Abstract
Malaria-associated bacteremia accounts for up to one third of deaths from severe malaria, and non-typhoidal Salmonella (NTS) has been reported as a major complication of severe malarial infection. Patients who develop NTS bacteremia during Plasmodium infection show higher mortality rates than individuals with malaria alone. Systemic bacteremia can be caused by a wound or translocation from epithelial sites. Although NTS is an intestinal pathogen, the contribution of bacterial translocation from the intestinal tract during Plasmodium infection is not well studied. Here, we investigated the integrity of intestinal barrier function of P. chabaudi-infected mice using large molecules and the intestinal pathogen, Salmonella. Intestinal histology and the adaptive immune response to malaria were also studied using light microscopy and flow cytometry. P. chabaudi infection compromised intestinal barrier function, which led to increased intestinal cellular infiltration. In addition, we observed increased serum lipopolysaccharide binding protein and leakage of soluble molecules from the intestine into the blood in infected mice. Plasmodium infection also increased intestinal translocation and dissemination of NTS to the liver. The adaptive immune response to P. chabaudi infection was also significantly impacted by NTS translocation. Reduced B and T cell activation were observed in co-infected animals, suggesting interference in the malarial-specific immune responses by bacteremia. These studies demonstrate that P. chabaudi infection induces a failure of the barrier function of the intestinal wall and enhanced intestinal bacterial translocation, affecting anti-malarial immunity.
Keywords: Malaria, Plasmodium, Translocation, Adaptive Immunity, Salmonella
Introduction
Approximately half of the world population is at risk of acquiring Plasmodium falciparum infection, an etiological agent of malaria, and malaria causes an estimated 445,000 annual deaths worldwide (WHO 2017). There is a malaria vaccine in Phase III clinical trials that shows 36.3% protection (95% confidence interval, CI), although the efficacy of the vaccine varies significantly among children of different age groups (Olotu et al. 2016). Gastrointestinal (GI) disturbance was reported in malaria-infected children who were admitted to hospitals in Nigeria, and 30% of admitted children tested positive for endotoxemia (Church et al. 2016). Children infected with P. falciparum are highly susceptible to invasive bacterial infections caused by enteric Gram-negative pathogens. In fact, children with severe malaria who develop systemic bacteremia have higher mortality rates than those without co-infection (Church et al. 2016). Systemic non-typhoidal Salmonella (NTS) is one such example of a complication of severe malarial infection (Church et al. 2014). Malaria infection, malnutrition, and anemia are the major risk factors for systemic NTS bacteremia in African children (Mooney et al. 2015). Normally, infection with NTS results in a self-limiting gastroenteritis with mild symptoms, manifested by abdominal pain, vomiting, and diarrhea (Eguale et al. 2015). However, bacteremia in combination with Plasmodium falciparum infection is more severe and may account for as many as one third of deaths from severe malaria (Nadjm et al. 2010), though little is known about mechanisms of interaction.
The intestinal epithelium provides a protective barrier against enteric pathogens (Vaishnavi 2013), and upon disruption exposes the underlying lamina propria to luminal antigens and microbes. However, human immunodeficiency virus (HIV) infection and other infections have been shown to drive increased intestinal permeability and dysbiosis, which are frequently associated processes. Interestingly, in HIV, microbial translocation from the gut drives significant immune activation and promotes disease (Brenchley et al. 2012). Therefore, it is an important question to ask whether malaria induces intestinal permeability or bacterial translocation, and if that process increases susceptibility to malaria infection or impairs immunity to malaria. Interestingly, the risk of infection with P. falciparum correlates with the composition of the fecal microbiota in Malian children (Yooseph et al. 2015). Furthermore, in several mouse models of malaria, the composition of the intestinal microbiota dramatically affects the growth of Plasmodium (Villarino et al. 2016). P. berghei ANKA infection of C57BL/6 mice induced intestinal dysbiosis with a decrease in Firmicutes and an increase in Proteobacteria during the peak of infection (Taniguchi et al. 2015). In this study, parasite-induced dysbiosis was independently associated with parasite growth, and progression of experimental cerebral malaria.
Sequestration of parasite in the microvasculature is a feature of most Plasmodium species, and is thought to be crucial for parasite survival by avoiding capture by phagocytes in the spleen. However, sequestration of P. falciparum infected red blood cells (iRBCs) on intestinal vascular endothelium in patients has been correlated with ischemia, increased gastrointestinal permeability, and malabsorption (Molyneux et al. 1989; Wilairatana et al. 1997), suggesting that sequestration plays a role in gut pathology. The rodent parasite P. chabaudi sequesters in blood vessels of the lungs and liver, but is not found concentrated in the blood vessels of the brain or intestine (Brugat et al. 2014). Therefore, this model affords the potential to investigate whether intestinal disturbance occurs in the absence of intestinal parasite sequestration. We observed that P. chabaudi did induce leakage of small molecules and intestinal bacteria into the circulation. Indeed, exposure of P. chabaudi-challenged mice to NTS resulted in rapid bacterial dissemination. We also found that NTS coinfection led to significant changes in the adaptive immune response to P. chabaudi, including a reduction in B and T cell activation. This suggests that the host immune response is exposed to systemic intestinal microbiota in Plasmodium infection to a significant degree.
