Abstract
Background:
The prognostic value of physical examination, its relation to quality of life (QoL), and influence of therapy in heart failure with preserved ejection fraction (HFpEF) is not well known.
Methods and Results:
We studied participants from the Americas with available physical examination (jugular venous distention, rales, and edema) at baseline in TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist). The association of the number of signs of congestion with the primary outcome (cardiovascular death or HF hospitalization), its individual components, and all-cause mortality was assessed using time-updated, multivariable-adjusted Cox regression analyses. We evaluated whether spironolactone improved congestion at 4-months, and whether improvement in congestion was related to QoL as assessed by Kansas City Cardiomyopathy Questionnaire clinical summary scores (KCCQ-OSS), and to outcomes. Among 1644 participants, 22%, 54%, 20%, and 4% had 0, 1, 2, and 3 signs of congestion, respectively, at baseline. After multivariable adjustment, each additional increase in sign of congestion was associated with a 30-60% increased risk of each outcome (p<0.001). Spironolactone reduced the total number of signs of congestion by −0.10 (p=0.005) signs, jugular venous distention (odds ratio 0.60, p=0.01) and edema (odds ratio 0.74, p=0.006) at 4-months compared to placebo. Each reduction in sign of congestion was independently associated with a 4.0 (95%CI: 2.4, 5.6) point improvement in KCCQ-OSS. When assessed simultaneously, time-updated, but not baseline, congestion predicted outcomes.
Conclusions:
In HFpEF, the physical exam provides independent prognostic value for adverse outcomes. Spironolactone improved congestion compared with placebo. Reducing congestion was independently associated with improved QoL and outcomes and is a modifiable risk factor.
Clinical Trials Registration:
ClinicalTrials.gov; Identifier:
Keywords: heart failure with preserved ejection fraction, physical exam, spironolactone, congestion
INTRODUCTION
Physical examination is the cornerstone of the evaluation and management of patients with heart failure (HF). Several studies have demonstrated the value of the cardiovascular physical exam in HF with reduced ejection fraction (HFrEF). The Studies of Left Ventricular Dysfunction (SOLVD) trial showed that jugular venous distention (JVD) was independently associated with progression of HF,1 while the Valsartan Heart Failure Trial (Val-HEFT) showed the value of the total number of signs of congestion in prognostication.2 Among patients with HF with preserved EF (HFpEF), effective therapeutic options are limited, and thus decongestion with diuretics is guideline-supported and is of utmost importance to improve symptoms, reduce pulmonary artery pressures, and improve right ventricular loading.3, 4 However, the value of the physical examination and the extent to which physical examination is responsive to therapy in HFpEF, is unknown.5
Therefore, we analyzed signs of congestion and their independent prognostic role in patients with HFpEF using data from the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) Trial. We also evaluated the effect of spironolactone on the physical exam, and whether improvement in the physical exam was associated with improved quality of life (QoL) and prognosis.
METHODS
TOPCAT study design and objectives
The data have been made available to other researchers for purposes of reproducing the results located on the National Institutes of Health website (https://biolincc.nhlbi.nih.gov/studies/topcat/). The design of the TOPCAT trial has been described in detail previously.6 Briefly, TOPCAT was a multi-center, international, randomized, double blind, placebo-controlled trial of spironolactone in adults with HFpEF recruited from over 270 clinical sites. The trial was funded by the National Heart, Lung, and Blood Institute as a contract with the Brigham and Women’s Hospital (Clinical Coordinating Center) and the New England Research Institute (Data Coordinating Center). Enrollment began in August 2006 and ended in January 2012, and the primary results of the trial were published in April 2014 (mean follow-up was 3.5 years).7 The primary aim was to determine whether treatment with spironolactone, compared with placebo, can produce a clinically meaningful reduction in the composite outcome of cardiovascular mortality, aborted cardiac arrest, or HF hospitalization in adults with symptomatic HF and documented LVEF ≥ 45%. All study participants provided written informed consent.
Inclusion criteria for TOPCAT included age ≥ 50 years; diagnosis of HF based on at least one HF symptom at the time of study screening and at least one HF sign within the 12 months prior to screening; LVEF ≥ 45% (per local reading); at least 1 HF hospitalization in the 12 months prior to study screening (“hospitalization stratum”) or B-type natriuretic peptide (BNP) > 100 pg/mL or N-terminal pro-BNP > 360 pg/mL in the absence of an alternative explanation for elevated natriuretic peptide level (“BNP stratum”) within the 60 days prior to screening; and serum potassium < 5.0 mmol/L prior to randomization.6 There were multiple exclusion criteria for TOPCAT, including severe chronic kidney disease (defined as estimated glomerular filtration rate <30 mL/min per 1.73 m2 or serum creatinine ≥2.5 mg/dL), severe systemic illness with a life expectancy of less than 3 years, a history of significant hyperkalemia, and recent myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention.6
For the main analysis, we excluded patients from Russia or Georgia (N=1678), given substantial regional variation described previously8 as well as significant missing complete physical exam data (93% missing, mostly due to missing JVD). We performed a subanalysis in the Russia/Georgia cohort, which is presented in this supplement. In the Americas, we also excluded those with incomplete data on the physical exam at baseline (N=123), leaving 1644 participants for analysis. The presence of JVD, rales, and edema was assessed by investigators in a “yes”/”no” format at each study visit throughout the trial, including 16 visits from baseline to 6 years.6
Type and doses of loop diuretic medication were collected in the study intake forms. For ease of display, furosemide dose equivalents were calculated via multiplication factors (2x for torsemide and 40x for bumetanide).9
Study outcomes
Endpoints for the present study include the time to the TOPCAT primary outcome (death from cardiovascular causes, aborted cardiac arrest, or HF hospitalization), cardiovascular mortality, all cause mortality, and HF hospitalization. All HF hospitalizations were adjudicated by a clinical endpoint committee at Brigham and Women’s Hospital, blinded to study drug assignments, and according to pre-specified criteria.6 We also assessed QoL at the baseline to 4-month visits using two validated instruments.10 First, we used the Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OSS), a HF specific assessment scored on a scale from 0 to 100, with higher scores indicating fewer symptoms and physical limitations associated with HF.11 We also used the EQ5D Visual analog scale (EQ5D-VAS), which assesses generic health-related QoL symptoms on scale from 0 to 100, with 100 representing the best possible health-related QoL.12
Statistical analysis
For descriptive purposes, baseline characteristics are summarized by the total number of signs and by individual signs (edema, rales, and JVD) using median and 25th-75th percentile. Categorical variables are presented as counts and percentages. Cuzack’s non-parametric trend test13 and chi-squared tests for trend were performed as appropriate.
