Abstract
This study aimed to research the effects of different dietary concentrate to forage (C:F) ratio on growth performance, rumen fermentation and bacteria diversity of barn feeding Tibetan sheep. The experiment contains fiver treatments (HS1, HS2 HS3, HS4 and HS5; n = 8, respectively) based on dietary C:F ratios 0:100, 15:85, 30:70, 45:55, and 60:40, respectively. The ruminal bacterial community structure was investigated through high-throughput sequencing of 16S rRNA genes in V4 hypervariable region. The results showed that increasing dietary concentrate feed level from 0% to 60% exerted a positive effect on DMI, BW gain, gain rate and feed conversation ratio (FCR) in Tibetan sheep. The increases dietary concentrate feed level elevatedNH3-N, propionate and valerate concentrations, whereas, reduced molar ratio of acetate to propionate (A/P ratio) (P < 0.05). For rumen bacterial diversity, increases in dietary concentrate content contributed to lower alpha diversity indexes including Shannon wiener, Chao1 and observed species, meanwhile, significantly increased the abundances of the phylum Bacteroidetes and the genus Prevotella_1 (P < 0.05). In conclusion, increases dietary concentrate content improved the growth performance and Tibetan sheep fed diets of 45% concentrate obtained a better performance; the inclusion of concentrate in feed changed rumen fermentation from acetate fermentation to propionate fermentation, and improved the energy utilization efficiency of Tibetan sheep; the increased in concentrate content significantly reduced rumen bacteria diversity and changed the abundance of some core bacteria.
Keywords: 16S rRNA, Tibetan sheep, Concentrate-to-forage ratio, Rumen bacterial diversity, Rumen fermentation parameter, Growth performance
Introduction
Tibetan sheep (Ovis aries) live exclusively on the Qinghai Tibetan Plateau (QTP) with a altitude greater than 3,000 (An, Dong & Dong, 2005), they have adapted well to extremely harsh environment and ingested grasses as their sole source of nutrition (Sasaki, 1994; Wiener et al., 2003). Under traditional management, Tibetan sheep mainly grazed on natural pasture without concentrate supplementation, and always suffered seasonal live-weight variations and viciously cycled in “alive in summer, strong in autumn, thin in winter, tired in spring”, due to seasonal fluctuations in natural pasture supply and the contradiction between herbage supply and livestock’s requirement on the alpine pasture (Dong et al., 2006; Xue, Zhao & Zhang, 2005; Xu et al., 2017). During growing season (June to Oct.), natural pasture can provide enough herbage for livestock, the local people only drive livestock to alpine pasture for grazing. During non-growing season (Nov to May) and vegetation green-up periods, grazing livestock shared low performance mainly attributed to the decrement in herbage supply (both in quantity and quality). In addition, irrational grazing during this period destroyed regular growth of alpine plants and function recovery of alpine pasture. Previous studies have confirmed that spring short term rest-grazing is an effective measure for the functional recovery of alpine meadows ( Li et al., 2014; Li et al., 2017; Ma et al., 2017), the local government encourages pastoralists to feed their livestock in warm shed during spring grazing break. However, few studies concerning growth performance of barn feeding Tibetan sheep during late non-growing and vegetation green-up periods were conducted, but which was quiet important for providing pastoralists guidance in livestock breeding.
The rumen microorganisms play an important role in the digestion of proteins, carbohydrates, starch, sugars and fats, which provides energy and proteins to the host by producing volatile fatty acids and bacterial proteins through anaerobic fermentation (Ceconi et al., 2015; Jiang et al., 2015) and finally affects ruminants performance. The rumen microorganism can be affected by many factors, such as diet, hosts and geographic region (Clark, 1975; Lee et al., 2012). The dietary nutrition level is a major factor affecting rumen microbial diversity, healthy status and production capacity of ruminants (Clark, 1975). The effect of diet on the structure of rumen microbial communities has been widely investigated in Yak, Tan sheep, Holstein dairy cows, Mehshana buffalo and goats (Pitta et al., 2014; Jiao et al., 2015; Pitta et al., 2016; Wang et al., 2016; Xue et al., 2016). However, as the dominant ruminant and living foundation for local herdsman, the studies of Tibetan sheep have only focused on the growth performance, slaughter performance and economic benefit under different dietary supplementation (Baruah et al., 2012; Chen et al., 2015b; Dodd, Mackie & Cann, 2011; Feng et al., 2013; Lee et al., 2012; Xu et al., 2017), but few comprehensive studies focused on the growth performance, rumen fermentation and rumen microbial communities of Tibetan sheep.
Therefore, this study aimed to investigate the growth performance, rumen fermentation and rumen bacterial diversity of Tibetan sheep under different dietary concentrate-to-forage ratios during late non-growing and green-up periods. We hypothesized that different C: F ratios could affect growth performance, rumen fermentation and rumen bacterial diversity in Tibetan sheep. These results will be of great importance for providing guidance for local herdsmen in Tibetan sheep breeding and for future research on rumen microbial metabolism in Tibetan sheep.
Materials and Methods
Animals and experimental design
This study was conducted at Haibei Demonstration Zone of Plateau Modern Ecological Animal Husandry Scientific and Technology in Haibei Prefecture, Qinghai Province, China (36°54′N, 100°56′E), from April 2016 to July 2016. All animal care procedures were consistent with the guidelines from the Institution of Animal Care and the Ethics Committee of the Northwest Institute of Plateau Biology, Chinese Academy of Sciences (NWIPB20160302). The processing of the samples after collection was performed strictly in accordance with the guidelines of NWIPB.
A total of 40 female yearling Tibetan sheep with familiar body conditions (21.39 ± 1.18 kg BW, and not under current antibiotic treatment) were randomly divided into five treatments (8 in each) under different C:F ratios (on dry matter basis):HS1(0:100), HS2 (15:85), HS3 (30:70), HS4 (45:55) and HS5 (60:40), respectively. All sheep were allocated into 5 pens within a warm shed. The concentrate and oat hay were manually mixed and fed (dry matter) based on 3.5% BW of Tibetan sheep. Before study, all the animals were fed the experimental diet for a 15-day adaptation phase. During formal 80 day experiment, the experimental animals were separately fed twice dairy, at 8:00 am and 5:00 pm. The diets, salt brick and clean water were available throughout the entire experiment. The ingredients and nutrient compositions of the animal diet are shown in Table 1 and Table S1. The concentrate feed used in this study was produced by Menyuan Yongxing Ecological Agricultural Development Co., Ltd., and oat hay are harvested, bundled and stored in the year 2015.
Table 1. The nutritional composition of the whole diets among different treatments (% DM basis).
| Items | Groupsa | ||||
|---|---|---|---|---|---|
| HS1 | HS2 | HS3 | HS4 | HS5 | |
| Ingredient (%) | |||||
| Concentrate feedsc | 0 | 15 | 30 | 45 | 60 |
| Oats hay | 100 | 85 | 70 | 55 | 40 |
| Nutrient content | |||||
| DMb | 93.42 | 92.70 | 91.82 | 91.23 | 90.35 |
| CP | 6.31 | 7.69 | 9.37 | 10.38 | 11.94 |
| Starch (mg/g) | 25.58 | 26.95 | 32.55 | 33.72 | 39.37 |
| EE | 2.13 | 2.21 | 2.36 | 2.37 | 2.49 |
| ADF | 34.1 | 30.68 | 27.33 | 23.79 | 19.64 |
| NDF | 57.64 | 52.04 | 44.85 | 39.05 | 33.12 |
| Calcium | 0.35 | 0.40 | 0.48 | 0.55 | 0.61 |
| Magnesium | 0.24 | 0.27 | 0.32 | 0.35 | 0.42 |
| Phosphorus | 0.22 | 0.27 | 0.33 | 0.38 | 0.45 |
Notes.
The tratments HS1, HS2, HS3, HS4 and HS5 refer to the C:F ratios of 0:100, 15:85, 30:70, 45:55 and 60:40, respectively.
DM, dry matter; CP, crude protein; EE, ether extract; ADF, acid detergent fibre; NDF, neutral detergent fibre.
Manufactured by Menyuan Yongxing Ecological Agricultural Development Co., Ltd. Contained corn (48%), wheat (30%), soybean meal (7%), colza cake (6%) cottonseed meal (5%), salt (1%), pre-mix (1%), CaHPO4 (1%) and CaCO3 (1%).
