Abstract
PURPOSE:
Patients undergoing breast augmentation are treated with multiple combinations of medications for pain control including ketorolac, liposomal bupivacaine, bupivacaine, and intravenous and oral narcotics. There is no current consensus on the optimal combination; therefore all are used at the discretion of the surgeon.
METHODS:
This was a single-center, retrospective study. Total number of patients included was 132. Comparisons were made between four groups: bupivacaine only (B); bupivacaine and liposomal bupivacaine (BL); bupivacaine and liposomal bupivacaine plus intraoperative ketorolac (BLKi); bupivacaine and liposomal bupivacaine plus post-operative ketorolac (BLKp). Average pain scores immediately postoperative and prior to discharge were recorded and correlated to percentage of patients who received narcotic in the PACU. Additional end points noted were side effects including nausea and time spent in PACU postoperatively.
RESULTS:
Those receiving intraoperative ketorolac had the lowest pain on discharge (p <0.0001) and the lowest percentage of patients receiving narcotics (p = 0.009) out of all four groups. There was no significant difference between the four groups in terms of time spent in PACU, pain immediately after the procedure, or amount of antiemetic given. No bleeding complications were noted for those who did or did not receive ketorolac.
CONCLUSION:
When given options for pain control in breast augmentation, intraoperative ketorolac should be considered, as its inclusion was significant in decreasing use of narcotics and pain upon discharge. Addition of other costly drugs such as liposomal bupivacaine may not provide additional benefit in the immediate postoperative setting for procedures with a short recovery period such as breast augmentation.
INTRODUCTION
Over the past four decades there has been an increasing number of outpatient procedures due to advances in anesthetic and surgical techniques and escalating healthcare costs. Improvement in anesthesia enabled patients to regain consciousness more quickly with fewer after effects and better analgesics for relief of pain.1 Discharge of patients undergoing outpatient procedures often depends on recovery from anesthesia. Factors that will delay discharge include nausea, vomiting, and unrelieved pain. There are significant consequences of inadequate perioperative pain that include a delay in recovery, a delayed return to normal daily living, and reduced patient satisfaction2. Traditional systemic methods of pain control include opioids; however, the adverse outcomes associated with opioids continue to be a major problem in outpatient anesthesia. Potential side effects include altered mental status, narcotic dependence, nausea, emesis, impairment of bowel function, constipation, and respiratory depression.3 Multimodal analgesics, where local and intravenous analgesics are combined, is a preferred over monotherapies due to lower pain scores, reduced opioid usage and opioid-related adverse effects.
Aim of this study:
Breast augmentation was the most common cosmetic surgical procedure (286,000) as per American Society of Plastic surgeon 2014 Plastic Surgery statistics Report. Our goal is to provide adequate postoperative analgesia after outpatient breast augmentation while meeting the criteria for discharge. We will evaluate the efficacy of multimodal therapy comparing four different techniques: bupivacaine only (B); bupivacaine and liposomal bupivacaine (BL); bupivacaine and liposomal bupivacaine plus intraoperative ketorolac (BLKi); bupivacaine and liposomal bupivacaine plus post-operative ketorolac (BLKp). We hypothesize that intraoperative use of ketorolac will produce prolonged analgesia, decrease postoperative supplemental narcotic use, and facilitate discharge from PACU. We also hypothesize that intraoperative use of liposomal bupivacaine will produce prolonged analgesia in PACU.
Materials and methods
Study population
This was a single-center, retrospective study that analyzed patients that underwent elective outpatient surgery for breast augmentation. All patients were female and 18 years or older. Patients that underwent surgery for this procedure over a seven-year period were included (2009– 2015). Those who did not receive a combination of bupivacaine and liposomal bupivacaine with or without ketorolac were excluded.
Two surgeons performed all operations with similar techniques. Data was collected via a database located at the Outpatient surgery center where the procedures were performed. This database held all scanned medical records pertaining to the perioperative care of the patient. The study was approved by the Biomedical Institutional Review Board of Tulane University New Orleans with waiver of consent.