Materials and Methods
Mice, parasites, bacteria, and in vivo experiments
Wild-type (WT) and congenic Ly5.1 C57Bl/6J (Jackson, Bar Harbor, ME), female mice 8–12 weeks old were used in all experiments. Stocks of Plasmodium chabaudi chabaudi (AS)- infected RBCs (iRBCs) (Jean Langhorne, Francis Crick Institute, London, UK) were stored at −80°C. Thin blood smears stained with Differential Quik Stain Kit (Siemens Healthcare diagnostic Inc, Newark, DE) or Giemsa stain (Ricca chemical company, Arlington, TX) were used to assess parasitemia using light microscopy. Parasite stock was thawed and 105 P. chabaudi (AS)-infected erythrocytes diluted in in Krebs glucose (1800 mg/l) were injected intraperitoneally (i.p.), diluted in normal saline (200 μL). Mice were first fasted for 4 hours then infected with 108 CFU Salmonella enterica serovar Typhimurium 14028 (American Type Culture Collection, Manassas, VA) in 5% Sodium Bicarbonate by oral gavage (100 μL). Splenocytes were collected from CBir1 T cell Receptor Transgenic (Cbir1 Tg) mice, labeled with 5(6)-Carboxyfluorescein ester (CFSE)- analog cell trace violet (CTV) and 9.4 × 106 cells were then i.p. transferred to each mouse. CD4+Cbir1 Tg CFSE+ cells are specific for an immunodominant microbiota flagellin antigen (Cong et al. 2009; Wu et al. 2016). All experiments were carried out in accordance with the protocols approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee.
Intestinal Permeability Assays
Fluorescein isothiocyanate (FITC)-dextran (3,000–5,000 molecular weight, MW) was injected into 8 hr-fasted mice (50–125 mg/ml, in 100 μL, by oral gavage) and then blood was collected by cardiac puncture, 4 hr post-gavage into tubes containing 10 μl of heparin. Blood was centrifuged at 4°C, 12,000g, for 3 min. Extracted plasma was analyzed for FITC-dextran concentration with a fluorescence spectrophotometer (FLUOstar Omega Microplate Reader – BMG LABTECH, Germany) at the excitation wavelength of 485 nm and the emission wavelength of 520 nm. Standard curves for calculating the FITC-dextran concentration in the samples were obtained by diluting FITC-dextran in non-treated plasma diluted with phosphate-buffered saline (PBS) (1:1 [vol/vol]). Lipopolysaccharide binding protein (LPSBP) ELISA (enzyme-linked immunosorbent assay) was performed on plasma from various days post-infection as per manufacturer’s instructions (Hycult Biotech, Plymouth Meeting, PA).
Histopathological Assessment
Small intestines were excised into PBS, and then fixed in 10% neutral buffered formalin for 24 hrs. Paraffin embedded samples were sectioned and stained with hematoxylin and eosin (H&E, UTMB Pathology Department Histopathology Core). Blinded histopathology evaluation was performed by two trained readers. For each tissue section, the number of cells within the lamina propria were counted in the two most affected areas at least 10 crypts apart, and the scores were averaged. The average counts were used to determine the mean value for each group.
Bacteriology
Blood samples were collected from mice by cardiac puncture into tubes containing 10 μl of diluted heparin. Aliquots of samples from blood and liver and spleen homogenates were serially diluted in PBS and then plated on Salmonella-Shigella (SS) agar for bacterial growth and enumeration.
Flow cytometry
Single-cell suspensions of splenocytes were made in hydroxyethyl piperazineethanesulfonic acid (HEPES)-buffered Hanks’ Balanced Salt Solution (HBSS) (Mediatech, Manassas, VA), and then depleted of erythrocytes by incubation in RBC lysis buffer (eBioscience, San Diego, CA). Cells were stained in PBS with 2% fetal bovine serum (FBS) (Sigma, St. Louis, MO) and 0.1% sodium azide with anti-CD16/32 (2.4G2) antibodies (Abs) (BioXCell, West Lebanon, NH) for Fc receptor blocking, followed by a combination of other PerCP-Cy5.5, PE/cyanine 7 (Cy7), PE/Cy5, Allophycocyanin (APC), or APC/efluor780–conjugated Abs (all from eBioscience); or CD62L PE-Texas Red (Invitrogen, Life Technologies, Grand island, NY). Cells were collected on a LSRII Fortessa using FACSDiva software (BD Biosciences, San Jose, CA) and analyzed in FlowJo (version 9.7, Tree Star, Ashland, OR). Compensation was performed in FlowJo using single-stained splenocytes (using CD4 in all colors). Data from each mouse was analyzed and averages and standard error of the mean (SEM) calculated in Microsoft Excel, and presented in Prism (GraphPad, La Jolla, CA).
