Abstract
Background
Studies have documented a temporal increase in incidence for several cancers in US young adults aged 25 to 39-years, including noncardia gastric cancer, colorectal cancer, and distant-stage breast cancer. To further characterize trends in young adults, we assessed age-specific and stage-specific incidence trends from 1975 to 2015, overall (all malignant cancers combined), and for 18 and 16 cancer sites in women and men, respectively.
Methods
We used US population-based data from the Surveillance, Epidemiology, and End Results Program to obtain overall and site-specific cancer incidence rates by sex and age group. We individually analyzed cancer sites with an incidence rate of at least 5 per 100 000 in 2015, accounting for greater than 90% of all cancer diagnoses. We estimated annual percent changes (APCs) using segment log-linear regression performed with joinpoint software; multiple permutation testing was used to identify inflection points. We forecasted overall cancer incidence through 2030 using age-period-cohort regression models.
Results
Based on trends occurring after the most recent joinpoint inflection point, overall cancer incidence increased by 1.15% (95% CI = 1.01% to 1.28%) per year in 25- to 39-year-old women and by 0.46% (95% CI = 0.17% to 0.75%) per year in 25- to 39-year-old men; APCs were of much lower magnitude in the older age groups (eg, 70- to 84-year-old women APC = −0.31%, 95% CI = −0.42% to −0.20%). We forecasted that overall cancer incidence will increase by an additional 11% by 2030 in 25- to 39-year-old women, and by an additional 12% in 25- to 39-year-old men. Ten of the 18 cancers assessed in 25- to 39-year-old women and 7 of the 16 cancers in 25- to 39-year-old men have been statistically significantly increasing over time. We found that the increase in incidence for young adults is stage specific for some cancers (eg, only nonlocalized breast cancer has increased in 25- to 39-year-old women).
Conclusion
Cancer incidence is increasing in young adults, particularly in young women.
Examining temporal trends in age-specific cancer incidence as reflected by annual percent change (APC) estimates reveal patterns that may help forecast future disease burden as well as help in developing hypotheses regarding etiology. Three recent studies, each examining a different cancer using US population-based data from the Surveillance, Epidemiology, and End Results (SEER) Program, have documented an increase in incidence in young adults, aged 25- to 39-years, compared with older age groups (1–3). Anderson et al. reported a 2.7% (95% confidence interval [CI] = 1.5% to 3.9%) per year increase in noncardia gastric cancer incidence in 25- to 39-year-old white women and men from 1977 to 2006 (1). Bailey et al. reported a 2.0% (95% CI = 1.5% to 2.5%) per year increase in colorectal cancer incidence in 20- to 34-year-old women and men of all races from 1975 to 2010 (2). Johnson et al. reported a 2.1% (95% CI = 1.6% to 2.6%) per year increase in distant-stage breast cancer incidence in 25- to 39-year-old women of all races from 1976 to 2009 (3). For each of these cancers, the APC in incidence was higher for individuals younger than 40 years compared with older adults. Importantly, increases occurred in age groups that are not routinely screened for the cancers that have population-screening tests like breast and colorectal cancers, thereby minimizing the possibility that the increase is screening related. Recently, Sung et al. used age-period-cohort modeling to examine recent (1995–2014) temporal trends by age and year of birth for 30 common cancers and found that incidence is increased in young adults for a number of cancers (4). However, this study did not consider sex-specific (for cancers commonly occurring both in women and men) or stage-specific incidence trends, and models did not allow for changing trends during the study period. In this study, we used joinpoint regression, a flexible modeling approach that allows for the identification of changes that affect different age groups in different years, to assess trends in the incidence of all malignant (excludes in situ) cancer diagnoses (hereafter referred to as overall cancer incidence) by age group at diagnosis using SEER data from 1975 to 2015. We also compared incidence trends by age group at diagnosis for 20 individual cancer sites (18 in women and 16 in men), which together account for greater than 90% of all cancer diagnoses, and we assessed stage-specific trends for cancers with increasing incidence in young adults.
Methods
We used the SEER 9 registries database (SEER 9) to obtain annual cancer incidence rates from 1975 to 2015, age-adjusted to the 2000 US standard population. SEER registries have a case ascertainment rate of 98%, and SEER 9 captures 9.4% of the US population including registry data from Connecticut, Iowa, New Mexico, Utah, Hawaii, Detroit, San Francisco-Oakland, Seattle-Puget Sound, and Atlanta (5). We used SEER 9 data for our main analysis because it provides the longest temporal window to assess incidence trends. As a sensitivity analysis, we estimated APCs in overall cancer incidence using the expanded SEER 13 (1992–2015) and SEER 18 (2000–2015) registries, which produced estimates aligning with SEER 9 results (Supplementary Table1, available online). We included all malignant cancers, regardless of histologic confirmation, in our main analysis, and we conducted a sensitivity analysis restricted to histologically confirmed cancers, which did not change conclusions.
