Abstract
The ability to adapt to aversive stimuli is critical for mental health. Here, we investigate the relationship between habituation to startling stimuli and startle-related activity in medial frontal cortex as measured by EEG in both healthy control participants and patients with Parkinson disease (PD). We report three findings. First, patients with PD exhibited normal initial startle responses but reduced startle habituation relative to demographically matched controls. Second, control participants had midfrontal EEG theta activity in response to startling stimuli, and this activity was attenuated in patients with PD. Finally, startle-related midfrontal theta activity was correlated with the rate of startle habituation. These data indicate that impaired startle habituation in PD is a result of attenuated midfrontal cognitive control signals. Our findings could provide insight into the frontal regulation of startle habituation.
INTRODUCTION
One of the most fundamental adaptive responses observed across phyla is the habituation or reduction of physiological responses to repeated aversive stimuli (Chen, Aksan, Anderson, Grafft, & Chapleau, 2014). Habituation can occur at the level of single neurons (Castellucci, Pinsker, Kupfermann, & Kandel, 1970) or via cortical modulation of basic response circuits (McDowell et al., 2006; Bubser & Koch, 1994; Koch & Bubser, 1994). Habituation thus indicates primitive learning about the intensity, timing, and outcome of the stimulus, which enables individuals to anticipate and/or devaluate future stimuli and conserve energy and effort (Chen et al., 2014; Koch & Schnitzler, 1997). The reliability and interpretability of this response make it a highly useful probe into neural disorders, particularly if affected mechanisms of this global response can be differentiated.
Failure of habituation is associated with worse mental health. Individuals who showed sustained skin conductance response (reflecting sympathetic-mediated sweating) to aversive noise bursts had a higher probability to develop posttraumatic stress disorder later in life (Pole et al., 2009). Reduced habituation in eye-blink startle responses (measured at the orbicularis oculi) and skin conductance response are also commonly found in patients with anxiety disorders and posttraumatic stress disorder (Jovanovic, Norrholm, Sakoman, Esterajher, & Kozaric-Kovacic, 2009; Shalev et al., 2000; Raskin, 1975; Lader & Wing, 1964). Finally, patients with Parkinson disease (PD) have attenuated habituation to startling stimuli (Nieuwenhuijzen, Horstink, Bloem, & Duysens, 2006; Matsumoto et al., 1992; Sandrini, Alfonsi, Martignoni, Horowski, & Nappi, 1985; Messina, Di Rosa, & Tomasello, 1972; Penders & Delwaide, 1971).
The precise neurobiological mechanisms underlying habituation to aversive stimuli are unclear. In humans, neuroimaging studies have implicated diverse networks in the cerebellum, medial frontal cortex (MFC)/cingulate, parietal cortex, and amygdala in habituation (Hare et al., 2008; Frings et al., 2006; McDowell et al., 2006). In rodents, regulation of startle responses in the prepulse inhibition paradigm involves the MFC (Koch & Schnitzler, 1997). Furthermore, this regulation depends on MFC dopaminergic signaling via D 1-type dopamine receptors (Ellenbroek, Budde, & Cools, 1996; Bubser & Koch, 1994). The MFC has a well-documented role in the regulation of emotional processing (Botvinick & Cohen, 2014; Ochsner, Silvers, & Buhle, 2012; Zelazo & Cunningham, 2007), leading to the hypothesis that the MFC is involved in startle habituation and is dysfunctional in patients with decreased dopamine.
We tested this idea by collecting scalp EEG during a startle habituation paradigm with an aversive noise burst in patients with PD and demographically matched controls. In this paradigm, a loud and aversive noise burst of 103 dB—equivalent to standing on platform by passing train—triggers strong eye-blink and autonomic nervous system responses. The magnitude of these responses reduces over time during repeated exposures to the same noise burst (Chen et ai., 2014; Jovanovic et al., 2009; Geyer & Braff, 1982). Because the intervals between the noise bursts varied (between 10 and 30 sec) and were unknown to the participants, the anticipation of oncoming noise bursts can be stressful.
Frontal dysfunction is common in PD (Narayanan, Rodnitzky, & Uc, 2013; Cools & D’Esposito, 2011; Ibarretxe-Bilbao, Junque, Marti, & Tolosa, 2011; Cools, Gibbs, Miyakawa, Jagust, & D’Esposito, 2008; Lange, Paul, Robbins, & Marsden, 1993) and associated with executive dysfunction (Ibarretxe-Bilbao et al., 2009; Nagano-Saito et al., 2005; Lewis, Dove, Robbins, Barker, & Owen, 2003). We focused on startle-related theta activity (4–7 Hz) recorded from midfrontal electrodes, which previous research has shown to reflect cognitive control implemented by the MFC (Parker, Chen, Kingyon, Cavanagh, & Narayanan, 2014, 2015; Cavanagh & Frank, 2014; Cavanagh & Shackman, 2014; Sauseng, Hoppe, Klimesch, Gerloff, & Hummel, 2007; Sauseng, Klimesch, Schabus, & Doppelmayr, 2005; Ishii et al., 1999). We found that patients with PD had attenuated startle habituation and attenuated midfrontal theta activity. These data provide insight into how the MFC may be involved in modulating responses to aversive stimuli.
EXPERIMENTAL PROCEDURES
Participants
Ten patients with PD were recruited from the movement disorders clinic or a patient registry administered by Dr. Ergun Uc at the Department of Neurology, University of Iowa (Uc et al., 2014)—seven women and three men, aged 63.69 ± 4.40 (mean ± SEM) years with 16.40 ± 0.52 years of education. All patients with PD were currently taking levodopa (n =8) or the dopamine agonist pramipexole (n = 2), with a levodopa equivalent dose of 630.80 ± 99.76 mg (range = 200–1100 mg). When on medication, the patients were less than Hoehn and Yahr stage III, and their Unified Parkinson’s Disease Rating Score, Part III (UPDRS III) was 9.22 ± 2.37 (range = 3–24). All patients with PD were not demented at the time of evaluation with Montreal Cognitive Assessment (MOCA) scores of 27.60 ± 0.50 (all ≥ 26) and did not have other confounding neurological or psychiatric disorders (Table 1).
Table 1.