Data collection
For the purpose of this study, information was collected regarding type of analgesia used intravenously or intramuscularly by anesthesia and subcutaneously by the surgeon intraoperatively. This data was combined with the medications given intravenously and by mouth in the post anesthesia care unit (PACU). Timing of the narcotics given post operatively was also analyzed.
Comparisons were made between four groups: bupivacaine only (B); bupivacaine and liposomal bupivacaine (BL); bupivacaine and liposomal bupivacaine plus intraoperative ketorolac (BLKi); bupivacaine and liposomal bupivacaine plus post-operative ketorolac (BLKp). Outcomes of interest were number of patients that required narcotics in the PACU, amount of narcotic received post operatively, amount of antiemetic required post operatively, pain scores upon arrival to PACU, pain scores upon discharge, time in PACU, and complications. The generic unidimensional numeric rating scale was used with “no pain” represented by a score of 0 and “worst imaginable pain” represented by a score of 10. Patient age, BMI, and weight were also assessed. All complications after surgery were recorded to determine rate of hematoma formation within each set of patients.
Initial query of the database of all patients undergoing breast augmentation during appropriate time period identified 132 patients. Division based on combination of analgesic resulted in the following: bupivacaine only (N=59); bupivacaine and liposomal bupivacaine (N=26); bupivacaine and liposomal bupivacaine plus intraoperative ketorolac (N=34); liposomal bupivacaine and bupivacaine plus post-operative ketorolac (N=13). Patients were excluded if they had a subglandular augmentation. All remaining patients included in the study received a submuscular augmentation.
Technique
Primary breast augmentation was performed in all patients in the subpectoral plane. Patients were first seen and marked preoperatively. After appropriate induction of anesthesia, skin prepping, and sterile draping the operation was initiated with injection of local anesthetic into the incision and as an intercostal nerve block, specific to each group as detailed below. An incision was then made at the inframammary fold or periareolar. Dissection was performed such that a subpectoral plane was created using a dual plane technique with complete division of pectoralis origins along the inferior border.. Once adequate hemostasis was achieved, injection of local anesthetic into the breast pocket was performed. After implant selection, pocket was irrigated, and permanent saline or silicone implants were placed. No drains were used.
The mean implant volume for the study population was 363cc with a range of 175cc-550cc. There was no difference in the mean volume of implant placed between the groups. All implants were round and smooth. Out of 132 patients, 68 (51.5%) had saline implants and 64 (48.5%) had silicone implants. Inframammary incisions were used in 100 out of 132 patients (75.8%) and periareolar incisions were placed in 32 out of 132 patients (24.2%).
The injection of local anesthetic for each group was as follows:
Patients in the B group (Figure 1) were given a mean of 40 ml of bupivacaine with 0.25% epinephrine per patient and this was injected into three areas: 1) into the marked incision prior to incision 2) intercostal nerve block prior to incision 3) into the breast pocket after dissection.
Figure 1.
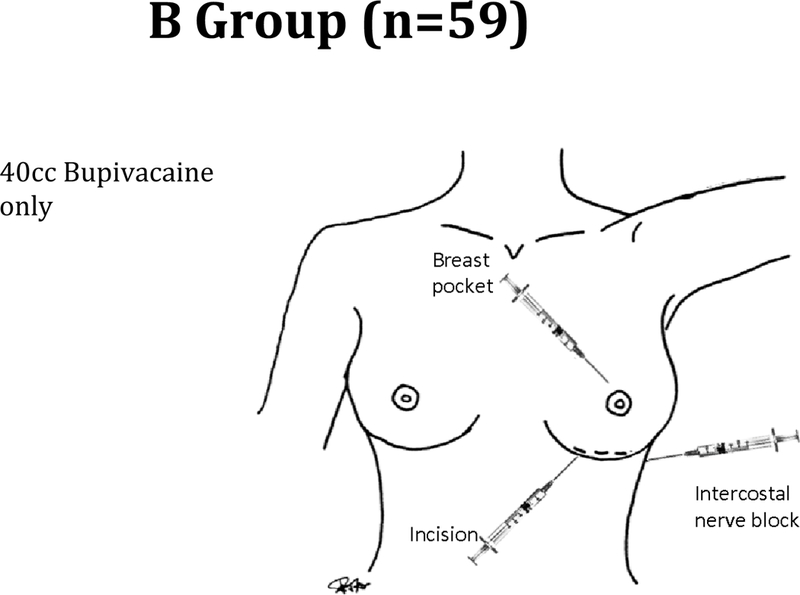
Patients in the B group were given a mean of 40 ml of bupivacaine with 0.25% epinephrine per patient and this was injected into three areas: 1) into the marked incision prior to incision 2) intercostal nerve block prior to incision 3) into the breast pocket after dissection.