Statistical Analysis
We estimated APCs using segment log-linear regression performed with joinpoint software version 4.6.0.0 from the National Cancer Institute; multiple permutation testing was used to identify inflection points in trends (6, 7). Models allowed for 0 to 5 joinpoints and required a minimum of 2 observations between each end of inflection points. Year of diagnosis was specified as the independent variable predicting log-transformed age-adjusted annual cancer incidence rates stratified by age group at diagnosis based on 15-year intervals (25–39, 40–54, 55–69, and 70–84 years). Cases diagnosed before age 25 years were excluded from analyses because of small case counts for individual cancer sites, which is due to the fact that there are substantial differences in the frequency and histology of cancers occurring in older adolescents and young adults (age 18–25 years) (8). APCs were also calculated for individual cancer sites classified using SEER’s site recode International Classification of Diseases for Oncology, third revision/World Health Organization-2008 (ICD-O-3/WHO-2008) 2008 variable. Cancers were selected based on our criteria that each site in our analysis had at least five cases per 100 000 adults (25- to 84-year-old men and women) in 2015. For cancers with increasing incidence in young adults, we also estimated APCs further stratified by stage at diagnosis, if available (eg, excludes leukemia), for the time trend after the most recent inflection point identified by joinpoint regression using data for all stages combined. Stage at diagnosis was defined using the SEER historic stage A definition (ie, localized, regional, and distant) (9). Statistically significant APCs were determined using a two-sided test based on the t distribution (H0: APC = 0).
To predict overall cancer incidence through 2030, we used an age-period-cohort regression model implemented through the Nordpred package in R software (10) (https://www.kreftregisteret.no/en/Research/Projects/Nordpred/Nordpred-software/) with input data aggregated into eight 5-year calendar periods (1976–1980 to 2011–2015) and 18 5-year age groups. National population projected data from 2016 to 2030 were obtained from the US Census Bureau (11). Separate models were fit for women and men, and predictions were based on trends over the entire 1975–2015 period. To offset exponential increases or decreases in incidence rates, we used the Power-5 link function (12). Assuming that trends are not likely to continue indefinitely, the drift component was reduced by 25% and 50% in the second and third calendar periods, respectively.
Results
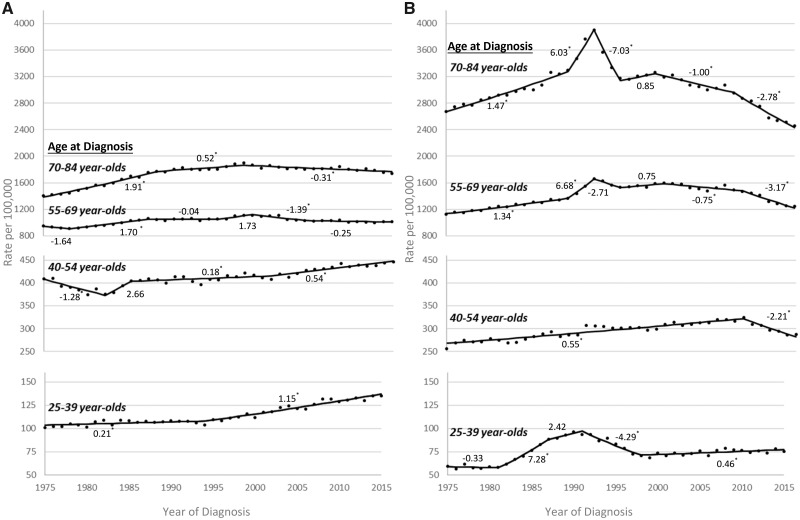
Age-specific overall cancer incidence rates and APCs from 1975 to 2015, stratified by sex, are presented in Figure 1. Although overall cancer incidence was more than threefold higher in the older age groups of women and men compared with the youngest age group (case counts available in Supplementary Table 2, available online), incidence increased at a much higher rate in the youngest adults during the most recent time trend. In the 25- to 39- and 40- to 54-year-old age groups, the overall cancer incidence rate was higher each year for women (Figure 1A) compared with men (Figure 1B); conversely, higher rates were observed for men compared with women for ages 55 and older. In 25- to 39-year-old women, overall cancer incidence statistically