Abstract
1. Purpose of review:
Members of the Cullin family act as scaffolds in E3 ubiquitin ligases and play a central role in mediating protein degradation. Interactions with many different substrate-binding adaptors permit Cullin-containing E3 ligases to participate in diverse cellular functions. In the kidney, one well-established target of Cullin-mediated degradation is the transcription factor Nrf2, a key player in responses to oxidative stress. The goal of this review is to discuss more recent findings revealing broader roles for Cullins in the kidney.
2. Recent findings:
Cullin 3 acts as the scaffold in the E3 ligase regulating Nrf2 abundance, but was more recently shown to be mutated in the disease Familial Hyperkalemic Hypertension. Studies seeking to elucidate the molecular mechanisms by which Cullin 3 mutations lead to dysregulation of renal sodium transport will be discussed. Disruption of Cullin 3 in mice unexpectedly causes polyuria and fibrotic injury suggesting it has additional roles in the kidney. We will also review recent transcriptomic data suggest that other Cullins are also likely to play important roles in renal function.
3. Summary:
Cullins form a large and diverse family of E3 ubiquitin ligases that are likely to have many important functions in the kidney.
Keywords: ubiquitination, kidney fibrosis, renal carcinoma, Cullin
Introduction
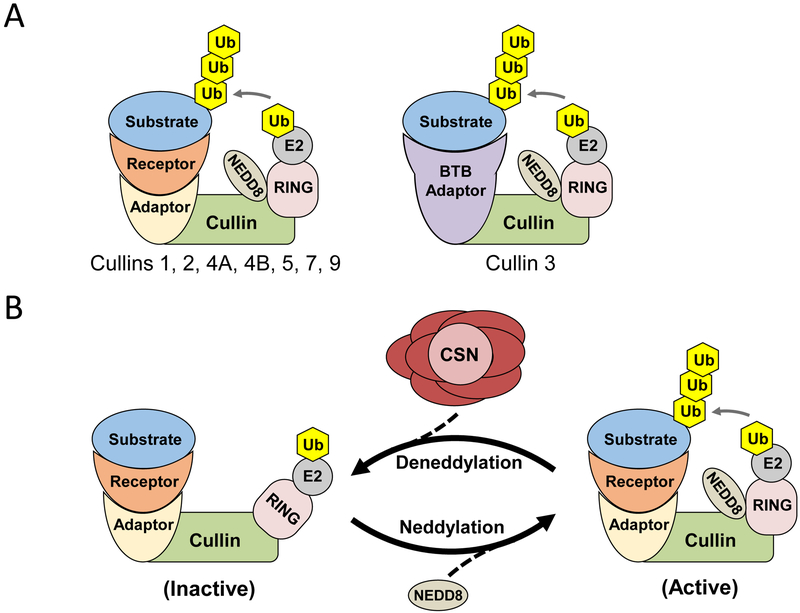
Members of the Cullin family of proteins act as scaffolds in complexes that mediate the covalent attachment of ubiquitin to proteins (ubiquitination), which targets the protein for proteasomal degradation. There are eight members of the Cullin family in humans (Cullins 1, 2, 3, 4A, 4B, 5, 7, and 9/PARC), each characterized by a carboxy-terminal Cullin homology domain. Together with a RING protein, the Cullins form Cullin-Ring Ligases (CRL), with the RING functioning as a docking site for ubiquitin-conjugating enzymes (E2s) (Figure 1A). CRLs also contain a substrate adaptor that binds to the more variable amino-terminal of the Cullin and to a substrate-specific receptor that determines the target. One notable exception is Cullin 3, which interacts with BTB (Broad complex, Tramtrack, Bric-a-brac) proteins, which serve as both adaptor and substrate receptor (Figure 1A). Many substrate-receptors have been identified, making the CRLs the largest family of E3 ubiquitin ligases, with over 400 members. The modular nature of CRL complexes is reflected by their diverse roles in proliferation, genomic stability, development, and signal transduction. CRL activity is stimulated by conformational changes induced by covalent attachment of the ubiquitin-like molecule Nedd8, a process called neddylation (Figure 1B). Nedd8 is removed from CRLs by the COP9 signalosome (CSN), a large 8 subunit protein complex. Importantly, cycling of neddylation and deneddylation is required to maintain CRL integrity. Very little is known about the roles of CRLs in the kidney, but interest has increased recently after recent studies suggesting that CRL activators may be protective during renal injury, and the identification of CRL mutations that cause hypertension in humans. Here, we will summarize recent findings regarding the roles of CRLs in normal renal physiology and in disease, and discuss evidence for additional roles for CRLs in the kidney.
Figure 1. Cullin Ring Ligase (CRL) components and regulation.
