Abstract
Purpose of Review
Improved tolerability and outcomes after hematopoietic cell transplantation (HCT), along with the availability of alternative donors, have expanded its use. With this growth, and the development of additional cellular therapies, we also aim to increase effectiveness, efficiency, and the quality of the care provided. Fundamentally, the goal of value-based care is to have better health outcomes with streamlined processes, improved patient experience, and lower costs for both the patients and the health care system. HCT and cellular therapy treatments are multiphase treatments which allow for interventions at each juncture.
Recent Findings
We present a summary of the current literature with focus on program structure and overall system capacity, coordination of therapy across providers, standardization across institutions, diversity and disparities in care, patient quality of life, and cost implications.
Summary
Each of these topics provides challenges and opportunities to improve value-based care for HCT and cellular therapy patients.
Keywords: Value-base care, Hematopoietic cell transplantation, CAR-T cell, Cellular therapy
Introduction
Over 20,000 hematopoietic cell transplantations (HCT) are performed annually in the USA and are often the only known curative therapy for some patients with high-risk malignant and non-malignant hematologic diseases. In addition, chimeric antigen receptor T cell (CAR T cell) therapy has been recently approved as an option for some leukemia and lymphoma patients who have relapsed after HCT or may not qualify for HCT due to refractory or persistent disease. Both of these modalities are resource intensive and can have potentially life-threatening side effects, but the risks are often outweighed by the potential long-term disease-free survival.
Both autologous and allogeneic HCTs are a several step process including referral from the treating oncologist and achieving appropriate disease control prior to transplantation; determination of insurance coverage; medical clearance; acquisition of the cells from the patient (autologous) or a donor (allogeneic); the HCT hospitalization including chemotherapy, monitoring while cytopenic, and count recovery allowing for discharge; convalesce; and long-term survivorship. Each of these phases lends itself to opportunities and challenges in improving care [1].
Value-based care is defined as “the practice of medicine incorporating the highest level of evidence-based data with the patient-perceived value conferred by healthcare interventions for the resources expended [2].” As the HCT field has embraced incorporating quality into the standard of care and the volume of HCT annually continues to increase, the aim of this review is to summarize the available literature and identify areas for further advancing value-based care in hematopoietic cell transplantation and the use of cellular therapies. Much of the focus will be on the USA; however, these concepts can apply broadly worldwide.
What Is Value-Based Care?
Value-based care requires a combination of improved health outcomes through better processes of care, enhanced patient experience, and reduced costs (Fig. 1) [3, 4]. Operationally, the measurement of such value then can then be broken down into these pieces. The process of care related to the health care system is both the physical structures and the operational teams delivering care. The patient experience relates to both the symptom burden caused by the treatments and the ease at which they navigate the system. Finally, cost can be evaluated from different perspectives, the financial impact on the patient, the health care system costs mediated between the payers and providers, and the societal costs determined by these choices.
Fig. 1.
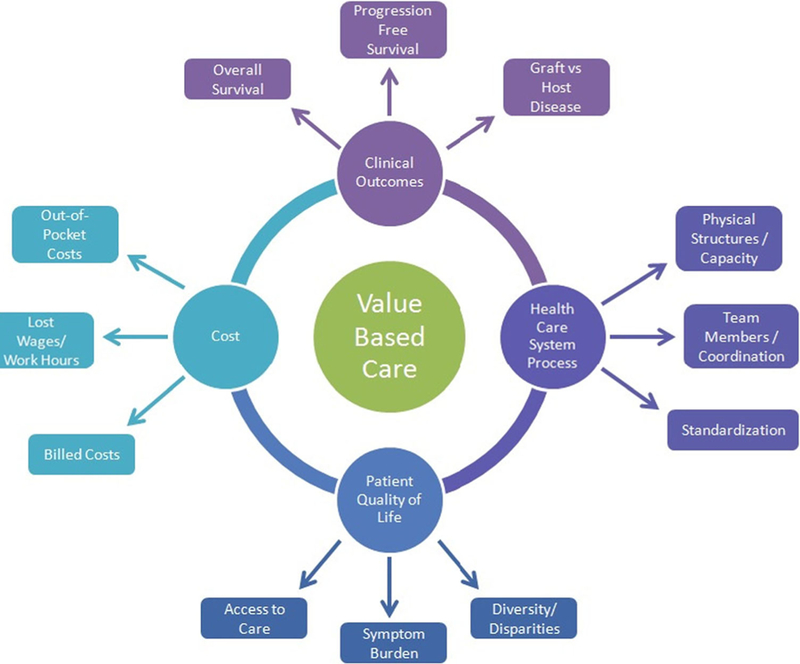
Conceptual diagram of value-based care in hematopoietic cell transplantation
Structure and System Capacity
In the modern transplant era, the vast majority of patients will have a donor, thereby removing this aspect as an obstacle to care. Furthermore, with better outcomes, more patients reach the survivorship phase and require monitoring for late effects [5]. The challenges of increasing volume and value now include the availability of personnel, physical space, and overall system capacity.
The National Marrow Donor Program (NMDP) along with the American Society for Blood and Marrow Transplant (ASBMT) conducted a 3-year symposium, the System Capacity Initiative, starting in 2009 to evaluate how to accommodate the increasing number of patients who qualify for an HCT [6, 7]. A wide variety of stakeholders including HCT physicians, advanced practice providers (APP), nurses, administrators, payers, and professional organizations discussed the barriers to meet the anticipated growth. In terms of the physician workforce, they identified that a comprehensive database of HCT physicians did not exist. Furthermore, their calculations showed that an additional 1300 HCT physicians would be needed by 2020 to meet the projected growth, but that this could be difficult due to minimal exposure early in training, lack of HCT specific certification, and the challenge of work/life balance [8]. Similar shortages and challenges were identified for the APPs and nurses. Many of these initiatives have occurred since that time, primarily along the lines of early exposure during training across all types of providers, the availability of advanced HCT training/fellowships, and the electronic centralizing of available resources for education and career opportunities.