significantly increased by 0.21% per year (95% CI = 0.03% to 0.39%) from 1975 to 1994 and by 1.15% per year (95% CI = 1.01% to 1.28%) from 1995 to 2015. In 40- to 54-year-old women, overall cancer incidence statistically significantly decreased up until 1982 (1975–1982 APC = −1.28%, 95% CI = −1.90% to −0.66%) and then increased in subsequent years (2001–2015 APC = 0.54%, 95% CI = 0.38% to 0.69%). Overall cancer incidence decreased during the most recent time trend for 55- to 69-year-old (2005–2015 APC = −0.25%, 95% CI = −0.49% to 0.00%) and 70- to 84-year-old (1998–2015 APC = −0.31%, 95% CI = −0.42% to −0.20%) women. In 25- to 39-year-old men, a peak in overall cancer incidence occurred around 1990, which tracks with the acquired immune deficiency syndrome (AIDS) epidemic in the United States (13,14); overall cancer incidence then increased by 0.46% per year (95% CI = 0.17% to 0.75%) from 1998 to 2015. Overall cancer incidence increased in earlier years, but statistically significantly decreased during the most recent time trend for 40- to 54-year-old (2009–2015 APC = −2.21%, 95% CI = −2.95% to −1.46%), 55- to 69-year-old (2009–2015 APC = −3.17%, 95% CI = −3.87% to −2.47%), and 70- to 84-year-old (2008–2015 APC = −2.78%, 95% CI = −3.31% to −2.25%) men. The spike in overall cancer incidence observed around 1992 in the two oldest age groups of men coincides with the introduction of the prostate-specific antigen test (15). The APC, 95% confidence interval, and interval years for each joinpoint segment presented in Figure 1 are available in the supplemental materials (Supplementary Table 3, available online).
Figure 1.
Trends in overall cancer incidence rates by sex, age group, and year of diagnosis, Surveillance, Epidemiology, and End Results 9 database registries, 1975–2015. A) Annual overall cancer incidence rates, age-adjusted to the 2000 US standard population, and annual percent change (APC) for each joinpoint segment for women by age group at diagnosis. B) Annual overall cancer incidence rates, age-adjusted to the 2000 US standard population, and APC for each joinpoint segment for men by age group at diagnosis. The APC for each joinpoint segment is provided above or below the trend line. *Statistically significant APCs (H0: APC = 0; P < .05 for a two-sided test based on the t distribution).
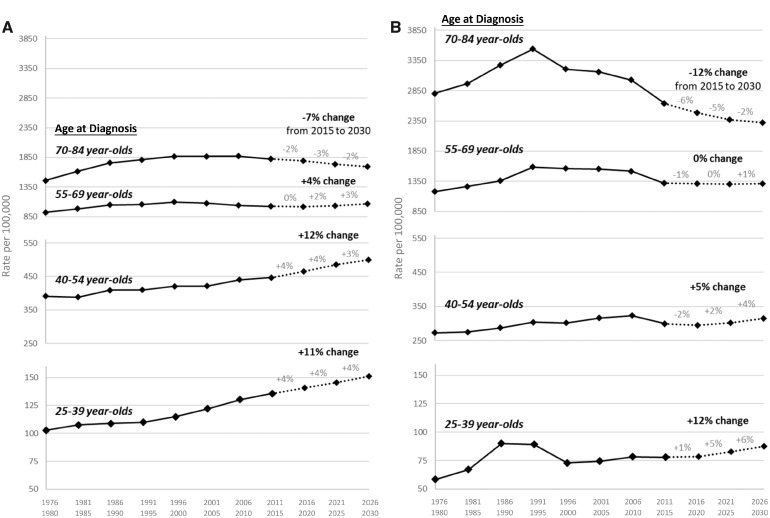
Overall cancer incidence rates forecasted through the year 2030 are presented in Figure 2. Based on our forecasting models, we predict that overall cancer incidence will increase by 11% over the next 15 years for 25- to 39-year-old women, by 12% for 40- to 54-year-old women, and by 4% for 55- to 69-year-old women. In contrast, our forecasting models predict that overall cancer incidence will decrease by 7% over the next 15 years for 70- to 84-year-old women. For men, we predict that overall cancer incidence will increase by 12% over the next 15 years for 25- to 39-year-olds and by 5% for 40- to 54-year-olds. Overall cancer incidence is predicted to remain stable over the next 15 years for 55- to 69-year-old men and decrease by 12% for 70- to 84-year-old men. The age-period-cohort models that we used for forecasting provided a good fit of the data (see Supplementary Figures 1 and 2, available online), and forecasting models excluding Kaposi sarcoma cases in men resulted in comparable predictions (see Supplementary Figure 3, available online).