Measurement and sampling
The diets and oats in each treatment were weighed and recorded daily to estimate individual dry matter intake (DMI). Diet samples of approximately 500 g from each treatment were collected, dried at 60 °C, ground through a 1-mm sieve and stored in a vacuum dryer for nutritional analysis. To measure animal growth performance, Tibetan sheep were weighed before feeding using an electronic balance at the beginning and end of formal experiment. We selected four Tibetan sheep from each treatment group for rumen fluid sampling. Specifically, on 80th day, rumen content samples were obtained 3–4 h after the morning feeding using a stomach tube attached to an electric pump, which has been confirmed to yield similar results for rumen microorganisms and fermentation parameters as sampling using a rumen cannula (Ramos-Morales et al., 2014). The rumen contents were filtered through four layers of sterilized gauze. Approximately 67 mL of liquid was obtained from the rumen of each Tibetan sheep. The rumen fluid was separated into three samples, one (approximately 2 mL) was transferred into sterilized freezing tubes and stored in liquid nitrogen for DNA extraction; the second sample (approximately 15 mL) was immediately used to measure the pH; and the third sample (approximately 50 mL) was used for the assessment of rumen fermentation parameters, including ammonia nitrogen (NH3-N) and rumen volatile fatty acids (VFAs).
Chemical analysis and calculation
The feed samples were fried in an oven at 135 °C for 3 h to obtain the DM (AOAC, 1990; Method No. 930.15). The total N was detected using Kjeldahl method; the crude protein content was calculated as 6.25 × N (Method No. 984.13); the ether extract (EE) was measured using the Soxhlet system (Method No. 954.02); the acid detergent fiber (ADF) and neutral detergent fiber (NDF) of diet were analysed using method described by Soest, Robertson & Lewis (1991); and the starch was measured according to PRC national standard (GB 5009.9-2016).
The body weight gain (BW gain), gain rate, average daily BW gain (ADG) and feed conversion ratio (FCR) were calculated according to the following equations:
where BWf and BWi are the final and initial body weights (kg), respectively, t is the experimental time (d), and DMconsume is the total feed consumed during the experiment (kg DM). The ruminal fluid pH was measured using a portable pH meter (PHSJ-3F; Precision Instruments Company, Shang Hai, China). For VFAs measurements, the rumen fluid was centrifuged at 12,000 g for 15 min, and the VFAs in the supernatant were analysed using a gas chromatograph Agilent 7890A (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with a polar capillary column (DB-WAX, 30 m × 0.25 mm × 0.25 µm) and a flame ionization detector (FID, temperature set at 250 °C). The temperature-programmed conditions were as follows: the temperature was maintained at 60 °C for 2 min, increased to 180 °C at rate of 10 °C/min, and increased to 250 °C at rate of 20 °C/min; the shunt ratio was 20:1; the flow rate was 1 mL/min; and the inlet temperature was 200 °C. The NH3-N content in the supernatant was quantified using a continuous flow analyser (SEAL Auto Analyser 3, SEAL Analystical, Norderstedt, Germany) described by Rhine et al. (1998).
DNA extraction and PCR amplification
Genomic DNA was directly extracted from 0.2 g of each semisolid-state sample using cetyl trimethylammonium bromide (CTAB) method (Porebski, Bailey & Baum, 1997). The DNA quality was assessed via 2% agarose gel elcectrophoresis, and metagenomic DNA concentratios were determined with a NanoDrop 2000 (Thermo Fisher, Waltham, MA, USA). The DNA was then diluted to 1 ng/µL using sterile water and stored at −4 °C for PCR amplification.
For PCR amplification, the V4 hypervariable region of the bacterial 16S rRNA gene was amplified using the universal primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′- GGACTACHVGGGTWTCTAAT-3′) with unique barcodes. PCRs were performed in 25 µL reactions consisting of 12.5 µL Phusion® High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA, USA), one µL of forward and reverse primers, one µL of template DNA and 9.5 µL of autoclaved distilled water. The thermal cycling program consisted of initial denaturation at 94 °C for 3 min, 30 cycles of denaturation at 94 °C for 40 s, annealing at 56 °C for 1 min, and elongation at 72 °C for 1 min and a final incubation step at 72 °C for 10 min. For PCR product quantification and qualification, we obtained mixtures of the same volumes of 1 × loading buffer (containing SYBR Green from Shanghai, SanoBio) and the PCR products were exmined ona 2% agarose gel electrophoresis. Samples with a bright band between 400 and 450 bp were mixed and purified using a Qiagen Gel Extraction Kit (Qiagen, Germany). Sequencing libraries were then generated using a TruSeq® UltraTM DNA Library Prep Kit for Illumina (NEB, Ipswich MA, USA) following the manufacturer’s recommendations, and the index codes were then added. The library quality was assessed using a Qubit® 2.0 fluorometer (Thermo Scientific) and an Agilent Bio analyzer system. The library was sequenced on an Illumina HiSeq PE250 platform (Novogene, Beijing, China).
Analysis of sequencing data
The paired-end reads were assigned to samples based on their unique barcodes, and the barcodes and primer sequences were then trimmed. The raw reads were filtered according to the following rules: Removing reads containing more than 10% of unknown nucleotides (N); removing reads containing less than 80% of bases with quality (Q-value) >20. The FLASH (version 1.2.7) was then used to merge paired-ends reads as raw tags with a minimum overlap of 10bp and mismatch error rates of 2% (Magoc & Salzberg, 2011). The noisy sequences of raw tags were filtered by QIIME (version 1.9.1) pipeline to obtain the high-quality clean tags (Caporaso et al., 2010). The reads were then compared with a Gold database using the UCHIME algorithm to detect and remove the chimaera sequences (Edgar et al., 2011). All the sequences were analysed using Uparse software (version 7.0.1001), and sequences with greater than or equal 97% similarity were assigned to the same operational taxonomic unit (OTU). Taxonomic information for each representative sequence was annotated using the Greengenes database based on the RDP classifier algorithm (version 2.2) (Wang et al., 2007).
Statistical analysis
Alpha and species diversity indexes, including the Shannon-Wiener indexe, Chao1 index, Good’s coverage and observed species were calculated by Qiime and graphed using Origin (version 8.0). The correlation among growth performance, rumen fermentation parameters and bacteria diversity were analyzed using SPSS (version 17.0), and heat map of correlations were also prepared using Origin. The beta diversity based on weighted UniFrac distance, was visualized through a principal coordinate analysis (PCoA). One-way ANOVA with Tuckey’s test was performed to compare the differences in relative abundances among different treatments, and Duncan’s multiple comparison test was used to determine the effects of dietary C: F ratios on BW gain, gain rate, ADG, DMI, DM consume and FCR using SPSS (version 17.0). Effects were considered significant at P < 0.05. The results are shown as the means ± SEMs.
Results
Growth performance of Tibetan sheep
As shown in Table 2, treatments HS4 and HS5 significantly increased BW gain, gain rate and ADG, whereas reduced FCR as compared to treatments HS1 and HS2 (P < 0.05). Tibetan sheep fed diet in group HS1 showed the lowest growth performance. No significant difference were detected in BW gain and FCR between treatments HS4 and HS5, and the initial BW showed no notably difference among the five groups (P = 0.196).
Table 2. The effects of different dietary C: F ratios on the growth performance of Tibetan sheep.
| Items | Groupsa | p-value | ||||
|---|---|---|---|---|---|---|
| HS1 | HS2 | HS3 | HS4 | HS5 | ||
| Initial BW (kg) | 21.31 ± 0.92 | 22.13 ± 3.06 | 21.25 ± 1.69 | 19.88 ± 1.1.81 | 22.19 ± 2.34 | 0.196 |
| Final BW(kg) | 23.81 ± 1.25d | 26.44 ± 3.07cd | 28.38 ± 2.23bc | 29.75 ± 2.62b | 32.94 ± 3.49a | <0.01 |
| BW gain (kg) | 2.50 ± 0.76d | 4.31 ± 1.92c | 7.13 ± 1.71b | 9.88 ± 1.43a | 10.75 ± 1.79a | <0.01 |
| Gain rate (%) | 11.73 ± 3.50d | 19.93 ± 6.54c | 33.78 ± 8.63b | 49.98 ± 7.13a | 48.59 ± 7.56a | <0.01 |
| ADG (g/d) | 31.25 ± 9.45d | 53.91 ± 12.91c | 89.06 ± 21.33b | 123.44 ± 17.91a | 134.38 ± 22.41a | <0.01 |
| DMI (g/d | 670.30 ± 45.26c | 719.16 ± 120.09bc | 741.21 ± 71.22abc | 807.68 ± 32.29ab | 851.93 ± 98.86a | <0.01 |
| DM consume (kg) | 53.62 ± 3.62c | 57.53 ± 8.98bc | 59.30 ± 5.70bc | 64.61 ± 4.98ab | 68.15 ± 7.91a | <0.01 |
| FCR | 23.86 ± 9.75a | 13.94 ± 3.38b | 8.92 ± 3.06c | 6.67 ± 1.15c | 6.44 ± 1.00c | <0.01 |
Notes.