With increased patients, consideration also has to be given to the capacity within facilities. Between 2005 and 2009, the NMDP Transplant Center Network Renewal Survey found that the number of allogeneic HCT increased by 30%, while the bed capacity increased by 17%, with a major portion of the increase from small- to medium-sized HCT centers [9]. Furthermore, Thiessen polygon boundaries were constructed to group transplant centers into market areas and determine the potential unmet need by the currently available programs [6]. Due to the geographical distributions of these centers, patients are often asked to remain locally for several months after HCT, and accommodations are also limited.
To further quantify the availability of sufficient infrastructure and appropriate models of care, a 42-item web-based survey was administered to medical directors of HCT centers evaluating their provider characteristics, team structure and processes, transplantation unit structure and resources, and medical center attributes [10]. The majority of programs incorporated APPs and pharmacists, but with a lower percentage including medical residents or fellows. Furthermore, most reported dedicated hospital beds for HCT patients and about half described an outpatient transplant option. Overall, Majhail et al. showed that there was considerable variability within the 84 adult and 53 pediatric programs participating, with a portion due to the annual transplant volume. However, they note that even programs with similar volumes and outcomes used their resources in different ways. Nivison-Smith et al. recently published evidence from Australia and New Zealand showing the constraints and variation are not unique to the USA and are likely more limiting in other countries [11].
Proposals of how to expand personnel capacity have included the incorporation of allied health professionals and transitioning aspects of care to other team members. One such strategy in HCT programs has been the increased involvement of the pharmacists for medication management via collaborative practice agreements [12, 13]. Laws controlling the degree of involvement vary by state and range from not allowing collaboration at all (in six states) to modification of existing therapy to starting new therapy, and even allowing reimbursement for these services, which was made possible by the Centers for Medicare & Medicaid Services (CMS) recognizing pharmacists as medical staff in the hospital setting in 2012 [14]. More recently, pharmacists conducted a study through the American Society of Clinical Oncology Quality Oncology Practice Initiative (ASCO QOPI) and found that 38% of the included measures had potential for pharmacist impact [15]. In the HCT setting, pharmacist collaboration is extremely helpful given the number of medications per day at various schedules, the monitoring of therapeutic immunosuppression levels, and the complexity of the drug-drug interactions. In furthering value-based care, this model allows for improved efficiency of responding to clinical changes and standardization within institutions, as well as between programs around the country. Furthermore, as the pharmacist has the discussion of these changes, the physician or APPs can evaluate the next patient, thereby increasing the overall capacity of the program.
Team members critical for superior patient outcomes include occupational and physical therapists, dieticians, case managers, and social workers, among others. The Social Work Workforce Group conducted a survey of HCT social workers which similarly found variability between programs in the scope of practice and responsibilities [16]. While they note challenges including not enough time to provide services and the emotional drain of taking care of chronically ill patients, the ability to offer care throughout the transplant process enhanced the provider and patient experience. Each team member therefore contributes in a way to improve care, with the need for additional providers as there is programmatic growth.
Coordination and Standardization
Another aspect of value-based care is the coordination and standardization of process to improve outcomes and avoid medical errors. As described above, the care of a single HCT patient requires a multitude of providers from their initial diagnosing and treating hematologist/oncologist to the HCT team after referral and back to the primary care physician managing chronic follow-up care. Furthermore, the health care system and insurance considerations along this pathway are complex. In addition, from early post-HCT day-to-day management to long-term survivorship, there are many opportunities for increasing consistency of care. Much standardization in the HCT field is monitored by the Foundation for the Accreditation of Cellular Therapy (FACT) or the Joint Accreditation Committee ISCT-EBMT (JACIE).
Due to a need for consistent nomenclature in clinical practice, research, and billing, LeMaistre et al. proposed definitions for the episodes of care for HCT patients [17]. They focused on the type and timing of the infusion and associated these with the available International Classification of Diseases 9 Procedure Codes (ICD-9), Current Procedural Terminology (CPT), and Medicare Severity Diagnosis Related Group (MS-DRG) codes. These definitions were expanded by an ASBMT taskforce to delineate episodes of HCT care in 2015 when the need arose to define routine costs in clinical trials that would be covered by payers as required by the Patient Protection and Affordable Care Act [18]. The transplant process was divided into evaluation, pre-transplantation workup, the transplantation event (conditioning through day 30–120 after HCT), and follow-up care.
On the basis of this prior work and through discussion, the Care Coordination Working Group facilitated by the National Marrow Donor Program/Be The Match in 2016 described the involved stakeholders and their primary goals and challenges along the phases of the transplant process, which they divided into the phases defined in 2015 [19]. In addition, they proposed elements for a care coordination model in HCT, which incorporated increased dialog between providers, patients, and other stakeholders, increased patient access, and education, improving the use of health care information technology to augment traditional patient care. Specifically, some of the suggestions included patient navigators, telemedicine post-transplant care [20], survivorship clinics, self-management support including peer-to-peer conversations and social media, and standardized evidence-based care pathways. Finally, they discussed the importance of metrics to document the effectiveness of interventions and provide basis for further improvement.