Figure 2.
Projections of overall cancer incidence trends through 2030 by sex, age group, and year of diagnosis, Surveillance, Epidemiology, and End Results 9 database registries, 1975–2015. A) Overall cancer incidence rates projected through 2030 for women by age group at diagnosis. B) Overall cancer incidence rates projected through 2030 for men by age group at diagnosis. The overall percent change from 2015 to 2030 and the percent change for each 5-year interval (eg, 2016–2020) are provided above the trend line.
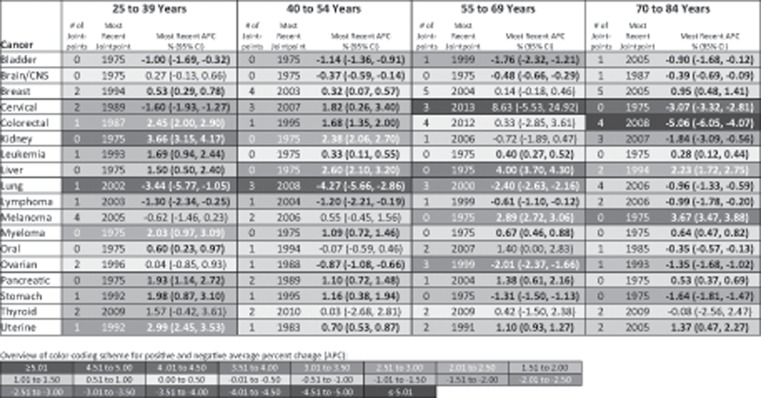
Figure 3 presents APCs in site-specific cancer incidence in women for the time period after the most recent joinpoint inflection point. Ten of the 18 cancer sites assessed in 25- to 39-year-old women statistically significantly increased in incidence during the most recent time trend. This included breast (1994–2015 APC = 0.53%, 95% CI = 0.29% to 0.78%), colorectal (1987–2015 APC = 2.45%, 95% CI = 2.00% to 2.90%), kidney (1975–2015 APC = 3.66%, 95% CI = 3.15% to 4.17%), leukemia (1993–2015 APC = 1.69%, 95% CI = 0.94% to 2.44%), liver (1975–2015 APC = 1.50%, 95% CI = 0.50% to 2.40%), myeloma (1975–2015 APC = 2.03%, 95% CI = 0.97% to 3.09%), oral (1975–2015 APC = 0.60%, 95% CI = 0.23% to 0.97%), pancreatic (1975–2015 APC = 1.93%, 95% CI = 1.14% to 2.72%), stomach (1992–2015 APC = 1.98%, 95% CI = 0.87% to 3.10%), and uterine (1992–2015 APC = 2.99%, 95% CI = 2.45% to 3.53%) cancer. Thyroid cancer also increased by more than 1% per year since 2009, although this trend was not statistically significant. Except for oral cancer, these same cancers also statistically significantly increased in 40- to 54-year-old women, as did cervical cancer. However, the magnitude of the annual increase was greater in 25- to 39-year-old women compared with 40- to 56-year-old women, except for liver cancer. In 55- to 69-year-old women, six cancers statistically significantly increased during the most recent time trend including leukemia, liver, melanoma, myeloma, pancreatic, and uterine cancer. These cancers also increased in incidence in 70- to 84-year-old women, in addition to breast cancer. As age group at diagnosis increased, so did the number of cancers for which there was a statistically significant decrease in incidence after the most recent joinpoint inflection point; four cancers statistically significantly decreased in 25- to 39-year-old women (bladder, cervical, lung, and lymphoma) compared with 10 cancers in 70- to 84-year-old women (bladder, brain, cervical, colorectal, kidney, lung, lymphoma, oral, ovarian, and stomach).
Figure 3.