A) CRL ubiquitin E3 ligases consist of a Cullin, which acts as a protein scaffold, and a RING E2 ubiquitin ligase which covalently attaches ubiquitin (Ub) to a substrate, tagging it for proteasomal degradation. Most CRLs contain an adaptor protein that interacts with the Cullin and a substrate receptor (left). In Cullin 3-containing CRLs, the adaptor and receptor function is performed by a single protein, which usually contains a BTB domain (right). Neural precursor cell Expressed Developmentally Downregulated-8 NEDD8 is a small ubiquitin-like protein that regulates CRL activity. B) Covalent attachment of NEDD8 by one of two conjugating enzymes (UBC12 and UBE2F) results in activation of the CRL. NEDD8 is removed by the 8 subunit COP9 signalosome (CSN) complex, in which JAB1/CSN5 is the deneddylating enzyme, leading to CRL deactivation. Cycling of neddylation and deneddylation is essential for stable CRL activity. Note that some CRLs (e.g. those containing CUL1, CUL3 and CUL4) dimerize, which may be required for activity.
1). Cullin 3 dysfunction in Familial Hyperkalemic Hypertension
Cullin 3 (CUL3) is highly conserved and expressed in all human organs. Its disruption results in embryonic lethality [1, 2]. CUL3 is absent from the glomerulus, but is expressed along all tubule segments, with highest protein levels in proximal tubule [3]. Whole exome sequencing revealed that autosomal dominant mutations in Cul3 cause the most severe form of the disease Familial Hyperkalemic Hypertension (FHHt, also called pseudohypoaldosteronism type II) [4]. The pathophysiology of FHHt is primarily related to inappropriate activation of the NaCl cotransporter (NCC), expressed along the distal convoluted tubule (DCT), since NCC blockade with thiazide-like diuretics reverses the disease phenotype. FHHt-causing Cul3 mutations occur at splice sites and lead to excision of exon 9 from the mRNA, resulting in a mutant CUL3 protein lacking amino acids 403–459 (referred to here as CUL3-Δ9). Mutations in genes encoding With No-Lysine [K] (WNK) kinases, WNK1 and WNK4, or the CUL3 adaptor KLHL3 also cause FHHt [4, 5, 6], and the study of this rare disease has provided significant insight into the mechanisms by which the distal nephron regulates Na+ and K+ homeostasis. WNK4, the predominant active WNK expressed in DCT, phosphorylates and activates the downstream Ste20p-related Proline Alanine-rich Kinase (SPAK), which then directly phosphorylates the amino-terminus of NCC, leading to its activation (recently reviewed in [7] and see Figure 2). Under normal conditions the CUL3-KLHL3 CRL targets WNK4 for proteasomal degradation. In FHHt, mutant WNK4 is unable to interact with KLHL3 [8, 9], or mutant KLHL3 is unable to interact with WNK4 [8, 9] or with CUL3 depending on the precise mutation, ultimately increasing WNK4 stability. CUL3-Δ9 also increases WNK4 abundance but the precise mechanism remains controversial and will be discussed in detail here. Regardless of the gene mutated the net result is excessive phosphorylation and activation of NCC.
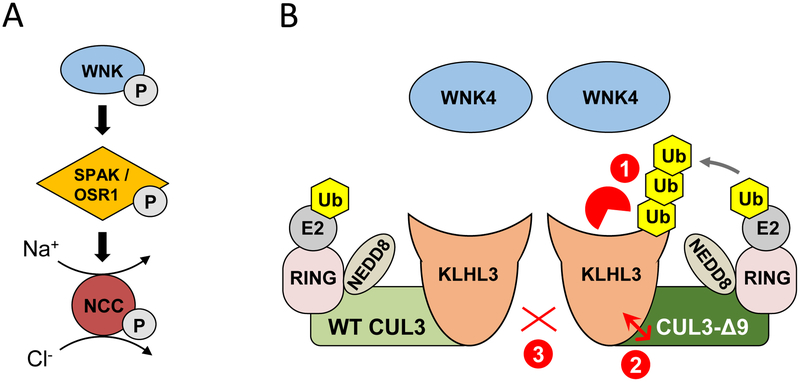
Figure 2. Normal and abnormal regulation of the renal Na+-Cl− cotransporter (NCC).
A) NCC is activated via phosphorylation by SPAK or the related kinase OSR1, which are in turn phosphorylated and activated by WNK kinases. B) Dimers of wild type (WT) CUL3-containing CRLs suppress NCC activation by mediating WNK4 degradation (not shown). Cul3 mutations cause the human disease Familial Hyperkalemic Hypertension (FHHt) due skipping of exon 9 during RNA splicing, leading to expression of CUL3-Δ9. Several mechanisms by which CUL3-Δ9 cause FHHt have been proposed. 1) CUL3-Δ9 inappropriately tags the WNK substrate adaptor KLHL3 for degradation; 2) CUL3-Δ9 has increased affinity for KLHL3, and sequesters it in non-functional CRLs; 3) WT and CUL3-Δ9 interact weakly. In each case, the loss of functional CRLs would result in accumulation of WNK4, and hyperactivation of NCC.