Statistics:
Where indicated, experiments were analyzed by one-way analysis of variance (ANOVA), followed by the Student’s t test in Prism (Graphpad, San Diego, CA): *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Results
P. chabaudi infection compromises the intestinal barrier
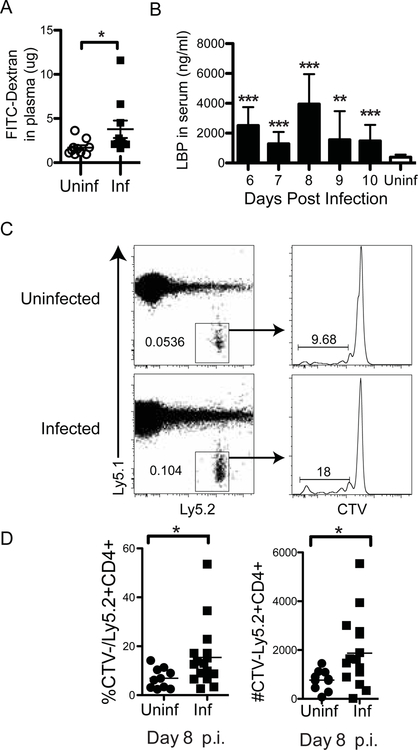
Based on data in humans showing increased systemic bacteremia in patients infected with P. falciparum, we hypothesized that malaria induces increased permeability of the intestinal wall. To test the integrity of the intestinal barrier, C57Bl/6J mice were infected with P. chabaudi and dextran was administrated into the gastrointestinal (GI) tract via gavage. We then tested for leakage of dextran into the blood. Due to the large molecular size of FITC-dextran (3,000–5,000 MW), this molecule can be used as a marker of paracellular transport and mucosal barrier dysfunction (Joly et al. 2014). Mice infected with P. chabaudi showed significant increase in plasma FITC-dextran concentrations at 8 days post P. chabaudi infection compared to non-infected mice (Fig. 1a). The presence of LPSBP, which binds LPS and promotes LPS signaling through Toll-like receptor (TLR)-4 and CD14, is a highly sensitive indicator of LPS from the GI tract in the blood (Fang et al. 2014). Therefore, to test gut barrier function, we also measured the concentration of LPSBP in the serum of infected animals, and uninfected controls. P. chabaudi-infected mice showed significant increases in plasma LPSBP over uninfected control mice on all days 6–10 p.i. Though there was significant variation among animals, there is a potential peak on day 8 p.i., which aligns with the peak of P. chabaudi infection (Fig. 1b).
Figure 1.
Gut leakage occurs in P. chabaudi infection. (A) C57Bl/6 mice were infected with P. chabaudi malarial parasite. At day 8 p.i., mice that had been fasted for 8 hrs were gavaged with FITC-Dextran. Plasma samples were collected 4 hr post-gavage and FITC-dextran concentration was analyzed. (B) Blood from infected animals was sampled daily and tested for LPSBP by ELISA. Age-matched uninfected mice were used for comparison. (C) Adoptive transfer of commensal bacterial flagellin-specific CD4+CFSE+ CBir1 Tg T cells (naïve, 9.4×106), into congenic Ly5.1 C57Bl/6 animals was performed followed by infection with P. chabaudi. Splenocytes were analyzed by flow cytometry at day 8 p.i. for proliferation (CTV-) of CBir1 Tg T cells (Ly5.2+) indicating exposure to commensal bacteria that have translocated from the intestinal lumen into the periphery. Representative dot plots show transferred Ly5.2+ cells gated on CD4+ lymphocytes. Representative histogram shows proliferation (CTVlo/-) of transferred CBir1 T cells (Ly5.2+). (D) Quantification of percent and number of divided CBir1 Tg T cells is shown as a measure of bacterial translocation. Experiments were repeated once with 8 mice per infected (Inf) group and 2–8 mice in uninfected (Uninf) group. Statistically significant differences between groups are indicated with *p ≤ 0.05.
Due to the variation, we confirmed that leakage of dextran and elevated levels of LPSBP in the blood indicated intestinal bacterial translocation induced by P. chabaudi infection by using an additional specific test for intestinal bacteria in the circulation. We took advantage of the Cbir1 T cell receptor (TCR) transgenic (Tg) animals, which have TCRs specific for Cbir1 flagellin, an antigen of commensal A4 bacteria which is a member of the family Lachnospiraceae (Cong et al. 2009; Wu et al. 2016). While not all animals have this specific commensal, Cbir Tg T cells injected intraperitoneally (i.p.) will only proliferate in the spleen if these specific intestinal commensals have escaped into the bloodstream from the GI tract (Feng et al. 2010). Therefore, we labeled Cbir1 TCR Tg splenocytes with a 5(6)-Carboxyfluorescein (CFSE) analog and adoptively transferred them (9.4 × 106) into congenic Ly5.1 C57Bl/6J animals (i.p.) to test their proliferation over the next 8 days during malarial infection. Flow cytometry of splenocytes at 8 d.p.i. revealed that transferred Cbir1 Tg CD4+ T cells divided (CFSElo/-) in infected mice (Fig. 1c). This is shown by the CFSE fluorescence, which decreases by half with each T cell division and becomes negative after about eight divisions. We observed that the Cbir1 Tg T cells did indeed proliferate more in infected animals than in uninfected mice (Fig. 1c, and 1d), indicating increased bacterial translocation. Taken together, the data demonstrate that malaria infection increases the permeability of the intestinal epithelium which allows luminal antigens and microbes to translocate into the lamina propria and then into the systemic circulation.