In the acute phase of the HCT, a large portion of time and medical decision-making surrounds the complicated medication regimen for immunosuppression, infection prophylaxis, and maintenance therapies to prevent relapse. However, all of this effort is undone if a patient is not taking the prescribed drugs, which may be due to many reasons including fatigue, nausea, pill burden, and confusion due to frequent changes. Morrison et al. reviewed the available literature focusing on medication adherence in the first 100 days after HCT [21]. In the five included studies, the adherence rate was 33 to 94.7%, which were measured by self-reporting, pill counts, and electronic bottle cap monitoring, and decreased over time. The authors conclude that further research is needed to identify barriers and to then test strategies to increase medication compliance.
With the improvement in long-term outcomes, the HCT community now also has a growing population of patients in the final phase along the HCT pathway, and survivorship issues provide ample areas for instituting and improving value-based care, partly due to more easily measurable metrics. One challenge for this field is that there is less robust evidence due to more retrospective or cross-sectional studies without adequate control groups. To identify the gaps in understanding and the available infrastructure, the National Cancer Institute and National Heart, Lung, and Blood Institute sponsored a 12-month initiative including clinicians, researchers, patient advocacy groups, and representatives from ASBMT, Center for International Blood and Marrow Transplantation Research (CIBMTR), European Society for Blood and Marrow Transplantation (EBMT), and the National Cancer Institute’s National Clinical Trials Network [22•] and published a series of papers on immune dysfunction [23], cardiovascular disease [24], secondary malignancies [25], patient-centered outcomes [26], health care delivery [27], and research design in survivorship [28]. Overall, they found many areas for improvement and suggestions for future interventions and research.
Diversity and Disparities
While an extensive discussion of HCT outcome disparities due to race, gender, and socioeconomic status is beyond the scope of this review, these factors are important to a discussion of value-based care as additional focused interventions are likely needed to counterbalance their effects. Using the Surveillance, Epidemiology, and End Results (SEER) cancer registry between 1997 and 2002, Joshua et al. report that HCT is more frequently used to treat leukemia, lymphoma, and multiple myeloma in Caucasians than in African Americans, and that women were less likely than men to receive an autologous HCT when controlling for age and disease factors [29]. Worse outcomes after HCT have been seen in patients with lower health literacy and increased comorbidity, those lacking social support, and those residing in more rural areas [29–34]. Across all racial groups, patients with median incomes in the lowest quartile (< $34,700) had worse overall survival (RR 1.15; 95% CI 1.04–1.26, p = 0.005) and higher risks of treatment-related mortality (RR 1.21; 1.07–1.36, p = 0.002) [30].
In part, some of these poorer results may be due to a delay in referral to a transplant center [35, 36] or other barriers to access [16], and efforts are ongoing to improve early referral for HCT [37]. More recently, higher income (p = 0.004), ability to work (p < 0.001), and having a partner (p = 0.021) were associated with better mean Lee chronic graft-versus-host disease (GVHD) symptom scores and quality of life (QoL), but not overall survival or non-relapse mortality in patients in the Chronic GVHD Consortium Improving Outcomes Assessment study [33]. More research is therefore needed into the relationship between biology, social factors, and outcomes.
Quality of Life/Patient-Reported Outcomes
Beyond addressing infrastructure, process, and social issues, a key component of value-based care is the improvement in QoL, which can be measured either by standardized questionnaires or by validated patient-reported outcome surveys [38, 39]. Studies of QoL after HCT have been more common in pediatrics [40–44]. More recently, assessment of QOL has been incorporated prospectively into clinical trials and observational studies in adult patients [45–53]. Overall, QoL decreases during the acute post-HCT period, with the worse QoL at the nadir of the blood cell counts, and improves with count recovery with stabilization around 1 year after HCT.
Several different QoL measures exist, and there is little consensus on which to include in studies. Pidala et al. describe the various multi-item measures that have been used or validated in the HCT population [39]. They also comment on the importance of longitudinal evaluation with repeated testing at different time points along the HCT journey. Long-term outcomes have been evaluated by Bevans et al. who enrolled patients who had survived to 3 years after HCT for annual QoL assessment [54] and summarized by the Patient-Centered Outcomes Working Group described above [26]. Additionally, mail and phone surveys have been conducted as a secondary data analysis using HSCT survivors identified from the CIBMTR, which showed physical symptoms were more associated with physical health-related QOL (HRQOL), while depressive symptoms and psychosocial factors impacted mental HRQOL more than physical HRQOL [49].
Alternatively, primarily two instruments have been used to collect patient-reported outcomes (PROs), the MD Anderson Symptom Inventory (MDASI) [53], and the National Cancer Institute Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (NCI PRO-CTCAE) [55]. These symptom scales differ from traditional quality of life measures in recall time (over the prior 24 h instead of 1 week) and in purpose (as these tend to focus on particular symptoms as opposed to global measures). In the autologous HCT setting, fatigue, pain, lack of appetite, disturbance in sleep, and drowsiness were the five most bothersome symptoms within the first 30 days after HCT when quantified by the MDASI [56]. Several studies have attempted interventions to improve symptom burden measured by the MDASI [51, 52, 56], with true acupuncture more efficacious than sham acupuncture in reducing nausea, lack of appetite, and drowsiness (p = 0.042, 0.025, and 0.01, respectively), as well as decreasing the amount of pain medications needed.
Overall, the use of traditional QoL scales or patient-reported outcome measures allow for better monitoring of symptom burden and suggest areas for interventions to improve care. In order to advance value-based care in HCT and cellular therapies, broader use of standardized and validated instruments allows for comparison across centers and between processes. Finally, as we increase the long-term success as measured by standard survival outcomes, metrics focusing on the patient’s journey and recovery allow for greater tolerability of the HCT and augment value from the patient perspective.