Several models for CUL3-Δ9-mediated FHHt have been proposed, all of which could result in decreased CRL activity (Figure 2). First, we demonstrated in vitro that CUL3-Δ9 is more neddylated than WT CUL3, and displays greater affinity for KLHL3 and other substrate adaptors than WT CUL3 [3]. Importantly, we demonstrated that CUL3-Δ9 inappropriately promotes KLHL3 degradation leading us to propose that this contributes to specific loss of the CRL along the DCT. In their studies of vascular effects of CUL3-Δ9, the Sigmund group demonstrated that CUL3-Δ9 and WT CUL3 form weak dimers, and CUL3-Δ9 strongly binds multiple adaptors with varying effects on adaptor degradation (none, increased, or decreased) [10]. Finally, the Kurz group reported that CUL3-Δ9 can trigger its own degradation, confirmed by the extremely low levels of CUL3-Δ9 they detected in a knock-in mouse model with Cul3 exon 9 deletion [11]. These mice displayed the FHHt phenotype, with elevated WNK4 abundance, leading to the hypothesis that FHHt arises from CUL3 haploinsufficiency. However, we reported that mice heterozygous for CUL3 do not phenocopy FHHt, while heterozygotes expressing CUL3-Δ9 from a transgene do [•12, 13]. We and the Sigmund group confirmed the finding that CUL3-Δ9 abundance is extremely low in vivo [•12, 14], supporting the idea that it induces its own degradation and suggesting it must exert additional effects in vivo to cause FHHt. While the Kurz group did not detect lower KLHL3 abundance in their FHHt mouse model, the Uchida group reported >70% lower KLHL3 abundance in an independently-generated model [•15]. Recent in vitro work has provided new insight into the actions of CUL3-Δ9. When WT and CUL3-Δ9 were overexpressed together in HEK293 cells, KLHL3 abundance was low and WNK4 abundance was high [16], similar to the Uchida group’s observation in CUL3-Δ9 knock-in mice. Increasing the amount of transfected KLHL3 to normalize KLHL3 abundance caused degradation of WNK4. However, this was not observed when only CUL3-Δ9 was expressed. These data suggest that 1) CUL3-Δ9 is unable to form a functional CRL with KLHL3 and 2) CUL3-Δ9-mediated KLHL3 degradation prevents WT CUL3 from degrading WNK4, consistent with the FHHt phenotype of KLHL3 KO mice [17]. Further studies are required to fully resolve the mechanism by which CUL3-Δ9 increases abundance of WNK4, and determine why CUL3-Δ9 disruption of the CRL causes a phenotype that in the kidney is primarily limited to the DCT.
2). The CSN in normal and abnormal NCC regulation
Deneddylation of CRLs through interactions with the CSN is essential to maintain normal CRL function [18]. The Kurz group reported that CUL3-Δ9 had decreased binding to CSN subunits CSN5/JAB1 and CSN8, contributing to its hyperneddylation [11]. It was more recently shown that the CSN binds to the α/β1 domain of CUL3 which is adjacent (C-terminal) to the 4HB domain deleted in the CUL3-Δ9 mutation [16], suggesting that the mutation alters protein folding. Loss of CSN function can prevent the degradation of CRL substrates. In many cases this loss of function was caused by anomalous activity of the CRL toward substrate adaptors [19, 20, 21, 22]. The CSN was recently disrupted in vivo using a kidney-specific inducible mouse model targeting the key catalytic subunit JAB1 [•23]. KLHL3 abundance was lower but Keap1 abundance was not, indicating selective degradation. Consistent with a selective effect on KLHL3, the WNK-SPAK-NCC pathway was activated, but the phenotype did not resemble FHHt due to effects on other segments, including injury and polyuria (see below). NCC activation typically leads to increased NCC abundance [11, •12, •15, 24] but JAB1 disruption unexpectedly resulted in dramatically lower abundance [•23], suggesting JAB1 plays a direct role in modulating NCC levels.
3). CUL3 and renal injury
The CUL3-Keap1-Nrf2 pathway, which regulates oxidative stress responses, is the most extensively studied CRL in kidney. The CUL3-Keap1 CRL constitutively degrades Nrf2 under basal conditions. Accumulation of reactive oxygen species interrupts the interaction between Keap1 and Nrf2 preventing its degradation [25], though CUL3-mediated degradation of Keap1 may also play a role [26]. Nrf2 is a transcription factor that induces multiple anti-oxidant and anti-inflammatory genes. The generation of reactive oxygen species is a key mechanism in acute kidney injury and its progression to chronic kidney disease (CKD) [27]. Several animal and clinical studies have sought to exploit the protective effects of Nrf2 to develop new treatments for CKD patients. Recent animal work showed that Nrf2 activation with dimethyl fumarate reduced renal injury from nephrolithiasis by preventing inflammation and oxidative stress [28]. The most extensive clinical study was performed in 2011 (BEACON) using bardoxolone, which modifies Keap1and thus prevents Keap1-Nrf2 interaction, in patients with Type II Diabetes Mellitus and stage 4 CKD [29]. However, the trial was terminated due to an increased hospitalization rate from cardiovascular events. More recent post-hoc analyses of BEACON have showed that bardoxolone caused significant weight loss [30] and increased eGFR [31]. More recent trials have examined the benefits of bardoxolone methyl in diabetic kidney disease, Alport syndrome and polycystic kidney disease. A comprehensive review of Nrf2 activators in the treatment for disease focusing on the kidney, including animal and clinical experiments can be found in [32].
