Summary
Through their effects on the intestinal epithelium and immunity, gut microbes and their fermentative byproducts can influence metabolic and psychological health parameters in patients with AN. Integrative therapies that restore gut microbial health may also benefit individuals with conditions in which gut microbial dysbiosis manifests, as in T1D, as individuals in this population experience difficulties with weight stabilization and altered metabolic traits and are vulnerable to developing symptoms of disordered eating.
Although the clinical implications of the brain-gut-microbiota axis are not yet fully understood in AN, targeted pro- and antibiotics represent two mechanisms by which augmenting the gut microbiota can serve as an ancillary therapy for lessening severity of bloating and discomfort during treatment. Specifically, antibiotics could be used to eliminate known pathogens that disrupt intestinal integrity, while targeted probiotics may help to restore beneficial species known to promote gut epithelial health. Thus, we conclude that controlled studies investigating use of such novel therapies, including FMT, should be undertaken as part of an interdisciplinary approach to address metabolic and psychological factors that influence acute and long-term health outcomes in AN and T1D. We highlight again that work on the role of the intestinal microbiota in eating disorders is both limited and confined to AN. As is commonly the case, biological research in eating disorders starts with AN before progressing to the other eating disorders presentations. Yet, in many ways, eating disorders are model conditions on in which to explore the gut-brain axis given the centrality of eating and metabolic factors to the illnesses. We encourage investigators to expand on this early work by conducting studies on the other eating disorders (both in youth and adults) to develop a more comprehensive picture of the role that the intestinal microbiota plays in the development and maintenance of and recovery from these debilitating illnesses.
Keywords: Anorexia nervosa, diabetes, microbiome, microbiota, metabolism
Introduction
The three most common eating disorders are anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED). Other presentations such as avoidant and restrictive food intake disorder (AFRID), purging disorder (PD), and night eating syndrome (NES) also exist and can be debilitating, yet we know virtually nothing about the role of the intestinal microbiota in these disorders. In fact, of the primary eating disorders, our understanding of the intestinal microbiota is limited to research on AN, which is the focus of this review. According to the Diagnostic Statistical Manual of Mental Disorders, 5th edition (DSM-5), AN is characterized by three diagnostic criteria:
restriction of energy intake relative to requirements leading to significantly low body weight,
fear of weight gain or becoming fat, or persistent behaviors to avoid weight gain despite low weight, and
disturbances in self-perceived weight and shape or persistent lack of recognition of the seriousness of the low weight.
Two subtypes—restricting AN (AN-R) and AN binge-purge (AN-BP) exist. Individuals with AN-R reach dangerous low weights primarily through restriction of food intake and/or excessive exercise or physical activity. Those with AN-BP also experience episodes of binge eating and/or purging as typical of BN.
Prevalence and etiology
The lifetime prevalence of AN has been reported to be between 1 and 4% of the population and the illness disproportionately affects women3. One 2007 case-control study from a cohort of Finnish women determined that the lifetime prevalence of AN according to DSM-IV criteria was 2.2%, which was higher than in previous studies that had ascertained cases of AN on the basis of hospital records alone3. Indeed, only about one-third of patients with eating disorders are ever detected by the healthcare system3, demonstrating the importance of clarifying ascertainment strategy when discussing prevalence of eating disorders.
Furthermore, the new, expanded criteria for a diagnosis of AN in DSM-5 have necessitated a recalculation of prevalence. A Japanese study reported that the prevalence of lifetime AN increased by 60% in the transition to DSM-5, with evidence of somewhat lower severity associated with the DSM-5 cases4. A US study of 391 predominantly Caucasian (76.5%) female (91%) participants reported a jump from 14% (DSM-IV) to 20% (DSM-5) percent of participants classified as having AN5. Although compelling, the results of both studies should be interpreted with caution as they are much higher than other estimates. A 2.5% increase in AN prevalence was reported in the shift from DSM-IV to DSM-5 criteria in a Portuguese sample of female high school and university students6. While the increased flexibility of the new criteria are favorable for insurance reimbursement in that they will allow a greater number of individuals to be treated, those who are classified as having AN may now present as a more heterogeneous group in terms of severity and metabolic phenotype, and should therefore be evaluated at the individual level when considering novel therapies such as those that will be discussed in this review.
In terms of risk for developing AN, both genetic and environmental factors are implicated7. Indeed, replicated twin studies have estimated the heritability of AN to be around 50–60%8. Results from a recent genome-wide association study (GWAS) suggest both psychiatric and metabolic etiological factors9. Thus, advances in AN treatment may need to focus not only on psychiatric, but also metabolic factors.
Treatment and prognosis
Current treatments for AN are often ineffective or lead to partial recovery, with frequent relapse, especially among adults10,11. Adolescents generally fare better, although relapse following full recovery occurs in between 7.1% and 21% of patients12–14. Further, relapse has been defined inconsistently, and follow-up lengths and treatment modalities have varied. With respect to recovery rate, one study estimated that 66.8% of patients maintained normal weight and menstrual cycle while abstaining from binge-eating and purging behaviors for a minimum of one year post-treatment15.
Therapeutic renourishment remains the cornerstone of the treatment of AN. A review describing developments in the treatment of AN recognized family based therapy (FBT) as being beneficial for youth, whereas for adults, a number of psychological approaches including cognitive behavioral therapy (CBT), specialist supportive clinical management, and interpersonal psychotherapy (IPT) have been recommended16. No medications have been found to be effective in the treatment of AN11.