Demographic Characteristics, PD Status, and Cognitive and Executive Function
| Patients with PD | Control Participants | t | P | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Sex | 7F, 3M | 8F, 4M | .62 | |
| Handedness | 9R, 1B | 11R, 1L | .71 | |
| Age (years) | 63.69 (4.40) | 62.55 (3.03) | 0.22 | .83 |
| Education (years) | 16.40 (0.52) | 15.58 (0.60) | 0.45 | .35 |
| PD status | ||||
| UPDRS IIIa | 9.22 (2.37) | – | ||
| Hoehn-Yahr | 1.6 (1.1) | |||
| Levodopa equivalent dose (mg) | 630.80 (99.76) | |||
| Cognitive function: general | ||||
| MOCAb | 27.60 (0.50) | 28.83 (0.42) | 1.90 | .07 |
| Executive function | ||||
| Trail Making (Parts B-A)a | 50.09 (12.31) | 28.35 (4.67) | −1.77 | .09 |
| Stroopb | 2.30 (1.50) | 0.77 (3.41) | −0.39 | .70 |
| Verbal Fluencyb | 38.50 (3.18) | 48.50 (4.13) | 1.86 | .08 |
| Digit Spanb | 16.90 (0.96) | 19.50 (1.08) | 1.76 | .09 |
| Wisconsin Card Sort Testb | 5.00 (0.67) | 5.75 (0.25) | 1.05 | .31 |
| Executive Function Compositeb | −0.24 (0.14) | 0.27 (0.15) | 2.26 | .03 |
Values are mean (SEM).
B = bilateral; F = female; L = left; M = male; R = right.
Lower score = better performance.
Higher score = better performance.
Twelve age- and education-matched individuals without a history of neurological conditions or current psychoactive medication were recruited from the Iowa City area—eight women and four men, aged 62.55 ± 3.03 years with 15.58 ± 0.60 years of education. MOCA scores of these control participants were 28.83 ± 0.42 (all ≥ 26). Table 1 shows demographic characteristics of all participants and disease status of the patients with PD. The numbers of patients and control participants are comparable with previous studies in the same topic (Putzki, Graf, Stude, Diener, & Maschke, 2008; Nieuwenhuijzen et al., 2006).
Startle Task
The startle task consisted of an aversive noise burst (8192-Hz white noise) presented at 103 dB for 50 msec and repeated 18 times. Time intervals between the noise bursts ranged from 10 to 30 sec (Figure 1A). The noise bursts were presented via over-ear headphones that covered the ear. We instructed participants to gaze at a fixation cross on the center of a screen and alerted them that they would hear noise bursts.
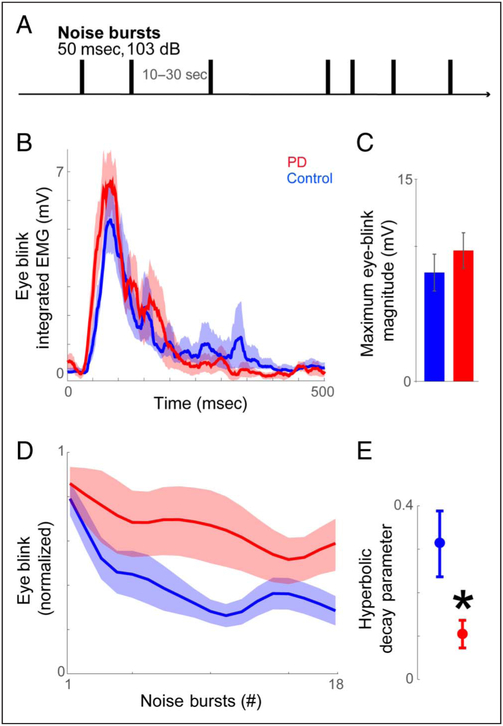
Figure 1.
Patients with PD had less startle habituation to aversive stimuli. (A) In the startle task, participants were presented with a burst of aversive noise (103 dB, 50 msec) repeated at the intervals of 10–30 sec. (B) Eye-blink response to the very first sample noise burst (unnormalized data). Patients with PD exhibited similar eye-blink responses as control participants. (C) An independent-samples t test confirmed the observation that the magnitude of the initial eye-blink responses did not statistically differ between groups (p = .42). (D) Across successive presentations of aversive stimuli, patients with PD exhibited stronger eye-blink responses and (E) had a lower rate of decay in those responses than control participants. All variance is mean ± SEM. *p < .05.
Executive Function Assessment
We assessed executive function using a battery of neuropsychological tests. First, we used the Trail Making Test Parts A and B, which examine the ability to switch between two mental sets. The difference in time to complete Parts A and B was scored (Tombaugh, 2004). Second, we used the Stroop task, which examines the ability to inhibit dominant responses; the interference score was used (Gyurak et al., 2009; Delis, Kaplan, & Kramer, 2001). Third, we used the Verbal Fluency, which examines cognitive flexibility: Participants generated words that began with F, A, and S (1 min for each letter). The total number of words generated was scored (Gyurak et al., 2009; Delis et al., 2001). Fourth, we used Digit Span, which examines working memory. Participants repeated two to nine digits in either the forward or backward orders. The number of correct repetitions was scored (Wechsler, 2008; Baldo, Shimamura, Delis, Kramer, & Kaplan, 2001). Finally, we used the Wisconsin Card Sort Test, which examines the ability to “learn new rules” and “shift from old to new rules” based on dynamic feedback provided by the experimenter (Anderson, Damasio, Jones, & Tranel, 1991; Grant & Berg, 1948). The number of completed categories was scored. The scores of each test of the patient and control groups are shown in Table 1. For Trail Making, higher scores correspond to worse performance. For other tests, higher scores correspond to better performance.
We constructed a composite index of executive function from the neuropsychological tests described above according to methods described previously (Parker et al., 2015). Briefly, we first transformed each test score into z scores based on our entire sample (n = 22), with higher z scores corresponding to better/worse performance relative to other participants in this study. We averaged the five z scores for each participant to form the composite score (for the Trail Making, we reversed the z score before averaging). Higher composite scores corresponded to better executive function.