While Nrf2 activation is considered beneficial, prolonged activation can be damaging, causing hepatic fibrosis [33], and promoting tumor cell growth and chemoresistance in multiple cancers [34]. Recently, progressive kidney injury was observed in two mouse models with dysregulated CRL activity, with both displaying accumulation of substrates including Nrf2. CUL3 knockout mice developed proximal tubule injury that progressed to renal fibrosis and eventually mouse death [3, •35]. In contrast to what was observed following renal ischemia-reperfusion injury, CUL3 disruption caused DNA damage and cell proliferation prior to induction of KIM-1, an early marker of renal injury [•35]. This suggests these effects may induce the injury, possibly via an effect on the CUL3 substrate cyclin E. It was also shown that high grade fibrosis in humans and injury in several mouse models was associated with lower CUL3 abundance. Kidney-specific disruption of the CRL regulator the COP9 signalosome also caused progressive renal injury leading to CKD presumably from CRL substrate accumulation [•23]. However, the renal injury in these models is unlikely to arise primarily from Nrf2 activation since kidney-specific Keap1 deletion in mice, which would lead to NRF2 accumulation, caused hydronephrosis but not CKD [36]. Other harmful effects of Keap1 inhibition include exacerbation of CKD progression, as observed after administration of a bardoxolone analogue to rats following 5/6 nephrectomy, [37] and increased kidney weight in a mouse model of Type 2 diabetes with incomplete global deletion of Keap1 [38]. Thus, exclusive Nrf2 activation can be damaging to the kidney but other factors, such as cell cycle dysregulation, must be present to cause CKD.
4). Cullins and renal cell carcinoma
Since CRLs mediate degradation of many oncogenes and tumor suppressors, CRL dysregulation has been associated with the development of tumors [39]. In contrast, inhibition of CRL neddylation (and hence activation) to lower degradation of tumor suppressors has been explored as a therapeutic approach in several cancers. The neddylation inhibitor MLN4924 has been tested in several phase I trials, including acute myeloid leukemia [40] and metastatic melanoma [41]. One limitation of this approach is that MLN4924 prevents neddylation of all eight Cullins, increasing the possibility of side effects. Indeed, there is also strong evidence that loss of CRL function plays a role in tumorigenesis in the kidney [42]. Renal cell carcinoma (RCC) accounts for 3.8% of all adult tumors in the United States with an estimated 65,340 new cases in 2018 [43]. In the inherited Von Hippel Lindau (VHL) syndrome, mutations in the Cullin 2 substrate receptor VHL cause clear cell RCC (ccRCC) [44]. VHL mutations destabilize it, leading to impaired proteasomal degradation of the transcription factor Hypoxia-Induced Factor-1∝ (HIF-1∝). This leads to inappropriate activation of glycolytic and angiogenic pathways, and results in highly vascularized tumors. It was recently proposed that stabilization of mutant VHL by supplementation with arginine, which promotes refolding of the mutant protein, may serve as a therapy [45]. Studies of newly generated mouse models of ccRCC may yield further insights into pathways regulated by VHL in the kidney [••46, 47]. The focus of CUL2-VHL research has been on RCC, and the roles of this complex in mediating physiological renal responses to hypoxia remain to be determined.
Dysfunction of CUL3-containing CRLs has also been implicated in renal cell carcinoma. Several studies have reported an association of loss-of-function mutations in CUL3 and Keap1, which result in Nrf2 activation, with papillary renal cell carcinoma [48]. Lower mRNA abundance of CUL3 and the substrate adaptors RhoBTB1 and RhoBTB3 has also been reported in renal cell carcinoma [49]. Upregulation of several CSN components including JAB1 was observed in ccRCC [42] raising the possibility that other CRLs are dysregulated in RCC. A recent study by Schleifer and colleagues using RNAseq data from The Cancer Genome Atlas revealed that expression of KLHL family of CUL3 substrate adaptors is especially altered (higher or lower depending on the specific KLHL) compared with normal tissue in several renal carcinomas [•50]. While KLHL5 was not dramatically altered, cell culture experiments showed that knockdown of KLHL5 expression increased sensitivity to several anticancer compounds, especially those blocking PI3 kinase signaling. These data raise the possibility that KLHL5 modulates PI3 kinase signaling in the normal kidney, and the general dysregulation of KLHLs in renal cancer also point to broader roles for CUL3 in normal renal physiology.
5). Other roles for Cullin 3 CRLs in the kidney
While the study of FHHt has revealed that the CRL containing CUL3-KLHL3 is a key regulator of NCC in disease, there is also evidence that the pathway is regulated in normal physiology. The Lifton group reported that KLHL3 can be phosphorylated at serine 433 via protein kinase C, and that this could be induced by angiotensin II [51]. Phosphorylation at serine 433 prevented interactions with WNK4, providing a mechanism by which NCC could be activated by angiotensin II to promote Na+ retention. The phosphatase calcineurin was shown to play a role in dephosphorylation of KLHL3 at serine 433 [••52], which may be relevant to inactivation of NCC in states of hyperkalemia [53, 54, 55]. This pathway may also be relevant in the development of salt-sensitive hypertension in Type II diabetes since PKC-dependent serine 433 phosphorylation was elevated in diabetic mice, leading to NCC activation [••56]. Inhibition of the sodium glucose cotransporter 2 (SGLT2) lowered KLHL3 phosphorylation.