The treatments HS1, HS2, HS3, HS4 and HS5 refer to concentrate-to-forage ratios of 0:100,15:85, 30:70, 45:55 and 60:40. DMI, dry matter intake; DM consume, DMI ± t, t is experimental time (d), BW, body weight; ADG, average daily BW gain. FCR, feed conversion ratio. Values in the same row with different superscripts are significantly different (P < 0.05).
Rumen fermentation parameters
As shown in Table 3, the NH3-N, propionate and valerate concentrations in treatments HS4 and HS5 were significantly higher than that of other three groups (P < 0.05). There was a decreasing trend for total VFA, acetate and isovalerate concentrations, but no significant differences were detected among the groups. The increases in dietary concentrate level significantly decreased A:P ratio (P < 0.05). The ruminal pH tended to decrease, but no significant difference was detected (P > 0.05), and the average pH value was approximately 6.61.
Table 3. The effects of different dietary C: F ratios on the rumen fermentation parameters of Tibetan sheep.
| Parameters | Groupsa | Pb | ||||
|---|---|---|---|---|---|---|
| HS1 | HS2 | HS3 | HS4 | HS5 | ||
| Ammonia nitrogen (mg/L) | 46.20 ± 12.37c | 46.20 ± 12.37c | 64.00 ± 3.88b | 67.10 ± 8.21b | 107.06 ± 15.07a | <0.05 |
| Acetate (%) | 77.82a | 71.70b | 64.10b | 60.14 ± 8.55b | 64.55 ± 2.15b | 0.32 |
| Propionate (%) | 12.60 ± 0.71b | 16.02 ± 0.18b | 22.79 ± 1.13a | 26.81 ± 3.60a | 29.38 ± 0.95a | <0.05 |
| Butyrate (%) | 8.25 ± 0.38 | 10.66 ± 1.73 | 11.30 ± 0.40 | 11.51 ± 2.20 | 12.02 ± 0.40 | 0.23 |
| Isovalerate (%) | 0.79 ± 0.10a | 0.92 ± 0.07a | 0.83 ± 0.02a | 0.58 ± 0.03b | 0.72 ± 0.09b | 0.23 |
| Valerate (%) | 0.52 ± 0.02b | 0.70 ± 0.04b | 0.98 ± 0.03b | 1.11 ± 0.04a | 1.50 ± 0.12a | <0.05 |
| Total volatile fatty acids (mmol/L) | 74.94 ± 10.76 | 66.12 ± 2.95 | 65.21 ± 1.00 | 67.44 ± 1.56 | 61.24 ± 7.67 | 0.11 |
| Acetate to propionate ratio (A:P) | 6.38 ± 1.55a | 4.47 ± 0.16a | 2.85 ± 0.24b | 2.24 ± 0.39b | 2.19 ± 0.69b | <0.05 |
| pH | 6.86 ± 0.01 | 6.83 ± 0.03 | 6.83 ± 0.04 | 6.63 ± 0.06 | 6.42 ± 0.10 | 0.41 |
Notes.
The treatments HS1, HS2, HS3, HS4 and HS5 refer to concentrate to forage ratios of 0:100, 15:85, 30:70, 45:55 and 60:40, respectively.
Values in the same row with different superscripts are significantly different (P < 0.05).
Sequencing and taxonomic composition of the rumen bacterial community
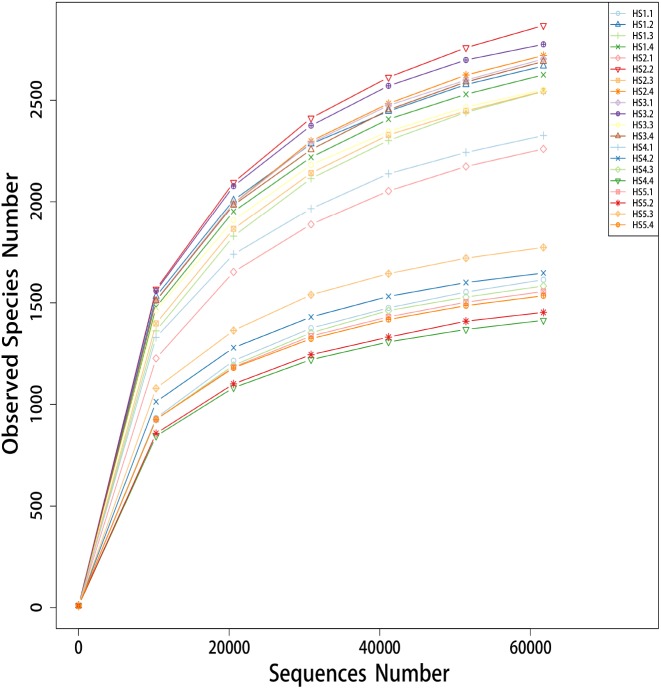
A total of 1,497,607 PE reads were obtained, and 1,461,673 clean reads from 20 different samples were generated after quality control (Table S2). All the sequences were aligned and clustered into OTUs using a 97% sequence identity as a cut-off, which yielded a total of 49,216 OTUs (Table S2). Rarefaction curves were generated with a 3% cut of for comparisons of species and richness as shown in Fig. 1. All the curves of the observed species numbers tended to saturate to a plateau at the minimum sequence number of 60,000 tags. In addition, the HS4 and HS5 groups tended to cluster together with a lower observed species number than the HS1, HS2 and HS3 groups.
Figure 1. The rarefaction analysis anomg the 20 different samples.
The group HS1 samples included sheep HS1.1, HS1.2, HS1.3 and HS1.4. The treatment HS2 samples included sheep HS2.1, HS 2.2, HS2.3 and HS4. The treatment HS3 samples included sheep HS3.1, HS3.2 HS3.3 and HS3.4. The treatment HS4 samples included HS4.1, HS4.2, HS4.3 and H4.4. The treatment HS5 samples included HS5.1, HS5.2, HS5.3 and H5.4. The treatment HS1, concentrate to forage ratio 0:100; HS2, concentrate to forage ratio 15:85; HS3, concentrate to forage ratio 30:70; HS4, concentrate to forage ratio 45:55; HS5, concentrate to forage ratio 60:40.
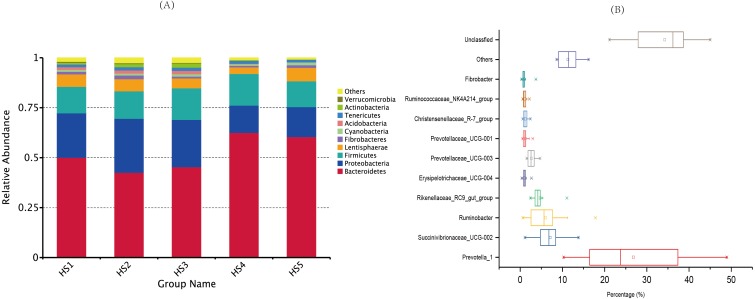
The taxonomic analysis detected a total of 41 phyla (Table S3), and the most abundant phyla were Bacteroidetes (52.18%), Proteobacteria (20.34%) and Firmicutes (14.34%). The top ten phyla, which exhibited the highest relative abundances, were prevalent in all the samples, accounting for nearly 98% of all sequences (Fig. 2A). The moderate abundant phyla included Fibrobacteres (1.25%), Cyanobacteria (1.04%), Acidobacteria (0.96%),Tenericutes (1.49%), Actinobacteria (0.96%) and Verrucomicrobia (0.47%). The other known phyla accounted for 1.48%, whereas the remaining sequences that were unclassified accounted for 0.26%.
Figure 2. Dominant bacterial phylum in individual samples and the shared genera across the ruminal samples.
(A) The top 10 shared bacterial taxonomic composition across ruminal samples at the phylum level. (B) The top 10 shared bacterial toxonomic composition across ruminal samples. Percentage is shown on the X-axis. The box represent the interquartile range between the first and the third quartiles, and the symbol “— —” represent the max value, “×” represent the variation range from 1% to 99% and “□” represent the mean value. The treatment HS1, concentrate to forage ratio 0:100; HS2, concentrate to forage ratio 15:85; HS3, concentrate to forage ratio 30:70; HS4, concentrate to forage ratio 45:55; HS5, concentrate to forage ratio 60:40.