The association between the number of signs and individual signs with the efficacy outcomes was assessed using time-updated, crude and multivariable-adjusted Cox regression. We analyzed the physical exam as a continuous, time-updated variable since this yielded the lowest Akaike Information Criteria for cardiovascular death compared with models using the baseline physical exam or modeling the signs of congestion as a categorical variable. Multivariable models were adjusted for baseline covariates known to have previous prognostic importance in TOPCAT analyses as well as those with clinical relevance, pre-specified based upon face validity.14 Covariates included age, sex, white race, New York Heart Association (NYHA) class, atrial fibrillation, diabetes mellitus, smoking, peripheral artery disease, serum creatinine, heart rate, ejection fraction, and randomization to spironolactone. Given the physical exam can be challenging in the setting of certain comorbidities, we performed interaction analyses between specific signs of congestion with body-mass index (BMI) and chronic lung diseases. Since the physical exam might inform a physician’s decision to hospitalize patients, and therefore inflate the relationship between signs of congestion and HF-related outcomes, we performed a sensitivity analysis. For the primary endpoint and HF hospitalization, we censored participants at the time of hospitalization for those hospitalized for HF on the same date as a clinic visit.
We next determined the treatment effect of spironolactone on the change in physical exam from baseline to the 4-month visit. The 4-month visit was chosen to allow enough time for the effect of the randomization treatment to be determined while minimizing study drop out. It also permitted related analysis of QoL scores, which were also collected at that visit. Post-randomization changes from baseline were compared using linear regression, controlling for treatment allocation and baseline physical exam as independent variables. We further controlled for clinical covariates that were independently associated with the change in the number of physical exam signs using backward stepwise regression at a significance level of 0.05 (baseline physical exam, serum creatinine, systolic blood pressure, diastolic blood pressure, diabetes mellitus, atrial fibrillation on electrocardiogram, BMI, and heart rate). Finally, to determine whether reducing congestion improved prognosis, we simultaneously entered both the baseline and time-updated signs of congestion into models assessing the relationship with the efficacy outcomes. We tested for an interaction between change in baseline and time-updated signs. Analyses were performed using STATA version 12, and a two-sided p-value < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Table 1 lists the baseline characteristics of the 1644 participants from the Americas with available baseline physical exam data, stratified by number of signs of congestion: 21.8% had no signs, 54.4% had 1 sign, 19.6% had 2 signs, and 4.1% had 3 signs. The majority (71%) had edema. Increasing congestion was associated with more advanced NYHA class, higher BMI, heart rate, and loop diuretic dose, and lower QoL scores and hemoglobin (p<0.05 for all comparisons), though there was no difference in atrial fibrillation. Supplementary Table 1 shows natriuretic peptide levels by number of signs of congestion among a small subsample of participants enrolled from the TOPCAT BNP stratum (401 with B-type natriuretic peptide levels and 240 with N-terminal pro B-type natriuretic peptide levels). There was no statistically significant difference in natriuretic peptide levels in this subanalysis. Supplementary Table 2 shows clinical characteristics by presence of individual signs of congestion (JVD, edema, and rales), demonstrating overall similar trends as observed in Table 1.
TABLE 1.
Baseline Clinical Characteristics by Number of Physical Exam Signs of Congestion
| 0 Signs N=359 |
1 Sign N=895 |
2 Signs N=322 |
3 Signs N=68 |
P-value* | |
|---|---|---|---|---|---|
| Edema, n (%) | 0 (0%) | 797 (89.1%) | 303 (94.1%) | 68 (100%) | |
| Jugular venous distention, n (%) | 0 (0%) | 44 (4.9 %) | 188 (58.4%) | 68 (100%) | |
| Rales, n (%) | 0 (0%) | 54 (6.0 %) | 153 (47.5%) | 68 (100%) | |
| Age, years | 72.8 [63.9, 79.5] | 71.6 [63.6, 79.2] | 73.4 [65.2, 79.8] | 75.6 [66.0, 81.9] | 0.09 |
| Female, n (%) | 179 (49.9%) | 449 (50.2%) | 163 (50.6%) | 27 (39.7%) | 0.46 |
| White race, n (%) | 285 (79.4%) | 697 (77.9%) | 248 (77.0%) | 52 (76.5%) | 0.42 |
| NYHA Class III or IV, n (%) | 91 (25.3%) | 273 (30.5%) | 164 (51.1%) | 45 (67.2%) | <0.001 |
| Enrollment through HF hospitalization stratum, n (%) | 233 (65 %) | 457 (51 %) | 179 (56 %) | 39 (57 %) | 0.045 |
| Physical Characteristics | |||||
| Systolic blood pressure (mmHg) | 126 [114, 139] | 129 [118, 138] | 130 [118, 139] | 127 [115, 139] | 0.36 |
| Diastolic blood pressure (mmHg) | 70 [62, 80] | 70 [62, 80] | 71 [62, 80] | 68 [61, 73] | 0.45 |
| Body mass index (kg/m2)** | 31.2 [26.6, 35.4] | 33.2 [28.5, 38.9] | 32.9 [27.8, 39.1] | 34.3 [27.2, 38.5] | 0.001 |
| Heart rate (beats/min) | 68 [60, 75] | 68 [61, 76] | 69 [62, 76] | 69 [61, 75] | 0.044 |
| Comorbidities, n (%) | |||||
| Hypertension | 324 (90.3%) | 803 (89.7%) | 294 (91.6%) | 60 (88.2%) | 0.86 |
| Atrial fibrillation | 175 (48.7%) | 353 (39.4%) | 137 (42.7%) | 27 (39.7%) | 0.10 |