Procedure
Patients with PD were asked to take their medication as usual on the day of evaluation. Participation involved one laboratory visit. All examinations were conducted in the morning. Informed consent was obtained upon arrival. Participants listened to some sample loud sounds (i.e., the same aversive white noise as in the startle task) immediately after informed consent and setup for EEG recording. Participants’ eye-blink response to the very first noise burst was analyzed to index the capability of responding to our testing stimulus. Between sample sounds and the startle task, participants performed other tasks for approximately ~1.5 hr (Parker et al., 2015)- Before the startle task, participants rested for 3 min. The startle task lasted for 8 min. After the task, a battery of neuropsychological tests and MOCA (original version) were administered. In addition, motor status of patients with PD was evaluated by UPDRS III by a movement disorders physician. All participants were debriefed at the end of their research participation. Five patients with PD returned for “off” testing in the practically defined off-levodopa period at least 12 hr after their last dose of levodopa.
Data Acquisition and Processing
EMG data were recorded at 1000 Hz using the BIOPAC system. Two Ag-AgCl electrodes were placed on the skin surface overlaying the orbicularis oculi muscle (impedance < 5 kΩ). EMG data were offline filtered in Acqknowledge software 4.1 using a 30- to 500-Hz band-pass filter and then integrated and rectified (Blumenthal et al., 2005)- Eye-blink stardes were defined as the maximum change in EMG in a time epoch of 300 msec after the onset of each noise burst (Figure 1B). One patient with PD exhibited relatively delayed eye-blink startle responses; therefore, we extended the time window to 500 msec for this participant as patients with PD can have delayed eye blinks (Vidailhet, Rothwell, Thompson, Lees, & Marsden, 1992). We limited the time window to 200 msec for two control participants who had a rare second eye blink at ~250 msec. Across all participants and all epochs, we excluded two epochs with spontaneous eye blinks during or immediately before the noise-elicited eye-blink startle response.
To examine participant’s capability of responding to our testing stimuli, we examined eye-blink responses to the veiy first sample noise burst (Figure 1B). We analyzed startle habituation by normalizing the data by converting the magnitude of eye blinks from all 18 trials to the percentage of response relative to the first noise burst of the task. The normalization process controls for individual differences in eye-blink reactivity to the noise bursts. Epochs with eye blink exceeding 3–5 SD away from the group average were excluded as outliers (across all participants, the total n of excluded epoch = 8). Data were smoothed at three bins for plotting only (Figure 1D). To compute the rate of startle habituation, for each participant, the normalized eye-blink startle responses over time were fit with a hyperbolic function. The fit took the form of
| (1) |
where a was the start value (always scaled to 100), x, was each time interval (1–18), and r was the rate of decay. The decay rate was fit by minimizing the sum of squared error across time points using the simplex method for gradient descent (MATLAB function fmincon.m) with 40 separate starting points (MATLAB function rmsearch.m). All parameters for r were compared with simulations and were fit by grid search (which yielded identical outcomes). Higher decay parameters correspond to faster/stronger startle habituation (Figure 1E).
EEG
We recorded EEG data on a Nihon Kohden system (Tokyo, Japan) with a sampling rate of 500 Hz. EEG data were recorded from 21 channels based on the 10–20 system (Fz, Cz, Pz, FP1/2, F3/4, C3/4, P3/4, F7/8, T3/4, T5/6, O1/2, and M1/2, where F is frontal, C is central, P is parietal, T is temporal, O is occipital, and M is mastoid). We also recorded left-eye vertical EOG and ground electrodes (forehead). Impedance of all electrodes was below 5 kΩ. Continuous data were parsed into 2500-msec epochs (−500 to 2000 msec relative to noise onset) and rereferenced to the mathematical average of the two mastoid channels (Ml/2). Bad channels across the entire electrode montage were interpolated. Eye blinks and eye movements were removed after independent component analysis from FEGLAB (UC San Diego; Delorme & Makeig, 2004). Continuous EEG data were filtered by a 50-Hz low-pass filter.
ERPs were analyzed using an epoch from −100 to 2000 msec relative to the onset of the noise bursts (Ford et al., 1995; Putnam & Roth, 1990). For ERPs, continuous EEG data were filtered <20 Hz, and data from 0 to 2000 msec were baseline-corrected by the data from −100 to 0 msec. Visual inspection suggested that the 3PQ and P2 components peaked between 100 to 180 and 180 to 300 msec, respectively (Figure 2A). Minimum (JSTl) and maximum (P2) amplitudes were obtained from the corresponding time windows to index each component.
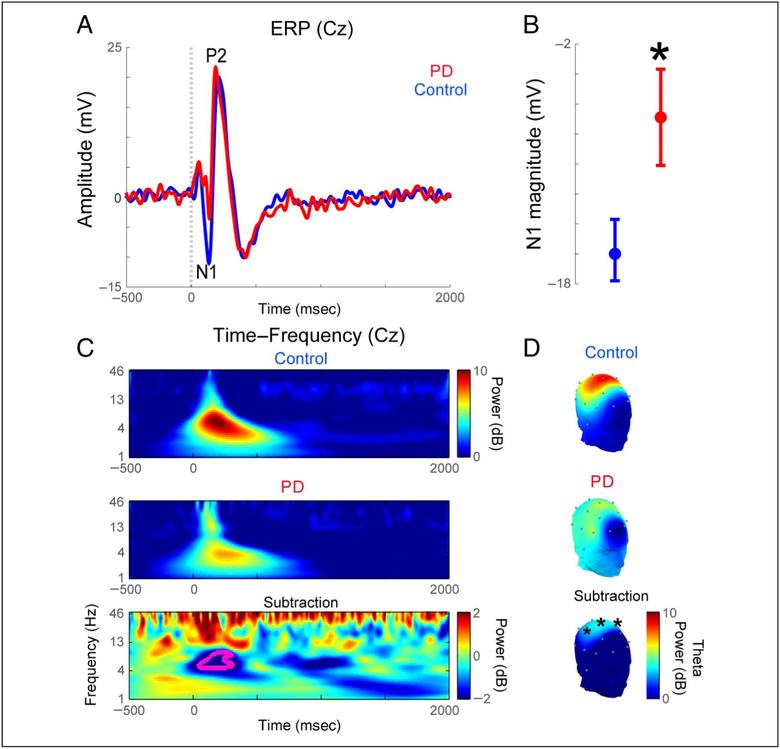
Figure 2.