At the genus level, we detected 129 distinct genera across all the groups (Table S4), and 10 genera whose relative abundances more than 1% were perceived as the most important bacteria that affecting the rumen environment and digestive system (Fig. 2B). The genus with relatively high across all the samples were Prevotella_1 (26.81%), Succinivibrionaceae_UCG-002 (7.11%), Ruminobacter (6.03%), Rikenellaceae_RC9_gut_group (4.73%), Prevotellaceae_UCG-003 (2.65%). Minor genera, such as Prevotellaceae_UCG-001, Erysipelotrichaceae_UCG-004, Fibrobacter, Christensenellaceae_R-7_group and Ruminococcaceae_NK4A214_group accounted for 1.23%, 1.09%, 1.01%, 1.22 and 1.09% of the sequences, respectively. The other known genera accounted for 11.32% of the sequences, whereas sequences that were unclassified accounted for 34.23% of the sequences.
Effect of diets with different C:F ratios on the bacterial community
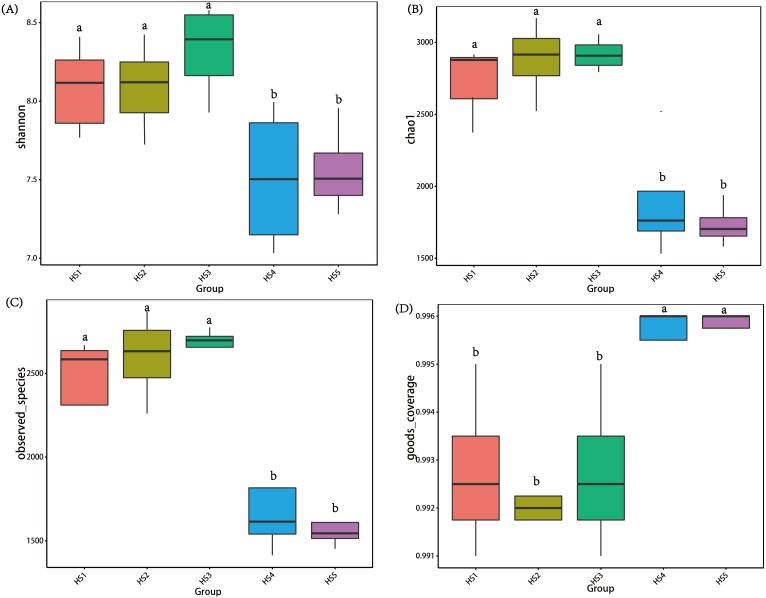
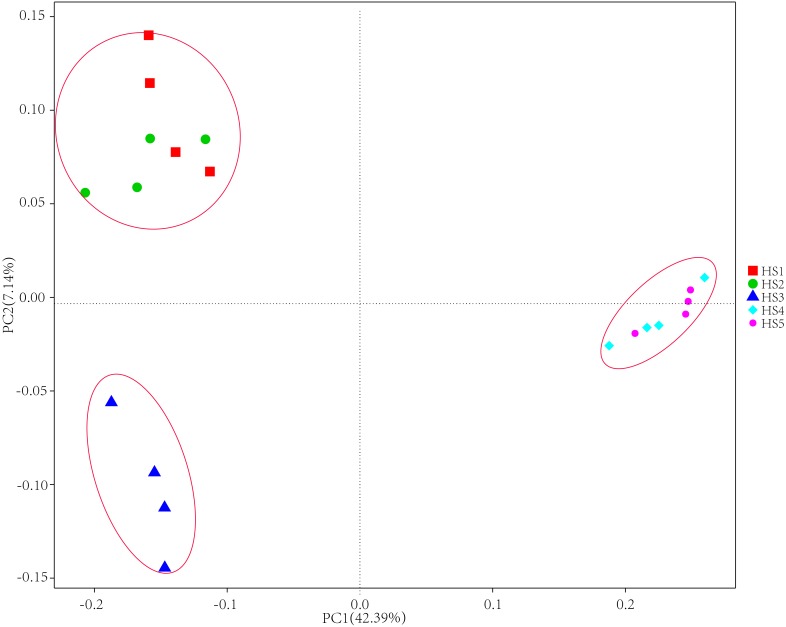
To determine alpha diversity, we calculated Shannon-Wiener, Chao 1, observed species and Good’s coverage indexes, as shown in Fig. 3. The indexes of Shannon-Wiener, Chao 1 and observed species in treatments HS1, HS2 and HS3 were significantly higher than treatments HS4 and HS5 (Figs. 3A, 3B and 3C). Good’s coverage among five treatment were greater than 99% (Fig. 3D). As shown in Fig. 4, the PCoA result showed that the rumen bacterial communities of the five treatments were mainly classified into three clusters (Fig. 4). The HS1 and HS2 treatments clustered very closely together, the HS4 and HS5, and the HS3 treatments formed two clusters. In addition, HS3 treatment were closer to the cluster composed of the HS1 and HS2 treatments, which only represented 7.14% of the variability obtained with PC2.
Figure 3. The differences in bacteria community diversity and richness indexes among different feeding treatment.
(A) The Shannon-Wiener diversity among each treatment. (B) The Chao1 richness estimator among each treatment. (C) The observed species in each treatment. (D) Good’s coverage in samples among each treatment. The treatment HS1, concentrate to forage ratio 0:100; HS2, concentrate to forage ratio 15:85; HS3, concentrate to forage ratio 30:70; HS4, concentrate to forage ratio 45:55; HS5, concentrate to forage ratio 60:40.
Figure 4. The principal coordinate analysis (PCoA) using weighted UniFrac metrics.
The treatment HS1, concentrate to forage ratio 0:100; HS2, concentrate to forage ratio 15:85; HS3, concentrate to forage ratio 30:70; HS4, concentrate to forage ratio 45:55; HS5, concentrate to forage ratio 60:40.
The relative abundance of bacterial taxa was used to describe the impact of diets with different C:F ratios on bacterial community. At the phylum level, the top eight phylum (relative abundance >1%) were analyzed in Table S5. The ruminal compositions of the HS1 to HS3 treatments contained a significantly lower relative abundance of Bacteroidetes, whereas there were higher relative abundances of Proteobacteria, Acidobacteria, and Actinobacteria than those of the HS4 and HS5 treatments (P < 0.05). No significant differences were found in the other phyla (P > 0.05). At the genus level, the top 12 genera (relative abundance >1%) were analyzed in Table S6. Treatments HS4 and HS5 shared a higher relative abundance of Prevotella_1 than the other three treatments (P < 0.05). No significant differences were found in the rest of genera (p > 0.05).
Relationships between rumen bacterial diversity and host growth performance
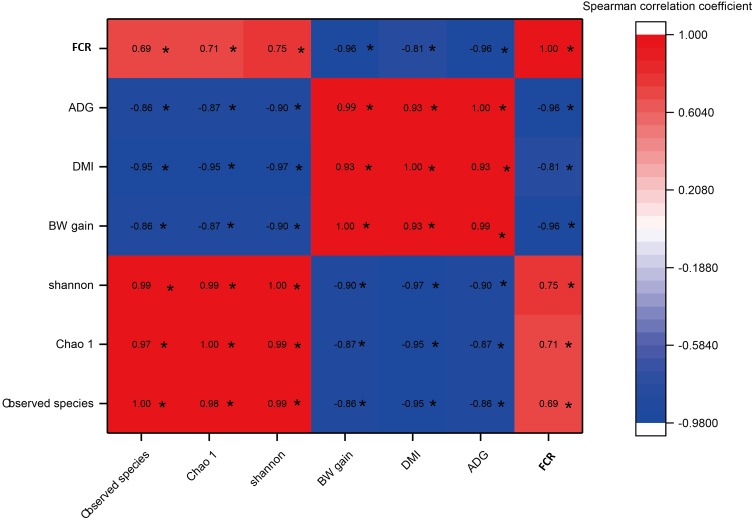
As shown in Fig. 5, a significant negative correlation was found between bacterial diversity (including the Shannon-Wiener, Chao1, and observed species indexes) and growth performance (including BW gain, ADG, and DMI) with increases in the C:F ratio (P < 0.05). FCR showed a negative correlation with increases in the DMI, BW gain and ADG and a positive correlation with the Shannon-Wiener, Chao1 and observed species indexes. In addition, growth performance showed significant positive correlations with increases in the dietary C:F ratio (P < 0.05).
Figure 5. The heat map of the correlations between bacterial diversity and growth performance of Tibetan sheep.
DMI, dry matter intake; ADG, average daily body weight gain; FCR, feed conversion ratio; BW again, body weight gain. The treatment HS1, concentrate to forage ratio 0:100; HS2, concentrate to forage ratio 15:85; HS3, concentrate to forage ratio 30:70; HS4, concentrate to forage ratio 45:55; HS5, concentrate to forage ratio 60:40. *Significant at P < 0.05.