Site-specific cancer incidence trends for women by age group at diagnosis based on the annual percent change (APC) after the most recent joinpoint inflection point, Surveillance, Epidemiology, and End Results 9 database registries, 1975–2015. Incidence rates are age-adjusted to the 2000 US standard population. Cancer types are classified based on SEER’s site recode International Classification of Diseases for Oncology, third revision/World Health Organization-2008 variable: bladder = urinary bladder; brain/CNS = brain and other central nervous system; cervical = cervix uteri; kidney = kidney and renal pelvis; liver = liver and intrahepatic bile duct; lung = lung and bronchus; oral = oral cavity and pharynx; uterine = corpus and uterus, not otherwise specified. Positive APCs, which indicate increasing incidence over time, are in red (color shading darkens with increasing values); negative APCs, which indicate decreasing incidence over time, are in blue. Statistically significant APCs (H0: APC = 0; P < .05 for a two-sided test based on the t distribution) are bold. CI = confidence interval.
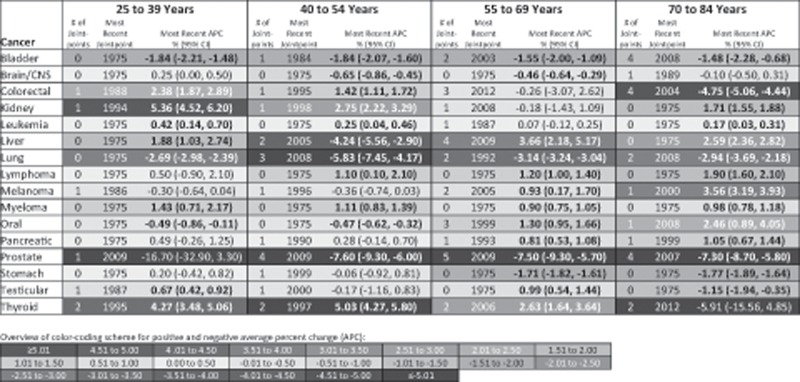
Figure 4 presents APCs in site-specific cancer incidence in men for the time period after the most recent joinpoint inflection point. Seven of the 16 cancer sites assessed in 25- to 39-year-old men statistically significantly increased in incidence during the most recent time trend. This included colorectal (1988–2015 APC = 2.38%, 95% CI = 1.87% to 2.89%), kidney (1994–2015 APC = 5.36%, 95% CI = 4.52% to 6.20%), leukemia (1975–2015 APC = 0.42%, 95% CI = 0.14% to 0.70%), liver (1975–2015 APC = 1.88%, 95% CI = 1.03% to 2.74%), myeloma (1975–2015 APC = 1.43%, 95% CI = 0.71% to 2.17%), testicular (1987–2015 APC = 0.67%, 95% CI = 0.42% to 0.92%), and thyroid (1995–2015 APC = 4.27%, 95% CI = 3.48% to 5.06%) cancer. In 40- to 54-year-old men, there was an increase in incidence of colorectal, kidney, leukemia, and myeloma, although the APCs for these cancers were observed at a lesser magnitude compared with what was observed for men younger than 40 years; lymphoma also increased in this age group of men. A greater number of cancers statistically significantly increased after the most recent joinpoint inflection point in the two oldest compared with two youngest age groups of men. In 55- to 69-year-old men, there was a statistically significant increase in liver, lymphoma, melanoma, myeloma, oral, pancreatic, testicular, and thyroid cancer; in 70- to 84-year-old men, there was a statistically significant increase in kidney, leukemia, liver, lymphoma, melanoma, myeloma, oral, and pancreatic cancer. However, as was observed in women, a greater number of cancers statistically significantly decreased in incidence after the most recent joinpoint inflection point in older compared with younger men. For example, three cancers decreased in incidence in 25- to 39-year-old men (bladder, lung, and oral cancer) compared with six cancers in 70- to 84-year-old men (bladder, colorectal, lung, prostate, stomach, and testicular cancer).
Figure 4.
Site-specific cancer incidence trends for men by age group at diagnosis based on the (APC) annual percent change after the most recent joinpoint inflection point, Surveillance, Epidemiology, and End Results 9 database registries, 1975–2015. Incidence rates are age-adjusted to the 2000 US standard population. Cancer types are classified based on SEER’s site recode International Classification of Diseases for Oncology, third revision/World Health Organization-2008 variable: bladder = urinary bladder; brain/CNS = brain and other central nervous system; kidney = kidney and renal pelvis; liver = liver and intrahepatic bile duct; lung = lung and bronchus; oral = oral cavity and pharynx. Positive APCs, which indicate increasing incidence over time, are in red (color shading darkens with increasing values); negative APCs, which indicate decreasing incidence over time, are in blue. Statistically significant APCs (H0: APC = 0; P < .05 for a two-sided test based on the t distribution) are bold. CI = confidence interval.