In addition to leading to hyperactivation of NCC and renal injury (fibrosis and hydronephrosis), disruption of CUL3 pathways in mice has revealed additional unexpected roles. Both inducible kidney-specific (KS)-CUL3 [3] and KS-JAB1 [•23] KO mice displayed severe polyuria, with a 5-fold and 2-fold increase in urine output respectively. In both cases this was associated with a dramatic reduction in abundance of the vasopressin-regulated water channel Aquaporin 2. The precise molecular mechanisms involved remain to be determined. The CUL3 adaptor KLHL2 has been shown to mediate WNK degradation in vitro [57]. In mice, KLHL2 is expressed exclusively in the renal medulla [58], and KLHL2 knockout mice display greater abundance of medullary WNK4 than wild type mice. These mice did not display electrolyte abnormalities, but whether activation of the Na+-K+-2Cl− cotransporter 2 (NKCC2), which is activated via WNK4 [59], or blood pressure were increased was not determined. CUL3-KLHL3 adaptors may also regulate K+ secretion along the CNT/CCD more directly since WT KLHL3 lowered ROMK plasma membrane abundance in COS-7 cells [9]. Importantly, FHHt-causing mutant KLHL3 did not have this effect. The O’Shaughnessy group further reported that WT KLHL3 or CUL3 could inhibit WNK4-mediated ROMK internalization in Xenopus laevis oocytes, but FHHt-causing mutants could not [60]. These data suggest a loss of ROMK internalization may contribute to hyperkalemia in FHHt, but the O’Shaughnessy group did not observe lower surface abundance in their CUL3-Δ9 knock-in mouse model [60].
6). Transcriptomics suggest additional roles for Cullins in the kidney
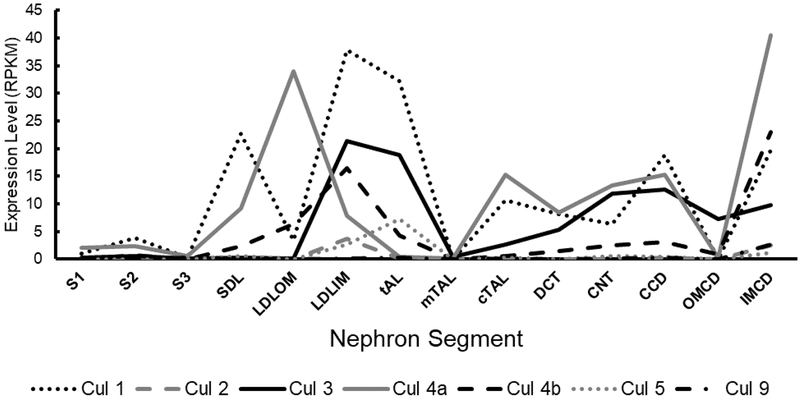
Recent advances in genomics technology have allowed precise molecular profiling of Cullin expression along the nephron [61]. Beyond the CUL3-Keap1-Nrf2 axis, the study of CUL3 has primarily focused on the DCT, but it is important to note that CUL3 is expressed in additional segments of the nephron (Figure 3). Relatively speaking, CUL3 is most highly expressed in the loop of henle and thick ascending limb. Moreover, CUL3 is not the most abundantly expressed Cullin along the nephron. Several other members of the Cullin family are expressed in different segments, with the highest expression belonging to CUL1. Further examination of the segment-specific expression pattern of CUL3 and JAB1, which show different overlap in expression in the DCT but less overlap in other segments may offer insight into why CSN disruption causes a similar, but not identical phenotype to CUL3 disruption [3, •23]. Additionally, single-cell and single-nuclear RNA sequencing may help to further refine the expression pattern in epithelial sub-types in the future [62, ••63].
Figure 3. Segment-specific expression of cullins in the kidney.
Transcriptomics on microdissected rat nephron segments was performed by the Knepper lab [61]. Expression is shown as the normalized value of Reads Per Kilobase of transcript, per Million mapped reads (RPKM). S1, first segment of the proximal tubule; S2, second segment of the proximal tubule; S3, third segment of the proximal tubule; SDL, short descending limb of the loop of Henle; LDLOM, long descending limb of the loop of Henle in the outer medulla; LDLIM, long descending limb of the loop of Henle in the inner medulla; tAL, thin ascending limb of the loop of Henle; mTAL, medullary thick ascending limb of the loop of Henle; cTAL, cortical thick ascending limb of the loop of Henle; DCT, distal convoluted tubule; CNT, connecting tubule; CCD, cortical collecting duct; OMCD, outer medullary collecting duct; IMCD, inner medullary collecting duct.
Conclusion
The discovery that the CUL3-KLHL3 CRL plays a central role in regulating activity of NCC has expanded interest in the roles of CRLs in the kidney beyond the CUL3-Keap1-Nrf2 pathway. Recent discoveries showing that KLHL3 can be phosphorylated in physiologically relevant contexts in the kidney further emphasizes the importance of this CRL. Progress has been made in determining the mechanisms underlying FHHt caused by CUL3-Δ9, but further in vivo studies are needed. CRL disruption has revealed roles in renal fibrosis and urine concentrating mechanisms, but the precise molecular mechanisms remain to be determined. Transcriptomics has revealed that several cullins are abundantly expressed along the nephron, suggesting additional unidentified roles for CRLs in the kidney.