P. chabaudi infection may increase systemic dissemination of Salmonella Typhimurium
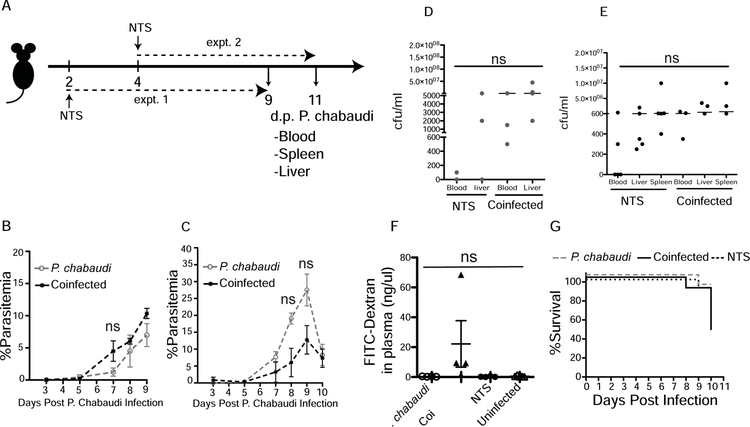
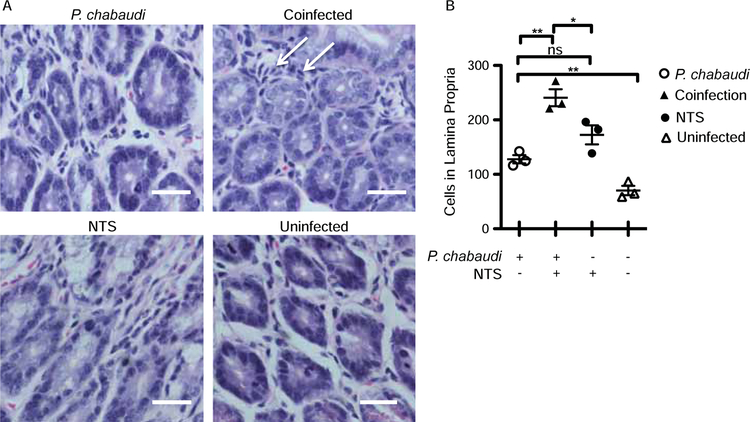
As we show that bacterial translocation is increased during P. chabaudi infection, we next tested whether dissemination of S. Typhimurium increased during malaria infection. We gavaged P. chabaudi-infected mice with S. Typhimurium at either 2 or 4 days post infection with the malaria parasite (Fig. 2a). There were no significant differences in parasitemia between the Plasmodium-infected and the co-infected animals in the two experiments (Fig. 2b and 2c). Bacterial load in the blood, livers, and spleens of Salmonella (alone) or co-infected mice were measured 7 d.p.i. with NTS (or days 9 or 11 after Plasmodium infection). The co-infected animals showed a trend towards higher bacterial load compared to Salmonella-infected mice in two separate experiments, which did not reach significance (Fig. 2d, and 2e). The FITC-dextran assay for intestinal permeability showed leakage of dextran in the plasma from the intestinal lumen only in co-infected mice, while the P. chabaudi- and NTS-only infected groups had serum concentrations of dextran below the level of detection (Fig. 2f). While only one mouse in each of the P. chabaudi- and NTS-only groups died, 50% of the mice in the co-infected group reached the humane end point one day prior to the end of the experiments and were sacrificed due to their symptoms of salmonellosis (Fig. 2g). Coincident with increased gut leakage, co-infected mice showed a significant increase in the infiltration of cells in the lamina propria compared to P. chabaudi- or NTS-only infected mice (Fig. 3a, and 3b). Collectively, these data suggest that Plasmodium infection led to compromised intestinal barriers that increased dissemination of an intestinal pathogen in some animals, even in the absence of local parasite sequestration.
Figure 2.
Co-infection with S. Typhimurium increases gut leakage in mice challenged with P. chabaudi. C57Bl/6J mice were infected with P. chabaudi, and co-infected mice received S. Typhimurium gavage either 2 or 4 days post P. chabaudi infection. The animals were sacrificed 9 or 11 days post malarial parasite infection, respectively, and both experiments ended 7 days post NTS infection. (A) Diagram showing the experimental design of experiment 1 and experiment 2 (expt. 1 and expt. 2). (B, C) Plasmodium parasite growth is shown in both experiments (expt.1 and expt.2, respectively). (D) Bacterial loads in blood and liver are shown at day 7 post NTS infection from (expt.1). (E) Bacterial loads in blood, liver and spleen are shown at day 7 post NTS infection (expt.2). (F) FITC-Dextran gavage followed by plasma collection 4 hr later on day 9 post malarial parasite infection (expt.1) allowed detection of leakage of the intestinal wall shown as FITC fluorescence in plasma. (G) Survival curve of infected and co-infected animals, combined from two experiments (expt.1 and expt.2). Experiments were repeated once with 5 mice per group.