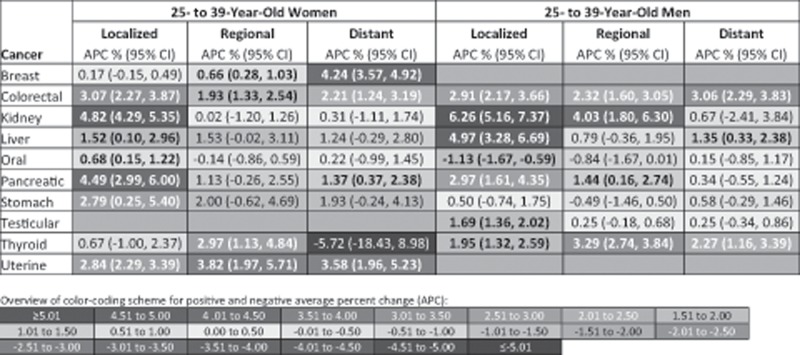
In Figure 5, stage-specific APCs for the time period after the most recent joinpoint inflection point are provided for cancers with increasing incidence over time in young adults. The increase in incidence was seen across all stages of disease for most of these cancers, with some exceptions. For example, we found that only localized stage disease increased (APCs >0.5% per year) for kidney and oral cancer in young women and for testicular cancer in young men. Conversely, only nonlocalized stage disease increased for breast cancer in young women. We identified some stage specific differences in APCs between women and men. For example, whereas thyroid cancer statistically significantly increased across all diagnostic stages for men, only regional-stage disease statistically significantly increased for women.
Figure 5.
Site- and stage-specific cancer incidence trends for 25- to 39-year-old women and men based on the annual percent change (APC) after the most recent joinpoint inflection point, Surveillance, Epidemiology, and End Results 9 database registries, 1975–2015. APCs are based on the time trend after the most recent inflection point identified by joinpoint regression using data for all stages combined. Refer to Figures 3 and 4 for the year of the most recent inflection point for each cancer. Incidence rates are age-adjusted to the 2000 US standard population. Cancer types are classified based on SEER’s site recode International Classification of Diseases for Oncology, third revision/World Health Organization-2008 variable: kidney = kidney and renal pelvis; liver = liver and intrahepatic bile duct; oral = oral cavity and pharynx; uterine = corpus and uterus, NOS. Positive APCs, which indicate increasing incidence over time, are in red (color shading darkens with increasing values); negative APCs, which indicate decreasing incidence over time, are in blue. Statistically significant APCs (H0: APC = 0; P < .05 for a two-sided test based on the t distribution) are bold. CI = confidence interval.
Discussion
We demonstrated that cancer incidence has varied over time by age group at diagnosis. Whereas cancer incidence rates in older adults continue to be substantially higher, younger adults, and particularly young women, have been disproportionately affected by rising cancer incidence in recent decades (as reflected by the APC). Overall cancer incidence statistically significantly increased during the most recent time trend for 25- to 39-year-old women, 40- to 54-year-old women, and 25- to 40-year-old men, which was determined by the most recent joinpoint inflection point. The rate of increase in 25- to 39-year-old women, which was greater than 1% per year since 1994, was twofold higher than the rate of increase in the other two groups (40- to 54-year-old women and 25- to 39-year-old men). Overall cancer incidence decreased during the most recent time trend for 55- to 84-year-old women and 40- to 84-year-old men.
Site-specific cancer incidence trends further illustrated the concerning rise in cancer incidence in young women younger than 40 years. Not only was there a greater number of cancers for which incidence statistically significantly increased during the most recent time trend in younger compared with older women, but also the magnitude of the annual increase was higher in younger compared with older women for many cancers. For example, there was a twofold higher increase in leukemia and uterine cancer in the youngest compared to the three older age groups of women during the most recent time trend. Both for women and men younger than 40 years, there was only a handful of cancers with a statistically significant decrease in incidence during the most recent time trend; only four cancers (bladder, cervical, lung, and lymphoma) statistically significantly decreased in 25- to 39-year-old women, and only three cancers statistically significantly decreased in 25- to 39-year-old men (bladder, lung, and oral). By comparison, 10 cancers decreased in the oldest age group of women and 6 cancers decreased in the oldest age group of men during the most recent time trend.