AN remains the deadliest of any psychiatric illness. A systematic review calculated weighted annual mortality for AN as 5.10 deaths (95% CI, 3.99–6.14) per 1000 person-years, of which 1.3 deaths were attributed to suicide17. In a subsequent study, suicide was verified as a leading cause of death among individuals with AN18. Other factors contributing to high mortality rates in AN include susceptibility to infection, dehydration, and electrolyte imbalance19. This highlights a pressing need for novel safe and effective treatments for AN.
Gut microbial health influences psychology and behavior
Multiple biologically plausible pathways have been proposed to explain the relationship between intestinal microbiota and neurological processes, including microbial production and modulation of neurotransmitters, short-chain fatty acids (SCFA), brain-derived neurotrophic factor (a protein with signaling properties in the central nervous system and periphery), inflammation, and the hypothalamic-pituitary axis (implicated in depression through secretion of hormones that stimulate cortisol production)20. A growing body of research—in both animals and humans—supports the concept of a brain-gut-microbiota axis21–25, whose signals to physiologic and neurologic processes include those related to eating behavior and mood.
Associations between gut microbiota and AN
Alterations in gut microbial composition and diversity
Gut microbial diversity varies inversely with severity of disease states, including in AN26 and T1D27,28, among others. This is especially true when comparing patients with AN pre- and post-treatment and to normal weight (NW) healthy controls26,29. Interestingly, reduced enteric microbial diversity has been associated with higher depression in AN29. The link between gut microbial dysbiosis and depression in AN could be an important one, as a diagnosis of major depressive disorder was found to be a predictor of long-term AN-R persistence in one study (OR 5.87, P=0.01)30.
The caloric restriction characteristic of AN invites speculation that this illness allows microorganisms capable of surviving in a calorie-poor environment to thrive31. For instance, multiple studies suggest that the methane-producing archeon Methanobrevibacter smithii is more abundant in AN26,32,33. This increased abundance may represent an adaptive response to very low energy intake. Indeed, M. smithii metabolizes the hydrogen produced during bacterial fermentation, preventing the build- up of hydrogen gas and allowing increased transformation of nutrients into calories32,33, which may benefit the host during nutrient-limited conditions. In addition, a significant decrease in the abundance of the phylum Bacteroidetes was reported among AN patients at baseline compared with NW controls, which persisted following weight restoration (FDR-adjusted P < 0.15 at baseline and P < 0.05 at follow-up)26. On the other hand, the phylum Firmicutes was significantly elevated in post-treated AN patients, but not at baseline, compared to NW controls (FDR-adjusted P < 0.05). Another bacterial phylum, Actinobacteria, was significantly elevated in AN patients relative to controls prior to and following weight restoration (FDR-adjusted P < 0.05 for both). Ultimately, there is little consistency as to which microbial phyla are associated with AN before or after clinical refeeding, most likely due to differing regional populations and treatment regimes.
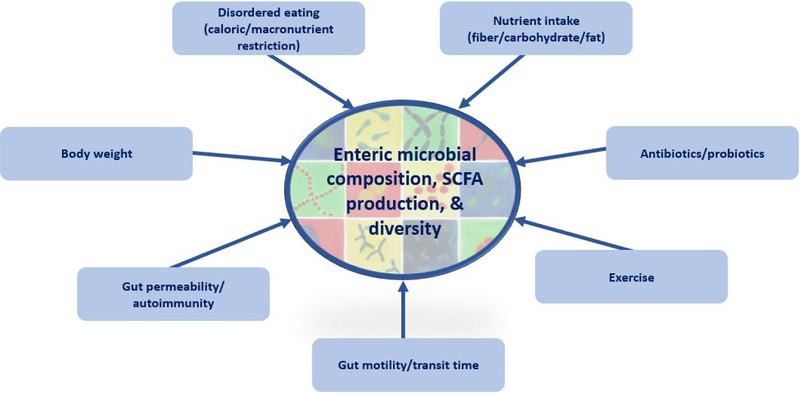
Notably, Mack et al. found that relative abundance of several mucin degraders— whose activities may be pathological for the gut epithelium—was increased among AN patients compared to healthy controls at baseline (FDR-adjusted P < 0.15 for Verrucombia in AN group vs. controls). However, this difference was no longer statistically significant following treatment26. The difference in abundance of mucindegraders was also significantly reduced from baseline to follow-up among AN patients (FDR-adjusted P < 0.05 for Verrucombia)26, suggesting that certain gut microbial changes may serve as benchmarks for recovery. (Figure 1)
Figure 1:
Factors that influence gut microbial composition, SCFA production, and diversity in anorexia nervosa.
Delayed gastrointestinal motility
Methane production by M. smithii can contribute to constipation and slowed gastrointestinal motility, allowing more time for energy harvest from the diet32,34. This theory derives further support from studies demonstrating positive associations between M. smithii counts and either BMI or weight gain32,35,36, as well as associations between breath methane and high BMI34,37,38. As mentioned previously, the abundance of this archeon is elevated in AN patients, although research is needed to clarify the role of M. smithii in influencing energy metabolism and weight status among individuals with or without this illness. Arguably, M. smithii might be an ally in weight restoration, insofar as it allows for increased fermentation of carbohydrates to SCFAs that can provide energy to the host. However, SCFA are likely not a significant source of energy, as fermentation of 32 to 42 grams of indigestible carbohydrate would produce SCFA equivalent to only two to four percent of daily energy needs39. M. smithii might also compete for utilization of SCFAs, thereby limiting any potential benefit. Moreover, as M. smithii contributes to slowed intestinal motility and constipation, it may discourage food intake and thereby interfere with weight restoration.