Patients with PD had less midfrontal theta activity to aversive stimuli. (A, B) Analysis of ERPs at Cz around noise bursts revealed a significantly weaker N1 in patients with PD. (C) Time-frequency analyses of EEG (Cz) revealed significantly lower theta activity (4–7 HZ) 100–200 msec after the delivery of noise bursts in patients with PD’. (D) Scalp topography of startle-related theta activity (100–200 msec after noise burst, 4–7 Hz) in controls and patients with PD. Significant differences were only seen for electrodes Cz, C3, and C4. All variance is mean ± SEM. *p < .05.
Time-Frequency and Statistical Analysis
Time-frequency measures were computed by multiplying the fast Fourier transformed (FFT) power spectrum of single-trial EEG data with the FFT power spectrum of a set of complex Morlet wavelets defined as a Gaussian-windowed complex sine wave: , where t was time,f was frequency (which increased from 1 to 50 Hz in 50 logarithmically spaced steps), and a defined the width (or “cycles”) of each frequency band, set according to 4/(2 πf) and taking the inverse FFT. The end result of this process is identical to time-domain signal convolution, and it resulted in estimates of instantaneous power (the magnitude of the analytic signal). Each epoch was then cut in length (−500 to 2000 msec). Power was normalized by conversion to a decibel (dB) scale [10 × logl0(powert/powerbaseline)], allowing a direct comparison of effects across frequency bands. The baseline for each frequency consisted of the average power from 500 to 300 msec before the onset of the noise bursts.
To test the hypothesis that startle habituation was related to medial frontal dysfunction, we focused on electrode Cz (Figure 2C), although all EEG electrodes were included in this analysis (Figure 2D). Scalp topography was performed by interpolating startle-related theta over the entire scalp based on a spline of 19-electrode EEG montages (excluding FP1/FP2), Statistical significance against the baseline was computed via a paired t test and is indicated by contours in the time-frequency plots (Figure 2C), with a minimum threshold of a 500-pixel cluster size.
RESULTS
Patients with PD Had Lower Executive Function
Executive function was indexed by a composite score of all listed tests in Table 1. An independent-samples t test revealed that patients with PD had significantly worse executive function than control participants (−0.24 vs. 0.27; t(20) = 2.26, p = .035; Table 1). Although patients with PD scored lower than control participants across all neuropsychological tests (except Stroop), the between-group difference was not statistically significant in any single test, likely reflecting the multidimensional nature of executive deficits in PD resulting in greater between-group differences when using composite scores of executive function (Litvan et al., 2012).
Patients with PD Had Normal Initial Eye-blink Response to an Aversive Noise Burst
To examine participants’ capability of responding to our testing stimulus, we analyzed nonnormalized eye-blink responses to the very first sample noise burst. As shown in Figure 1B, patients with PD had similar eye-blink responses to the noise burst as control participants. A t test confirmed this observation: The magnitudes of eye-blink responses did not statistically differ between patients with PD and control participants (PD/control participants = 9.5/7.9 mV; t(20) = 0.83, p = .42; Figure 1C). To further test the null hypothesis that eye-blink responses of control participants were equivalent to those of patients with PD, we performed a Bayesian t test and found no credible difference between the two groups (95% highest density interval [−1.22, 9.59]; Bayes factor in favor of the null hypothesis = 55000; Kruschke, 2013).
Patients with PD Had Less Startle Habituation
As shown in Figure 1D and E, patients with PD had stronger and sustained eye-blink responses in responding to successive noise bursts (Noise bursts 2–18; t(20) = 2.78, p = .012). In both controls and patients with PD, eye-blink responses decayed or were weaker on the 18th versus 1st noise burst (Figure 1D; controls: paired t( 11) = 15.50, p = 10−9; for patients with PD: paired t(9) = 2.95, p = .016). We then calculated the rate of hyperbolic decay in eye blinks and found that the rate of decay was significantly lower in patients with PD than in controls (0.11 vs. 0.32;t(20) = 2.35, p = .029; Figure 1E). These data are consistent with prior evidence that patients with PD have decreased startle habituation (Nieuwenhuijzen et al., 2006; Matsumoto et al., 1992; Sandrini et al., 1985; Messina et al., 1972; Penders & Delwaide, 1971).
Patients with PD Have Dysfunctional MFC Activity during Startle Habituation
To examine the neural basis underlying decreased startle habituation in PD, we first examined the ERPs time-locked to the onset of noise bursts at midfrontal sites. The N1 wave, corresponding to the first negative voltage deflection at the vertex (Cz) ~100 msec after the noise, was significantly weaker in patients with PD than in control participants (−6.88 vs. −15–75; t(20) = 2.40, p = .026; Figure 2A and B). By contrast, the two groups did not significantly differ in P2 amplitude (22.58 vs. 25,14; t(20) = 0.96, p = ,34; 95% highest density interval [−12.3, 4.7]; Bayes factor in favor of the null hypothesis = 3–5 × 10lb).
Next, we examined non-phase-locked spectral power in theta bands from midfrontal sites (electrode Cz; 4–7 Hz) that have consistently been linked to cognitive control (Parker et al., 2015; Cavanagh & Frank, 2014; Cavanagh & Shackman, 2014; Sauseng et al., 2005, 2007; Ishii et al., 1999). Time-frequency analysis revealed markedly diminished theta activity immediately after the noise bursts in patients with PD relative to controls (5,7 vs. 8.6;t(20) = 2.26, p = .035; Figure 2C). Notably, MFC Nl ERPs were significantly correlated with midfrontal theta activity (r = .45, p = .036). A subset of patients with PD had a second visit and performed the same startle task when off levodopa medication for at least 12 hr. Midfrontal theta activity was not statistically different in on-versus-off levodopa (n = 5, 5.45 vs, 3–66; paired t(4) = 0.90, p = .43; 95% highest density interval [−12.1, 16.2]; Bayes factor in favor of the null hypothesis = 92.6). Given the small sample size, the on-off comparison suggests no consistent effect of levodopa on midfrontal theta activity.
Scalp topography of whole-brain EEG revealed significant differences in startle-related theta activity between controls and patients with PD only for midfrontal electrodes (Cz as above; C3: t(20) = 2.46, p = .023; C4:t(20’) = 2.13, p = .045; Figure 2D). Notably, although localizing activity at these sites to a specific cortical area is challenging, activity at these electrodes can correspond with medial regions of the frontal cortex (Nieuwenhuis, Holroyd, Mol, & Coles, 2004). Together, these results indicate that MFC theta activity is attenuated in patients with PD.