Increasing overall cancer incidence in young adults, particularly in young women, is concerning, especially given that current trends are projected to continue in future years. The scope of this trend is underscored by the number and diversity of cancers for which there were statistically significant increases in incidence in adults younger than 40 years after the most recent joinpoint inflection point. Young adults have more potential years of life affected by disease compared with older adults and, for many cancers, prognosis is worse for younger compared with older adults (3,16). Further, for most cancers, routine screening is not currently recommended for young adults (17). Thus, whereas participation in cancer screening may account for incidence patterns in older age groups, they are unlikely to explain the higher APCs in those younger than 40 years. For example, in the youngest age group of 25- to 39-year-old women and men, for which all individuals are below screening age, we found that colorectal incidence increased by more than 1.9% per year across all stages of disease. Although it is possible that the increase for some cancers may be from noncancer medical screening (eg, computed topography scan), if this were to be the major explanation for observed trends then we would expect some of this increase to be reflected in the stage distribution of the cancers, which we did not consistently observe. For example, for breast and uterine cancer, we found that the APCs were higher in magnitude for nonlocalized disease. This suggests that the increase in cancer incidence for young adults cannot be fully explained by increased detection, but instead reflects a true increase in risk, at least for some cancers.
Hereditary factors alone cannot explain temporal trends in cancer incidence rates over just a few decades. Increasing incidence trends thus require further investigation into changes in secular patterns to environmental exposures including changes to environmental carcinogens, diet, and physical activity, as well as other health indicators, including obesity and infection burden (18–21). In particular, risk factors that are shared across cancers should be considered. For example, obesity is associated with increased risk of several cancers, including postmenopausal breast, colorectal, kidney, pancreatic, stomach, and thyroid (22). Obesity rates have increased in US adults over the past several decades (23), and correspondingly, we found an increase in incidence over time in several obesity-associated cancers in young adults. However, not all cancers with increasing incidence over time in young adults are associated with obesity (24). In fact, obesity is associated with a decreased risk of premenopausal breast cancer (24), which has increased by 0.53% per year (and by 4.24% for distant-stage disease) in 25- to 39-year-old women since 1994. Therefore, young adult cancer incidence trends cannot be fully explained by obesity trends, and so temporal changes in other exposures must also be considered. Given the early age of disease onset, exposures occurring earlier in life, including in utero and early childhood, should also be considered when assessing cancer etiology in young adults.
The decreasing trends in lung cancer in adults support the potential impact of identifying and intervening on modifiable risk factors of cancer. From 1975 to 1994, lung cancer incidence rates statistically significantly increased in 55- to 85-year-old women and 70- to 84-year-old men, but decreased or remained stable in younger age groups. From 1995 to 2015, lung cancer incidence rates decreased across all age groups except 70- to 84-year-old women. These trends track with smoking patterns in the United States in which daily cigarette smoking peaked during the 1970s in male smokers and during the 1980s in female smokers, before decreasing in the two sexes in parallel (25). Other incidence trends, such as the decrease in Kaposi sarcoma (26) in young men and cervical cancer in young women, provide additional examples of the potential impact of cancer prevention and policy initiatives aimed at modifiable risk factors of disease.
The overall inferences of our results are strengthened by our use of population-based data, assessment of incidence for cancer overall and for 20 individual sites in women and men, and consideration of stage-specific and time-period-specific incidence trends. We were limited by registry data, which lacked individual-level data on exposures and other important factors such as family history of disease. We also used relatively broad tumor categories for some cancer sites (eg, stomach included cardia and noncardia). The use of more narrowly defined tumor groups may help us further understand age-specific incidence trends. Further, we note that case counts were small for some stage-specific cancers, which prevented us from conducting jointpoint analyses stratified by stage at diagnosis. Instead, we assessed stage-specific trends based on the most recent inflection point identified in the joinpoint analysis of all stages combined, which allows for direct comparisons to be made across stages. Finally, because of the already broad scope of our analysis, we did not assess temporal trends in cancer incidence further stratified by other potentially relevant factors such as geography, race and ethnicity, or socioeconomic status. Such factors should be considered in future analyses of cancer incidence trends, particularly in light of some evidence suggesting larger racial disparities in cancer mortality in young adults compared with older age groups (27).
In closing, our findings underscore the need for continued and accelerated research on cancer etiology in young adults and point to the potential role of environmental and behavioral exposures throughout the life course in explaining age-specific cancer incidence trends. We note that although we identified several cancers for which incidence is statistically significantly increasing over time, we also found examples of cancers with decreasing incidence trends, particularly in older adults, which may be attributed to more effective screening practices and/or decreasing exposure to cancer risk factors over time. Such findings provide promise that similar progress can be made toward reducing cancer incidence in young adults if the necessary attention and resources are devoted to this understudied population. This includes a reevaluation of screening guidelines for younger adults, particularly those at higher risk of cancer, as well as increased investment in prevention research focused on modifiable risk factors in early life.