Relationships between rumen fermentation parameter and microorganisms
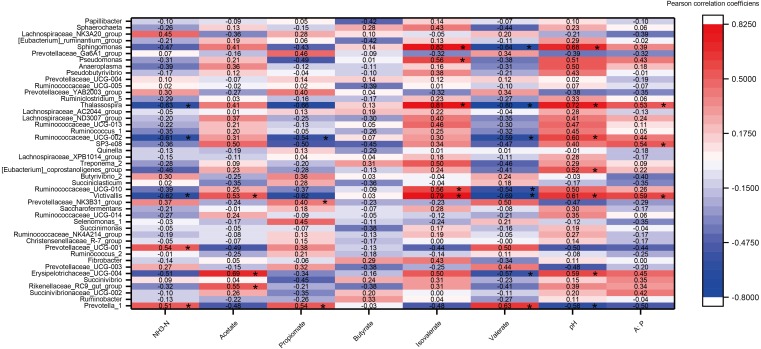
A heat map of the correlations between the top 45 genera (relative abundance >0.1%) and rumen fermentation was constructed (Fig. 6). A total of 360 correlation coefficients were generated, and 33 of these coefficients (9.17% of the total correlation coefficients) showed significant correlations (P < 0.05). The fermentation parameters pH, acetate, isovalerate and A:P were significantly positively correlated with most genera (e.g., Victivalls, Thalassospira and Sphaerochaeta), whereas valerate and NH3 showed a negative correlation with most genera (e.g., Victivalls, Ruminococcaceae_UCG_02, and Thalassosphira). Prevotella_1, which was the most abundant genera, was significantly positively (P < 0.05) correlated with NH3, propionate and valerate and negatively correlated with pH. No significant correlation were detected between butyrate and the top 45 genera.
Figure 6. The heat map of the correlations between bacterial diversity and growth performance of Tibetan sheep.
DMI, dry matter intake; ADG, average daily body weight gain; FCR, feed conversion ratio; BW again, body weight gain. The treatment HS1, concentrate to forage ratio 0:100; HS2, concentrate to forage ratio 15:85; HS3, concentrate to forage ratio 30:70; HS4, concentrate to forage ratio 45:55; HS5, concentrate to forage ratio 60:40. *Significant at P < 0.05.
Discussion
Growth performance of barn-fed Tibetan sheep
Previous studies mainly aimed to ensure that Tibetan sheep live through grazing breaks (Ma, 2008; Xie et al., 2014), and there is little knowledge on the BW gain and feed efficiency of Tibetan sheep fed diets with different C:F ratios during late non-growing and vegetation green-up periods. Our results revealed that increases in dietary concentrate feed levels within a certain range (0% to 45%) exerted a positive effect on the feed intake, BW gain and FCR of Tibetan sheep during spring grazing breaks. This finding can be mainly attributed to the fact that high-concentrate diet contains more digestive energy and nonstructural carbohydrates (Wang et al., 2015), which leads to increased nutrient intake, faster digestion through the digestive tract, and then improved growth performance and higher feed efficiency (Haddad & Ata, 2009; Haddad, 2005).
Under traditional grazing, Tibetan sheep exhibit low growth performance (ADG = 36∼55 g/d) during spring, and more seriously, unsustainable grazing during this period decreases the plant species richness and biomass in alpine pasture (Ma, 2008). In contrast, shortened rest-grazing increases the standing herbage biomass by 77%∼189% (Li et al., 2017; Ma et al., 2017). Herein, we confirmed that a diet with diet with a 45% concentration supplementation significantly improves the performance of Tibetan sheep. Therefore, the use of spring rest-grazing combined with barn feeding could increase livestock performance and promote the functional recovery of alpine pasture.
Effect of different C: F ratios on rumen fermentation parameters
The pH, NH3-N concentration and VFA molar ratio are the main internal environmental indicators of rumen fermentation. If the rumen pH was in the range of 6.2 to 7.0, the ecological environment of rumen microorganisms could be relatively stable, which could ensure normal rumen fermentation (Weng, 2013; Zhang et al., 2013). In our study, the ruminal pH ranged from 6.42 to 6.86, and this finding indicated that the increased proportion of concentrate did not induce acidosis, which is usually defined as a decrease in the rumen pH to less than the threshold value of 6.0 (Nocek, 1997). The ruminant pH values obtained in the present study are inconsistent with previous studies (Cerrillo, Russell & Crump, 1999; Chen et al., 2015a). The pH value can be affected by many factors, such as higher doses of flour or starches (Lettat et al., 2010; Minuti et al., 2014), the time from concentrate feed supplementation to ruminal liquid collection (Lettat et al., 2010), and the insertion depth of the rumen catheter (Li et al., 2009). In our study, increases in the dietary concentrate level tended to decrease the ruminal pH, but no significant difference was detected (P > 0.05), which could be due to the starch content. The HS1 and HS2 groups had a lower starch content (25.58 and 26.95 mg/g, respectively), which led to a higher pH value, whereas the HS3, HS4 and HS5 groups had a higher starch content (32.55, 33.72 and 39.37mg/g, respectively), which resulted in a relatively lower pH value. The dietary C:F ratios did not significantly affect the total volatile fatty acids, which is in accordance with previous research (Chen et al., 2015a). This result might be due to the ability of the rumen system that can adapt to appropriate dietary C:F ratios through the self-adjustment of rumen microorganisms. Ammonium nitrogen which is the final product through the decomposition of protein and nonprotein in feed, and it is also the main nitrogen source for the synthesis of bacterial protein by rumen microorganisms. In our study, high concentrate feed level elevated NH3-N concentration, which is consistent with the former study (Reddy & Reddy, 1985; Yang, Beauchemin & Rode, 2001). The high dietary concentrate increased nitrogen content in the rumen, thereby increasing ruminal ammonia nitrogen (Moorby et al., 2006). As for individual VFA concentrations in current study, diets with high-concentrate levels slightly reduced acetate concentration, whereas significantly increased propionate proportion, thereby resulting in a significant reduction of A:P ratio. (Andrade & Schmidely, 2006; Polyorach, Wanapat & Cherdthong, 2014). From the point of view of energy utilization, a decrease in the A:P ratio reflects an improvement in the feed energy utilization efficiency, which also explains the improved growth performance of Tibetan sheep in the high-concentrate groups (HS4 and HS5).
Core bacterial communities in the rumen
Although rumen microbial community composition varies with diet and host, a ‘core microbiome’ is found across a wide geographical range (Henderson et al., 2015). The dominance of Bacteroidetes or Proteobacteria could be attributable to variations in diet, environment, hosts and farming practices over a wide geographical range (Amato et al., 2013; Henderson et al., 2013). In our study, different dietary concentrate level induced Bacteroidetes (55.02%) to become the most abundant phyla, followed by Proteobacteria (22.10%), and this microbial distribution of major phyla was similar to that obtained in previous studies on Yaks (Chen et al., 2015a). These results might be related to the functions of rumen bacteria. Members of the phylum Bacteroidetes have a greater ability to degrade protein and carbohydrates than species belonging to Proteobacteria (Huo, Zhu & Mao, 2014; Pitta et al., 2014). Our study also found that the relative abundance of Firmicutes was the third largest phylum and was lower than that of Proteobacteria. Similar results were observed in bovines and cattle during the transition from forage to concentrate (Jami et al., 2013). However, Xue et al. (2016) observed that the most abundant phyla in the rumen of natural pasture-grazing Tibetan sheep are Bacteroidetes, followed by Firmicutes. One possible reason for this difference is that the hosts investigated by Xue et al. (2016) were grazed on natural pasture and that the forage nutrition and types differed from those in our study, and these differences might have resulted in a higher abundance of Firmicutes. Additionally, the phylum Fibrobacteres, with an abundance less than 1.5%, comprised only a small fraction of the community composition compared with those of other phyla, and these results agree with previous studies (Petri et al., 2013; Wang et al., 2016).
In our study, although different C:F ratio did not change the core structure of the rumen microbiome, the relative abundances of Bacteroidetes and Proteobacteria showed noticeable shifts at phylum level. Tibetan sheep fed high-concentrate diets significantly increased the relative abundance of Bcateroidetes, whereas, reduced the relative abundance of Proteobacteria. Previous research revealed that Bacteroidetes were the major rumen microorganisms in degrading non-fibrous carbohydrates and contained genes related to the degradation of non-fibrous polysaccharides (Russell & Diez-Gonzalez, 1997). In our study, high concentrate feed contained more non-fibrous carbohydrates and polysaccharides, thus increasing the abundance of Bacteroidetes. At the genus level, Prevotella_1 was the predominant genus, and the relative abundances of this genus significantly increased with increases in dietary C:F ratio. This finding was consistent with that previously obtained by Stevenson & Weimer (2007). The high abundance of this genus can perhaps be explained from two points of view. Firstly, it if possible that this bacterial genus has a wide metabolic niche due to genetic relatedness or to the high genetic variability that enable this genus to occupy different ecological niches within the rumen. Secondly, Prevotella_1 strains play an important role in the degradation and utilization of plant noncellulosic polysaccharides, protein, starch and xylans. The increase in the abundance of this genus could be attributed to dietary nutrition changes, such as increased dietary protein and starch. In addition, Prevotella_1 is also considered to be associated with propionic acid production (Strobel, 1992), which might also explain the increasing proportion of propionate obtained with increase in the C:F ratio.