Figure 3.
Malarial parasite-infected animals showed increased cellularity within the lamina propria. C57Bl/6 mice were infected with P. chabaudi, and co-infected mice received Salmonella Typhimurium gavage 4 days post malaria infection and were sacrificed 11 days post malarial infection (expt.2). Sections representing the jejunum of the small intestine were stained with H&E. (A) Representative image (40X) showing a cross-section of the lamina propria in the jejunum. Arrows indicate cellular infiltration. The scale bar represents 25mm. (B) Quantification of the number of cells in the lamina propria of sections of the jejunum. Statistically significant differences between groups are indicated with *p ≤ 0.05 and **p ≤ 0.01.
NTS co-infection inhibits the adaptive immune response to Plasmodium
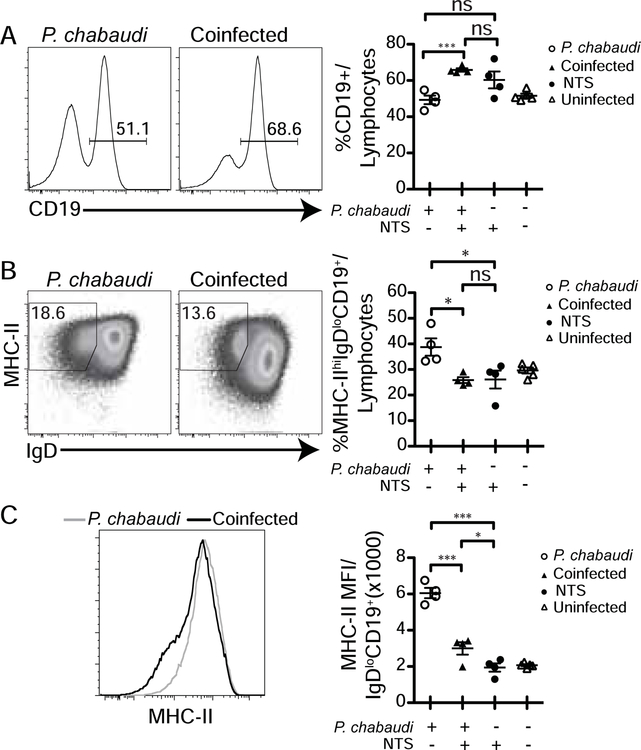
In order to test the effect of bacterial translocation of NTS on the immune response to P. chabaudi, we evaluated B cell activation by flow cytometry. We found an increase in the fraction of B cells in the lymphocyte gate in the spleen of co-infected animals compared to P. chabaudi-infected mice (Fig. 4a). However, NTS co-infection caused a significant decrease in the frequency of activated B cells out of total B cells (MHCIIhi IgD−, Fig. 4b), and there was less upregulation (mean fluorescent intensity: MFI) of MHCII on the surface of activated (CD19+IgD−) B cells (Fig. 4c). As B cell activation is less in co-infected animals than in response to P. chabaudi infection alone, we conclude that exposure to NTS results in a reduction in B cell activation in response to P. chabaudi (Fig. 4c).
Figure 4.
Co-infection with S. Typhimurium increases proportion of B cells, but decreases their activation in mice. C57Bl/6J mice were infected with P. chabaudi. Co-infected mice received NTS gavage 2 days post malarial infection, and were sacrificed 9 days post parasite infection, which is 7 days post NTS infection (expt.1). (A) Representative flow cytometry histogram showing fraction of CD19+ B cells of splenic lymphocytes, and graph showing quantification in all mice. (B) Representative dot plots showing fraction of CD19+ B cells having been activated (MHCIIhi IgD−) and graph summarizing fraction of B cells that are activated in all mice. (C) Representative histogram of MHCII gated on IgD− CD19+ splenic lymphocytes with MFI quantified for all mice. Statistically significant differences between groups are indicated with *p ≤ 0.05 and ***p ≤ 0.001.