| Diabetes mellitus | 145 (40.4%) | 413 (46.1%) | 139 (43.3%) | 32 (47.1%) | 0.34 |
| Myocardial Infarction | 76 (21.2%) | 179 (20.0%) | 65 (20.2%) | 15 (22.1%) | 0.94 |
| COPD | 48 (13.4%) | 156 (17.4%) | 53 (16.5%) | 13 (19.1%) | 0.19 |
| Asthma | 26 (7.2 %) | 111 (12.4%) | 37 (11.5%) | 6 (8.8 %) | 0.2 |
| Stroke | 33 (9.2 %) | 81 (9.1 %) | 25 (7.8 %) | 8 (11.8%) | 0.93 |
| Peripheral arterial disease | 40 (11.1%) | 107 (12.0%) | 30 (9.3 %) | 12 (17.6%) | 0.78 |
| Current smoker | 26 (7.2 %) | 59 (6.6 %) | 23 (7.1 %) | 5 (7.4 %) | 0.98 |
| Quality of Life Scales | |||||
| KCCQ-OSS | 67 [48, 80] | 59 [40, 77] | 54 [34, 71] | 50 [30, 73] | <0.001 |
| EQ-5D VAS | 70 [50, 80] | 65 [50, 80] | 60 [50, 75] | 60 [50, 80] | 0.001 |
| Medication Use, n (%) | |||||
| ACE-I and/or ARB | 283 (79.1%) | 713 (79.7%) | 259 (80.4%) | 48 (70.6%) | 0.56 |
| Beta-blocker | 291 (81.3%) | 717 (80.1%) | 237 (73.6%) | 54 (79.4%) | 0.045 |
| Any calcium channel blocker | 128 (35.8%) | 348 (38.9%) | 140 (43.5%) | 20 (29.4%) | 0.38 |
| Dihydropyridine calcium channel blocker | 107 (29.8%) | 276 (30.8%) | 114 (35.4%) | 16 (23.5%) | 0.57 |
| Loop diuretic | 282 (78.6%) | 695 (77.7%) | 257 (79.8%) | 59 (86.8%) | 0.23 |
| Furosemide equivalent loop diuretic dose (mg/day)^ | 40 [20, 80] | 40 [20, 80] | 40 [20, 80] | 40 [40, 80] | 0.002 |
| Thiazide diuretic | 64 (17.8%) | 187 (20.9%) | 66 (20.5%) | 19 (27.9%) | 0.11 |
| Laboratory Testing | |||||
| Creatinine (mg/dL) | 1.1 [0.9, 1.4] | 1.1 [0.9, 1.4] | 1.1 [0.9, 1.4] | 1.1 [0.9, 1.4] | 0.48 |
| Hemoglobin (mg/dL) | 13.1 [11.8, 14.2] | 12.8 [11.7, 13.9] | 12.7 [11.6, 13.7] | 12.1 [10.7, 13.3] | < 0.001 |
| Electrocardiographic and Imaging Data | |||||
| Ejection fraction (%) | 58 [51, 63] | 58 [51, 64] | 60 [55, 65] | 60 [54, 63] | 0.06 |
| ECG Left ventricular hypertrophy (%) | 40 (14.2%) | 76 (11.7%) | 28 (11.4%) | 9 (16.1%) | 0.70 |
| ECG atrial fibrillation (%) | 91 (32.4%) | 224 (34.6%) | 84 (34.3%) | 20 (35.7%) | 0.58 |
NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; ACE-I, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; HF, heart failure; KCCQ-OSS, Kansas City Cardiomyopathy Questionnaire Overall Summary Score; EQ5D-VAS, EQ5D visual analog scale.
Continuous variables shown as median (25th-75th percentile).
P-value shown for trend.
Body mass index not corrected to “dry weight”.
Furosemide dose equivalents were calculated as the following: bumetanide 1 mg or torsemide 20 mg were considered equivalent to 40 mg of furosemide.
Supplementary Table 3 shows the frequency of common combinations of signs of congestion. Most participants (78.2%) had at least one sign of congestion. Common combinations of signs included edema only (48.5%), JVD and edema (10.3%), rales and edema (8.2%), and all three signs concurrently (4.1%). Only rarely did JVD or rales occur in the absence of edema.
Subanalysis of congestion at baseline and the 4-month visit in Russia and Georgia
Supplementary Table 4 demonstrates the frequency of signs of congestion among participants in Eastern Europe both at baseline and at the 4 month visit. Edema and rales were present in 47.9% and 13.7% at baseline. There was significant missing data for JVD. Loss of edema and rales, when present at baseline, was common (64.8% and 85.7%, respectively).
Association between time-updated physical exam signs and adverse cardiovascular outcomes
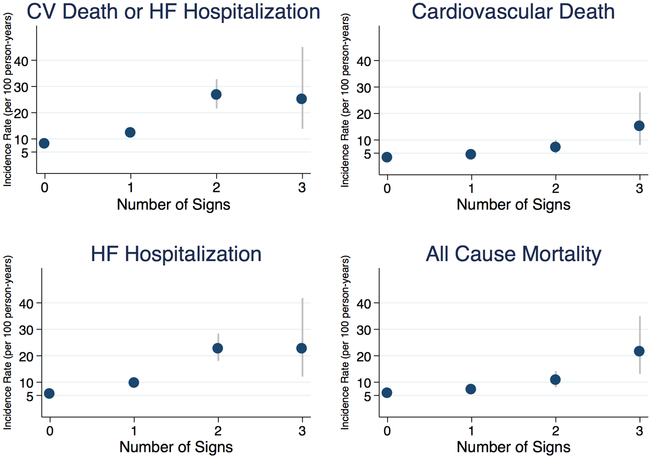
The incidence rates for each outcome showed a generally graded increase in risk with each additional clinical sign (Figure 1 and Supplementary Table 5). The crude and multivariable-adjusted hazard ratios (HRs) for the study outcomes, stratified by time-updated signs of congestion, with 0 signs designated as the referent group, are shown in Table 2. Each additional increase in exam sign was independently associated with a 40–80% increased risk of each outcome (p<0.001 for all outcomes). After multivariable adjustment, congestion was still associated with the primary outcome (HR 1.45, 95% CI: 1.28–1.63) with similar findings noted for other outcomes (p<0.001 for all comparisons). Spironolactone reduced the risk for the primary outcome independent of the baseline physical exam (p=0.64 for interaction).
Figure 1: Incidence Rates of Adverse Cardiovascular Events by Number of Signs of Congestion.
Incidence rates expressed per 100 person-years shown by time-updated number of signs of congestion. Increasing number of signs of congestion was associated with increasing risk for each cardiovascular outcome. The corresponding incidence rates are shown in Supplementary Table 5. CV, cardiovascular; HF, heart failure.
TABLE 2.
Crude and Adjusted Hazard Ratios for Efficacy Outcomes by Time-Updated Total Number of Physical Exam Signs
| Outcomes | Hazard ratio (95% CI) per sign increase | P-value |
|---|---|---|
| Composite endpoint | ||
| Crude model | 1.61 (1.41, 1.81) | <0.001 |
| Multivariable-adjustment model | 1.45 (1.28, 1.63) | <0.001 |
| Cardiovascular mortality | ||
| Crude model | 1.52 (1.28, 1.80) | <0.001 |
| Multivariable-adjustment model | 1.41 (1.18, 1.68) | <0.001 |
| Heart failure hospitalization | ||
| Crude model | 1.77 (1.56, 2.01) | <0.001 |
| Multivariable-adjustment model | 1.57 (1.37, 1.80) | <0.001 |
| All cause mortality | ||
| Crude model | 1.43 (1.25, 1.63) | <0.001 |
| Multivariable-adjustment model | 1.34 (1.17, 1.53) | <0.001 |
HR, hazard ratio; CI, confidence interval.