MFC Theta Is Related to Startle Habituation and Executive Function
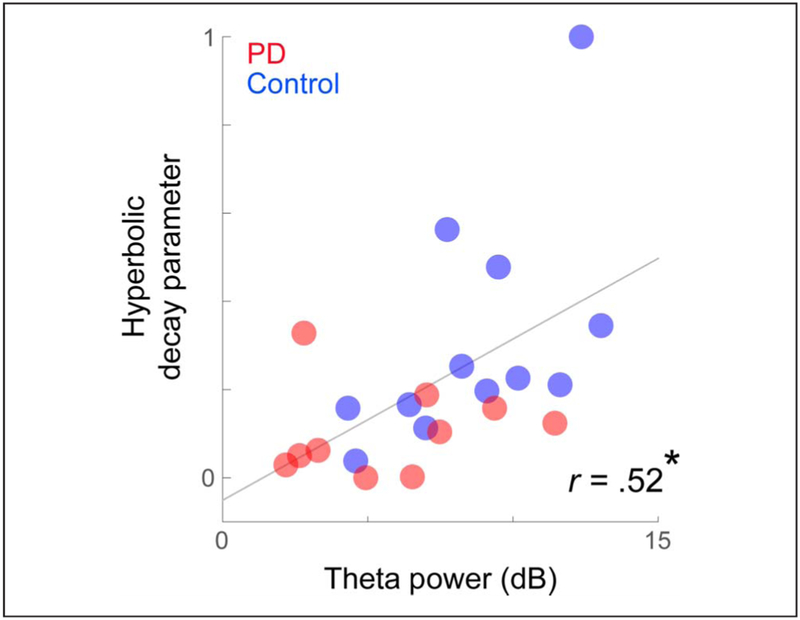
To examine if diminished MFC activity and startle habituation were related, we conducted bivariate Pearson correlation tests. Across all participants with PD and control participants, those with stronger theta signals showed greater eye-blink decay (r = .52, p = .012; Figure 3). Notably, executive function also trended to correlate with eye-blink decay (r = .40, p = .067); however, correlations between eye-blink decay and theta activity were significant even when executive function was included as a covariate in a linear regression (main effect of midfrontal theta on eye-blink habituation: F(18) = 4.80, p < .042; no effects of disease status, executive function, or interactions were found). There were no significant correlations with disease severity (as measured by UPDRS or Hoehn-Yahr; Table 1) or dopaminergic medication dose (Benamer et al., 2000). These data indicate that startle habituation could be linked with MFC theta activity and provide evidence that decreased startle habituation in PD is a result of impaired MFC-related signals.
Figure 3.
Startle habituation was correlated with midfrontal theta activity. Pearson’s correlation between theta activity and startle-related decay in eye blinks. Participants with higher theta activity showed stronger decay in eye-blink startle response. *p < .05.
DISCUSSION
We tested the hypothesis that the MFC is involved in starde habituation and that this response is dysfunctional in disorders with altered MFC function such as PD. We report three findings. First, patients with PD exhibited normal initial starde responses but reduced habituation. Second, patients with PD had lower startle-related midfrontal theta activity and smaller N1 ERP components. Finally, starde-related midfrontal theta was significantiy correlated with starde habituation. Our findings are in line with animal work implicating MFC dopamine in regulation of responses to aversive stimuli (Koch & Schnitzler, 1997; Ellenbroek et al., 1996; Bubser & Koch, 1994) and suggest that the MFC plays a critical role in modulating behavioral response to recurrent aversive stimuli, possibly by supporting systems for top-down control.
Previous literature has linked midfrontal theta activity to attention and unexpected events (Clayton, Yeung, & Cohen Kadosh, 2015; Fries, 2015; Wessel & Aron, 2013). In this study, patients with PD actually have sustained startle responses but less midfrontal theta activity. PD is a multifaceted disease involving cortical alpha-synuclein, genetic polymorphisms in dopamine receptors, mitochondrial abnormalities, and deficits in ascending neurotransmitters including norepinephrine and serotonin, and the most prominent of which is dopamine (Narayanan, Rodnitzky, et al., 2013). In response to startling stimuli, some combination of these processes in PD results in both sustained starding stimuli and less midfrontal theta activity. One explanation that can account for both observations is that midfrontal theta represents a signal of the need for cognitive control, such as the obligatory response that reactively readies eye-blink responses to naturalistic aversive stimuli (Cavanagh & Frank, 2014; Cavanagh & Shackman, 2014). Diminished midfrontal theta in patients with PD likely reflects a failure of primitive learning mechanisms regarding the stimulus-response association to startling stimuli.
Cortical dopamine is reliably disrupted in PD (Narayanan, Rodnitzky, et al., 2013; Cools & D’Esposito, 2011; Cools, Stefanova, Barker, Robbins, & Owen, 2002; Mattay et al., 2002; Deutch, 1993). In patients with PD, MFC low-frequency rhythms are markedly abnormal, and this effect can be replicated from focally depleting dopamine in medial frontal circuits (Parker et al., 2015) or by locally blocking D1 dopamine receptors (Parker et al., 2014). However, patients with PD are diverse with many reasons for cortical dysfunction such as cortical Lewy bodies or disturbances in nondopaminergic ascending projections (Zhang, Kim, & Narayanan, 2015; Narayanan, Rodnitzky, et al., 2013); thus, the effects we observe might not strictly be related to dopamine.
Oral levodopa can have complex effects on prefron-tal systems, making it difficult to establish responses of MFC theta to oral dopaminergic medication (Cools & D’Esposito, 2011; Pascual-Sedano et al., 2008). Midfrontal rhythms in patients with PD do not appear to be specifically responsive to levodopa (Mostile et al., 2015), and we did not see consistent modulation in our data. Because patients with PD had relatively low UPDRS III scores in this study (~9/108) and because cortical dopamine can have nonlinear effects, our patients may in fact be hyperdopaminergic when on levodopa medication (Cools & D’Esposito, 2011). Although this depends on diverse factors such as PD severity and nonmotor symptoms, the effects of levodopa on midfrontal activity detectable at scalp EEG may also reflect modulation of other brain structures, such as the striatum (Duchesne, Soucy, Masson, Chouinard, & Bedard, 2002). Detailed work in animal models in which dopamine can be modulated at individual sites is likely required to address the effect of levodopa on cortical networks (Duchesne et al., 2002; Gotham, Brown, & Marsden, 1988).