Key Points.
Transcriptomics has revealed expression of multiple Cullins along the nephron indicating they are likely to play many important roles in renal function.
Significant progress in determining the mechanisms by which mutations in Cullin 3 (CUL3) cause the disease Familial Hyperkalemic Hypertension, with effects on the substrate adaptor KLHL3 likely to be central.
The CUL3-Keap1-Nrf2 antioxidative pathway has been extensively studied in kidney disease, but more recent data suggest CUL3 dysregulation may cause renal injury through effects on the cell cycle.
KLHL substrate adaptors are more dramatically dysregulated in renal cancer than in other cancers, revealing their potential as therapeutic targets.
Several animal models suggest roles for CUL3 in Thick Ascending Limb and Collecting Duct, and together with KLHL3 it may also play a role in mediating the effects of calcineurin, and in promoting salt retention in diabetes.
Acknowledgments
Financial support and sponsorship: Funding was provided by NIH grants F32DK112531 to RJC and R01DK098141 to JAM, and American Heart Association Postdoctoral Fellowship 17POST33670206 to MZF.
This work was supported by funding from the National Institutes of Health (NIH) and American Heart Association
Footnotes
Conflicts of interest: none.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Singer JD, Gurian-West M, Clurman B, Roberts JM. Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev 1999; 13:2375–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEvoy JD, Kossatz U, Malek N, Singer JD. Constitutive turnover of cyclin E by Cul3 maintains quiescence. Mol Cell Biol 2007; 27:3651–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormick JA, Yang CL, Zhang C, et al. Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest 2014; 124:4723–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyden LM, Choi M, Choate KA, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 2012; 482:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson FH, Disse-Nicodeme S, Choate KA, et al. Human hypertension caused by mutations in WNK kinases. Science 2001; 293:1107–1112. [DOI] [PubMed] [Google Scholar]
- 6.Louis-Dit-Picard H, Barc J, Trujillano D, et al. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 2012; 44:456–460, S451-453. [DOI] [PubMed] [Google Scholar]
- 7.Hadchouel J, Ellison DH, Gamba G. Regulation of Renal Electrolyte Transport by WNK and SPAK-OSR1 Kinases. Annu Rev Physiol 2016; 78:367–389. [DOI] [PubMed] [Google Scholar]
- 8.Wakabayashi M, Mori T, Isobe K, et al. Impaired KLHL3-mediated ubiquitination of WNK4 causes human hypertension. Cell Rep 2013; 3:858–868. [DOI] [PubMed] [Google Scholar]
- 9.Shibata S, Zhang J, Puthumana J, et al. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proc Natl Acad Sci U S A 2013; 110:7838–7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibeawuchi SR, Agbor LN, Quelle FW, Sigmund CD. Hypertension-causing Mutations in Cullin3 Protein Impair RhoA Protein Ubiquitination and Augment the Association with Substrate Adaptors. J Biol Chem 2015; 290:19208–19217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schumacher FR, Siew K, Zhang J, et al. Characterisation of the Cullin-3 mutation that causes a severe form of familial hypertension and hyperkalaemia. EMBO Mol Med 2015; 7:1285–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •12.Ferdaus MZ, Miller LN, Agbor LN, et al. Mutant Cullin 3 causes familial hyperkalemic hypertension via dominant effects. JCI Insight 2017; 2(24). pii: 96700. doi: 10.1172/jci.insight.96700.This study showed that Cul3 mutations do no cause FHHt via haploinsufficency. Mice heterozygous for CUL3 did not display an FHHt phenotpye, but heterozygous mice expressing mutant CUL3 from a transgene did.
- 13.Ferdaus MZ, McCormick JA. Mechanisms and controversies in mutant Cul3-mediated familial hyperkalemic hypertension. Am J Physiol Renal Physiol 2018; 314:F915–F920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agbor LN, Ibeawuchi SC, Hu C, et al. Cullin-3 mutation causes arterial stiffness and hypertension through a vascular smooth muscle mechanism. JCI Insight 2016; 1:e91015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •15.Yoshida S, Araki Y, Mori T, et al. Decreased KLHL3 expression is involved in the pathogenesis of pseudohypoaldosteronism type II caused by cullin 3 mutation in vivo. Clin Exp Nephrol 2018; 22:1251–1257.This study provided in vivo evidence that the FHHt-causing CUL3 mutant promotes degradation of the substrate adaptor KLHL3.