Kamal et al. verified that intestinal motility, or gastric transit time is delayed among AN patients by measuring whole-gut and mouth to cecum transit time. As hypothesized, whole-gut transit time was significantly delayed among AN patients compared with healthy controls (P < 0.05), while mouth to cecum transit time also trended towards being increased, although the difference did not reach statistical significance40.
Implications for treatment
Delayed gastric transit time has been reported to elevate colonization of the gut by slow-growing mucin-degrading microbes, which may conceivably promote AN- associated bloating41. Moreover, patients may increase fiber consumption to relieve constipation-associated bloating, which can further exacerbate symptoms via gaseous byproducts of microbial fermentation42. Bloating symptoms may then disincentivize recovery and intensify fear of weight gain, although these byproducts of treatment can be circumvented through use of antibiotics. For instance, elimination of M. smithii using antibiotic rifaxamin has been shown to reduce bloating symptoms43, while other probiotics have relieved bloating symptoms in trials of functional bowel disorders44. However, their influence on indigenous mucin-degrading microbes remains unknown.
Intestinal permeability, autoimmunity, and effects on appetite
Alterations in gut permeability have been demonstrated in both human and mouse models of AN45. This may allow foreign invaders to activate the immune system and stimulate the production of autoantibodies that target neuropeptides, including anorexigenic (appetite-suppressing) α-melanocyte-stimulating hormone (α-MSH) and orexigenic (appetite-stimulating) ghrelin46–48. This particular phenomenon has been observed in healthy women and rats47.
In support of the concept that autoimmunity contributes to ED, Terashi et al. have speculated that changes in abundance and binding availability of ghrelin autoantibodies contribute to the elevated plasma ghrelin and ghrelin resistance characteristic of AN49,50. Additionally, autoantibodies to α-MSH were found in most AN patients and a positive correlation between autoantibody levels and ED pathology was detected51,52. In rodents, α-MSH autoantibodies were induced by caseinolytic protease B (ClpB) – an enzyme produced by Enterobacteriaceae, microbes observed at higher concentrations in AN patients by multiple research groups33,53,54. Associations between ClpB concentration and psychopathological traits have also been detected in AN patients53.
In contrast to other findings, reduced small intestinal permeability has also been reported among patients with AN55. It is possible that the effects of AN on intestinal permeability are site-specific; arguably, differential effects on the small intestine and the colon may reflect the role of gut microbiota in regulating permeability, as the highest concentrations of microbes occur in the colon44,56. At the same time, as Jésus et al.45 used a mouse model, their findings may not translate to humans. Moreover, the level of caloric restriction in these mice—typically 30 to 40 percent of total calories—may not match the level of caloric restriction common in individuals with AN, which itself is difficult to measure accurately.
Excessive exercise alters intestinal permeability and gut microbial composition
Exercise intensity and intestinal permeability are positively correlated57,58. The activity-based anorexia (ABA) mouse phenotype yields hyperactivity via limited access to food. Increased colonic permeability and altered tight junction protein expression have been demonstrated in ABA mice45. Higher levels of mucin-degrading Akkermansia muciniphila have also been observed in athletes. The investigators speculated that A. muciniphila may improve barrier function by mechanisms still not fully understood, whereas others hypothesized that increased levels of the microbe would compromise the mucus layer of the epithelium and thereby the integrity of the intestinal barrier59,60.
Studies of forced activity in rodents could reveal how excessive exercise could affect the gut microbiota in AN, as it may better approximate the compulsive, compensatory exercise associated with AN rather than voluntary exercise. For example, Allen et al. found that mice subjected to forced treadmill running (FTR) had greater microbial diversity and altered gut microbial composition relative to mice exposed to voluntary wheel running61. While increased gut microbial diversity is generally associated with better health, here it was related to an expansion of rare bacterial species. The FTR mouse feces also exhibited a predominance of taxa that have been linked to disease states.
SCFA
Role in human health
SCFA—dietary metabolites produced by gut microbial fermentation of indigestible dietary carbohydrates—are an emerging topic of interest in metabolic health and weight management. Butyrate is a widely studied SCFA that is known to stimulate goblet cell mucin synthesis, which promotes gut health by lubricating and protecting epithelial cells. Butyrate also serves as a salient energy source for the intestinal epithelium62. Interestingly, butyrate is primarily found in milk fat63. SCFA production could be reduced in AN due to avoidance of fat-containing food products (13% of calories consumed from fat have been noted in AN patients vs. 31% in controls)64.
Fecal SCFA are reduced in AN
Most studies have reported reduced fecal SCFA in AN patients compared with controls. Borgo et al. detected significantly lower fecal concentrations of total SCFA (P = .041), butyrate (P = .045), and propionate (P = .028); notably, their finding of decreased butyrate is consistent with decreased carbohydrate-fermenting genera Ruminococcus (p = .019), Roseburia (P = .037), and Clostridium (P = .031)33. Decreased acetate (P = .0003) and propionate (P = .001) were found in AN patients in Japan compared to healthy controls65. By contrast, Mack et al.26 reported comparable fecal concentrations of total SCFA, acetate, butyrate, and propionate in AN patients and controls. They nevertheless detected reduced butyrate as a percentage of total SCFA among AN patients on admission, compared with discharge and with normal weight controls, which concurred with a reduced abundance of butyrate-producing Roseburia. Further, butyrate concentration correlated with Roseburia abundance in all three groups. The inconsistencies across studies may reflect compositional differences that occur across geographical regions66,67.