Multivariable model adjusted for New York Heart Association class, diabetes mellitus, serum creatinine, heart rate, age, sex, white race, current smoking, atrial fibrillation, peripheral artery disease, ejection fraction, and randomization arm.
Supplementary Table 6 demonstrates that presence of each individual sign of congestion was associated with an increased risk for all events on crude analysis (even adjusting for the presence of other signs of congestion) with generally similar findings after multivariable adjustment. In a multivariable Cox analysis the HRs (95% CI) for the primary outcome for JVD, edema, and rales were 1.64 (1.27, 2.12), 1.35 (1.11, 1.64), and 1.42 (1.05, 1.92), respectively. All 3 signs of congestion equally predicted the primary outcome (p=0.65 for equality).
We performed interaction analyses for the primary outcome by conditions that may hinder clinical interpretation of the physical exam. BMI did not modify for the relationship between JVP and the primary outcome (interaction p=0.54), while a history of chronic obstructive pulmonary disease or asthma did not modify the relationship for rales and the primary outcome (interaction p=0.75). There was a significant interaction of BMI with edema, such that the relationship between edema and the primary outcome was stronger at higher BMI than at lower BMI (interaction p=0.023).
Because awareness of the physical exam might influence the decision to hospitalize patients, we performed a sensitivity analysis by censoring patients at the time of hospitalization after a clinic visit (Supplementary Table 7). There were 19 office visits when participants were subsequently hospitalized the same day for their first post-randomization HF event. The relationship between signs of congestion and the primary outcome and HF hospitalization remained robust to this adjustment (p<0.001 for all comparisons).
Treatment effect of spironolactone on congestion
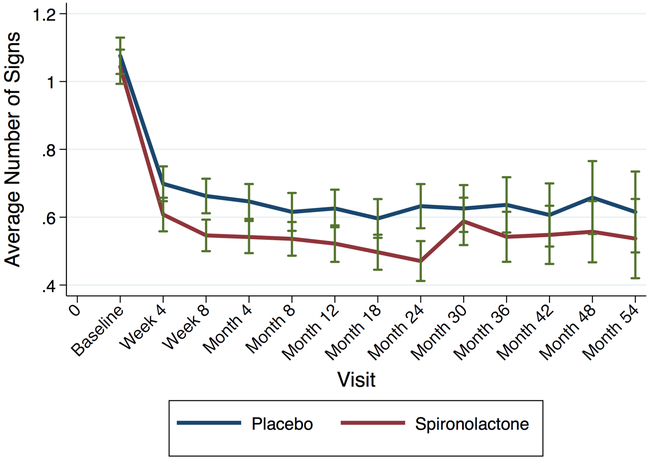
Figure 2 shows the number of physical exam signs throughout follow-up by treatment arm. Spironolactone improved signs of congestion compared to placebo during follow-up. Table 3 shows the effect of spironolactone on congestion at the 4-month visit compared to placebo among 1462 participants with available data. Spironolactone reduced the total number of signs of congestion by −0.10 (95% CI −0.16, −0.03, p=0.005) signs; JVD by 40% (odds ratio 0.60, 95% CI 0.41 – 0.88, p=0.01) and edema by 26% (odds ratio 0.74, 95% CI 0.60 – 0.92, p=0.006). The number needed to treat with spironolactone to reduce 1 physical exam sign was 10, while the numbers to treat to eliminate edema and JVD were approximately 15 and 27, respectively. Supplementary Table 8 shows the new presence or loss of sign of congestion between visits by treatment arm. For example, while there was no difference between treatment arms in new onset edema at the 4 month visit (among those without edema at baseline, p=0.37), spironolactone significantly reduced edema compared to placebo at the 4 month visit among those with edema at baseline (p=0.008).
Figure 2: Average Number of Signs of Congestion During Follow-up by Randomization Arm.
Spironolactone (red) reduced congestion more than placebo (blue) during the majority of follow-up, shown from baseline to the 54-month visit. Subsequent visits were truncated due to small number of individuals in both arms. Error bars denote 95% confidence intervals.
TABLE 3.
Between Treatment Analysis of Change in Physical Exam Sign Frequency at 4 Month
| Placebo N=727 |
Spironolactone N=735 |
Between Group Difference (95% CI)* |
Odds Ratio (95% CI)* |
P-value* | |
|---|---|---|---|---|---|
| Average number of physical exam signs | 0.65 ± 0.70 | 0.54 ± 0.65 | −0.10 (−0.16, −0.03) | − | 0.005 |
| Frequency of physical exam signs, n (%) | 0.007 | ||||
| 0 | 345 (47.5%) | 398 (54.2%) | − | − | |
| 1 | 303 (41.7%) | 281 (38.2%) | − | − | |
| 2 | 70 (9.6%) | 51 (6.9%) | − | − | |
| 3 | 9 (1.2%) | 5 (0.7%) | − | − | |
| Jugular venous distention, n (%) | 75 (10.2%) | 48 (6.5%) | − | 0.60 (0.41, 0.88) | 0.01 |
| Edema, n (%) | 369 (48.7%) | 319 (42.1%) | − | 0.74 (0.60, 0.92) | 0.006 |
| Rales, n (%) | 49 (6.6%) | 48 (6.4%) | − | 1.06 (0.69, 1.61) | 0.80 |
Adjusted for baseline exam.
Relationship between change in physical exam to QoL and outcomes
The relationship between change in the physical exam and change in QoL (assessed using KCCQ-OSS and EQ5D-VAS) is shown in Table 4. Each reduction of a physical sign was associated with a 4.4 (95% CI: 3.0, 5.7) increase in KCCQ-OSS, and 2.7 (95% CI: 1.4, 4.1) increase in EQ5D-VAS. These findings remained unchanged after adjusting for covariates associated with the change in the exam. Spironolactone also improved KCCQ-OSS at the 4-month visit irrespective of baseline congestion (p=0.80 for interaction).
TABLE 4.
Relationship between Reducing Congestion and Quality of Life at the 4-Month Visit.
| Change in Quality of Life Score Minimally-adjusted Model Beta-coefficient (95% CI)* |
P-value | Change in Quality of Life Score Fully-adjusted Model Beta-coefficient (95% CI)*† |
P-value | |
|---|---|---|---|---|
| KCCQ-OSS | ||||
| Total number of physical exam signs | 4.4 (3.0, 5.7) | <0.001 | 4.0 (2.4, 5.6) | <0.001 |
| Edema | 3.9 (1.8, 5.9) | <0.001 | 4.4 (1.9, 6.9) | <0.001 |
| Jugular venous distention | 6.5 (2.7, 10.3) | 0.001 | 6.0 (1.7, 10.2) | 0.006 |
| Rales | 4.7 (0.5, 8.9) | 0.029 | 3.0 (−2.0, 7.9) | 0.24 |
| EQ5D-VAS | ||||
| Total number of physical exam signs | 2.7 (1.4, 4.1) | <0.001 | 2.4 (0.7, 4.0) | 0.004 |
| Edema | 3.3 (1.5, 5.2) | <0.001 | 2.8 (0.5, 5.0) | 0.015 |
| Jugular venous distention | 3.9 (0.5, 7.3) | 0.026 | 3.9 (0.1, 7.8) | 0.044 |
| Rales | 2.0 (−1.8, 5.7) | 0.30 | 0.5 (−3.9, 4.9) | 0.82 |
CI, confidence interval; KCCQ-OSS, Kansas City Cardiomyopathy Questionnaire Overall Summary Score; EQ5D-VAS, EQ5D visual analog scale.