Patients in the BL group (Figure 2) were given a mixture of 20 ml of bupivacaine, 20 ml of liposomal bupivacaine with 0.25% epinephrine and 20 ml of 0.9% normal saline and this was injected into three areas: 1) into the marked incision prior to incision 2) intercostal nerve block prior to incision 3) into the breast pocket after dissection.
Figure 2.
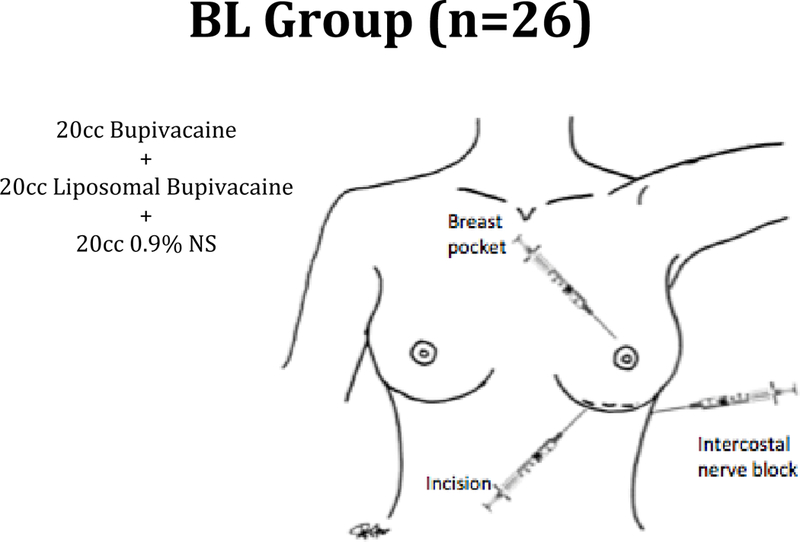
Patients in the BL group were given a mixture of 20 ml of bupivacaine, 20 ml of liposomal bupivacaine with 0.25% epinephrine and 20 ml of 0.9% normal saline and this was injected into three areas: 1) into the marked incision prior to incision 2) intercostal nerve block prior to incision 3) into the breast pocket after dissection.
Patients in the BLKi group (Figure 3) were given a mixture of 20 ml of bupivacaine, 20 ml of liposomal bupivacaine with 0.25% epinephrine and 20 ml of 0.9% normal saline and this was injected into three areas: 1) into the marked incision prior to incision 2) intercostal nerve block prior to incision 3) into the breast pocket after dissection. In addition ketorolac 30mg IV and 30mg IM were given postincisionally but prior to end of procedure.
Figure 3.
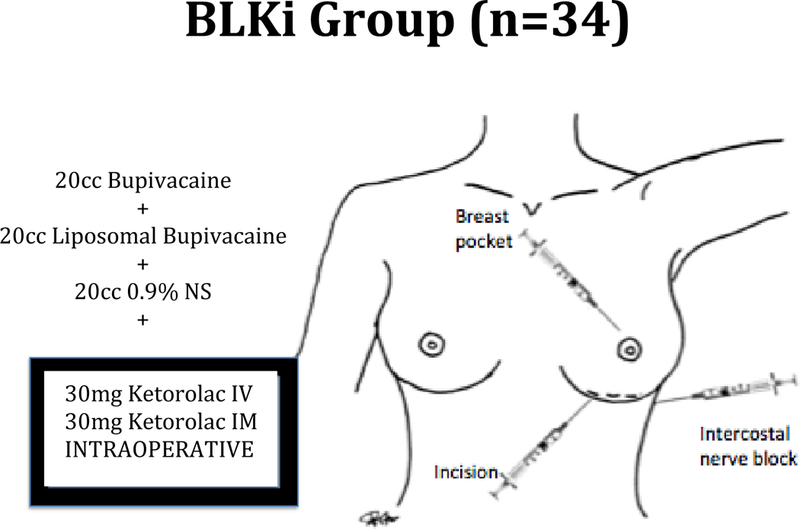
Patients in the BLKi group were given a mixture of 20 ml of bupivacaine, 20 ml of liposomal bupivacaine with 0.25% epinephrine and 20 ml of 0.9% normal saline and this was injected into three areas: 1) into the marked incision prior to incision 2) intercostal nerve block prior to incision 3) into the breast pocket after dissection. In addition ketorolac 30mg IV and 30mg IM were given postincisionally but prior to end of procedure.