To remedy reduced SCFA production in AN patients, some have proposed administering butyrate-producing Roseburia or supplementing directly with SCFAs26,68. Theoretically, increased intake of carbohydrates and prebiotic fibers would also enhance SCFA production. However, the bacterial fermentation of carbohydrates would also contribute to gas, bloating, and distention, producing physical discomfort after meals and potentially exacerbating body image concerns.
Parallels between AN and T1D
Energy dysregulation
Alterations in energy metabolism are central to both T1D and AN. Similar to the catabolic state that occurs due to starvation in AN69, severe weight loss is a feature of untreated T1D69,70. Even when treated, elevated resting energy expenditure (REE) has occurs in individuals with T1D relative to prediction equations for healthy individuals71,72. Although reduced REE occurs in underweight AN73, many patients experience hypermetabolism during refeeding for unknown reasons74.
Etiology, prevalence, and complications of disordered eating in T1D
It is tempting to speculate that the increased prevalence of disordered eating among individuals with T1D is a function of constant carbohydrate counting for blood glucose control and intense attention to weight. Although this behavior is initially medically-driven, food restriction, defined as restraint, or self-imposed resistance to food consumption75, is associated with undesirable shifts in behavior and metabolism. One such behavior includes insulin restriction76, which can lead to uncontrolled blood glucose77,78 and thus acute and chronic health complications. Schober et al. found that reasons most commonly reported for insulin omission included denial of the disease in situations with peers (30%), self-destructive behavior and suicidal ideation (28%), fear of severe hypoglycemia (24%), and intention to lose weight (15.5.%)79. Conversely, intentional insulin overdosing to enable binge eating has also been commonly reported among individuals with T1DM79. Elevated BMI may also result, as restraint can lead to uncontrolled overeating when individuals cease to limit their food intake80,81.
Furthermore, co-occurring T1D and ED may interact to synergistically worsen health outcomes. In one study, mortality via diabetes-related metabolic complications was increased with co-occurring T1D and AN, compared with either disorder alone (standardized mortality ratio 4.06, 8.86, and 14.5 for T1D, AN, and T1D and AN combined, respectively)82. Peveler et al. reported that among individuals with T1D, those with EDs had a higher baseline Hemoglobin A1c (HbA1c – a three-month measure of BG) than those without an ED (11.9 vs. 9.4 , P = 0.009)78. However, HbA1c was not associated with ED status at 8 to 12 year follow-up points, suggesting that in some instances, disordered eating behaviors may normalize following adolescence78.
A systematic review suggests that both BN and the combined presence of BN and AN are significantly elevated in patients with T1D compared with controls (both P < 0.05)2. Of 550 female patients with T1D, 1% had lifetime AN and 16.2% had lifetime BN83. Subthreshold disordered eating is also prevalent, with one study reporting a greater proportion of girls aged 9–14 with T1D reporting two or more unhealthy eating behaviors compared to non-diabetic controls (P < 0.0005)84.
Gut microbial dysbiosis in T1D
Although shifts in dietary behaviors rapidly and reliably alter the enteric microbial community, much of the literature linking T1D with changes in the gut microbiota has focused on infants and children proximal to T1D onset. Most27,28, but not all85 studies report reduced enteric microbial diversity among patients who develop autoimmunity to pancreatic islet cells compared to controls. Compositional differences were reported in two independent cohorts of Mexican and Finnish children displaying increased Bacteroides among T1D cases and Prevotella among controls86,87. Another research team found that two species from the Bacteroides phylum were significantly increased among Finnish T1D case children months before diabetes onset88. Yet other studies reported reduced abundance of Bifidobacterium in patients compared with controls89,90, although other compositional differences have been less consistently observed27,85,89.
Similar to AN, reduced fecal SCFA have been observed among individuals with T1D compared to controls. Despite a trend towards increased fiber consumption among individuals with T1D compared to non-diabetic controls in one study, control participants had increased levels of plasma acetate and propionate compared to the T1D group, although total fecal SCFA were similar91. This may suggest enhanced utilization of SCFA metabolites by individuals with T1D before they reach the plasma, perhaps in order to fulfill functions related to gut epithelial integrity.
The gut microbiota is associated with weight status and glycemia
It is important to note that gut microbial composition has also been found to shift reliably in association with changes in weight status and metabolic parameters— including glucose homeostasis—in both animal and human models, which is relevant considering the increased prevalence of overweight and obesity among individuals with T1D92. For instance, Rabot et al. showed that germ-free mice fed a high-fat diet were able to maintain euglycemia (normal blood glucose), although conventionally-raised mice with gut microbiota that had been allowed to colonize naturally, developed glucose intolerance and had higher plasma insulin concentrations in both a fed and 6-hour unfed state93. In a study with human participants, Nadal et al. found that changes in blood glucose significantly correlated with changes in proportions of gut microbial groups in adolescents participating in a weight loss intervention, regardless of weight loss outcome (P=0.006)94. Of note, diagnostic crossover is common in eating disorders, meaning that during the course of an individual’s illness, they may transition across diagnostic presentations (AN, BN, BED)95, which can entail considerable fluctuations in weight. No work has yet been done to understand how the gut microbiota may be implicated in these longitudinal changes in symptom presentation.