Expressed per decrease in, or absence of, physical exam sign. All analyses controlled for randomization arm and baseline physical exam.
Additionally adjusted for serum creatinine, systolic blood pressure, diastolic blood pressure, diabetes mellitus, atrial fibrillation on electrocardiogram, body mass index, and heart rate.
To understand whether reducing congestion is associated with improved prognosis, we simultaneously entered both the baseline and updated sign of congestion into regression models (Table 5). Time-updated congestion (p<0.001 for all comparisons), but not baseline congestion (p>0.20 for all comparisons), was associated with each of the efficacy outcomes, and multivariable adjustment yielded similar findings. There was no interaction between baseline and time-updated congestion. These findings imply that reducing congestion is associated with improved outcomes, and that baseline congestion is a modifiable risk factor.
TABLE 5.
Prognostic Value of Time-Updated Signs and Baseline Signs after Minimal and Fully Adjusted Cox Regression
| Outcomes | Minimally-adjusted Model HR (95% CI)* |
P-value | Fully-adjusted Model HR (95% CI)† |
P-value | Baseline sign × time-updated signs interaction P-value† |
|---|---|---|---|---|---|
| Composite endpoint | |||||
| Time-updated signs | 1.57 (1.38, 1.77) | <0.001 | 1.43 (1.26, 1.62) | <0.001 | 0.62 |
| Baseline signs | 1.08 (0.96, 1.23) | 0.21 | 1.05 (0.92, 1.19) | 0.50 | |
| Cardiovascular mortality | |||||
| Time-updated signs | 1.53 (1.28, 1.84) | <0.001 | 1.43 (1.18, 1.72) | <0.001 | 0.46 |
| Baseline signs | 0.96 (0.79, 1.17) | 0.70 | 0.95 (0.78, 1.16) | 0.64 | |
| HF hospitalization | |||||
| Time-updated signs | 1.71 (1.49, 1.97) | <0.001 | 1.55 (1.34, 1.79) | <0.001 | 0.82 |
| Baseline signs | 1.09 (0.95, 1.26) | 0.25 | 1.04 (0.90, 1.21) | 0.59 | |
| All cause mortality | |||||
| Time-updated signs | 1.44 (1.25, 1.65) | <0.001 | 1.35 (1.17, 1.55) | <0.001 | 0.02 |
| Baseline signs | 0.99 (0.85, 1.14) | 0.84 | 0.98 (0.84, 1.14) | 0.76 |
HF, heart failure; CI, confidence interval;
Baseline signs do not predict adverse events when simultaneously adjusted for time-updated signs of congestion.
Adjusted simultaneously for time-updated and baseline number of signs of congestion.
Additionally adjusted for New York Heart Association class, diabetes mellitus, serum creatinine, heart rate, age, white race, current smoking, atrial fibrillation, peripheral artery disease, ejection fraction, randomization arm, and sex.
DISCUSSION
In a large study of the physical exam in HFpEF outpatients, we demonstrated that physician-assessed congestion was independently prognostic of subsequent adverse outcomes, that the effect of spironolactone was consistent across the baseline physical exam, and spironolactone therapy improved clinical congestion compared with placebo during follow-up. Moreover, the change in the physical exam strongly related to patient-assessed QoL scales. Time-updated congestion was superior to baseline congestion in terms of risk prediction for all clinical outcomes (similar to a pattern observed with baseline and follow-up natriuretic peptides in HFrEF)15, implying that reducing congestion is associated with lower future risk of adverse events and that baseline congestion is a modifiable risk factor. Given the paucity of currently available data on the physical exam in HFpEF16 and its widespread clinic use, as well as limited therapeutic options, our findings support 1) the relevance of the physical exam in HFpEF as an independent prognostic marker, 2) the use of serial physical exams as targets of therapy to improve QoL and reduce risk for adverse cardiovascular events, including mortality, 3) use of decongestant therapy to improve symptoms, which is a 1C recommendation in professional society guidelines with previously limited supporting data 4, and 4) the ability to spironolactone to significantly reduce clinical congestion.
Several studies have evaluated the impact of congestion on outcomes in HFrEF in both inpatient and outpatient settings. For example, inpatient studies of advanced HF populations have demonstrated the prognostic relevance of congestion,17, 18 and although complete relief from congestion is not often achieved, improvement in congestion at discharge has been shown to be associated with improved outcomes.19, 20 Outpatient studies of in HFrEF complement our findings.1, 21 However, outpatient studies have generally not assessed the utility of serial exams and whether reducing congestion in the outpatient setting was associated with improved outcomes. Studying these questions is particularly important, since decongestion remains a target of outpatient care and has largely been assumed to associate with improved HF outcomes. In addition, concerns have been raised regarding decongestion with high doses of loop diuretics, which are associated with worse outcomes, though use of such therapy may simply be a marker of a sicker patient.22 Of course, decongestion is important not just to improve outcomes, but also to mitigate symptoms. Although these assertions may be intuitive, they are mostly anecdotal because of a paucity of data in chronic HF that quantifies this association. One relevant study showed short-term omission of diuretic therapy increased biomarker evidence of congestion.23 In our study, we document that reducing total and individual signs of congestion was associated with an improvement in HF related QoL (4–6 point increase the in KCCQ-OSS, values considered to indicate clinically significant improvement in QoL),24 with similar global improvement in the EQ5D-VAS scale.
Spironolactone significantly reduced overall congestion compared with placebo, the effect being most marked for JVD (40% reduction) followed by edema (26% reduction) at the 4-month visit. This translated to a number needed to treat with spironolactone of 10 to reduce 1 physical exam sign, and numbers to treat to eliminate edema and JVD of approximately 15 and 27, respectively. Several echocardiographic studies complement our findings and have demonstrated improvement in measures of elevated filling pressures, such as E/e’ ratio, with spironolactone.25-28 However, the TOPCAT echocardiographic substudy (N=239) was not able to confirm these findings, possibly because it was underpowered to detect such differences.29 Nevertheless, the TOPCAT echocardiographic substudy did show that improvements in several parameters that also reflect congestion (such as left atrial volume, E/A ratio, and pulmonary artery systolic pressure) were associated with improved prognosis. Similarly, we found that reducing congestion clinically was associated with improved QoL and outcomes.