The N1 component recorded at Cz was found to be reduced in patients with PD. The midline N1 wave has been previously implicated in effortful and adaptive control and is at least partially generated at the MFC, particularly the ACC (Narayanan, Cavanagh, Frank, & Laubach, 2013; Esposito, Mulert, & Goebel, 2009; Mulert et al., 2008; Mulert, Menzinger, Leicht, Pogarell, & Hegerl, 2005). The N1 wave may play an important role in early selective attention, particularly in the processing of emotional stimuli (Hajcak, Weinberg, MacNamara, & Foti, 2012; Coull, 1998). Previous studies have found that patients with PD with cognitive impairment had structural differences in the frontal cortex (Nagano-Saito et al., 2005; Pereira et al., 2014). Reduced N1 activity in patients with PD may reflect prefrontal abnormalities and deficits in effortful and adaptive control (Narayanan, Rodnitzky, et al., 2013) as well as disruption of early attentional processes for emotional stimuli (Dietz et al., 2013; Vieregge, Verleger, Wascher, Stuven, & Kömpf, 1994).
Neuronal oscillations in low frequencies are a mechanism of cognitive control in scenarios involving adaptation, error, uncertainty, conflict, and surprise (Cavanagh & Frank, 2014; Cavanagh & Shackman, 2014; Shackman et al., 2011). We found that midfrontal theta activity correlated with the rate of startle habituation. These findings suggest that MFC plays a critical role in modulating startle response during habituation. The MFC may contribute to starde habituation by supporting the systems essential for cognitive control of responses to aversive stimuli. Habituation to recurrent aversive stimuli may require aspects of cognitive control, including increased attention and working memory load to encode the intensity, duration, and frequency of the stimulus; inhibition/modulation of fear and anxiety triggered by the aversive stimulus; and anticipation of stimulus timing. Medial regions of the frontal cortex may implement these functions, particularly selective attention and working memory (Ochsner & Gross, 2014), temporal processing (Parker et al., 2014; Parker, Alberico, Miller, & Narayanan, 2013; Kim, Jung, Byun, Jo, & Jung, 2009), and anticipation of future events (Straube, Schmidt, Weiss, Mentzel, & Miltner, 2009; Straube, Mentzel, & Miltner, 2007).
Our study is somewhat limited by low-density clinical EEG recording, which obviates the ability to estimate generative sources in the frontal cortex (Figure 2D). However, this signal is robust and thus can be observed with limited montages, making it highly translatable for clinical or diagnostic purposes. We studied highly functional, nondemented patients with PD; therefore, the findings may not be generalized to other populations with PD. We did not perform a hearing test or quantify facial muscle function. Facial masking is a symptom of PD that may result in reduced eye blinks (Jankovic, 2008). Despite these concerns, patients with PD exhibited normal eye-blink responses to the very first noise burst (Figure 1B). Although our sample size is comparable with previous studies that allows us to detect between-group effects (Nieuwenhuijzen et al., 2006), the relative low n in each group limits the capability of examining the relationship between theta activity and startle habituation separately for the patient and control groups. Finally, in line with our past work, we interpret that startling stimuli are aversive (Chen et al., 2014), but we do not have evidence that patients with PD find noise bursts explicitly unpleasant.
In summary, our data revealed a novel neural mechanism of startle habituation. These data provide evidence that impaired startle habituation in PD is a result of attenuated midfrontal cognitive control signals. Our findings may provide a mechanistic insight into affective dysfunction in PD and be helpful in developing novel EEG-based biomarkers for emotional disorders in PD and other diseases involving MFC dysfunction.
Acknowledgments
We would like to acknowledge Ergun Uc for helping in participant recruitment, Nicholas Jones and Deanne Tadlock for technical support, Ruth Henson for assisting in participant scheduling, and Tracy Lukasiewicz for data entry. We thank all participants for their participation. This work was supported by National Institute of Neurological Disorders and Stroke (K08 NS078100 to N. S. N.) and National Institutes of Health Predoctoral Training Grant (T32-NS007421 to Dr. Daniel Tranel for trainee K. L. O.). K. L. O. was partially supported by the R. J. McElroy Trust.
REFERENCES
- Anderson SW, Damasio H, Jones D, & Tranel D (1991). Wisconsin Card Sorting Test performance as a measure of frontal lobe damage. Journal of Clinical and Experimental Neuropsychology, 13, 909–922. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP, Delis DC, Kramer J, & Kaplan E (2001). Verbal and design fluency in patients with frontal lobe lesions. Journal of the International Neuropsychological Society, 1, 586–596. [DOI] [PubMed] [Google Scholar]
- Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, & Grosset DG (2000). Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Movement Disorders, 15, 692–698. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, & Van Boxtel A (2005) Committee report: Guidelines for human starde eyeblink electromyographic studies. Psychophysiology, 42, 1–15. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, & Cohen JD (2014). The computational and neural basis of cognitive control: Charted territory and new frontiers. Cognitive Science, 38, 1249–1285 [DOI] [PubMed] [Google Scholar]
- Bubser M, & Koch M (1994). Prepulse inhibition of the acoustic starde response of rats is reduced by 6-hydroxydopamine lesions of the medial prefrontal cortex. Psychopharmacology, 113, 487–492. [DOI] [PubMed] [Google Scholar]
- Castellucci V, Pinsker H, Kupfermann I, & Kandel ER (1970). Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in aplysia. Science, 167, 1745–1748. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, & Frank MJ (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, & Shackman AJ (2014). Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. Journal of Physiology, Paris, 109, 3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K-H, Aksan N, Anderson S, Grafft A, & Chapleau MW (2014). Habituation of parasympathetic-mediated heart rate responses to recurring acoustic starde. Frontiers in Psychology, 5, 1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton MS, Yeung N, & Cohen Kadosh R (2015) The roles of cortical oscillations in sustained attention. Trends in Cognitive Sciences, 19, 188–195 [DOI] [PubMed] [Google Scholar]
- Cools R, & D’Esposito M (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry, 69, ell3–el25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, & D’Esposito M (2008). Working memory capacity predicts dopamine synthesis capacity in the human striatum. Journal of Neuroscience, 28, 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, & Owen AM (2002). Dopaminergic modulation of high-level cognition in Parkinson’s disease: The role of the prefrontal cortex revealed by PET. Brain, 125, 584–594. [DOI] [PubMed] [Google Scholar]