Genetics
The association between AN and T1D may reflect shared genetic variants, including those related to metabolism. An AN GWAS detected one genome-wide significant variant for AN9, which was previously found to be associated with T1D. Significant genetic correlations emerged between AN and multiple metabolic traits implicated in T1D, including insulin resistance, fasting insulin, fasting glucose, and cholesterol and lipid measures9. These findings are consistent with evidence of increased ED prevalence and disordered eating among individuals with T1D, as well as increased risk of autoimmune disorders, especially of endocrinological and gastroenterological types, among individuals with ED48. Considerably more work is essential to confirm and dissect the nature of this relationship. Larger sample sizes for AN GWAS are critical first steps for any more detailed analysis of the association.
Future directions
It is vital to consider genetic, metabolic, and psychological factors that influence AN and multifactorial disorders such as T1D, in which symptoms of disordered eating, energy dysregulation, gut microbial dysbiosis manifest. Fecal microbiota transplantation (FMT), or the transfer of fecal microbiota from healthy donors to diseased patients, is one potential treatment that is on the horizon for many disease states including T1D and AN96 based on its effectiveness at treating Clostridium difficile infections97. One challenge with respect to translational application of FMT to other disease states is donor screening, as systematic assessments of donor health have yet to be established. Furthermore, no standard exists for ideal gut microbial composition, although screening out individuals with pathogenic gut microorganisms is critical. However, preliminary evidence exists that FMT can improve metabolic phenotypes, including median rate of glucose disappearance and insulin sensitivity among males with metabolic syndrome (P < 0.05), which is relevant in light of obesity-associated insulin resistance that can develop in T1D98,99. Thus, experimenting with FMT and other adjunct therapies in treating symptoms of AN and T1D may provide insight into how the gut microbiota contribute to disease pathology and prognosis.
Synopsis.
Anorexia nervosa has poor prognosis and treatment outcomes, and is influenced by genetic, metabolic, and psychological factors. Gut microbes interact with gut physiology to influence metabolism and neurology, although potential therapeutic benefits remain unknown. Type 1 diabetes is linked to anorexia through energy dysregulation, which in both disease states is related to the gut microbiota, disordered eating, and genetics.
Key points.
Anorexia nervosa is highly refractory, and novel treatments are needed to improve prognosis.
The gut microbiota is dysregulated in anorexia nervosa, and may be a new avenue for research in reducing discomfort during refeeding.
Metabolism is often dysregulated in anorexia nervosa and type 1 diabetes, which share common genetic alterations, disordered eating patterns, and features of gut microbial dysbiosis.
Acknowledgments
Disclosure statement: Dr. Igudesman receives funding from 1UC4DK101132–01. Dr. Carroll is a Consultant for Vivilex; former consultant for Salix Pharmaceuticals, and receives funding from NIH (R21-AI125800–01-02); NIMH (R01-MN105684–03); and Arthritis Foundation (A17–1004-001). Dr. Mayer-Davis recieves funding from NIH (1UC4DK101132–01; 2R01DK077949–4; 1UC4DK108173–03; 2P30DK056350–16; 1DP3DK113358–02; R01DK115434–02), CDC (1U18DP006138–03), and Helmsley. Dr. Bulik is an author and royalty recipient from Pearson and Walker and grant recipient and Scientific Advisory Board member for Shire, and receives funding from Swedish Research Council (VR Dnr: 538–2013-8864); and NIH (R01, MN105684–03, PI Carroll). Dr. Sweeney has nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daria Igudesman, University of North Carolina at Chapel Hill, 135 Dauer Dr, Chapel Hill, NC 27599.
Megan Sweeney, University of North Carolina at Chapel Hill, 135 Dauer Dr, Chapel Hill, NC 27599.
Ian Carroll, University of North Carolina at Chapel Hill, 135 Dauer Dr, Chapel Hill, NC 27599.
Elizabeth Mayer-Davis, University of North Carolina at Chapel Hill, 135 Dauer Dr, Chapel Hill, NC 27599.
Cynthia M. Bulik, University of North Carolina at Chapel Hill, 101 Manning Drive, Chapel Hill, NC 27599.