Spironolactone, as a potassium-sparing diuretic acting in the distal nephron, excretes 1–2% of filtered sodium load, less than that achieved by loop diuretics.30 In addition, the placebo arm received loop diuretic therapy at the discretion of study investigators, and therefore a similar degree of decongestion would have been expected. Thus, it is possible that the decongestion observed with spironolactone may be multifactorial and include other, non-diuretic mechanisms, such as improvements in ventricular-vascular coupling or endothelial cell function,3, 6 as well as reduction in salt taste, since mineralocorticoid receptor activation augments salt taste through increases in central 11-bHSD2 levels.31, 32 The explanation for why spironolactone reduced congestion in TOPCAT compared to a lack of decongestion observed in a randomized trial of acutely decompensated HFrEF and HFpEF patients (Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure (ATHENA-HF)) may relate to the need for longer duration of treatment to achieve therapeutic levels of active spironolactone metabolites in order to observe effects on volume status.33
Alternative strategies to assess congestion and guide strategies for decongestion have emerged. Natriuretic peptides are commonly used biomarkers of volume status and prognosis in HFpEF.34 Yet, these biomarkers vary significantly by subgroups and may modestly reduced in obesity, a common comorbidity in HFpEF, thus affecting its clinical interpretation.34, 35 As such, there has been significant interest in identifying other, more reliable biomarkers to guide treatment, particularly in HFpEF.36 Decongestion guided by wireless pulmonary artery pressure monitoring has shown benefit in HFpEF, but this technology may not be available or appropriate for widespread use.37 Thus, the physical examination remains a cost-effective and critical tool in assessing and treating HFpEF patients.
The three signs studied are all important parts of the physical exam in patients with HF, although each sign carries its own limitations.38 JVD typically signifies elevated right sided filling pressures and is often present in those with elevated wedge pressure,17 though can be challenging to detect in some patients and requires experience and skill to perform accurately.39 Rales, a marker of left sided congestion, are relatively non-specific and might explain why spironolactone did not specifically reduce rales;40 in addition, they tend to be absent in most patients with chronic HF since increases in lymphatic drainage may limit its development.41 This might explain why many patients in our study who had JVD tended not to have rales. Edema, on the other hand, is often non-specific, as both obesity-related and unrelated conditions, including venous insufficiency, hypoalbuminemia, impaired lymphatic flow, or medication side effect (such as calcium channel blockers), could cause edema. In TOPCAT, edema was also common and present in 71% of patients at baseline. Interestingly, we found that the prognostic value of edema actually increased with higher BMI rather than being decreased because of non-specificity of edema in obese patients. In addition, while no individual sign perfectly captures clinical congestion, physical examination signs in aggregate remain useful in assessing congestion status in HFpEF. Indeed, the total number of signs of congestion powerfully predicted cardiovascular death in a graded fashion, and no threshold effect was observed in risk. Likewise, no single sign emerged as a stronger predictor of cardiovascular death than any other.
Increasingly, technology (such as near-infrared spectroscopy or ultrasound) has been employed to complement or replace elements of the physical exam.42, 43 For example, hand-held ultrasonography can evaluate volume status by interrogation of the inferior vena cava, yet its use is subject to availability, cost, and expertise.44 In some patients with a challenging physical exam, this tool may have a future role. However, we showed in our analysis that baseline BMI did not modify the prognostic utility of JVD. Further, office-based physical exams may decline with the rising use of telemedicine. While such technology may be more convenient, our findings suggest that physical exam still serves an important role and accurate assessment of these signs of congestion likely necessitates in-person evaluation of patients.
Strengths of our study include the large sample size, serial measurement of physical exams, comprehensive delineation of clinical and laboratory characteristics, rigorous follow-up, assessment of QoL using validated instruments, and adjudicate outcomes. There are some potential limitations. First, performance of the physical exam by the study investigators was not standardized and gold standard assessments of congestion were not employed. Nevertheless, our analysis reflects typical clinical practice and therefore increases generalizability of our findings. Additionally, random misclassification of exam signs would have also biased toward the null hypothesis. Second, signs of congestion may influence a physician’s decision to hospitalize patients and therefore could explain to some extent the relationship between the physical exam and HF hospitalization. However, we performed a sensitivity analysis to account for this potential bias and our findings were robust. In addition, signs of congestion powerfully predicted other outcomes that would be unaffected by a physician’s knowledge of the physical exam, including cardiovascular death and all cause mortality. Third, signs of congestion were dichotomized on study intake forms, and therefore we were unable to assess the relationship between grades of congestion and outcomes. Finally, TOPCAT did not collect serial natriuretic peptide levels, and therefore we could not compare the longitudinal value of the physical exam to these biomarkers.
In summary, in the largest study to date of the physical exam in HFpEF, signs of congestion independently predicted outcomes, and improvement in the physical exam was independently associated with improved QoL and prognosis, including a reduction in all cause mortality. The effect of spironolactone was consistent across the spectrum of baseline physical signs and significantly improved congestion compared to placebo. Our results demonstrate that the physical exam, a frequently utilized outpatient clinical assessment, remains highly relevant in the contemporary era.
Supplementary Material
WHAT IS NEW?
There is a paucity of data regarding the prognostic value of the physical examination, its relationship to quality of life, and influence of therapy in heart failure with preserved ejection fraction (HFpEF).
Using a time-updated analysis, we demonstrated that the total number of signs of congestion (jugular venous distention, rales, and edema) have independent prognostic utility. Reducing congestion was strongly associated with improved quality of life and lower risk for adverse events. Spironolactone significantly decreased congestion compared to placebo.
WHAT ARE THE CLINICAL IMPLICATIONS?
Our analysis has several clinical implications, including 1) the relevance of the physical exam in HFpEF as an independent prognostic marker, 2) the use of serial physical exams as targets of therapy to improve quality of life and reduce risk for adverse cardiovascular events, 3) use of decongestant therapy to improve symptoms (a recommendation in professional society guidelines with previously limited supporting data), and 4) the ability to spironolactone to significantly reduce clinical congestion.
Acknowledgments
FUNDING SOURCE
This work was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, contract HHSN268200425207C. The content of this article does not necessarily represent the views of the National Heart, Lung, and Blood Institute or of the Department of Health and Human Services.