- 16.Cornelius RJ, Zhang C, Erspamer KJ, et al. Dual gain and loss of cullin 3 function mediates familial hyperkalemic hypertension. Am J Physiol Renal Physiol 2018; 315:F1006–F1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki E, Susa K, Mori T, et al. KLHL3 Knockout Mice Reveal the Physiological Role of KLHL3 and the Pathophysiology of Pseudohypoaldosteronism Type II Caused by Mutant KLHL3. Mol Cell Biol 2017; 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubiel D, Rockel B, Naumann M, Dubiel W. Diversity of COP9 signalosome structures and functional consequences. FEBS Lett 2015; 589:2507–2513. [DOI] [PubMed] [Google Scholar]
- 19.Denti S, Fernandez-Sanchez ME, Rogge L, Bianchi E. The COP9 signalosome regulates Skp2 levels and proliferation of human cells. J Biol Chem 2006; 281:32188–32196. [DOI] [PubMed] [Google Scholar]
- 20.He Q, Cheng P, He Q, Liu Y. The COP9 signalosome regulates the Neurospora circadian clock by controlling the stability of the SCFFWD-1 complex. Genes Dev 2005; 19:1518–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wee S, Geyer RK, Toda T, Wolf DA. CSN facilitates Cullin-RING ubiquitin ligase function by counteracting autocatalytic adapter instability. Nat Cell Biol 2005; 7:387–391. [DOI] [PubMed] [Google Scholar]
- 22.Cope GA, Deshaies RJ. Targeted silencing of Jab1/Csn5 in human cells downregulates SCF activity through reduction of F-box protein levels. BMC Biochem 2006; 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •23.Cornelius RJ, Si J, Cuevas CA, et al. Renal COP9 Signalosome Deficiency Alters CUL3-KLHL3-WNK Signaling Pathway. J Am Soc Nephrol 2018; 29:2627–2640.This study described the phenotype of mice lacking JAB1, the catalytic subunit of the COP9 signalosome. The data suggest that FHHt-causing CUL3 mutations disrupt interactions with the deneddylating COP9 signalsosome.
- 24.Rosenbaek LL, Kortenoeven ML, Aroankins TS, Fenton RA. Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter NCC and subsequent clathrin-mediated endocytosis. J Biol Chem 2014; 289:13347–13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A 2002; 99:11908–11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang DD, Lo SC, Sun Z, et al. Ubiquitination of Keap1, a BTB-Kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathway. J Biol Chem 2005; 280:30091–30099. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol 2009; 297:F461–470. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Wang Q, Li C, et al. Inhibiting inflammation and modulating oxidative stress in oxalate-induced nephrolithiasis with the Nrf2 activator dimethyl fumarate. Free Radic Biol Med 2018; 134:9–22. [DOI] [PubMed] [Google Scholar]
- 29.de Zeeuw D, Akizawa T, Audhya P, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 2013; 369:2492–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chertow GM, Appel GB, Block GA, et al. Effects of bardoxolone methyl on body weight, waist circumference and glycemic control in obese patients with type 2 diabetes mellitus and stage 4 chronic kidney disease. J Diabetes Complications 2018; 32:1113–1117. [DOI] [PubMed] [Google Scholar]
- 31.Chin MP, Bakris GL, Block GA, et al. Bardoxolone Methyl Improves Kidney Function in Patients with Chronic Kidney Disease Stage 4 and Type 2 Diabetes: Post-Hoc Analyses from Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Study. Am J Nephrol 2018; 47:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamawaki K, Kanda H, Shimazaki R. Nrf2 activator for the treatment of kidney diseases. Toxicol Appl Pharmacol 2018; 360:30–37. [DOI] [PubMed] [Google Scholar]
- 33.Ni HM, Woolbright BL, Williams J, et al. Nrf2 promotes the development of fibrosis and tumorigenesis in mice with defective hepatic autophagy. J Hepatol 2014; 61:617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018; 34:21–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •35.Saritas T, Cuevas CA, Ferdaus MZ, et al. Disruption of CUL3-mediated ubiquitination causes proximal tubule injury and kidney fibrosis. Sci Rep 2019; 9:4596.This study showed that inducible CUL3 disruption in mice results in a progressive renal injury that may be driven by dysregulateion of the cell cycle. It also provides evidence that CUL3 dysregulation may contribute to fibrosis in other mouse models, and in humans.
- 36.Noel S, Arend LJ, Bandapalle S, et al. Kidney epithelium specific deletion of kelch-like ECH-associated protein 1 (Keap1) causes hydronephrosis in mice. BMC Nephrol 2016; 17:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaziri ND, Liu S, Farzaneh SH, et al. Dose-dependent deleterious and salutary actions of the Nrf2 inducer dh404 in chronic kidney disease. Free Radic Biol Med 2015; 86:374–381. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Kulkarni SR, Donepudi AC, et al. Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes 2012; 61:3208–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gummlich L, Rabien A, Jung K, Dubiel W. Deregulation of the COP9 signalosome-cullin-RING ubiquitin-ligase pathway: mechanisms and roles in urological cancers. Int J Biochem Cell Biol 2013; 45:1327–1337. [DOI] [PubMed] [Google Scholar]
- 40.Swords RT, Watts J, Erba HP, et al. Expanded safety analysis of pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukemia and myelodysplastic syndromes. Blood Cancer J 2017; 7:e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatia S, Pavlick AC, Boasberg P, et al. A phase I study of the investigational NEDD8-activating enzyme inhibitor pevonedistat (TAK-924/MLN4924) in patients with metastatic melanoma. Invest New Drugs 2016; 34:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gummlich L, Kahne T, Naumann M, et al. New Insights Into the Mechanism of COP9 Signalosome-Cullin-RING Ubiquitin-Ligase Pathway Deregulation in Urological Cancers. Int Rev Cell Mol Biol 2016; 323:181–229. [DOI] [PubMed] [Google Scholar]
- 43.Cancer Stat Facts: Kidney and Renal Pelvis Cancer. NIH - NATIONAL CANCER INSTITUTE Surveillance, Epidemiology, and End Results Program: https://seer.cancer.gov/statfacts/html/kidrp.html
- 44.Clark PE. The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy. Kidney Int 2009; 76:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shmueli MD, Levy-Kanfo L, Haj E, et al. Arginine refolds, stabilizes, and restores function of mutant pVHL proteins in animal model of the VHL cancer syndrome. Oncogene 2019; 38:1038–1049. [DOI] [PubMed] [Google Scholar]
- ••46.Harlander S, Schonenberger D, Toussaint NC, et al. Combined mutation in Vhl, Trp53 and Rb1 causes clear cell renal cell carcinoma in mice. Nat Med 2017; 23:869–877.This study reported a new model of ccRCC involving deletion of VHL, the CUL2 substrate receptor. This model may provide a new tool to uncover new therapeutic strategies for a subset of human ccRCCs.