References
- 1.Jones JM, Lawson ML, Daneman D, Olmsted MP, Rodin G. Eating disorders in adolescent females with and without type 1 diabetes: cross sectional study. BMJ. 2000;320:1563–1566. [PMC free article] [PubMed] [Google Scholar]
- 2.Mannucci E, Rotella F, Ricca V, Moretti S, Placidi GF, Rotella CM. Eating disorders in patients with type 1 diabetes: a meta-analysis. J Endocrinol Invest. 2005;28:417–419. [DOI] [PubMed] [Google Scholar]
- 3.Keski-Rahkonen A, Mustelin L. Epidemiology of eating disorders in Europe: prevalence, incidence, comorbidity, course, consequences, and risk factors. Curr Opinion Psychiatry. 2016;29:340–345. [DOI] [PubMed] [Google Scholar]
- 4.Nakai Y, Fukushima M, Taniguchi A, Nin K, Teramukai S. Comparison of DSM-IV versus proposed DSM-5 diagnostic criteria for eating disorders in a Japanese sample. Eur Eat Disord Rev. 2013;21:8–14. [DOI] [PubMed] [Google Scholar]
- 5.Keel PK, Brown TA, Holm-Denoma J, Bodell LP. Comparison of DSM-IV versus proposed DSM-5 diagnostic criteria for eating disorders: reduction of eating disorder not otherwise specified and validity. Int J Eat Disord. 2011;44:553–560. [DOI] [PubMed] [Google Scholar]
- 6.Machado PP, Goncalves S, Hoek HW. DSM-5 reduces the proportion of EDNOS cases: evidence from community samples. Int J Eat Disord. 2013;46:60–65. [DOI] [PubMed] [Google Scholar]
- 7.Schaumberg K, Welch E, Breithaupt L, et al. The science behind the Academy for Eating Disorders’ Nine Truths About Eating Disorders. Eur Eat Disord Rev. 2017;25:432–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yilmaz Z, Hardaway JA, Bulik CM. Genetics and epigenetics of eating disorders. Adv Genom Genet. 2015;5:131–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan L, Yilmaz Z, Gaspar H, et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of anorexia nervosa. Am J Psychiatry. 2017;174:850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treasure J, Zipfel S, Micali N, et al. Anorexia nervosa. Nat Rev Dis Prim. 2015;1:15074. [DOI] [PubMed] [Google Scholar]
- 11.Zipfel S, Giel KE, Bulik CM, Hay P, Schmidt U. Anorexia nervosa: aetiology, assessment, and treatment. Lancet Psychiatry. 2015;2:1099–1111. [DOI] [PubMed] [Google Scholar]
- 12.Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: Survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int J Eat Disord. 1997;22:339–360. [DOI] [PubMed] [Google Scholar]
- 13.Lock J, Le Grange D, Agras WS, Moye A, Bryson SW, Jo B. Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Arch Gen Psychiatry. 2010;67:1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisler I, Simic M, Russell GF, Dare C. A randomised controlled treatment trial of two forms of family therapy in adolescent anorexia nervosa: A five-year follow-up. J Child Psychol Psychiatry. 2007;48:552–560. [DOI] [PubMed] [Google Scholar]
- 15.Keski-Rahkonen A, Hoek HW, Susser ES, et al. Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry. 2007;164:1259–1265. [DOI] [PubMed] [Google Scholar]
- 16.Watson H, Bulik C. Update on the treatment of anorexia nervosa: review of clinical trials, practice guidelines and emerging interventions. Psychol Med. 2013;43:2477–2500. [DOI] [PubMed] [Google Scholar]
- 17.Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68:724–731. [DOI] [PubMed] [Google Scholar]
- 18.Pompili M, Mancinelli I, Girardi P, Ruberto A, Tatarelli R. Suicide in anorexia nervosa: A meta-analysis. Int J Eat Disord. 2004;36:99–103. [DOI] [PubMed] [Google Scholar]
- 19.Zipfel S, Löwe B, Reas DL, Deter, H, Herzog W. Long-term prognosis in anorexia nervosa: lessons from a 21-year follow-up study. Lancet. 2000;355:721–722. [DOI] [PubMed] [Google Scholar]
- 20.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trend Neurosci. 2008;31:464–468. [DOI] [PubMed] [Google Scholar]
- 21.Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. 2011;12:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’mahony S, Clarke G, Borre Y, Dinan T, Cryan J. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. [DOI] [PubMed] [Google Scholar]
- 23.Rieder R, Wisniewski PJ, Alderman BL, Campbell SC. Microbes and mental health: a review. Brain Behav Immun. 2017. [DOI] [PubMed] [Google Scholar]
- 24.Sherwin E, Rea K, Dinan TG, Cryan JF. A gut (microbiome) feeling about the brain. Curr Opin Gastroenterol. 2016;32:96–102. [DOI] [PubMed] [Google Scholar]
- 25.van de Wouw M, Schellekens H, Dinan TG, Cryan JF. Microbiota-gut-brain axis: modulator of host metabolism and appetite. J Nutr. 2017;147:727–745. [DOI] [PubMed] [Google Scholar]
- 26.Mack I, Cuntz U, Grämer C, et al. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci Rep. 2016;6:26752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giongo A, Gano KA, Crabb DB, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostic AD, Gevers D, Siljander H, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleiman SC, Watson HJ, Bulik-Sullivan EC, et al. The intestinal microbiota in acute anorexia nervosa and during renourishment: relationship to depression, anxiety, and eating disorder psychopathology. Psychosom Med. 2015;77:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franko DL, Tabri N, Keshaviah A, et al. Predictors of long-term recovery in anorexia nervosa and bulimia nervosa: Data from a 22-year longitudinal study. J Psychiatr Res. 2018;96:183–188. [DOI] [PubMed] [Google Scholar]
- 31.Glenny EM, Bulik-Sullivan EC, Tang Q, Bulik CM, Carroll IM. Eating disorders and the intestinal microbiota: Mechanisms of energy homeostasis and behavioral influence. Curr Psychiatry Rep. 2017;19:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PloS One. 2009;4:e7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borgo F, Riva A, Benetti A, et al. Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PloS One. 2017;12:e0179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathur R, Kim G, Morales W, et al. Intestinal Methanobrevibacter smithii but not total bacteria is related to diet-induced weight gain in rats. Obesity (Silver Spring). 2013;21:748–754. [DOI] [PubMed] [Google Scholar]