- Coull JT (1998). Neural correlates of attention and arousal: Insights from electrophysiology, functional neuroimaging and psychopharmacology. Progress in Neurobiology, 55, 343–361. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH (2001). Delis-Kaplan Executive Function Scale. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Deutch AY (1993). Prefrontal cortical dopamine systems and the elaboration of functional corticostriatal circuits: Implications for schizophrenia and Parkinson’s disease. Journal of Neural Transmission: General Section, 91, 197–221. [DOI] [PubMed] [Google Scholar]
- Dietz J, Bradley MM, Jones J, Okun MS, Perlstein WM, & Bowers D (2013). The late positive potential, emotion and apathy in Parkinson’s disease. Neuropsychologia, 51, 960–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne N, Soucy JP, Masson H, Chouinard S, & Bedard MA (2002). Cognitive deficits and striatal dopaminergic denervation in Parkinson’s disease: A single photon emission computed tomography study using 123iodine-beta-CIT in patients on and off levodopa. Clinical Neuropharmacology, 25, 216–224. [DOI] [PubMed] [Google Scholar]
- Elienbroek BA, Budde S, & Cools AR (1996). Prepulse inhibition and latent inhibition: The role of dopamine in the medial prefrontal cortex. Neuroscience, 75, 535–542. [DOI] [PubMed] [Google Scholar]
- Esposito F, Mulert C, & Goebel R (2009). Combined distributed source and single-trial EEG-fMRI modeling: Application to effortful decision making processes. Neuroimage, 47, 112–121. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roth WT, Isaacks BG, White PM, Hood SH, & Pfefferbaum A (1995) Elderly men and women are less responsive to starding noises: Nl, P3 and blink evidence. Biological Psychology, 39, 57–80. [DOI] [PubMed] [Google Scholar]
- Fries P (2015) Rhythms for cognition: Communication through coherence. Neuron, 88, 220–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings M, Awad N, Jentzen W, Dimitrova A, Kolb FP, Diener HC, et al. (2006). Involvement of the human cerebellum in short-term and long-term habituation of the acoustic starde response: A serial PET study. Clinical Neurophysiology, 117, 1290–1300. [DOI] [PubMed] [Google Scholar]
- Geyer MA, & Braff DL (1982). Habituation of the blink reflex in normals and schizophrenic patients. Psychophysiology, 19, 1–6. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, & Marsden CD (1988). “Frontal” cognitive function in patients with Parkinson’s disease “on” and “off’ levodopa. Brain, 111, 299–321. [DOI] [PubMed] [Google Scholar]
- Grant DA, & Berg E (1948). A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. Journal of Experimental Psychology, 38, 404–411. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Goodkind M, Madan A, Kramer J, Miller B, & Levenson R (2009). Do tests of executive functioning predict ability to downregulate emotions spontaneously and when instructed to suppress? Cognitive, Affective & Behavioral Neuroscience, 9, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, & Foti D (2012). ERPs and the study of emotion In Emily SK & Steven JL (Eds.), The Oxford handbook of event-related potential components (pp. 441–474). Oxford: Oxford University Press. [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, & Casey BJ (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63, 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N, Junque C, Marti MJ, & Tolosa E (2011). Brain structural MRI correlates of cognitive dysfunctions in Parkinson’s disease. Journal of the Neurological Sciences, 310, 70–74. [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N, Junque C, Tolosa E, Marti M-J, Valldeoriola F, Bargallo N, et al. (2009). Neuroanatomical correlates of impaired decision-making and facial emotion recognition in early Parkinson’s disease. European Journal of Neuroscience, 30, 1162–1171. [DOI] [PubMed] [Google Scholar]
- Ishii R, Shinosaki K, Ukai S, Inouye T, Ishihara T, Yoshimine T, et al. (1999). Medial prefrontal cortex generates frontal midline theta rhythm. NeuroReport, 10, 675–679. [DOI] [PubMed] [Google Scholar]
- Jankovic J (2008). Parkinson’s disease: Clinical features and diagnosis. Journal of Neurology, Neurosurgery, and Psychiatry, 79, 368–376. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, & Kozaric-Kovacic D (2009). Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. International Journal of Psychophysiology, 71, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung AH, Byun J, Jo S, & Jung MW (2009). Inactivation of medial prefrontal cortex impairs time interval discrimination in rats. Frontiers in Behavioral Neuroscience, 3, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, & Bubser M (1994). Deficient sensorimotor gating after 6-hydroxydopamine lesion of the rat medial prefrontal cortex is reversed by haloperidol. European Journal of Neuroscience, 6, 1837–1845. [DOI] [PubMed] [Google Scholar]
- Koch M, & Schnitzler H-U (1997). The acoustic starde response in rats—Circuits mediating evocation, inhibition and potentiation. Behavioural Brain Research, 89, 35–49. [DOI] [PubMed] [Google Scholar]
- Kruschke JK (2013). Bayesian estimation supersedes the t test. Journal of Experimental Psychology: General, 142, 573–603. [DOI] [PubMed] [Google Scholar]
- Lader MH, & Wing L (1964). Habituation of the psychogalvanic reflex in patients with anxiety states and in normal subjects. Journal of Neurology, Neurosurgery and Psychiatry, 27, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KW, Paul GM, Robbins TW, & Marsden CD (1993). L-dopa and frontal cognitive function in Parkinson’s disease. Advances in Neurology, 60, 475–478. [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, & Owen AM (2003). Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. Journal of Neuroscience, 23, 6351–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. (2012). Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Movement Disorders, 27, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Noro H, Kaneshige Y, Chiba S, Miyano N, Motoi Y, et al. (1992). A correlation study between blink reflex habituation and clinical state in patients with Parkinson’s disease. Journal of the Neurological Sciences, 107, 155–159. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Tessitore A, Callicott JH, Bertolino A, Goldberg TE, Chase TN, et al. (2002). Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Annals of Neurology, 51, 156–164. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Brown GG, Lazar N, Camchong J, Sharp R, Krebs-Thomson K, et al. (2006). The neural correlates of habituation of response to starding tactile stimuli presented in a functional magnetic resonance imaging environment. Psychiatry Research: Neuroimaging, 148, 1–10. [DOI] [PubMed] [Google Scholar]
- Messina C, Di Rosa AE, & Tomasello F (1972). Habituation of blink reflexes in Parkinsonian patients under levodopa and amantadine treatment. Journal of the Neurological Sciences, 17, 141–148. [DOI] [PubMed] [Google Scholar]
