, et al. An aquaporin‐4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell‐volume control in astrocytes.Proc Natl Acad Sci U S A. 2011;108:2563‐2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mola MG, Sparaneo A, Gargano CD, et al. The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: a different point of view on the role of aquaporins.Glia. 2016;64:139‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kitchen P, Day RE, Taylor LHJ, et al. Identification and molecular mechanisms of the rapid tonicity‐induced relocalization of the aquaporin 4 channel.J Biol Chem. 2015;290:16873‐16881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mazzaferri J, Costantino S, Lefrancois S. Analysis of AQP4 trafficking vesicle dynamics using a high‐content approach.Biophys J. 2013;105:328‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Amlal H, Sheriff S, Soleimani M. Upregulation of collecting duct aquaporin‐2 by metabolic acidosis: role of vasopressin.Am J Physiol Cell Physiol. 2004;286:C1019‐C1030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Experimental design of methods used in this study.
Supplementary Figure 2 Immunopositivity of aquaporin 0, 3, 6 and within human IVD.
Supplementary Figure 3 Human IHC correlation. In human NP tissue AQP4 and 7% immunopostivity significantly correlated with grade of degeneration but not with age. Correlations determined using Spearman's rank correlation *P < 0.05.
Supplementary Figure 4 ICC/IF identifying AQP4 (green) and β‐tubulin (red) localization in human NP cells. Merged image shows colocalization of AQP4 and β‐tubulin (orange). Nuclei counterstained with DAPI(4',6‐diamidino‐2‐phenylindole) (blue). Scale bar 20 μm.
Supplementary Figure 5.Canine IHC correlation. In canine NP tissue AQP6 and 7 % immunopositivity significantly correlated with Thompson grade and age. Correlations determined using Spearman's rank correlation *P ≤ 0.05.
Supplementary Figure 6.Canine IVD tissue was analysed with the H & E grading system for the expression of AQP family members in canine NC and NP cells. Non‐degenerate (grade 0‐4), moderately degenerate (grade 4.1‐6.9), severely degenerate (grade 7‐12). Immunopositive cells were expressed as a percentage of total count; the median value is represented by the bars. Statistical analysis was performed on changes of percentage immunopositivity *P ≤ 0.05.
Supplementary Figure 7.H & E and Thompson grading systems for canine IVD tissue significantly correlate. Correlations determined using Spearman's rank correlation *P ≤ 0.05.
Supplementary Table 1 Human intervertebral disc sample information. Samples were separated into different grades, 0‐4 (Nondegenerate, ND), 4.1‐6.9 (Moderately‐degenerate, MD), and 7‐12 (severely‐degenerate, SD). X indicates samples used for direct extraction (DE) of RNA and RT‐qPCR and immunohistochemistry (IHC).
Supplementary Table 2 Canine sample information. Nonchondrodystrophic (NC) = 0, chondrodystrophic (C) = 1, not determined (ND).
Supplementary Table 3 (A) Antibody information used for IHC on human IVD tissue, (B) antibody information used for IHC on canine IVD tissue. All antibodies purchased from Abcam, Cambridge, UK. ab6720 (goat anti Rabbit) and ab7074 (rabbit anti mouse) secondary antibodies at 1:500 were used. After endogenous peroxidase blocking antigen retrieval methods were used. Enzyme: slides were added to 0.1% (w/v) α‐chymotrypsin (Sigma‐Aldrich) in 1×tris‐buffered saline (TBS) containing 1% (w/v) CaCl2 for 30 minutes at 37°C. Heat: slides were added to 50 mM tris, pH 9.5 (pre‐heated to 60°C) and irradiated at 40% power in a microwave oven (Sanyo) for 5 minutes, left to stand at room temperature for 1 minute and further irradiated for 5 minutes at 20% power. Slides were then cooled at room temperature for 15 minutes.
Supplementary Table 4 Probe IDs of TaqMan gene expression assays used in this study. All primers were purchased from Thermo Fisher Scientific (Paisley, UK).
Supplementary Table 5 (A) Antibody information used for ICC/IF on human NP cells and 3D‐cultured canine IVD cells.