- 35.Mbakwa CA, Penders J, Savelkoul PH, et al. Gut colonization with Methanobrevibacter smithii is associated with childhood weight development. Obesity (Silver Spring). 2015;23:2508–2516. [DOI] [PubMed] [Google Scholar]
- 36.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal– bacterial mutualism. Proc Natl Acad Sci U S A. 2006;103:10011–10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basseri RJ, Basseri B, Pimentel M, et al. Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol Hepatol (NY). 2012;8:22. [PMC free article] [PubMed] [Google Scholar]
- 38.Mathur R, Amichai MM, Mirocha JM, et al. Concomitant methane and hydrogen production in humans is associated with a higher body mass index. Gastroenterol. 2011;140:S-335. [Google Scholar]
- 39.Cummings JH. The large intestine in nutrition and disease. Institut Danone Brussels, Belgium; 1997. [Google Scholar]
- 40.Kamal N, Chami T, Andersen A, Rosell FA, Schuster MM, Whitehead WE. Delayed gastrointestinal transit times in anorexia nervosa and bulimia nervosa. Gastroenterol. 1991;101:1320–1324. [DOI] [PubMed] [Google Scholar]
- 41.Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2015:gutjnl-2015–309618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehler PS, Brown C. Anorexia nervosa–medical complications. J Eat Disord. 2015;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. [DOI] [PubMed] [Google Scholar]
- 44.Ringel-Kulka T, Palsson OS, Maier D, et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011;45:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jésus P, Ouelaa W, François M, et al. Alteration of intestinal barrier function during activity-based anorexia in mice. Clin Nutr. 2014;33:1046–1053. [DOI] [PubMed] [Google Scholar]
- 46.Fetissov SO, Déchelotte P. The putative role of neuropeptide autoantibodies in anorexia nervosa. Curr Opin Clin Nutr Metab Care. 2008;11:428–434. [DOI] [PubMed] [Google Scholar]
- 47.Fetissov SO, Sinno MH, Coëffier M, et al. Autoantibodies against appetite-regulating peptide hormones and neuropeptides: putative modulation by gut microflora. Nutrition. 2008;24:348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raevuori A, Haukka J, Vaarala O, et al. The increased risk for autoimmune diseases in patients with eating disorders. PLoS One. 2014;9:e104845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smitka K, Papezova H, Vondra K, Hill M, Hainer V, Nedvidkova J. The role of “mixed” orexigenic and anorexigenic signals and autoantibodies reacting with appetite-regulating neuropeptides and peptides of the adipose tissue-gut-brain axis: relevance to food intake and nutritional status in patients with anorexia nervosa and bulimia nervosa. Int J Endocrinol. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terashi M, Asakawa A, Harada T, et al. Ghrelin reactive autoantibodies in restrictive anorexia nervosa. Nutrition. 2011;27:407–413. [DOI] [PubMed] [Google Scholar]
- 51.Fetissov SO, Hallman J, Oreland L, et al. Autoantibodies against α-MSH, ACTH, and LHRH in anorexia and bulimia nervosa patients. Proc Natl Acad Sci U S A. 2002;99:17155–17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fetissov SO, Harro J, Jaanisk M, et al. Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc Natl Acad Sci U S A. 2005;102:14865–14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breton J, Legrand R, Akkermann K, et al. Elevated plasma concentrations of bacterial ClpB protein in patients with eating disorders. Int J Eat Disord. 2016;49:805–808. [DOI] [PubMed] [Google Scholar]
- 54.Million M, Angelakis E, Maraninchi M, et al. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes (Lond). 2013;37:1460. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Monteleone P, Carratu R, Carteni M, et al. Intestinal permeability is decreased in anorexia nervosa. Mol Psychiatry. 2004;9:76. [DOI] [PubMed] [Google Scholar]
- 56.Lam YY, Maguire S, Palacios T, Caterson ID. Are the gut bacteria telling us to eat or not to eat? reviewing the role of gut microbiota in the etiology, disease progression and treatment of eating disorders. Nutrients. 2017;9:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pals KL, Chang R-T, Ryan AJ, Gisolfi CV. Effect of running intensity on intestinal permeability. J Appl Physiol. 1997;82:571–576. [DOI] [PubMed] [Google Scholar]
- 58.Pugh JN, Impey SG, Doran DA, Fleming SC, Morton JP, Close GL. Acute high-intensity interval running increases markers of gastrointestinal damage and permeability but not gastrointestinal symptoms. Appl Physiol Nutr Metab. 2017;42:941–947. [DOI] [PubMed] [Google Scholar]
- 59.Monda V, Villano I, Messina A, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Queipo-Ortuño MI, Seoane LM, Murri M, et al. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PloS One. 2013;8:e65465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen JM, Berg Miller ME, Pence BD, et al. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol. 2015;118:1059–1066. [DOI] [PubMed] [Google Scholar]
- 62.Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18:190–195. [DOI] [PubMed] [Google Scholar]
- 63.Posati LP, Orr ML. Composition of foods: dairy and egg products--raw, processed, prepared. Agriculture Handbook-US Dept of Agriculture (USA) no 8–1. 1976. [Google Scholar]
- 64.Fernstrom MH, Weltzin TE, Neuberger S, Srinivasagam N, Kaye WH. Twenty-four-hour food intake in patients with anorexia nervosa and in healthy control subjects. Biol Psychiatry. 1994;36:696–702. [DOI] [PubMed] [Google Scholar]