Relationship between bacterial diversity and growth performance of Tibetan sheep
In the past 50 years, humans have drastically altered the diet that ruminants consume. The use of grain feed increases the productivity of animals and the economic benefit of animal husbandry. In our study, the diversity and richness was significantly lower in high dietary C:F ratios treatments than low dietary C: F ratios (HS1, HS2 and HS3). Lin et al. (2015) found that the Shannon diversity index of bacteria were higher in low concentrate diet group than in high concentrate diet group concentrate diet group (Lin et al., 2015). Liu et al. (2015) discovered the diversity of ruminal epithelial bacterial community from goats fed the hay diet were significantly higher than those fed the high-grain diet (Liu et al., 2015). Diet composition may effect on the diversity index of microorganisms.The increase in microbial diversity could be because low dietary concentrate diets provide a greater range of carbohydrate substrates (e.g., cellulose and many heteropolysaccharides) than high dietary concentrate treatments and/ or because microorganisms grow faster in high pH conditions (Hobson & Stewart, 2012).The growth performance results indicated that increases in the concentrate feed level from 0 to 45%, exerted a positive effect on the feed intake, BW gain, gain rate and FCR in Tibetan sheep. Therefore, based on our findings, we can conclude that improved growth performance is not linked to a higher diversity of rumen microorganisms. It is well known that a decrease in the bacterial diversity can induce the evolution of a certain bacterial group and thereby its dominance in the community. Thus, in our study, a decrease in bacteria diversity is associated with a higher dominance index (phyla Bacteroidetes and genera Prevotella_1), which ensured that the host could receive more nutrition and ultimately exhibits an improved growth performance. In addition, in our study, increases in the dietary C:F ratio from 0:100 to 30:70 were associated with increases in both the rumen bacterial diversity and the host growth performance, whereas, dietary C:F ratios of 45:55 and 60:40 could only promote growth performance of Tibetan sheep. Briesacher et al. (1992) observed that the digestive of the rumen is influenced by the number of bacterial species and the total abundance of bacteria. Wanapat et al. (2003) investigated the microorganisms in cattle and swamp buffaloes and observed that swamp buffaloes exhibit a stronger ability to digest cellulose than cattle due to their greater abundance of bacteria (1.6 × 109/mL) compared with that of cattle (1.36 × 109/mL). In our study, the HS3 group (C:F ratio 30:70) exhibited a richer microbial community and an increased in microbial diversity.Therefore, the inclusion of 30% concentrate in feed might be a good choice of diet that can be provided to Tibetan sheep. However, we did not perform digestibility experiments with all the treatments, which is a limitation of this study. Therefore, whether a rumen environment with a high bacterial richness and a high bacterial diversity is associated with a higher digestive capacity of Tibetan sheep remains to be investigated.
Conclusion
The different dietary C: F ratios affected the growth performance, rumen fermentation and rumen bacterial diversity of Tibetan sheep. Increasing the dietary concentrate feed level from 0% to 60% exerted a positive effect on the DMI, BW gain, gain rate and FCR of Tibetan sheep during late non-growing and green-up period, and Tibetan sheep fed with 45% concentrate level for barn feeding can result in a better improvement in animal performance. Moreover, high C:F ratios significantly increased ammonium nitrogen, reduced the A:P molar ratio and changed the composition of the bacterial community.
Supplemental Information
The treatment HS1= concentrate to forage ratio 0:100, HS2= concentrate to forage ratio 15:85, HS3= concentrate to forage ratio 30:70, HS4= concentrate to forage ratio 45:55, HS5=concentrate to forage ratio 60:40. DMI=dry matter intake; DM consume= DMI × t, t is experimental time(d), BW=body weight; ADG= average daily BW gain. FCR= feed conversion ratio.
The 2 sequences in the file represent 2 samples of Tibetan sheep in group HS1.
The 2 sequences in the file represent another 2 samples of Tibetan sheep in group HS1.
The 4 sequences in the file represent 4 samples of Tibetan sheep in group HS2.
The 4 sequences in the file represent 4 Tibetan sheep in group HS3.
The 4 sequences in the file represent 4 rumen samples of Tibetan sheep in group HS4.
The 4 sequences in the file represent 4 Tibetan sheep in group HS5.
Acknowledgments
We would like to thank Yinfa Ji for his help in sampling. We also thank Hanzhong Ji, senior livestock engineer from Haibei Prefecture, for providing the experiment site.
Funding Statement
This work was supported by the National Key R&D Plan (NO. 2016YFC0501905, NO. 2016YFC0501805), the “Strategic Priority Research Program” of CAS, the “Key Technology Support Program” of Qinghai province (No. 2018-S-2, 2015-SF-A4-2, 2016-NK-148, 2017-SF-A6, 2017-NK-153) and the Qinghai Innovation Platform Construction Project (No. 2017-ZJ-Y20) and NSFC (No. 31402120). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Hongjin Liu and Tianwei Xu conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper.
Shixiao Xu conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Li Ma approved the final draft, dr Li Ma helped to collect the samples and review the manuscript.
Xueping Han approved the final draft, dr Li Ma helped to review the manuscript.
Xungang Wang, Xiaoling Zhang and Xinquan Zhao approved the final draft.
Linyong Hu approved the final draft, dr Linyong Hu helped to collect the samples and review the manuscript.
Na Zhao approved the final draft, dr Na Zhao helped to collect the samples and review the manuscript.
Yongwei Chen approved the final draft, mr Yongwei Chen helped to collect the samples.
Li Pi contributed reagents/materials/analysis tools, approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All animal care procedures were consistent with the provision of the Institution of Animal Care and the Ethics Committee of the Northwest Institute of Plateau Biology, Chinese Academy of Sciences (NWIPB20160302).
Data Availability
The following information was supplied regarding data availability:
The sequencing data for the 16S rRNA genes are publicly available in the NCBI Short Read Archive: PRJNA477411.