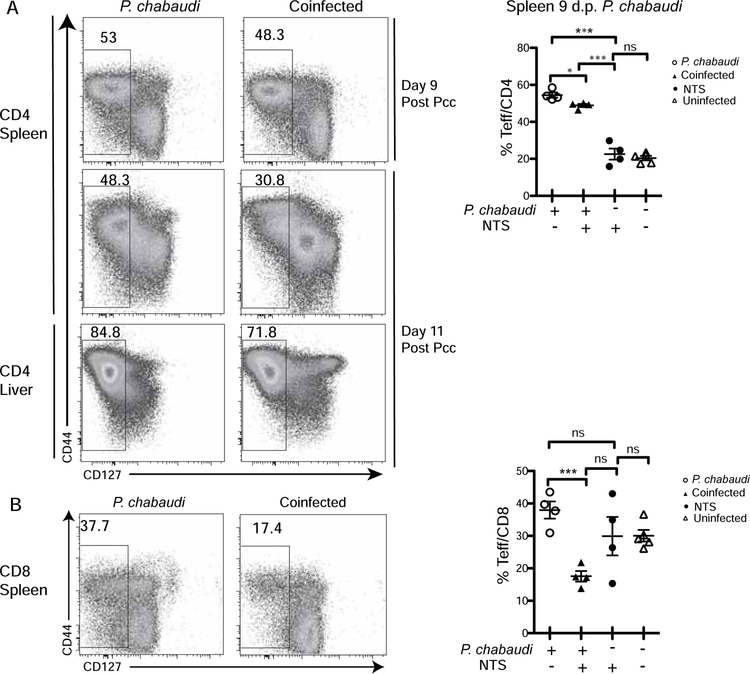
Generation of effector T cell cells was also investigated in the spleen by flow cytometry. Co-infection with S. Typhimurium significantly reduced CD4 effector T cell induction to P. chabaudi as defined by reduced CD127 downregulation on CD4 T cells in the spleen and liver (Fig. 5a). CD127 is the IL-7 receptor alpha chain, which is downregulated upon activation, while CD44 is slowly upregulated on T cells. This decrease was observed in the spleen on days 9 and 11 post Plasmodium infection, as well as in liver on day 9 post Plasmodium infection (not shown). P. chabaudi-induced CD8 T cell activation was also significantly inhibited upon co-infection with Salmonella, resulting in 54 % reduction of CD8 effector T cells (CD127−, Fig. 5b). These findings show that Plasmodium infection not only increases dissemination of NTS in co-infection, but also suggest that the dissemination of Salmonella may lead to a reduced acute malaria-specific immune response.
Figure 5.
Co-infection with S. Typhimurium compromises T cell activation. C57Bl/6J mice were infected with P. chabaudi and co-infected mice received NTS gavage either 2 or 4 days post parasite infection. The animals were sacrificed 9 or 11 days post P. chabaudi infection, respectively. (A) Representative dot plots of effector T cells (CD127−CD44int/hi) gated on splenic or hepatic lymphocytes (expt.1 and expt.2), and graph showing percentage in the spleens of P. chabaudi-infected mice on days 9 post infection (expt.1). (B) Representative dot plots and quantification of effector CD8 T cells (CD44hiCD127−) gated on splenic lymphocytes at 9 days post parasite infection, and graph summarizing fraction of effector CD8 T cells that are activated in all mice (expt.1). Statistically significant differences between groups are indicated with *p ≤ 0.05 and ***p ≤ 0.001.
Discussion
In the current study, we showed that P. chabaudi infection induces intestinal changes and gut barrier leakage, which become enhanced in the context of co-infection with Salmonella. Increased intestinal permeability (Chau et al. 2013) and bacterial translocation has also been shown in animals infected with P. yoelii (Chau et al. 2013; Mooney et al. 2015; Roux et al. 2010). Chau et al. also observed mastocytosis and elevated histamine levels which can directly increase intestinal permeability (Chau et al. 2013). A previous study showed detachment of the epithelium of the small intestine and dysbiosis with increased intestinal permeability in P. berghei ANKA infection, including sequestration in vessels of the intestinal villi (Taniguchi et al. 2015). Despite the fact that P. chabaudi does not specifically sequester in the gut of infected mice (Brugat et al. 2014), the parasite still induced intestinal barrier disruption and intestinal translocation of microbiota that was detectable in some animals. The present data in mice infected with P. chabaudi is consistent with increased bacterial translocation as indicated by high serum LPSBP, proliferation of microbiota antigen-specific (CBir1) T cells, and dextran leakage into the serum in some animals. Many Salmonella infected animals showed dissemination 7 days after infection with NTS, however mice co-infected with Salmonella and Plasmodium appeared to have increased systemic NTS dissemination. Though this did not reach statistical significance, all co-infected animals exhibited disseminated bacteremia, and more co-infected animals died. Infection with P. yoelii was reported to increase the susceptibility to Salmonella via alterations to the intestinal microbial community (Mooney et al. 2015). It is possible that the systemic increase in the bacterial loads in our co-infection model was due to increase in intestinal colonization of Salmonella. However, a recent study by the same group showed that infection of mice with P. yoelii partially rescued the systemic colonization of Salmonella with mutant defective for type III secretion system-2 (T3SS-2), (Lokken et al. 2018), suggesting a role for an increase in leakiness of the epithelium allowing increased bacterial escape during plasmodium infection. P. chabaudi-infected mice also showed intestinal pathology characterized by cellular infiltration of the lamina propria. Therefore, our data suggest that P. chabaudi infection, or the pro-inflammatory immune response to it, induces dissemination of intestinal microbiota, regardless of intestinal parasite sequestration.