Funding
RDK is supported by the National Institutes of Health, National Cancer Institute, Cancer Epidemiology Training Grant (grant number T32-CA009529).
Notes
Affiliations of authors: Department of Epidemiology, Mailman School of Public Health, Columbia University, New York, NY (RDK, WY, PT, MBT); Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, New York, NY (PT, MBT).
The authors have no conflicts of interest to disclose.
Supplementary Material
References
- 1. Anderson WF, Camargo MC, Fraumeni JF Jr, Correa P, Rosenberg PS, Rabkin CS.. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303(17):1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey CE, Hu CY, You YN.. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson RH, Chien FL, Bleyer A.. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309(8):800–805. [DOI] [PubMed] [Google Scholar]
- 4. Sung H, Siegel RL, Rosenberg PS, Jemal A.. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4(3):e136–e147. [DOI] [PubMed] [Google Scholar]
- 5. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Research Data, Nov 2017 Sub (1973-2015) <Katrina/Rita Population Adjustment>—Linked To County Attributes - Total U.S., 1969-2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018. www.seer.cancer.gov. Based on November 2017 submission.
- 6. Kim HJ, Fay MP, Feuer EJ, Midthune DN.. Permutation tests for joinpoint regression with applications to cancer rates [Erratum in: Stat Med 2001;20(4):655]. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 7. National Cancer Institute, Statistical Methodology and Applications Branch, Surveillance Research Program. Joinpoint Regression Program, Version 4.6.0.0. April 2018. https://surveillance.cancer.gov/joinpoint/. Accessed January 30, 2018.
- 8. Bleyer A, O’Leary M, Barr R, Ries L, eds. Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975-2000. Bethesda, MD: National Cancer Institute, NIH Pub. No. 06-5767; 2006. [Google Scholar]
- 9. Young JL Jr, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA, eds. SEER Summary Staging Manual - 2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute, NIH Pub. No. 01-4969; 2001. https://seer.cancer.gov/tools/ssm/ssm2000/intro.pdf. Accessed August 27, 2018. [Google Scholar]
- 10. R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.R-project.org/. Accessed October 3, 2018. [Google Scholar]
- 11. United States Census Bureau. 2017 National population projections table. 2018. https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html. Accessed January 5, 2019.
- 12. Møller B, Fekjaer H, Hakulinen T, et al. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med. 2003;22(17):2751–2766. [DOI] [PubMed] [Google Scholar]
- 13. Levine AM. AIDS-related malignancies: the emerging epidemic. J Natl Cancer Inst. 1993;85(17):1382–1397. [DOI] [PubMed] [Google Scholar]
- 14. Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20(12):1645–1654. [DOI] [PubMed] [Google Scholar]
- 15. Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer—Part I: evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91(12):1017–1024. [DOI] [PubMed] [Google Scholar]
- 16. Keegan TH, Ries LA, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122(7):1009–1016. [DOI] [PubMed] [Google Scholar]
- 17. Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68(4):297–316. [DOI] [PubMed] [Google Scholar]
- 18. Briefel RR, Johnson CL.. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. [DOI] [PubMed] [Google Scholar]
- 19. Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crimmins EM, Finch CE.. Infection, inflammation, height, and longevity. Proc Natl Acad Sci U S A. 2006;103(2):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nelson MC, Neumark-Stzainer D, Hannan PJ, Sirard JR, Story M.. Longitudinal and secular trends in physical activity and sedentary behavior during adolescence. Pediatrics. 2006;118(6):e1627–e1634. [DOI] [PubMed] [Google Scholar]
- 22. Donohoe CL, Lysaght J, O'Sullivan J, Reynolds JV.. Emerging concepts linking obesity with the hallmarks of cancer. Trends Endocrinol Metab. 2017;28(1):46–62. [DOI] [PubMed] [Google Scholar]
- 23. Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–570. [DOI] [PubMed] [Google Scholar]
- 24. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M.. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. [DOI] [PubMed] [Google Scholar]
- 25. Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eltom MA, Jemal A, Mbulaiteye SM, Devesa SS, Biggar RJ.. Trends in Kaposi’s sarcoma and non-Hodgkin’s lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst. 2002;94(16):1204–1210. [DOI] [PubMed] [Google Scholar]
- 27. Tehranifar P, Goyal A, Phelan JC, et al. Age at cancer diagnosis, amenability to medical interventions, and racial/ethnic disparities in cancer mortality. Cancer Causes Control. 2016;27(4):553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.