Costs
Beyond the quality of care as delineated by the components above, the other side of value is cost. The increased volume of HCT adds financial burden to the health care system, both to the patient and caregivers and to the payers. Several researchers have evaluated these aspects of the HCT process.
Through a retrospective questionnaire of 268 HCT patients at the Mayo Clinic, most of whom had insurance, Khera et al. report that 73% of patients noted their illness affected their finances; 47% had increased financial burdens including their household income decreasing by > 50%, selling/mortgaging home, or withdrawing money from retirement accounts; and 3% declared bankruptcy [57]. A portion of this financial hardship is due to the out-of-pocket costs of medications after HCT. Farnia et al. estimate the burden on Medicare patients using copayment amounts attached to the categories of standard medications after HCT [58] and found that patients may pay from $6700 to $8000 for 6 months worth of medications while enrolled in a Medicare Advantage plan, which would increase greatly if the patient develops complications requiring longer term therapies. Given the inverse association between financial toxicity and quality of life, treatment adherence, and survival [59], solutions to these problems from the payer, pharmaceutical, and policy perspectives have a significant potential to improve care.
An early step in the pathway to HCT is the determination of insurance coverage, which can be widely variable between public and private payers, as well as within different plans by the same payer [60, 61]. As such, the Financial Working Group of the NMDP System Capacity Initiative examined the HCT benefit across payers. They created a consensus package that would avoid major coverage gaps while maintaining good outcomes, which included the donor search, cell acquisition and procurement, cell infusion, hospital care, travel and lodging, prescription medications, and routine care while allowing participation on clinical trials, though acknowledged that the majority of plans do not meet these standards [62•].
Studies that evaluate the actual cost of HCT have been heterogeneous, with variable time frames and most being single center and conducted outside the USA [63–65]. However, an initial autologous HCT hospitalization costs on average between $36,000 and $88,000, while an average allogeneic HCT costs $200,000. To better characterize the cost in the USA, Majhail et al. used a longitudinal administrative claims database [66]. The median 100-day total cost for an autologous HCT was $99,899 (interquartile range (IQR), $73,914–140,555), and for allogeneic HCT was $203,026 (IQR, $141,742–316,426), with more than 75% of costs occurred during the initial transplant hospitalization.
The next step is the combined evaluation of costs and outcomes for comparison between two strategies as done in cost-effectiveness or a cost-utility analysis [65, 67–69]. These have been less frequently conducted in the HCT setting, but as an example, Shah et al. examining the cost-effectiveness of autologous HCT for elderly patients with multiple myeloma compared to non-HCT strategies using the SEER-Medicare database found a longer median overall survival with HCT with an incremental cost-effectiveness ratio (ICER) of $72,852 per life-year gained, which is in the range of interventions considered cost-effective [68]. Similarly, using a decision analysis model, Pandya et al. determined that early autologous HCT had a benefit of 1.96 quality-adjusted life years compared to delayed HCT and was potentially cost-effective [69]. Overall, defining the costs and conducting comparison evaluations between therapeutic options are the first steps to determining areas for improvement.
CAR T cell Therapy
Tisagenlecleucel (Kymriah, Novartis) and axicabtagene ciloleucel (Yescarta, Kite Pharmaceuticals) are recently FDA-approved CAR T cell therapies for B cell acute lymphoblastic leukemia (B-ALL) and non-Hodgkin lymphoma (NHL), respectively, with impressive clinical outcomes, but also high costs. As such, the issue of value-based care is also relevant with the expected upsurge of use for these therapies. Many of the concerns surrounding HCT from risk of complications, to QoL, to insurance coverage and billing issues are also applicable in the CAR T cell discussion.
While there are limited clinical results and minimal QoL data for CAR T cells, the financial aspects have been more widely discussed as the price for a single treatment of tisagenlecleucel is $475,000 and for axicabtagene ciloleucel is $375,000, with Novartis attempting outcomes-based pricing with a plan to charge for tisagenlecleucel only if treated patients go into remission within 1 month [70, 71]. Outcomes-based (or value-based) pricing is not a new idea, but has not been broadly adopted in the health care industry as of yet. The goal is to modulate the unsustainable price increases in newer agents by setting the price based on how well the medication or intervention works in a particular situation. In practice, this can be done by setting a price for an indication, charging that price only if a particular outcome is reached, or a rebate if the patient achieves the target metric. Partly, the challenge remains what the metric and goal should be for each case.
Furthermore, the prices are for the cellular therapy only and do not account for the costs of the hospitalization or complications, such as the anticipated cytokine release syndrome. One of the early issues with HCT reimbursement was the lack of appropriate billing codes, prompting creation over time of appropriate codes and the transition from fee-for-service to bundled payments for a defined HCT episode [61, 72]. If a code does not exist, there is no routine method for a claim to be entered by a provider or paid by an insurance company, so in an effort to standardize efforts until more appropriate codes are created, the ASBMT has conducted an extensive evaluation and presented guidance on the most suitable options [73].
The Institute for Clinical and Economic Review recently released its draft evidence report regarding CAR T cell therapy for B cell cancers [74••]. They present a detailed analysis of the clinical data, as well as cost-effectiveness models for both approved CAR T cells. While there will likely be debate over the created model both for the comparators chosen and the treatment course, they found the use of tisagenlecleucel in B-ALL provides clinical benefit in terms of gains in quality-adjusted and overall survival over clofarabine and the majority of time is cost-effective at a willingness-to-pay threshold of $150,000/QALY. The use of axicabtagene ciloleucel in NHL also provided clinical benefit in terms of gains in quality-adjusted and overall survival over chemotherapy, but at the same threshold, was cost-effective in only 68% of the model’s iterations. Overall, these analyses are important to be conducted, but likely will have to be adjusted as the outcomes, processes, and costs become more apparent.