- Mostile G, Nicoletti A, Dibilio V, Luca A, Pappalardo I, Giuliano L, et al. (2015) Electroencephalographic lateralization, clinical correlates and pharmacological response in untreated Parkinson’s disease. Parkinsonism & Related Disorders, 21, 948–953. [DOI] [PubMed] [Google Scholar]
- Mulert C, Menzinger E, Leicht G, Pogarell O, & Hegerl U (2005) Evidence for a close relationship between conscious effort and anterior cingulate cortex activity. International Journal of Psychophysiology, 56, 65–80. [DOI] [PubMed] [Google Scholar]
- Mulert C, Seifert C, Leicht G, Kirsch V, Erd M, Karch S, et al. (2008). Single-trial coupling of EEG and fMRI reveals the involvement of early anterior cingulate cortex activation in effortful decision making. Neuroimage, 42, 158–168. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, et al. (2005). Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology, 64, 224–229 [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Cavanagh JF, Frank MJ, & Laubach M (2013). Common medial frontal mechanisms of adaptive control in humans and rodents. Nature Neuroscience, 16, 1888–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Rodnitzky RL, & Uc EY (2013). Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Reviews in the Neurosciences, 24, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijzen PHJA, Horstink MW, Bloem BR, & Duysens J (2006). Starde responses in Parkinson patients during human gait. Experimental Brain Research, 171, 215–224. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Holroyd CB, Mol N, & Coles MG (2004). Reinforcement-related brain potentials from medial frontal cortex: Origins and functional significance. Neuroscience & Biobehavioral Reviews, 28, 441–448. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, & Gross JJ (2014). The neural bases of emotion and emotion regulation: A valuation perspective In Gross JJ (Ed.), Handbook of emotion regulation (2nd ed., pp. 23–42). New York: The Guilford Press. [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Alberico SL, Miller AD, & Narayanan NS (2013). Prefrontal D1 dopamine signaling is necessary for temporal expectation during reaction time performance. Neuroscience, 255, 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Chen KH, Kingyon JR, Cavanagh JF, & Narayanan NS (2014). Dl-dependent 4 Hz oscillations and ramping activity in rodent medial frontal cortex during interval timing. Journal of Neuroscience, 34, 16774–16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Chen K-H, Kingyon JR, Cavanagh JE, & Narayanan NS (2015). Medial frontal ~4 Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. Journal of Neurophysiology, 114, 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Sedano B, Kulisevsky J, Barbanoj M, Garcia-Sanchez C, Campolongo A, Gironell A, et al. (2008). Levodopa and executive performance in Parkinson’s disease: A randomized study. Journal of the International Neuropsychological Society, 14, 832–841. [DOI] [PubMed] [Google Scholar]
- Penders CA, & Delwaide PJ (1971). Blink reflex studies in patients with Parkinsonism before and during therapy. Journal of Neurology, Neurosurgery, and Psychiatry, 34, 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Svenningsson P, Weintraub D, Brønnick K, Lebedev A, Westman E, et al. (2014). Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology, 82, 2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, & Marmar CR (2009). Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory starde responses. Biological Psychiatry, 65, 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam LE, & Roth WT (1990). Effects of stimulus repetition, duration, and rise time on starde blink and automatically elicited P300. Psychophysiology, 27, 275–297. [DOI] [PubMed] [Google Scholar]
- Putzki N, Graf K, Stude P, Diener HC, & Maschke M (2008). Habituation of the auditory startle response in cervical dystonia and Parkinson’s disease. European Neurology, 59, 172–178. [DOI] [PubMed] [Google Scholar]
- Raskin M (1975). Decreased skin conductance response habituation in chronically anxious patients. Biological Psychology, 2, 309–319. [DOI] [PubMed] [Google Scholar]
- Sandrini G, Alfonsi E, Martignoni E, Horowski R, & Nappi G (1985) Effects of lisuride on blink reflex habituation in Parkinson disease. European Neurology, 24, 374–379– [DOI] [PubMed] [Google Scholar]
- Sauseng P, Hoppe J, Klimesch W, Gerloff C, & Hummel FC (2007). Dissociation of sustained attention from central executive functions: Local activity and interregional connectivity in the theta range. European Journal of Neuroscience, 25, 587–593. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, & Doppelmayr M (2005). Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. International Journal of Psychophysiology, 57, 97–103. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, & Davidson RJ (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12, 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Peri T, Brandes D, Freedman S, Orr SP, & Pitman RK (2000). Auditory starde response in trauma survivors with posttraumatic stress disorder: A prospective study. American Journal of Psychiatry, 157, 255–261. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel H-J, & Miltner WHR (2007). Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. Neuroimage, 37, 1427–1436. [DOI] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel H-J, & Miltner WHR (2009). Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage, 44, 975–981. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN (2004). Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology, 19, 203–214. [DOI] [PubMed] [Google Scholar]
- Uc EY, Doerschug KC, Magnotta V, Dawson JD, Thomsen TR, Kline JN, et al. (2014). Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology, 83, 413–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidailhet M, Rothwell JC, Thompson PD, Lees AJ, & Marsden CD (1992). The auditory starde response in the Steele-Richardson-Olszewski syndrome and Parkinson’s disease. Brain, 115, 1181–1192. [DOI] [PubMed] [Google Scholar]
- Vieregge P, Verleger R, Wascher E, Stüven F, & Kömpf D (1994). Auditory selective attention is impaired in Parkinson’s disease—Event-related evidence from EEG potentials. Cognitive Brain Research, 2, 117–129. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2008). Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV). San Antonio, TX: NCS Pearson. [Google Scholar]
- Wessel JR, & Aron AR (2013). Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. Journal of Neuroscience, 33, 18481–18491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, & Cunningham WA (2007). Executive function: Mechanisms underlying emotion regulation In Lewis MD, Haviland-Jones JM, & Barrett LF (Eds.), Handbook of emotion regulation (pp. 135–158). New York: Guilford Press. [Google Scholar]
- Zhang Q, Kim YC, & Narayanan NS (2015) Disease-modifying therapeutic directions for Lewy-Body dementias. Frontiers in Neuroscience, 9, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]