Patients in the BLKp group (Figure 4) were given a mixture of 20 ml of bupivacaine, 20 ml of liposomal bupivacaine with 0.25% epinephrine and 20 ml of 0.9% normal saline and this was injected into three areas: 1) into the marked incision prior to incision 2) intercostal nerve block prior to incision 3) into the breast pocket after dissection. In addition ketorolac 30mg IV and 30mg IM were given at the end of procedure.
Figure 4.
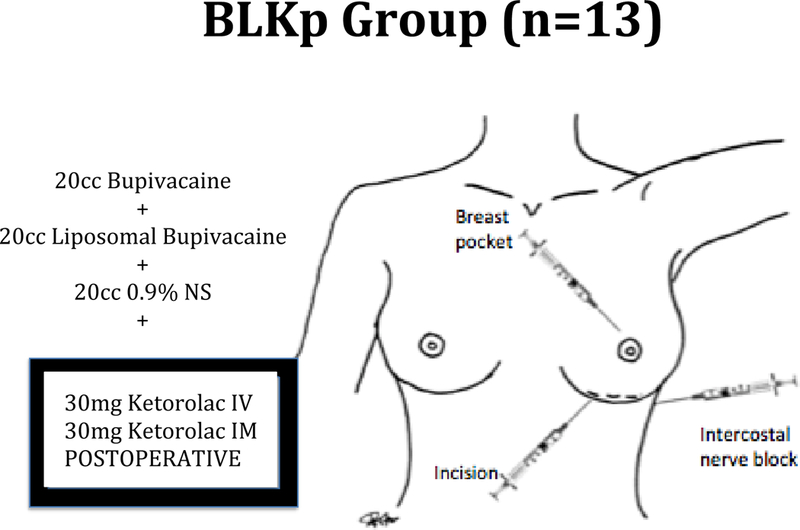
Patients in the BLKp group were given a mixture of 20 ml of bupivacaine, 20 ml of liposomal bupivacaine with 0.25% epinephrine and 20 ml of 0.9% normal saline and this was injected into three areas: 1) into the marked incision prior to incision 2) intercostal nerve block prior to incision 3) into the breast pocket after dissection. In addition ketorolac 30mg IV and 30mg IM were given at the end of procedure.
It should be noted that the use of liposomal bupivacaine as a regional nerve block is off-label and has not been approved by the FDA.
Statistical Analysis
For the continuous measures, data were analyzed using ANOVA methods. When assumptions of normality were not met, results were verified by analysis with the Kruskal-Wallis test. For categorical measures, differences in frequencies were assessed using Pearson’s chis-squared test. Sub-hypotheses were assessed using Bonferroni-adjusted p-values.
RESULTS
132 patients were analyzed in this study and fell into one of four groups: group B (n = 59), group BL (n=26), group BLKi (n=34) and group BLKp (n=13). There were no significant differences in age or BMI across the groups, and all breast augmentations took place in the same surgical center. Procedures were all performed by one of two surgeons. BLKi patients had lower pain scores upon discharge at 1.15 (p<0.0001). Additionally, the smallest number of patients requiring narcotics in PACU was also found in the BLKi group at 29.4% (10 out of 34 patients) (p=0.0094). There was no significant difference between the groups when comparing time in PACU, pain immediately post-operative, number of patients that received antiemetics post-operatively and time in the PACU for patients before they needed pain medications (Table 1).