Discussion
Modern indications, the availability of alternative donors, and progress in conditioning regimens, supportive care, and survivorship allow for expanded use of HCT and cellular therapies with improved outcomes. According to an Agency for Health Care Research and Quality report, HCT generated the most rapid increase in total hospital costs from 2004 to 2007 with a growth rate of 84.9% and 1.3 billion dollars spent in 2007 [75]. With this increase in cost, it behooves us to optimize value, defined in this sense as the health outcomes achieved per dollar spent. Frameworks put forward by the American Society of Clinical Oncology and the National Comprehensive Cancer Network, as well as others, aim to streamline management to increase value. Additionally, newer payment models such as the patient-centered medical home defined by the Medicare Access and Children’s Health Insurance Plan Reauthorization Act (MACRA) offer suggestions for enhancing value by delivering well-coordinated care [76].
The multiphase HCT process is complex due in part to the transitions of care with the required communication between providers, the availability and access to an HCT center with the requisite specialized care team, and the extensive financial and caregiver support needed from the evaluation phase through long-term survival. These challenges may be slightly different for the CAR T cell therapies, but both procedures remain resource and cost-intensive high-risk high-rewards strategies. In the HCT field, there has been much work in outlining the opportunities for intervention to improve value-based care as described above (Table 1).
Table 1.
Examples of challenges and solutions for value-based care in hematopoietic stem cell transplantation
| Value-based care element | Challenges | Current and potential future solutions |
|---|---|---|
| Structure and system capacity | ||
| Availability of personnel | • Not enough physician, APPs, SW, pharmacists, rehabilitation therapists for projected numbers • Limited time for each provider to see patient |
• Exposure to HCT early in training • Advanced training • Burnout prevention • Collaborative practice agreements with allied health team members |
| Physical space | • Limited inpatient bed capacity and clinic space • Minimal accommodations for post-HCT care |
• Increase outpatient HCT • Residence space owned/leased by hospital |
| Coordination and standardization | ||
| Terminology | • Different definitions for the phases of care | • Standard definitions have been created by expert consensus |
| Care coordination | • Local providers and HCT center providers unaware of each other’s plans • Multiple appointments and visits with different team members and testing |
• Improving the use of health care information technology to increase communication between providers |
| Medication adherence | • Significant pill burden • Frequent medication and dosing changes |
• Telemedicine visits to discuss medication changes and confirm adherence • Self-management support • Electronic monitored pill boxes |
| Survivorship | • Few known outcomes to monitor for • Loss to follow-up |
• Increase research in long-term outcomes • Increase survivorship clinics and coordination with primary care providers |
| Diversity and disparities | ||
| Access to care | • Delays in HCT referral • Distance to nearest HCT center • Socioeconomic, race, and gender differences |
• Improved education on referral timing • Pathways established between outside practices and HCT centers • Funding for those with limited resources |
| Quality of life | ||
| PRO measurements | • Significant symptom burden through HCT phases | • Increase monitoring of QoL and PRO • Interventions to target major side effects |
| Costs | ||
| Out-of-pocket costs | • High-cost immunosuppression and antimicrobial medications • Multiple visits for extended periods of time after HCT—copays, parking, etc. |
• Common post-HCT medications included in bundle with insurance • Funding for those with limited resources |
| Lost work/wages | • In addition to patient, caregiver out of work for long period of time | • Hired trained caregiver added into HCT bundle |
| Billing/reimbursement | • Lack of appropriate billing codes or distinction for newer types of treatment • National push for frameworks and alternative care models |
• Creation of new codes in timely manner • Standardized bundled care including all essential components of HCT • New care models such as patient-centered medical homes, value-based pricing |
| Outcomes | • Limited meaningful and actionable measures | • Consensus agreement on and validation of HCT measures outside of survival and graft-versus-host disease |
APP, advanced practice providers; HCT, hematopoietic stem cell transplantation; PRO, patient-reported outcomes; QoL, quality of life; SW, social workers
The optimization of care can be measured by outcome, process, and structural metrics, with some well defined in the HCT setting [1]. The most concrete of these is the center-specific CIBMTR report providing the observed and expected 1-year survival based on case mix [77]. Standardization and reporting of additional metrics may be the next step in expanding value-based care across HCT centers. Additionally, value-based purchasing, which combines the concepts of bundled payments and pay-for-performance, may be on the horizon with narrowing payer networks and the labeling of “centers of excellence [72],” which will require adaptations in both structure and process. Finally, the aim of increasing value-based care is not limited to the HCT and cell therapy settings, as evidenced by a similar discussion surrounding kidney transplantation [78], and it will likely behoove the transplant community to more broadly examine the progress in other fields and possibly apply proposed solutions to HCT given the evolving health care legislative climate.