As severe malaria patients with systemic bacteremia have a higher rate of mortality (Church et al. 2016), several studies have examined the mechanisms mediating the increased susceptibility to bacteremia during malaria; however, few have focused on the effects of the bacterial infection on the immune response to malaria. In addition, these studies to date have focused only on innate immunity. Dysfunctional macrophages (Van et al. 2013) and neutrophils (Cunnington et al. 2012) have been shown to play important roles in increasing susceptibility to bacteremia in malaria co-infection settings. Adaptive immunity to Salmonella may also be affected by P. falciparum malaria, as mice immunized with a live-attenuated Salmonella vaccine strain showed a reduced protective response to Salmonella infection during malaria (Mooney et al. 2015). A recent study suggested that malaria patients with bacteremia have lower parasitemia (Davenport et al. 2016). However, in animal models, we and others, have not detected a significant difference in Plasmodium parasitemia when animals infected with the malarial parasite alone were compared to co-infected animals (Chau et al. 2013).
Our work also uncovered a previously unknown effect of Salmonella infection on the adaptive immune response to Plasmodium. We showed that S. Typhimurium infection was associated with a significant decrease in B cell activation in response to P. chabaudi. Furthermore, our data showed that Plasmodium/Salmonella co-infection resulted in inhibition of malarial parasite-associated T cell activation. Malaria generally induces profound effector T cell expansion to control parasite proliferation and regulate pathology (Spence et al. 2012). However, our data indicate that co-infected mice generated significantly fewer of both CD4 and CD8 effector T cells (CD127− CD44hi) compared to animals infected with P. chabaudi alone. Therefore, our data suggest that Salmonella co-infection impacts the adaptive immune response to Plasmodium and contribute to intestinal changes that increase the chances of pathogenic bacteremia.
In conclusion, this study showed that infection of mice with P. chabaudi compromises intestinal barrier functions, characterized by increased barrier permeability and increased infiltration of cells in the lamina propria. Sequestration of Plasmodium in the gut wall microvasculature has been proposed as a mechanism of malarial gut pathology. However, P. chabaudi sequesters in other organs, but does not accumulate in the gut vasculature, suggesting that these effects are independent of local parasite adhesion. P. chabaudi infection specifically promoted NTS bacterial dissemination from the intestinal tract, and co-infection led to an inhibited adaptive immune response to parasite. Both of these findings contribute to our understanding of malarial pathology in the presence of a varied gut microbiome, which also has been shown to contribute to parasite control.
Acknowledgements
We would like to thank Mark Griffin in the Department of Microbiology and Immunology Flow Cytometry and Cell Sorting Core Facility for his kind assistance. In addition, we appreciate the members of the Chopra lab for generous help.
Funding information
Funding was provided by UTMB Department of Internal Medicine, Division of Infectious Diseases, and the Saudi Arabian Cultural Mission, Jazan University.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards and protocols approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee.
Conflict of interest
The authors declare that they have no competing interests.
References
- Brenchley JM, Douek DC (2012) Microbial translocation across the GI tract. Annu Rev Immunol 30:149–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugat T, Cunningham D, Sodenkamp J, Coomes S, Wilson M, Spence PJ, Jarra W, Thompson J, Scudamore C, Langhorne J (2014) Sequestration and histopathology in Plasmodium chabaudi malaria are influenced by the immune response in an organ-specific manner. Cell Microbiol 16:687–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau JY, Tiffany CM, Nimishakavi S, Lawrence JA, Pakpour N, Mooney JP, Lokken KL, Caughey GH, Tsolis RM, Luckhart S (2013) Malaria-associated L-arginine deficiency induces mast cell-associated disruption to intestinal barrier defenses against nontyphoidal Salmonella bacteremia. Infect Immun 81:3515–3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Nyamako L, Olupot-Olupot P, Maitland K, Urban BC (2016) Increased adhesion of Plasmodium falciparum infected erythrocytes to ICAM-1 in children with acute intestinal injury. Malar J 15:54-016-1110-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J, Maitland K (2014) Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: a systematic review. BMC Med 12:31-7015-12-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO (2009) A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A 106:19256–19261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington AJ, Njie M, Correa S, Takem EN, Riley EM, Walther M (2012) Prolonged neutrophil dysfunction after Plasmodium falciparum malaria is related to hemolysis and heme oxygenase-1 induction. J Immunol 189:5336–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport GC, Hittner JB, Otieno V, Karim Z, Mukundan H, Fenimore PW, Hengartner NW, McMahon BH, Kempaiah P, Ong’echa JM, Perkins DJ (2016) Reduced