Table 1.
B Group = bupivacaine only; BL Group = bupivacaine and liposomal bupivacaine; BLKi Group = ketorolac given intraoperatively plus bupivacaine and liposomal bupivacaine; BLKp = ketorolac given postoperatively plus bupivacaine and liposomal bupivacaine. Variables listed above include age, BMI, time spent in the pacu (post anesthesia care unit), pain score immediately upon PACU arrival, pain score upon discharge, timing of narcotic administration, amount of meperidine given (mg), patients receiving meperidine, and patients receiving antiemetics.
| B (n = 59) |
BL (N = 26) |
BLKi ( N = 34) |
BLKp (N = 13) |
p |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| age | 30.8 | 9.9 | 31.9 | 9.3 | 32.1 | 8.4 | 28.1 | 6.9 | 0.5309 |
| BMI | 21.7 | 2.7 | 22.5 | 4.4 | 22.3 | 3.0 | 20.8 | 2.3 | 0.4604 |
| Time pacu | 90.9 | 18.7 | 93.8 | 23.7 | 86.4 | 16.9 | 96.9 | 12.9 | 0.5644 |
| Pain po (0–10) | 0.56 | 1.74 | 0.23 | 1.18 | 0.53 | 1.94 | 0.00 | 0.00 | 0.6832 |
| Pain dc (0–10) | 2.22 | 1.91 | 2.81 | 1.30 | 1.15 | 1.78 | 3.08 | 1.32 | <0.0001 |
| Time meds | 33.9 | 19.5 | 38.6 | 37.2 | 24.6 | 12.1 | 41.4 | 23.2 | 0.4085 |
| Amount of meperidine given (mg) in PACU | 37.1 | 18.9 | 34.2 | 18.6 | 34.0 | 32.0 | 23.2 | 8.6 | |
| N = 32 | N = 19 | N = 10 | N = 7 | 0.5521 | |||||
| N | % | N | % | N | % | N | % | p | |
| Received Meperidine | 33 | 55.9 | 19 | 73.1 | 9 | 26.5 | 7 | 53.8 | 0.0044 |
| Received Antiemetics | 30 | 50.8 | 15 | 44.1 | 9 | 26.5 | 6 | 46.2 | 0.0706 |
DISCUSSION
Breast augmentation has steadily increased in popularity and parallel to it, the need to optimize postoperative pain control, mitigate cost and decrease adverse side effects.4 Placement of the implant into the retromuscular pocket has also increased pain associated with the procedure due to the partial detachment of the sternal and costal origins of the pectoralis major muscle. In our study, we have aimed to demonstrate the best combination of drugs for optimal pain control in this patient population.
Studies show that pain is triggered by sensitization of neurons in the pain pathway. This sensitization can be either central or peripheral.5 During tissue injury such as surgery, cytokine and prostaglandins are produced. This increases inflammation and therefore increases excitability of central neurons in the spinal cord giving the sensation of pain.6 During surgery, massive stimulation occurs of peripheral afferent fibers.7,8 Other studies also show that C-fibers are activated by brief noxious stimulation, and this may alter central neural function by prolonging their alteration. 9,10 General anesthesia attenuates this stimulation but does not prevent it.8 Traditional analgesic regimens have involved narcotics both intravenously or orally; however, systemic opioid use alone is insufficient to block nociceptive neurons and prevent central sensitization.10, 11 In addition, there are side effects such as postoperative nausea and vomiting. The spare use of opioids can decrease this effect as well as sedation, disorientation10,12 respiratory depression, urinary retention, ileus13,14,15 headache, constipation, sleep disturbance, altered mental status, as well tolerance and abuse that often occur. To reduce use, other modes of analgesia such as NSAIDs and local anesthetics are advised.