Increasing value-based care for hematopoietic cell transplantation and cellular therapies is a laudable goal, which has been taken on by many of the stakeholders involved. Physicians, advanced practice providers, pharmacists, and allied health professionals, as well as the ASBMT, NMDP, CIBMTR, and additional professional societies, have devoted the considerable time and resources to the investigations summarized in this review. The development and implementation of proposed interventions are now needed to continue to advance and improve outcomes, quality of life, and overall delivery of care.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Gunjan L. Shah, Nandita Khera, and Sergio Giralt declare no conflict of interest. Navneet Majhail is a consultant for Anthem, Inc.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Majhail NS. Optimizing quality and efficiency of healthcare delivery in hematopoietic cell transplantation. Curr Hematol Malig Rep 2015;10:199–204. [DOI] [PubMed] [Google Scholar]
- 2.Brown M, Brown G, Sharma S. Evidence-based to value-based medicine Chicago: AMA Press; 2005. [Google Scholar]
- 3.Conrad DA. The theory of value-based payment incentives and their application to health care. Health Serv Res 2015;50:2057–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porter ME. What is value in health care? NEJM 2010;363:2477–81. [DOI] [PubMed] [Google Scholar]
- 5.Hashmi SK, Bredeson C, Duarte RF, et al. National Institutes of Health Blood and Marrow Transplant Late Effects Initiative: The Healthcare Delivery Working Group Report. BBMT 2017;23:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denzen EM, Majhail NS, Stickney Ferguson S, et al. Hematopoietic cell transplantation in 2020: summary of year 2 recommendations of the National Marrow Donor Program’s System Capacity Initiative. BBMT 2013;19:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majhail NS, Murphy EA, Denzen EM, et al. The National Marrow Donor Program’s Symposium on Hematopoietic Cell Transplantation in 2020: a health care resource and infrastructure assessment. BBMT 2012;18:172–82. [DOI] [PubMed] [Google Scholar]
- 8.Neumann JL, Mau L-W, Virani S, Denzen EM, Boyle DA, Boyle NJ, et al. Burnout, moral distress, work–life balance, and career satisfaction among hematopoietic cell transplantation professionals. BBMT 2017; 10.1016/j.bbmt.2017.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majhail NS, Murphy EA, Omondi NA, Robinett P, Gajewski JL, LeMaistre CF, et al. Allogeneic transplant physician and center capacity in the United States. BBMT 2011;17:956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majhail NS, Mau LW, Chitphakdithai P, et al. National survey of hematopoietic cell transplantation center personnel, infrastructure, and models of care delivery. BBMT 2015;21:1308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nivison-Smith I, Milliken S, Dodds AJ, Gottlieb D, Kwan J, Ma DDF, et al. Activity and capacity profile of transplant physicians and centers in Australia and New Zealand. BBMT 2018;24:169–74. [DOI] [PubMed] [Google Scholar]
- 12.Merten JA, Shapiro JF, Gulbis AM, et al. Utilization of collaborative practice agreements between physicians and pharmacists as a mechanism to increase capacity to care for hematopoietic stem cell transplant recipients. BBMT 2013;19:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komanduri KV. Pharmacists and physicians in hematopoietic stem cell transplantation: advances and opportunities to use collaborative practice agreements to improve care. BBMT 2013;19:505–8. [DOI] [PubMed] [Google Scholar]
- 14.Medicare and Medicaid Programs. Reform of hospital and critical access hospital conditions of participation. Fed Regist 2012;77: 29034–76. To be codified at 42 CFR x482 and x48 [PubMed] [Google Scholar]
- 15.Vulaj V, Hough S, Bedard L, Farris K, Mackler E (2018) Oncology pharmacist opportunities: closing the gap in quality care JOP JOP2017026666. [DOI] [PubMed] [Google Scholar]
- 16.Stickney Ferguson S, Randall J, Dabney J, Kalbacker ME, Boyle N, Thao V, et al. Perceived workforce challenges among clinical social workers in hematopoietic cell transplantation programs. BBMT 2017; 10.1016/j.bbmt.2017.12.793. [DOI] [PubMed] [Google Scholar]
- 17.LeMaistre CF, Farnia S, Crawford S, McGuirk J, Maziarz RT, Coates J, et al. Standardization of terminology for episodes of hematopoietic stem cell patient transplant care. BBMT 2013;19:851–7. [DOI] [PubMed] [Google Scholar]
- 18.Majhail NS, Giralt S, Bonagura A, Crawford S, Farnia S, Omel JL, et al. Guidelines for defining and implementing standard episode of care for hematopoietic stem cell transplantation within the context of clinical trials. BBMT 2015;21:583–8. [DOI] [PubMed] [Google Scholar]
- 19.Khera N, Martin P, Edsall K, Bonagura A, Burns LJ, Juckett M, et al. Patient-centered care coordination in hematopoietic cell transplantation. Blood Adv 2017;1:1617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khera N, Gooley T, Flowers MED, Sandmaier BM, Loberiza F, Lee SJ, et al. Association of distance from transplantation center and place of residence on outcomes after allogeneic hematopoietic cell transplantation. BBMT 2016;22:1319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison CF, Martsolf DM, Wehrkamp N, Tehan R, Pai ALH. Medication adherence in hematopoietic stem cell transplantation: a review of the literature. BBMT 2017;23:562–8. [DOI] [PubMed] [Google Scholar]
- 22. •.Battiwalla M, Hashmi S, Majhail N, Pavletic S, Savani, Shelburne N. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: developing recommendations to improve survivorship and long-term outcomes. BBMT 2017;23:6–9.First in series of papers describing the National Institutes of Health HCT Late Effects Initiative process and recommendations.