DISCLOSURES
Dr Rouleau is a consultant for Novartis, Bayer and Astra-Zeneca. Dr Pfeffer has received consulting fees from Aastrom, Abbott Vascular, Amgen, Cerenis, Concert, Daiichi Sankyo, Fibrogen, Genzyme, GlaxoSmithKline, Hamilton Health Sciences, Medtronic, Merck, Novo Nordisk, Roche, Salix, Sanderling, Sanofi Aventis, Serono, Servier, and Teva, as well as research grants from New England Research Institute via subcontract from the National Institutes of Health, Amgen, Celladon, Novartis, and Sanofi-Aventis. The Brigham and Women’s Hospital has patents for the use of inhibitors of the renin-angiotensin system in selected survivors of myocardial infarction with Novartis Pharmaceuticals on which Dr Pfeffer is a coinventor. His share of the licensing agreement is irrevocably transferred to charity. Dr Desai has received consulting fees from Novartis, AstraZeneca, Abbott, Relypsa, Boston Scientific, Corvidia, Boehringer Ingelheim, DalCor Pharma, and Signature Medical as well as research grants from Novartis. Dr Lewis has received research grants from the National Heart, Lung, and Blood Institute, Novartis, and Sanofi Aventis. Dr Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), and serves on advisory boards for AstraZeneca, Bayer AG, and Baxter Healthcare. Dr S. Shah has received research grants from the American Heart Association, National Institutes of Health, Actelion, AstraZeneca, Corvia, and Novartis, and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Gilead, Ironwood, Merck, MyoKardia, Novartis, Pfizer, Sanofi, and United Therapeutics. Dr Sweitzer has received research grants from the National Institutes of Health, Merck and Novartis. Dr Pitt reports receiving consulting fees from Amorcyte, AstraZeneca, Aurasense, Bayer, BG Medicine, Gambro, Johnson & Johnson, Mesoblast, Novartis, Pfizer, Relypsa, and Takeda; receiving research grant support from Forest Laboratories; and holding stock in Aurasense, Relypsa, BG Medicine, and Aurasense. Dr Pitt also reports a pending patent related to site-specific delivery of eplerenone to the myocardium. Dr Solomon has received consulting fees from Novartis and Bayer and research grants from the National Heart, Lung, and Blood Institute. The other authors report no conflicts.
Contributor Information
Senthil Selvaraj, Division of Cardiology, Department of Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA.
Brian Claggett, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Sanjiv J. Shah, Division of Cardiology, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL.
Inder S. Anand, VA Medical Center and University of Minnesota, Minneapolis, MN.
Jean L. Rouleau, Department of Medicine, Montreal Heart Institute, University of Montreal, Quebec, Canada.
Akshay S. Desai, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Eldrin F. Lewis, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Muthiah Vaduganathan, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Stephen Y. Wang, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Bertram Pitt, University of Michigan, Ann Arbor, MI.
Nancy K. Sweitzer, Sarver Heart Center, University of Arizona College of Medicine, Tucson.
Marc A. Pfeffer, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Scott D. Solomon, Cardiovascular Division, Department of Medicine, Brigham and Women’s Hospital, Boston, MA
REFERENCES
- 1.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345:574–581 [DOI] [PubMed] [Google Scholar]
- 2.Wong M, Staszewsky L, Carretta E, Barlera S, Latini R, Chiang YT, Glazer RD, Cohn JN. Signs and symptoms in chronic heart failure: Relevance of clinical trial results to point of care-data from val-heft. Eur J Heart Fail. 2006;8:502–508 [DOI] [PubMed] [Google Scholar]
- 3.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-specific treatment of heart failure with preserved ejection fraction: A multiorgan roadmap. Circulation. 2016;134:73–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 acc/aha/hfsa focused update of the 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart failure society of america. Circulation. 2017;136:e137–e161 [DOI] [PubMed] [Google Scholar]
- 5.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259 [DOI] [PubMed] [Google Scholar]
- 6.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O’Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: A randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J. 2011;162:966–972 e910 [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Pitt B, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;371:181–182 [DOI] [PubMed] [Google Scholar]
- 8.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (topcat) trial. Circulation. 2015;131:34–42 [DOI] [PubMed] [Google Scholar]
- 9.Vardeny O, Claggett B, Kachadourian J, Desai AS, Packer M, Rouleau J, Zile MR, Swedberg K, Lefkowitz M, Shi V, McMurray JJV, Solomon SD. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: The paradigm-hf trial. Eur J Heart Fail. 2019;21:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis EF, Kim HY, Claggett B, Spertus J, Heitner JF, Assmann SF, Kenwood CT, Solomon SD, Desai AS, Fang JC, McKinlay SA, Pitt BA, Pfeffer MA, Investigators T. Impact of spironolactone on longitudinal changes in health-related quality of life in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2016;9:e001937. [DOI] [PubMed] [Google Scholar]
- 11.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the kansas city cardiomyopathy questionnaire: A new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255 [DOI] [PubMed] [Google Scholar]
- 12.Brazier J, Jones N, Kind P. Testing the validity of the euroqol and comparing it with the sf-36 health survey questionnaire. Qual Life Res. 1993;2:169–180 [DOI] [PubMed] [Google Scholar]
- 13.Cuzick J A wilcoxon-type test for trend. Stat Med. 1985;4:87–90 [DOI] [PubMed] [Google Scholar]
- 14.Selvaraj S, Claggett B, Shah SJ, Anand I, Rouleau JL, Desai AS, Lewis EF, Pitt B, Sweitzer NK, Pfeffer MA, Solomon SD. Systolic blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: An analysis of the topcat trial. Eur J Heart Fail. 2018;20:483–490 [DOI] [PubMed] [Google Scholar]
- 15.Kubanek M, Goode KM, Lanska V, Clark AL, Cleland JG. The prognostic value of repeated measurement of n-terminal pro-b-type natriuretic peptide in patients with chronic heart failure due to left ventricular systolic dysfunction. Eur J Heart Fail. 2009;11:367–377 [DOI] [PubMed] [Google Scholar]
- 16.Damy T, Kallvikbacka-Bennett A, Zhang J, Goode K, Buga L, Hobkirk J, Yassin A, Dubois-Randé JL, Hittinger L, Cleanland JG, Clark AL. Does the physical examination still have a role in patients with suspected heart failure? Eur J Heart Fail. 2011:1340–1348 doi: 10.1093/eurjhf/hfr12 [DOI] [PubMed] [Google Scholar]