References
- Amato et al. (2013).Amato KR, Yeoman CJ, Kent A, Righini N, Carbonero F, Estrada A, Gaskins HR, Stumpf RM, Yildirim S, Torralba M, Gillis M, Wilson BA, Nelson KE, White BA, Leigh SR. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME Journal. 2013;7:1344–1353. doi: 10.1038/ismej.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, Dong & Dong (2005).An D, Dong X, Dong Z. Prokaryote diversity in the rumen of yak (Bos grunniens) and Jinnan cattle (Bos taurus) estimated by 16S rDNA homology analyses. Anaerobe. 2005;11:207–215. doi: 10.1016/j.anaerobe.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Andrade & Schmidely (2006).Andrade PVD, Schmidely P. Influence of percentage of concentrate in combination with rolled canola seeds on performance, rumen fermentation and milk fatty acid composition in dairy goats. Livestock Science. 2006;104:77–90. doi: 10.1016/j.livsci.2006.03.010. [DOI] [Google Scholar]
- Baruah et al. (2012).Baruah K, Paul V, Ahmed F, Chakravarty P, Deori S, Sarma H, Saikia A. Effect of strategic feed supplementation on growth performance and nutrient utilization in yak calves during winter. Indian Journal of Animal Sciences. 2012;82(2):220–222. doi: 10.1016/j.applanim.2012.01.004. [DOI] [Google Scholar]
- Briesacher et al. (1992).Briesacher S, May T, Grigsby K, Kerley M, Anthony R, Paterson J. Use of DNA probes to monitor nutritional effects on ruminal prokaryotes and Fibrobacter succinogenes S85. Journal of Animal Science. 1992;70:289–295. doi: 10.2527/1992.701289x. [DOI] [PubMed] [Google Scholar]
- Caporaso et al. (2010).Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceconi et al. (2015).Ceconi I, Ruiz-Moreno M, DiLorenzo N, DiCostanzo A, Crawford GI. Effect of urea inclusion in diets containing corn dried distillers grains on feedlot cattle performance, carcass characteristics, ruminal fermentation, total tract digestibility, and purine derivatives-to-creatinine index. Journal of Animal Science. 2015;93:357–369. doi: 10.2527/jas.2014-8214. [DOI] [PubMed] [Google Scholar]
- Cerrillo, Russell & Crump (1999).Cerrillo MA, Russell JR, Crump MH. The effects of hay maturity and forage to concentrate ratio on digestion kinetics in goats. Small Ruminant Research. 1999;32:51–60. doi: 10.1016/s0921-4488(98)00153-9. [DOI] [Google Scholar]
- Chen et al. (2015b).Chen Y-B, Lan D-L, Tang C, Yang X-N, Li J. Effect of DNA extraction methods on the apparent structure of yak rumen microbial communities as revealed by 16s rdna sequencing. Polish Journal of Microbiology. 2015b;64:29–36. [PubMed] [Google Scholar]
- Chen et al. (2015a).Chen GJ, Song SD, Wang BX, Zhang ZF, Peng ZL, Guo CH, Zhong JC, Wang Y. Effects of forage: concentrate ratio on growth performance, ruminal fermentation and blood metabolites in housing-feeding yaks. Asian-Australasian Journal of Animal Sciences. 2015a;28:1736–1741. doi: 10.5713/ajas.15.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark (1975).Clark HJ. Lactational responses to postruminal administration of proteins and amino acids. Journal of Dairy Science. 1975;58:1178–1197. doi: 10.3168/jds.S0022-0302(75)84696-0. [DOI] [PubMed] [Google Scholar]
- Dodd, Mackie & Cann (2011).Dodd D, Mackie RI, Cann IK. Xylan degradation, a metabolic property shared by rumen and human colonic Bacteroidetes. Molecular Microbiology. 2011;79:292–304. doi: 10.1111/j.1365-2958.2010.07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2006).Dong QM, Zhao XQ, Ma YS, Xu SX, Li QY. Live-weight gain, apparent digestibility, and economic benefits of yaks fed different diets during winter on the Tibetan plateau. Livestock Science. 2006;101:199–207. doi: 10.1016/j.Lnivprodsci.2005.11.009. [DOI] [Google Scholar]
- Edgar et al. (2011).Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng et al. (2013).Feng BF, Zhao XQ, Dong QM, Xu SX, Zhao L, Cao J. The effect of feed supplementing and processing on the live weight gain of tibetan sheep during the cold season on the Qinghai-Tibetan Plateau. Journal of Animal & Veterinary Advances. 2013;12:312–315. doi: 10.3923/javaa.2013.312-315. [DOI] [Google Scholar]
- Haddad (2005).Haddad SG. Effect of dietary forage: concentrate ratio on growth performance and carcass characteristics of growing Baladi kids. Small Ruminant Research. 2005;57:43–49. doi: 10.1016/j.smallrumres.2004.05.001. [DOI] [Google Scholar]
- Haddad & Ata (2009).Haddad SG, Ata MA. Growth performance of lambs fed on diets varying in concentrate and wheat straw. Small Ruminant Research. 2009;81:96–99. doi: 10.1016/j.smallrumres.2008.11.015. [DOI] [Google Scholar]
- Henderson et al. (2015).Henderson G, Cox F, Ganesh S, Jonker A, Young W, Janssen PH. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Scientific Reports. 2015;6:14567. doi: 10.1038/srep19175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson et al. (2013).Henderson G, Cox F, Kittelmann S, Miri VH, Zethof M, Noel SJ, Waghorn GC, Janssen PH. Effect of DNA extraction methods and sampling techniques on the apparent structure of cow and sheep rumen microbial communities. PLOS ONE. 2013;8(9):e74787. doi: 10.1371/journal.pone.0074787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson & Stewart (2012).Hobson PN, Stewart CS. The rumen microbial ecosystem. Springer Science & Business Media; 2012. [Google Scholar]
- Huo, Zhu & Mao (2014).Huo W, Zhu W, Mao S. Impact of subacute ruminal acidosis on the diversity of liquid and solid-associated bacteria in the rumen of goats. World Journal of Microbiology & Biotechnology. 2014;30:669–680. doi: 10.1007/s11274-013-1489-8. [DOI] [PubMed] [Google Scholar]
- Jami et al. (2013).Jami E, Israel A, Kotser A, Mizrahi I. Exploring the bovine rumen bacterial community from birth to adulthood. Isme Journal Multidisciplinary Journal of Microbial Ecology. 2013;7:1069–1079. doi: 10.1007/s11274-013-1489-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2015).Jiang S, Yang Z, Yang W, Li Z, Zhang C, Liu X, Wan F. Diets of differentially processed wheat alter ruminal fermentation parameters and microbial populations in beef cattle. Journal of Animal Science. 2015;93:5378–5385. doi: 10.2527/jas.2015-9547. [DOI] [PubMed] [Google Scholar]
- Jiao et al. (2015).Jiao J, Huang J, Zhou C, Tan Z. Taxonomic identification of ruminal epithelial bacterial diversity during rumen development in goats. Applied and Environmental Microbiology. 2015;81:3502–3509. doi: 10.1128/AEM.00203-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2012).Lee HJ, Jung JY, Oh YK, Lee SS, Madsen EL, Jeon CO. Comparative survey of rumen microbial communities and metabolites across one caprine and three bovine groups, using bar-coded pyrosequencing and 1H nuclear magnetic resonance spectroscopy. Applied & Environmental Microbiology. 2012;78:5983–5993. doi: 10.1128/AEM.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettat et al. (2010).Lettat A, Noziere P, Silberberg M, Morgavi DP, Berger C, Martin C. Experimental feed induction of ruminal lactic, propionic, or butyric acidosis in sheep. Journal of Animal Science. 2010;88:3041–3046. doi: 10.2527/jas.2010-2926. [DOI] [PubMed] [Google Scholar]
- Li et al. (2017).Li LQ, Ma YS, Li SX, Wang XL, Wang YL, Jing ML, Li SY, Nian Y, Han HL. Effects of rest-grazing in the regreen-up period on moderately degreaded steppification meadow of Qilian Mountain. Pratacultural Science. 2017;10:2016–2022. doi: 10.11829/j.issn.1001-0629.2017-0200. [DOI] [Google Scholar]
- Li et al. (2009).Li M, Penner GB, Hernandez-Sanabria E, Oba M, Guan LL. Effects of sampling location and time, and host animal on assessment of bacterial diversity and fermentation parameters in the bovine rumen. Journal of Applied Microbiology. 2009;107:1924–1934. doi: 10.1111/j.1365-2672.2009.04376.x. [DOI] [PubMed] [Google Scholar]
- Li et al. (2014).Li Y-J, Yan Z, Zhao J-N, Gang L, Hui W, Xin L, Yang D-L. Effects of rest grazing on organic carbon storage in stipa grandis steppe in Inner Mongolia, China. Journal of Integrative Agriculture. 2014;13:624–634. doi: 10.1016/S2095-3119(13)60720-0. [DOI] [Google Scholar]
- Lin et al. (2015).Lin B, Henderson G, Zou C, Cox F, Liang X, Janssen PH, Attwood GT. Characterization of the rumen microbial community composition of buffalo breeds consuming diets typical of dairy production systems in Southern China. Animal Feed Science and Technology. 2015;207:75–84. doi: 10.1016/j.anifeedsci.2015.06.013. [DOI] [Google Scholar]
- Liu et al. (2015).Liu JH, Bian GR, Zhu WY, Mao SY. High-grain feeding causes strong shifts in ruminal epithelial bacterial community and expression of Toll-like receptor genes in goats. Frontiers in Microbiology. 2015;6 doi: 10.3389/fmicb.2015.00167. Article 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma (2008).Ma HY. Biomass measurement and Tibetan sheep barn supplementing experiment during spring rest-garzing period in alphine pasture aera. Prataculture & Anim Husbandry. 2008;6:34–35. [Google Scholar]