- 23.Gea-Banacloche J, Komanduri KV, Carpenter P, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: the Immune Dysregulation and Pathobiology Working Group Report. BBMT 2017;23:870–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armenian SH, Chemaitilly W, Chen M, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: the Cardiovascular Disease and Associated Risk Factors Working Group Report. BBMT 2017;23:201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morton LM, Saber W, Baker KS, Barrett AJ, Bhatia S, Engels EA, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: the Subsequent Neoplasms Working Group Report. BBMT 2017;23:367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bevans M, El-Jawahri A, Tierney DK, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: the Patient-Centered Outcomes Working Group Report. BBMT 2017;23:538–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashmi SK, Bredeson C, Duarte RF, et al. National Institutes of Health Blood and Marrow Transplant Late Effects Initiative: the Healthcare Delivery Working Group Report. BBMT 2017;23: 717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw BE, Hahn T, Martin PJ, Mitchell SA, Petersdorf EW, Armstrong GT, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: the Research Methodology and Study Design Working Group Report. BBMT 2017;23:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshua TV, Rizzo JD, Zhang M-J, Hari PN, Kurian S, Pasquini M, et al. Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer 2010;116:3469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker KS, Davies SM, Majhail NS, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. BBMT 2009;15:1543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao K, Darrington DL, Schumacher JJ, Devetten M, Vose JM, Loberiza FR. Disparity in survival outcome after hematopoietic stem cell transplantation for hematologic malignancies according to area of primary residence. BBMT 2007;13:1508–14. [DOI] [PubMed] [Google Scholar]
- 32.Majhail NS, Nayyar S, Santibañez MEB, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. BMT 2012;47:1385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton BK, Rybicki L, Arai S, et al. Association of Socioeconomic Status with chronic graft-versus-host disease outcomes. BBMT 2018;24:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ailawadhi S, Frank RD, Advani P, Swaika A, Temkit M’, Menghani R, et al. Racial disparity in utilization of therapeutic modalities among multiple myeloma patients: a SEER-medicare analysis. Cancer Med 2017;6:2876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majhail NS, Jagasia M. Referral to transplant center for hematopoietic cell transplantation. Hematol Oncol Clin North Am 2014;28: 1201–13. [DOI] [PubMed] [Google Scholar]
- 36.Pidala J, Craig BM, Lee SJ, Majhail N, Quinn G, Anasetti C. Practice variation in physician referral for allogeneic hematopoietic cell transplantation. BMT 2013;48:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preston E, Chinapen S, Borrill T, et al. Disease progression is main barrier to allogeneic hematopoietic stem cell transplantation (HCT) in patients with newly diagnosed and relapsed acute leukemia. BBMT Abstract 2018; 465. [Google Scholar]
- 38.Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr 2007;2007:16–21. [DOI] [PubMed] [Google Scholar]
- 39.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood 2009;114:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodgers C, Wills-Bagnato P, Sloane R, Hockenberry M. Health-related quality of life among children and adolescents during hematopoietic stem cell transplant recovery. J Pediatr Oncol Nurs 2015;32:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawitschka A, Güclü ED, Varni JW, Putz M, Wolff D, Pavletic S, et al. Health-related quality of life in pediatric patients after allogeneic SCT: development of the PedsQL Stem Cell Transplant module and results of a pilot study. BMT 2014;49:1093–7. [DOI] [PubMed] [Google Scholar]
- 42.Rodgers C, Wills-Bagnato P, Sloane R, Hockenberry M. Health-related quality of life among children and adolescents during hematopoietic stem cell transplant recovery. J Pediatr Oncol Nurs 2015;32:329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons SK, Tighiouart H, Terrin N. Assessment of health-related quality of life in pediatric hematopoietic stem cell transplant recipients: progress, challenges and future directions. Expert Rev Pharmacoecon Outcomes Res 2013;13:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y-M, Jaing T-H, Chen Y-C, Tang S-T, Li C-Y, Wen Y-C, et al. Quality of life after hematopoietic stem cell transplantation in pediatric survivors. Cancer Nurs 2016;39:502–9. [DOI] [PubMed] [Google Scholar]
- 45.Mosher CE, DuHamel KN, Rini C, Corner G, Lam J, Redd WH. Quality of life concerns and depression among hematopoietic stem cell transplant survivors. Support Care Cancer 2011;19:1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bevans MF, Mitchell SA, Barrett AJ, Bishop M, Childs R, Fowler D, et al. Function, adjustment, quality of life and symptoms (FAQS) in allogeneic hematopoietic stem cell transplantation (HSCT) survivors: a study protocol. Heal Qual Life Outcomes 2011;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton JG, Wu LM, Austin JE, Valdimarsdottir H, Basmajian K, Vu A, et al. Economic survivorship stress is associated with poor health-related quality of life among distressed survivors of hematopoietic stem cell transplantation. Psychooncology 2013;22:911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Wreede LC, Watson M, van Os M, Milligan D, van Gelder M, Michallet M, et al. Improved relapse-free survival after autologous stem cell transplantation does not translate into better quality of life in chronic lymphocytic leukemia: lessons from the randomized European Society for Blood and Marrow Transplantation-Intergroup study. Am J Hematol 2014;89:174–80. [DOI] [PubMed] [Google Scholar]
- 49.Kenzik K, Huang I-C, Rizzo JD, Shenkman E, Wingard J. Relationships among symptoms, psychosocial factors, and health-related quality of life in hematopoietic stem cell transplant survivors. Support Care Cancer 2015;23:797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pillay B, Lee SJ, Katona L, De Bono S, Burney S, Avery S. A prospective study of the relationship between sense of coherence, depression, anxiety, and quality of life of haematopoietic stem cell transplant patients over time. Psychooncology 2015;24:220–7. [DOI] [PubMed] [Google Scholar]