- 65.Morita C, Tsuji H, Hata T, et al. Gut dysbiosis in patients with anorexia nervosa. PLoS One. 2015;10:e0145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost F, Brummer RJ. The role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. [DOI] [PubMed] [Google Scholar]
- 69.Schocken DD, Holloway JD, Powers PS. Weight loss and the heart: effects of anorexia nervosa and starvation. Arch Intern Med. 1989;149:877–881. [PubMed] [Google Scholar]
- 70.Charlton MR, Nair KS. Role of hyperglucagonemia in catabolism associated with type 1 diabetes: effects on leucine metabolism and the resting metabolic rate. Diabetes. 1998;47:1748–1756. [DOI] [PubMed] [Google Scholar]
- 71.Nair K, Halliday D, Garrow J. Increased energy expenditure in poorly controlled type 1 (insulin-dependent) diabetic patients. Diabetologia. 1984;27:13–16. [DOI] [PubMed] [Google Scholar]
- 72.Rigalleau V, Lasseur C, Pecheur S, et al. Resting energy expenditure in uremic, diabetic, and uremic diabetic subjects. J Diabetes Complications. 2004;18:237–241. [DOI] [PubMed] [Google Scholar]
- 73.Krahn DD, Rock C, Dechert RE, Nairin KK, Hasse SA. Changes in resting energy expenditure and body composition in anorexia nervosa patients during refeeding. J Am Diet Assoc. 1993;93:434–438. [DOI] [PubMed] [Google Scholar]
- 74.Van Wymelbeke V, Brondel L, Marcel Brun J, Rigaud D. Factors associated with the increase in resting energy expenditure during refeeding in malnourished anorexia nervosa patients. Am J Clin Nutr. 2004;80:1469–1477. [DOI] [PubMed] [Google Scholar]
- 75.Ouwens MA, van Strien T, van der Staak CP. Tendency toward overeating and restraint as predictors of food consumption. Appetite. 2003;40:291–298. [DOI] [PubMed] [Google Scholar]
- 76.Lawrence JM, Liese AD, Liu L, et al. Weight-loss practices and weight-related issues among youth with type 1 or type 2 diabetes. Diabetes Care. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herpertz S, Albus C, Kielmann R, et al. Comorbidity of diabetes mellitus and eating disorders: a follow-up study. J Psychosom Res. 2001;51:673–678. [DOI] [PubMed] [Google Scholar]
- 78.Peveler RC, Bryden KS, Neil HAW, et al. The relationship of disordered eating habits and attitudes to clinical outcomes in young adult females with type 1 diabetes. Diabetes Care. 2005;28:84–88. [DOI] [PubMed] [Google Scholar]
- 79.Schober E, Wagner G, Berger G, et al. Prevalence of intentional under- and overdosing of insulin in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12:627–631. [DOI] [PubMed] [Google Scholar]
- 80.De Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–630. [DOI] [PubMed] [Google Scholar]
- 81.Martyn-Nemeth P, Quinn L, Hacker E, Park H, Kujath AS. Diabetes distress may adversely affect the eating styles of women with type 1 diabetes. Acta Diabetol. 2014;51:683–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nielsen S, Emborg C, Mølbak A-G. Mortality in concurrent type 1 diabetes and anorexia nervosa. Diabetes Care. 2002;25:309–312. [DOI] [PubMed] [Google Scholar]
- 83.Birk R, Spencer ML. The prevalence of anorexia nervosa, bulimia, and induced glycosuria in IDDM females. Diabetes Educ. 1989;15:336–341. [DOI] [PubMed] [Google Scholar]
- 84.Colton P, Olmsted M, Daneman D, Rydall A, Rodin G. Disturbed eating behavior and eating disorders in preteen and early teenage girls with type 1 diabetes: a case- controlled study. Diabetes Care. 2004;27:1654–1659. [DOI] [PubMed] [Google Scholar]
- 85.Murri M, Leiva I, Gomez-Zumaquero JM, et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brown CT, Davis-Richardson AG, Giongo A, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PloS One. 2011;6:e25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mejía-León ME, Petrosino JF, Ajami NJ, Domínguez-Bello MG, de La Barca AMC. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci Rep. 2014;4:3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davis-Richardson AG, Ardissone AN, Dias R, et al. Bacteroides dorei dominates gut microbiome prior to autoimmunity in Finnish children at high risk for type 1 diabetes. Front Microbiol. 2014;5:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Goffau MC, Fuentes S, van den Bogert B, et al. Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia. 2014;57:1569–1577. [DOI] [PubMed] [Google Scholar]
- 90.Soyucen E, Gulcan A, Aktuglu-Zeybek AC, Onal H, Kiykim E, Aydin A. Differences in the gut microbiota of healthy children and those with type 1 diabetes. Pediatr Int. 2014;56:336–343. [DOI] [PubMed] [Google Scholar]
- 91.De Groot PF, Belzer C, Aydin Ö, et al. Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PloS One. 2017;12:e0188475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes:“double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care. 2007;30:707–712. [DOI] [PubMed] [Google Scholar]
- 93.Rabot S, Membrez M, Bruneau A, et al. Germ-free C57BL/6J mice are resistant to high- fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948–4959. [DOI] [PubMed] [Google Scholar]
- 94.Nadal I, Santacruz A, Marcos A, et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes (Lond). 2009;33:758–767. [DOI] [PubMed] [Google Scholar]
- 95.Schaumberg K, Jangmo A, Thornton L, et al. Patterns of diagnostic flux in eating disorders: a longitudinal population study in Sweden. Psychol Med. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88. [DOI] [PubMed] [Google Scholar]
- 97.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079. [DOI] [PubMed] [Google Scholar]
- 98.Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2015;22:277–282. [DOI] [PubMed] [Google Scholar]
- 99.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterol. 2012;143:913–916. e917. [DOI] [PubMed] [Google Scholar]