- Ma et al. (2017).Ma YS, Li SX, Wang YL, Sun XD, Jing ML, Li SY, Li LX, W XL. Effect of rest-grazing in the green-up period on degraded vegetation in alpine. Acta Agrestia Sinica. 2017;2:291–295. doi: 10.11733/j.issn.1007-0435.2017.02.010. [DOI] [Google Scholar]
- Magoc & Salzberg (2011).Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuti et al. (2014).Minuti A, Ahmed S, Trevisi E, Piccioli-Cappelli F, Bertoni G, Jahan N, Bani P. Experimental acute rumen acidosis in sheep: consequences on clinical, rumen, and gastrointestinal permeability conditions and blood chemistry. Journal of Animal Science. 2014;92:3966–3977. doi: 10.2527/jas.2014-7594. [DOI] [PubMed] [Google Scholar]
- Moorby et al. (2006).Moorby JM, Dewhurst RJ, Evans RT, Danelo’n JL. Effects of dairy cow diet forage proportion on duodenal nutrient supply and urinary purine derivative excretion. Journal of Dairy Science. 2006;89:3552–3562. doi: 10.1016/j.livsci.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Nocek (1997).Nocek JE. Bovine acidosis: implications on laminitis. Journal of Dairy Science. 1997;80:1005–1028. doi: 10.3168/jds.S0022-0302(97)76026-0. [DOI] [PubMed] [Google Scholar]
- Petri et al. (2013).Petri RM, Schwaiger T, Penner GB, Beauchemin KA, Forster RJ, McKinnon JJ, McAllister TA. Characterization of the core rumen microbiome in cattle during transition from forage to concentrate as well as during and after an acidotic challenge. PLOS ONE. 2013;8(12):e83424. doi: 10.1371/journal.pone.0083424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitta et al. (2016).Pitta DW, Indugu N, Kumar S, Vecchiarelli B, Sinha R, Baker LD, Bhukya B, Ferguson JD. Metagenomic assessment of the functional potential of the rumen microbiome in Holstein dairy cows. Anaerobe. 2016;38:50–60. doi: 10.1016/j.anaerobe.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Pitta et al. (2014).Pitta D, Kumar S, Veiccharelli B, Parmar N, Reddy B, Joshi C. Bacterial diversity associated with feeding dry forage at different dietary concentrations in the rumen contents of Mehshana buffalo (Bubalus bubalis) using 16S pyrotags. Anaerobe. 2014;25:31–41. doi: 10.1016/j.anaerobe.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Polyorach, Wanapat & Cherdthong (2014).Polyorach S, Wanapat M, Cherdthong A. Influence of Yeast Fermented Cassava Chip Protein (YEFECAP) and roughage to concentrate ratio on ruminal fermentation and microorganisms using in vitro gas production technique. Asian-Australasian Journal of Animal Sciences. 2014;27:36–45. doi: 10.5713/ajas.2013.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porebski, Bailey & Baum (1997).Porebski S, Bailey LG, Baum BR. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Molecular Biology Reporter. 1997;15:8–15. doi: 10.1007/bf02772108. [DOI] [Google Scholar]
- Ramos-Morales et al. (2014).Ramos-Morales E, Arco-Pérez A, Martín-García AI, Yáñez Ruiz DR, Frutos P, Hervás G. Use of stomach tubing as an alternative to rumen cannulation to study ruminal fermentation and microbiota in sheep and goats. Animal Feed Science and Technology. 2014;198:57–66. doi: 10.1016/j.anifeedsci.2014.09.016. [DOI] [Google Scholar]
- Reddy & Reddy (1985).Reddy KJ, Reddy M. Effect of feeding complete feeds on various nitrogen fractions and total VFA concentrations in the rumen fluid of sheep. Indian Journal of Animal Sciences. 1985;55:819–823. doi: 10.1051/gse:19850410. [DOI] [Google Scholar]
- Rhine et al. (1998).Rhine ED, Mulvaney RL, Pratt EJ, Sims GK. Improving the berthelot reaction for determining ammonium in soil extracts and water. Soil Science Society of America Journal. 1998;62:473–480. doi: 10.2136/sssaj1998.03615995006200020026x. [DOI] [Google Scholar]
- Russell & Diez-Gonzalez (1997).Russell JB, Diez-Gonzalez F. The effects of fermentation acids on bacterial growth. Advances in Microbial Physiology. 1997;39:205–234. doi: 10.1016/s0065-2911(08)60017-x. [DOI] [PubMed] [Google Scholar]
- Sasaki (1994).Sasaki M. Yak: hardy multi-purpose animal of Asian highland. Asian Livestock (FAO); Bangkok: 1994. [Google Scholar]
- Soest, Robertson & Lewis (1991).Soest PJV, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Stevenson & Weimer (2007).Stevenson DM, Weimer PJ. Dominance of prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Applied Microbiology and Biotechnology. 2007;75:165–174. doi: 10.1007/s00253-006-0802-y. [DOI] [PubMed] [Google Scholar]
- Strobel (1992).Strobel HJ. Vitamin B12-dependent propionate production by the ruminal bacterium Prevotella ruminicola 23. Applied and Environmental Microbiology. 1992;58:2331–2333. doi: 10.1128/aem.58.7.2331-2333.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanapat et al. (2003).Wanapat M, Nontaso N, Yuangklang C, Wora-anu S, Ngarmsang A, Wachirapakorn C, Rowlinson P. Comparative study between swamp buffalo and native cattle in feed digestibility and potential transfer of buffalo rumen digesta into cattle. Asian Australasian Journal of Animal Sciences. 2003;16:504–510. doi: 10.5713/ajas.2003.504. [DOI] [Google Scholar]
- Wang et al. (2007).Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2015).Wang QL, Hua LM, Wang GZ, Liu L. Effect of defered grazing on grassland community characteristics and productivity in a lphine meadow. Acta Agrectir Sinica. 2015;23:1068–1072. [Google Scholar]
- Wang et al. (2016).Wang W, Li C, Li F, Wang X, Zhang X, Liu T, Nian F, Yue X, Li F, Pan X, La Y, Mo F, Wang F, Li B. Effects of early feeding on the host rumen transcriptome and bacterial diversity in lambs. Scientific Reports. 2016;6:32479. doi: 10.1038/srep32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng (2013).Weng XX. The study on rumen fermentation, volatile fatty acid absorption chacrcteristics and genen expression in dairly cows receiving different types od diets. Gansu Agricultural University; Lanzhou: 2013. [Google Scholar]
- Wiener et al. (2003).Wiener G, Han JL, Long RJ, Wiener G, Han JL, Long RJ. Regional Office for Asia and the Pacific of the Food and Agriculture Organization of the United Nations, Bangkok, ThailandOrigins, domestication and distribution of yak. 2003
- Xie et al. (2014).Xie J, Yan SM, Li J, Zhao YG, Wu TM, Shi Y, Guo XY. Effect of supplementary feeding of grazing Hulunbuir lacting ewes on lamb growth performance and ewe weight during the spring. Feed Industry. 2014;35:43–46. [Google Scholar]
- Xu et al. (2017).Xu T, Xu S, Hu L, Zhao N, Liu Z, Ma L, Liu H, Zhao X. Effect of dietary types on feed intakes, growth performance and economic benefit in Tibetan sheep and yaks on the Qinghai-Tibet Plateau during cold season. PLOS ONE. 2017;12(1):e0169187. doi: 10.1371/journal.pone.0169187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue et al. (2016).Xue D, Chen H, Chen F, He Y, Zhao C, Zhu D, Zeng L, Li W. Analysis of the rumen bacteria and methanogenic archaea of yak (Bos grunniens) steers grazing on the Qinghai-Tibetan Plateau. Livestock Science. 2016;188:61–71. doi: 10.1016/j.livsci.2016.04.009. [DOI] [Google Scholar]
- Xue, Zhao & Zhang (2005).Xue B, Zhao XQ, Zhang YS. Seasonal changes in weight and body composition of yak grazing on alpinemeadow grassland in the Qinghai-Tibetan plateau of China. Journal of Animal Science. 2005;83:1908–1913. doi: 10.2527/2005.8381908x. [DOI] [PubMed] [Google Scholar]
- Yang, Beauchemin & Rode (2001).Yang WZ, Beauchemin KA, Rode LM. Effects of grain processing, forage to concentrate ratio, and forage particle size on rumen pH and digestion by dairy cows. Journal of Dairy Science. 2001;84:2203–2216. doi: 10.3168/jds.S0022-0302(01)74667-X. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2013).Zhang T, Zhuang S, Dong WC, Chang GJ, Yan R, Wang T. Effects of different dietary concentrate to forage ratios on rumen fluid pH and VFA levels and blood VFA levels in dairy goats. Animal Husbandry and Veterinary Medicine. 2013;4:5–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The treatment HS1= concentrate to forage ratio 0:100, HS2= concentrate to forage ratio 15:85, HS3= concentrate to forage ratio 30:70, HS4= concentrate to forage ratio 45:55, HS5=concentrate to forage ratio 60:40. DMI=dry matter intake; DM consume= DMI × t, t is experimental time(d), BW=body weight; ADG= average daily BW gain. FCR= feed conversion ratio.
The 2 sequences in the file represent 2 samples of Tibetan sheep in group HS1.
The 2 sequences in the file represent another 2 samples of Tibetan sheep in group HS1.
The 4 sequences in the file represent 4 samples of Tibetan sheep in group HS2.
The 4 sequences in the file represent 4 Tibetan sheep in group HS3.
The 4 sequences in the file represent 4 rumen samples of Tibetan sheep in group HS4.
The 4 sequences in the file represent 4 Tibetan sheep in group HS5.
Data Availability Statement
The following information was supplied regarding data availability:
The sequencing data for the 16S rRNA genes are publicly available in the NCBI Short Read Archive: PRJNA477411.