Parasite Burden in Children with Falciparum Malaria and Bacteremia Coinfections: Role of Mediators of Inflammation. Mediators Inflamm 2016:4286576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguale T, Gebreyes WA, Asrat D, Alemayehu H, Gunn JS, Engidawork E (2015) Non-typhoidal Salmonella serotypes, antimicrobial resistance and co-infection with parasites among patients with diarrhea and other gastrointestinal complaints in Addis Ababa, Ethiopia. BMC Infect Dis 15:497-015-1235-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Xu Z, Wang GS, Ji FY, Mei CX, Liu J, Wu GM (2014) Directed evolution of an LBP/CD14 inhibitory peptide and its anti-endotoxin activity. PLoS One 9:e101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Wang L, Schoeb TR, Elson CO, Cong Y (2010) Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med 207:1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly Condette C, Khorsi-Cauet H, Morliere P, Zabijak L, Reygner J, Bach V, Gay-Queheillard J (2014) Increased gut permeability and bacterial translocation after chronic chlorpyrifos exposure in rats. PLoS One 9:e102217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E (2017) Dysbiosis and the immune system. Nat Rev Immunol 17:219–232 [DOI] [PubMed] [Google Scholar]
- Lokken KL, Stull-Lane AR, Poels K, and Tsolis RM, (2018) Malaria parasite-mediated alteration of macrophage function and increased iron availability predispose to disseminated non-typhoidal salmonella infection. Infect. Immun 9, e00301–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux ME, Looareesuwan S, Menzies IS, Grainger SL, Phillips RE, Wattanagoon Y, Thompson RP, Warrell DA (1989) Reduced hepatic blood flow and intestinal malabsorption in severe falciparum malaria. Am J Trop Med Hyg 40:470–476 [DOI] [PubMed] [Google Scholar]
- Mooney JP, Lokken KL, Byndloss MX, George MD, Velazquez EM, Faber F, Butler BP, Walker GT, Ali MM, Potts R, Tiffany C, Ahmer BM, Luckhart S, and Tsolis RM, (2015) Inflammation-associated alterations to the intestinal microbiota reduce colonization resistance against non-typhoidal salmonella during concurrent malaria parasite infection. Sci Rep 5, 14603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney JP, Lee SJ, Lokken KL, Nanton MR, Nuccio SP, McSorley SJ, Tsolis RM (2015) Transient Loss of Protection Afforded by a Live Attenuated Non-typhoidal Salmonella Vaccine in Mice Co-infected with Malaria. PLoS Negl Trop Dis 9:e0004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadjm B, Amos B, Mtove G, Ostermann J, Chonya S, Wangai H, Kimera J, Msuya W, Mtei F, Dekker D, Malahiyo R, Olomi R, Crump JA, Whitty CJ, Reyburn H (2010) WHO guidelines for antimicrobial treatment in children admitted to hospital in an area of intense Plasmodium falciparum transmission: prospective study. BMJ 340:c1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, Kaslow DC, Njuguna P, Marsh K, Bejon P (2016) Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N Engl J Med 374:2519–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux CM, Butler BP, Chau JY, Paixao TA, Cheung KW, Santos RL, Luckhart S, and Tsolis RM, (2010) Both hemolytic anemia and malaria parasite-specific factors increase susceptibility to nontyphoidal salmonella enterica serovar typhimurium infection in mice. Infect Immun 78: 1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence PJ, Langhorne J (2012) T cell control of malaria pathogenesis. Curr Opin Immunol 24:444–448 [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Miyauchi E, Nakamura S, Hirai M, Suzue K, Imai T, Nomura T, Handa T, Okada H, Shimokawa C, Onishi R, Olia A, Hirata J, Tomita H, Ohno H, Horii T, Hisaeda H (2015) Plasmodium berghei ANKA causes intestinal malaria associated with dysbiosis. Sci Rep 5:15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi C (2013) Translocation of gut flora and its role in sepsis. Indian J Med Microbiol 31:334–342 [DOI] [PubMed] [Google Scholar]
- Van Santen S, de Mast Q, Swinkels DW, van der Ven AJ (2013) The iron link between malaria and invasive non-typhoid Salmonella infections. Trends Parasitol 29:220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino NF, LeCleir GR, Denny JE, Dearth SP, Harding CL, Sloan SS, Gribble JL, Campagna SR, Wilhelm SW, Schmidt NW (2016) Composition of the gut microbiota modulates the severity of malaria. Proc Natl Acad Sci U S A 113:2235–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2017). World Malaria Report 2017 Switzerland, Geneva [Google Scholar]
- Wilairatana P, Meddings JB, Ho M, Vannaphan S, Looareesuwan S (1997) Increased gastrointestinal permeability in patients with Plasmodium falciparum malaria. Clin Infect Dis 24:430–435 [DOI] [PubMed] [Google Scholar]
- Wu W, Liu HP, Chen F, Liu H, Cao AT, Yao S, Sun M, Evans-Marin HL, Zhao Y, Zhao Q, Duck LW, Elson CO, Liu Z, Cong Y (2016) Commensal A4 bacteria inhibit intestinal Th2-cell responses through induction of dendritic cell TGF-beta production. Eur J Immunol 46:1162–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yooseph S, Kirkness EF, Tran TM, Harkins DM, Jones MB, Torralba MG, O’Connell E, Nutman TB, Doumbo S, Doumbo OK, Traore B, Crompton PD, Nelson KE (2015) Stool microbiota composition is associated with the prospective risk of Plasmodium falciparum infection. BMC Genomics 16:631-015-1819-3 [DOI] [PMC free article] [PubMed] [Google Scholar]