- 17.Drazner MH, Hellkamp AS, Leier CV, Shah MR, Miller LW, Russell SD, Young JB, Califf RM, Nohria A. Value of clinician assessment of hemodynamics in advanced heart failure: The escape trial. Circ Heart Fail. 2008;1:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, Stevenson LW. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–1804 [DOI] [PubMed] [Google Scholar]
- 19.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr., Grinfeld L, Udelson JE, Zannad F, Gheorghiade M, Investigators ET. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the everest trial. Eur Heart J. 2013;34:835–843 [DOI] [PubMed] [Google Scholar]
- 20.Lala A, McNulty SE, Mentz RJ, Dunlay SM, Vader JM, AbouEzzeddine OF, DeVore AD, Khazanie P, Redfield MM, Goldsmith SR, Bart BA, Anstrom KJ, Felker GM, Hernandez AF, Stevenson LW. Relief and recurrence of congestion during and after hospitalization for acute heart failure: Insights from diuretic optimization strategy evaluation in acute decompensated heart failure (dose-ahf) and cardiorenal rescue study in acute decompensated heart failure (caress-hf). Circ Heart Fail. 2015;8:741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caldentey G, Khairy P, Roy D, Leduc H, Talajic M, Racine N, White M, O’Meara E, Guertin MC, Rouleau JL, Ducharme A. Prognostic value of the physical examination in patients with heart failure and atrial fibrillation: Insights from the af-chf trial (atrial fibrillation and chronic heart failure). JACC Heart Fail. 2014;2:15–23 [DOI] [PubMed] [Google Scholar]
- 22.Pellicori P, Cleland JG, Zhang J, Kallvikbacka-Bennett A, Urbinati A, Shah P, Kazmi S, Clark AL. Cardiac dysfunction, congestion and loop diuretics: Their relationship to prognosis in heart failure. Cardiovasc Drugs Ther. 2016;30:599–609 [DOI] [PubMed] [Google Scholar]
- 23.Dovancescu S, Pellicori P, Mabote T, Torabi A, Clark AL, Cleland JGF. The effects of short-term omission of daily medication on the pathophysiology of heart failure. Eur J Heart Fail. 2017;19:643–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelkar AA, Spertus J, Pang P, Pierson RF, Cody RJ, Pina IL, Hernandez A, Butler J. Utility of patient-reported outcome instruments in heart failure. JACC Heart Fail. 2016;4:165–175 [DOI] [PubMed] [Google Scholar]
- 25.Daniel KR, Wells G, Stewart K, Moore B, Kitzman DW. Effect of aldosterone antagonism on exercise tolerance, doppler diastolic function, and quality of life in older women with diastolic heart failure. Congest Heart Fail. 2009;15:68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the randomized aldosterone antagonism in heart failure with preserved ejection fraction trial (raam-pef). J Card Fail. 2011;17:634–642 [DOI] [PubMed] [Google Scholar]
- 27.Kurrelmeyer KM, Ashton Y, Xu J, Nagueh SF, Torre-Amione G, Deswal A. Effects of spironolactone treatment in elderly women with heart failure and preserved left ventricular ejection fraction. J Card Fail. 2014;20:560–568 [DOI] [PubMed] [Google Scholar]
- 28.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B, Aldo DHFI. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The aldo-dhf randomized controlled trial. JAMA. 2013;309:781–791 [DOI] [PubMed] [Google Scholar]
- 29.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Deswal A, Anand IS, Fleg JL, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of changes in cardiac structure and function in heart failure with preserved ejection fraction and the impact of spironolactone. Circ Heart Fail. 2015;8:1052–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose BD. Diuretics. Kidney Int. 1991;39:336–352 [DOI] [PubMed] [Google Scholar]
- 31.Pitt B, Pedro Ferreira J, Zannad F. Mineralocorticoid receptor antagonists in patients with heart failure: Current experience and future perspectives. Eur Heart J Cardiovasc Pharmacother. 2017;3:48–57 [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto T, Fujii A, Saito N, Kondo H, Ohuchi A. Alteration of amiloride-sensitive salt taste nerve responses in aldosterone/nacl-induced hypertensive rats. Neurosci Res. 2016;108:60–66 [DOI] [PubMed] [Google Scholar]
- 33.Butler J, Anstrom KJ, Felker GM, Givertz MM, Kalogeropoulos AP, Konstam MA, Mann DL, Margulies KB, McNulty SE, Mentz RJ, Redfield MM, Tang WHW, Whellan DJ, Shah M, Desvigne-Nickens P, Hernandez AF, Braunwald E, National Heart L, Blood Institute Heart Failure Clinical Research N. Efficacy and safety of spironolactone in acute heart failure: The athena-hf randomized clinical trial. JAMA Cardiol. 2017;2:950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myhre PL, Vaduganathan M, Claggett BL, Anand IS, Sweitzer NK, Fang JC, O’Meara E, Shah SJ, Desai AS, Lewis EF, Rouleau J, Pitt B, Pfeffer MA, Solomon SD. Association of natriuretic peptides with cardiovascular prognosis in heart failure with preserved ejection fraction: Secondary analysis of the topcat randomized clinical trial. JAMA Cardiol. 2018;3:1000–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal b-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah SJ. The urgent need for biomarkers beyond b-type natriuretic peptide for the diagnosis and management of heart failure with preserved ejection fraction. JAMA Cardiol. 2018. doi: 10.1001/jamacardio.2018.3702. [DOI] [PubMed] [Google Scholar]
- 37.Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7:935–944 [DOI] [PubMed] [Google Scholar]
- 38.Thibodeau JT, Drazner MH. The role of the clinical examination in patients with heart failure. JACC Heart Fail. 2018;6:543–551 [DOI] [PubMed] [Google Scholar]
- 39.From AM, Lam CS, Pitta SR, Kumar PV, Balbissi KA, Booker JD, Singh IM, Sorajja P, Reeder GS, Borlaug BA. Bedside assessment of cardiac hemodynamics: The impact of noninvasive testing and examiner experience. Am J Med. 2011;124:1051–1057 [DOI] [PubMed] [Google Scholar]
- 40.Elder A, Japp A, Verghese A. How valuable is physical examination of the cardiovascular system? BMJ. 2016;354:i3309. [DOI] [PubMed] [Google Scholar]
- 41.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261:884–888 [PubMed] [Google Scholar]
- 42.Pellicori P, Shah P, Cuthbert J, Urbinati A, Zhang J, Kallvikbacka-Bennett A, Clark AL, Cleland JGF. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur J Heart Fail. 2019. doi: 10.1002/ejhf.1383. [DOI] [PubMed] [Google Scholar]
- 43.Pellicori P, Clark AL, Kallvikbacka-Bennett A, Zhang J, Urbinati A, Monzo L, Dierckx R, Anker SD, Cleland JGF. Non-invasive measurement of right atrial pressure by near-infrared spectroscopy: Preliminary experience. A report from the sica-hf study. Eur J Heart Fail. 2017;19:883–892 [DOI] [PubMed] [Google Scholar]
- 44.Barrett PM, Topol EJ. To truly look inside. Lancet. 2016;387:1268–1269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.