Local anesthetics have the ability to limit central sensitization by limiting impulses to afferent fibers and diminishing spinal cord stimulation and pain processing.8, 16 This can contribute to a significant pain-free period post-operatively. Bupivacaine has a half-life of 2.7 hours and this duration is prolonged when combined with epinephrine.12 It is also comparatively inexpensive and can be used to obtain this pain-free period.10, 17 An alternative that is even longer acting is liposomal bupivacaine. This liposomal formulation is administered intraoperatively and releases bupivacaine for up to 96 hours. This has been demonstrated in other studies as an advantageous addition to multimodal analgesia.18,19,20,21,22
Non-steroidal anti-inflammatory drugs have the ability to work both centrally and peripherally. The local inflammatory response from injury is reduced, attenuating peripheral sensitization, which also decreases spinal nociceptive processing.10 Centrally, they prevent spinal prostanoid synthesis. By reducing both peripheral and central sensitization, pain is reduced in the post-operative period, requiring fewer additional analgesics including decreased total dose of opioids even after major surgery.8, 16, 23 NSAIDS are likely to be inadequate when used alone.23,24,25 Other studies have demonstrated that a combination of NSAIDs such as ketorolac with other modalities including local anesthetics are safe and superior than the use of each product alone.8, 23, 24, 25
One study comparing intravenous ketorolac versus placebo for pain control after surgery found that NSAIDs combined with opioids resulted in not only less opioid consumption and better analgesia, but also decreased postoperative nausea, vomiting, and sedation26,27,28 This gives NSAIDs an advantage over opioids in the perioperative period.10 Ketorolac has even been shown to provide similar analgesic effect to morphine.29,30 For mild to moderate post-operative pain, NSAIDS should be the drug of choice. 13, 31
Multimodal analgesia has become the standard in clinical practice in recent decades in attempts to prevent peripheral and central stimulation and control postoperative pain.26, 32, 33, 34 This approach utilizes combinations of analgesic drugs to optimize pain relief. Drugs with different mechanisms of action can be used to target distinct pathways. This method of postsurgical pain control is preferred to singular methods. 18, 35, 36, 37
Numerous studies have shown that a multimodal regimen incorporating several different mechanisms of action has clear advantages over one that employs a single medication.38 In our study we looked at four different pain regimens; group B, group BL, group BLKi, and group BLKp. Our study shows that intraoperative ketorolac with liposomal bupivacaine and bupivacaine (BLKi) performed the best in terms of lowering narcotic usage and pain scores upon discharge.
Additionally, bupivacaine is much more cost-effective, as liposomal bupivacaine costs one hundred times more than standard bupivacaine.39
As discussed above, avoiding peripheral and central sensitization is an optimal method to decrease post injury pain. Preventive analgesia utilizes this theory by using analgesia prior to the painful stimulus to decrease the intensity of the pain.40 This has been demonstrated in animal studies with substantial results.5 Due to NSAIDs demonstrating antinociceptive effects both on peripheral and central neurons, ketorolac is an optimal drug for use in preventive analgesia. Local anesthesic can be combined for additional local, peripheral nociceptor blockade to attenuate sensitization prior to the surgical stimulus.6, 41
Based on the underlying principal of preemptive analgesia, ketorolac as well as local anesthetic should be given prior to incision for optimal pain prevention. Ketorolac should be given as preventive analgesia after primary hemostasis has been achieved.
Our study supports that the timing of ketorolac administration greatly influences outcomes. With regards to discharge pain scores and post-operative narcotic usage, our results suggest that intravenous ketorolac given systemically after surgery is less effective than ketorolac given intraoperatively, near the end of surgery. This is supported by a study in hand surgery. Here, preincisional use of both local anesthetics and NSAIDs as opposed to postincisional or post surgical were superior for adequate pain control.42,43
NSAIDs are still thought to be relatively contraindicated when used preincisionaly due to the effect on platelet function, which is crucial for surgical hemostasis.13 Overall, it is accepted for use preincisionally in small procedures, but is still unclear in larger more invasive procedures due to lack of sufficient evidence in its safety.16, 44 In the neurosurgical population, is has been associated with higher risk for postoperative hematoma.45 Others have reported significant blood loss intraopertively if ketorolac was given before “primary” hemostasis was achieved.46,47 There is currently no evidence to suggest any bleeding complications from use of ketorolac after hemostasis was achieved.48 It is commonly given near the end of surgery. 13 In our study, ketorolac was used postincisional in breast augmentation with no bleeding complications. In both clinical and laboratory studies, bleeding has been demonstrated due to a reversible inhibition of platelet aggregation causing prolonged bleeding times.30, 49 This, however, does not conclude that ketorolac is unsafe for use. In patients who are not otherwise coagulopathic, it has even been demonstrated as safe with respect to bleeding in surgery patients including those undergoing spinal procedure. 13, 50, 51, 52, 53.