- 51.Landau H, Wood K, Chung DJ, Koehne G, Lendvai N, Hassoun H, et al. Fractionated stem cell infusions for patients with plasma cell myeloma undergoing autologous hematopoietic cell transplantation. Leuk Lymphoma 2016;57:1781–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deng G, Giralt S, Chung DJ, Landau H, Siman J, Search B, et al. Acupuncture for reduction of symptom burden in multiple myeloma patients undergoing autologous hematopoietic stem cell transplantation: a randomized sham-controlled trial. Support Care Cancer 2018;26:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson KO, Giralt SA, Mendoza TR, Brown JO, Neumann JL, Mobley GM, et al. Symptom burden in patients undergoing autologous stem-cell transplantation. BMT 2007;39:759–66. [DOI] [PubMed] [Google Scholar]
- 54.Bevans MF, Mitchell SA, Barrett JA, et al. Symptom distress predicts long-term health and well-being in allogeneic stem cell transplantation survivors. BBMT 2014;20:387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dueck AC, Mendoza TR, Mitchell SA, Reeve BB, Castro KM, Rogak LJ, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 2015;1:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah N, Shi Q, Williams LA, et al. Higher stem cell dose infusion after intensive chemotherapy does not improve symptom burden in older patients with multiple myeloma and amyloidosis. BBMT 2016;22:226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khera N, Chang Y, Hashmi S, et al. Financial burden in recipients of allogeneic hematopoietic cell transplantation. BBMT 2014;20: 1375–81. [DOI] [PubMed] [Google Scholar]
- 58.Farnia S, Ganetsky A, Silver A, Hwee T, Preussler J, Griffin J, et al. Challenges around access to and cost of life-saving medications after allogeneic hematopoietic cell transplantation for Medicare patients. BBMT 2017;23:1387–92. [DOI] [PubMed] [Google Scholar]
- 59.Bestvina CM, Zullig LL, Rushing C, Chino F, Samsa GP, Altomare I, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pr 2014;10:162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Preussler JM, Farnia SH, Denzen EM, Majhail NS. Variation in medicaid coverage for hematopoietic cell transplantation. J Oncol Pr 2014;10:e196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maziarz RT, Driscoll D. Hematopoietic stem cell transplantation and implications for cell therapy reimbursement. Cell Stem Cell 2011;8:609–12. [DOI] [PubMed] [Google Scholar]
- 62. •.Maziarz RT, Farnia S, Martin P, Komanduri KV. Optimal benefits for hematopoietic stem cell transplantation: a consensus opinion. BBMT 2014;20:1671–6.Recommendations by the Financial Working Group of the National Marrow Donor Program-sponsored System Capacity Initiative on what to include in a bundled insurance payment.
- 63.Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood 2012;120:1545–51. [DOI] [PubMed] [Google Scholar]
- 64.Preussler JM, Mau L-W, Majhail NS, et al. Administrative claims data for economic analyses in hematopoietic cell transplantation: challenges and opportunities. BBMT 2016;22:1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. BBMT 2012;18:1620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Majhail NS, Mau LW, Denzen EM, Arneson TJ. Costs of autologous and allogeneic hematopoietic cell transplantation in the United States: a study using a large national private claims database. BMT 2013;48:294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SJ, Anasetti C, Kuntz KM, Patten J, Antin JH, Weeks JC. The costs and cost-effectiveness of unrelated donor bone marrow transplantation for chronic phase chronic myelogenous leukemia. Blood 1998;92:4047–52. [PubMed] [Google Scholar]
- 68.Shah GL, Winn AN, Lin P-J, et al. Cost-effectiveness of autologous hematopoietic stem cell transplantation for elderly patients with multiple myeloma using the surveillance, epidemiology, and end results-Medicare database. BBMT 2015;21:1823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pandya C, Hashmi S, Khera N, Gertz MA, Dispenzieri A, Hogan W, et al. Cost-effectiveness analysis of early vs. late autologous stem cell transplantation in multiple myeloma. Clin Transpl 2014;28:1084–91. [DOI] [PubMed] [Google Scholar]
- 70.Worcester S (2017) CAR T-cell therapy: moving from cost to value. In: Hematol. News https://www.mdedge.com/hematologynews/article/152563/all-acute-lymphoblastic-leukemia/car-t-cell-therapy-moving-cost-value Accessed 15 Jan 2018. [Google Scholar]
- 71.Bach PB, Giralt SA, Saltz LB. FDA approval of Tisagenlecleucel. JAMA 2017;318:1861–2. [DOI] [PubMed] [Google Scholar]
- 72.LeMaistre CF, Farnia SH. Goals for pay for performance in hematopoietic cell transplantation: a primer. BBMT 2015;21:1367–72. [DOI] [PubMed] [Google Scholar]
- 73.Farnia S, Shah J, Rinkle V, Gajewski J. ASBMTautologous CAR-T coding guidance 2017 [Google Scholar]
- 74. ••.Tice JA, Walsh JM, Otuonye I, Chapman R, Kumar V, Seidner M, Ollendorf DA, Pearson SD (2017) Chimeric antigen receptor T-cell therapy for B-cell cancers: effectiveness and value. Inst Clin Econ Rev 1–120.Draft evidence report by the Institute for Clinical and Economic Review summarizing currently known clinical data on CAR T cells and presenting economic models.
- 75.Stranges E, Russo C, Friedman B. Procedures with the most rapidly increasing hospital costs, 2004–2007 [HCUP statistical brief 82] Rockville, MD: 2009 [Google Scholar]
- 76.Gajewski JL, McClellan MB, Majhail NS, et al. Payment and care for hematopoietic cell transplantation patients: toward a specialized medical home for complex care patients. BBMT 2018;24:4–12. [DOI] [PubMed] [Google Scholar]
- 77.Transplantation C for B and M (2017) CIBMTR report of survival statistics for blood and marrow transplantation https://www.cibmtr.org/ReferenceCenter/SlidesReports/StatReport/Pages/index.aspx Accessed 15 Jan 2018. [Google Scholar]
- 78.Hippen BE, Maddux FW. Integrating kidney transplantation into value-based care for people with renal failure. Am J Transpl 2018;18:43–52. [DOI] [PubMed] [Google Scholar]