- 47.Hou W, Ji Z. Generation of autochthonous mouse models of clear cell renal cell carcinoma: mouse models of renal cell carcinoma. Exp Mol Med 2018; 50:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cancer Genome Atlas Research N, Linehan WM, Spellman PT, et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med 2016; 374:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berthold J, Schenkova K, Ramos S, et al. Characterization of RhoBTB-dependent Cul3 ubiquitin ligase complexes--evidence for an autoregulatory mechanism. Exp Cell Res 2008; 314:3453–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •50.Schleifer RJ, Li S, Nechtman W, et al. KLHL5 knockdown increases cellular sensitivity to anticancer drugs. Oncotarget 2018; 9:37429–37438.This study showed that multiple CUL3 substrate adaptors (KLHLs) are dysregulated in several forms of cancer, but most dramatically in renal carcinoma. It also raises the possibility that KLHL5 may play a role in modulating signaling through the PI3 Kinase pathway.
- 51.Shibata S, Arroyo JP, Castaneda-Bueno M, et al. Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proc Natl Acad Sci U S A 2014; 111:15556–15561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••52.Ishizawa K, Wang Q, Li J, et al. Calcineurin dephosphorylates Kelch-like 3, reversing phosphorylation by angiotensin II and regulating renal electrolyte handling. Proc Natl Acad Sci U S A 2019; 116:3155–3160.This study provided further evidence that phosphorylation of the CUL3 adaptor KLHL3 plays a central role in mediating degradation of WNK kinases and hence activation of the NaCl cotransporter NCC. It provides a possible mechanism by which the immunosuppressant tacrolimus can mimic FHHt as an unwanted side effect.
- 53.Shoda W, Nomura N, Ando F, et al. Calcineurin inhibitors block sodium-chloride cotransporter dephosphorylation in response to high potassium intake. Kidney Int 2017; 91:402–411. [DOI] [PubMed] [Google Scholar]
- 54.Terker AS, Zhang C, Erspamer KJ, et al. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 2016; 89:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terker AS, Zhang C, McCormick JA, et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 2015; 21:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••56.Ishizawa K, Wang Q, Li J, et al. Inhibition of Sodium Glucose Cotransporter 2 Attenuates the Dysregulation of Kelch-Like 3 and NaCl Cotransporter in Obese Diabetic Mice. J Am Soc Nephrol 2019.This study showed that dysregulation of KLHL3 phosphorylation may contribute to salt-sensitive hypertension in Type II diabetes.
- 57.Takahashi D, Mori T, Wakabayashi M, et al. KLHL2 interacts with and ubiquitinates WNK kinases. Biochem Biophys Res Commun 2013; 437:457–462. [DOI] [PubMed] [Google Scholar]
- 58.Kasagi Y, Takahashi D, Aida T, et al. Impaired degradation of medullary WNK4 in the kidneys of KLHL2 knockout mice. Biochem Biophys Res Commun 2017; 487:368–374. [DOI] [PubMed] [Google Scholar]
- 59.Terker AS, Castaneda-Bueno M, Ferdaus MZ, et al. With no lysine kinase 4 modulates sodium potassium 2 chloride cotransporter activity in vivo. Am J Physiol Renal Physiol 2018; 315:F781–F790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murthy M, Kurz T, O’Shaugnessy KM. ROMK expression remains unaltered in a mouse model of familial hyperkalemic hypertension caused by the CUL3Δ403-459 mutation. Physiol Rep 2016; 4: pii: e12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JW, Chou CL, Knepper MA. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J Am Soc Nephrol 2015; 26:2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu H, Kirita Y, Donnelly EL, Humphreys BD. Advantages of Single-Nucleus over Single-Cell RNA Sequencing of Adult Kidney: Rare Cell Types and Novel Cell States Revealed in Fibrosis. J Am Soc Nephrol 2019; 30:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••63.Park J, Shrestha R, Qiu C, et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 2018; 360:758–763.This study reported a single-cell RNA seq dataset from mouse kidney, and described novel transitional cell types in the distal nephron. This technique that is becoming more widely used in the study of normal and abnormal renal function in both animals and humans.