There is also a concern amongst the surgical community that ketorolac can cause acute kidney injury post-operatively. This is a rare event and occurs in approximately 1 in 1,000–100,000.54 Its use does not significantly elevate serum creatinine levels and can be used safely.55,56 Some studies have shown that the transient fall in postoperative GFR and thus renal function is insignificant enough to warrant its use preoperatively. 57
Studies have found that parenteral ketorolac is associated with upper GI adverse events including gastritis, duodenitis, peptic ulcers and hemorrhage compared to nonuse.58 Ketorolac should be avoided in patients with malignancy, coagulopathies, as well as chronic kidney disease.
This study found no significant difference in postoperative nausea, vomiting, renal injury or hemorrhage between these four groups.
Conclusion
Intraoperative ketorolac should be considered as part of the analgesic regimen for acute postoperative pain control in breast augmentation. Its inclusion was significant in decreasing patient use of narcotics and pain upon discharge. Other points to consider include issues of cost, overall patient satisfaction and safety as compared to narcotics. Addition of other costly drugs such as liposomal bupivacaine may not provide further benefit in the immediate postoperative setting for procedures with a short recovery period such as breast augmentation. Studies have found that compared to narcotics, intravenous ketorolac produced a lower overall mean cost per patient, as well as a higher average patient satisfaction score. 59
Limitations of this study include that this data has been retrospectively analyzed which may have more sources of bias than if it was studied prospectively. Additionally, two surgeons performed all operations. This study does not account for differences in skill, technique, and experience between the two.
Fig 5.
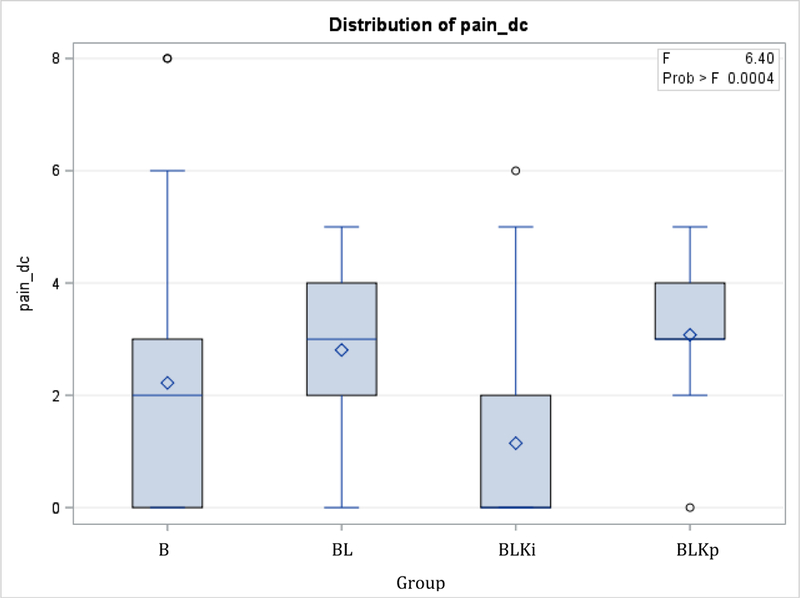
Pain score at time of discharge. In pairwise comparisons, BLKi < BL and BLKi < BLKp (all p < 0.05). B= bupivacaine, BL= bupivacaine + liposomal bupivacaine, BLKi= bupivacaine + liposomal bupivacaine + intraoperative ketorolac, BLKp= bupivacaine + liposomal bupivacaine + postoperative ketorolac.
Acknowledgments
Tulane University Department of Biostatistics and Bioinformatics is supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center
Footnotes
Financial disclosures
The authors have no financial or commercial associations that might create a conflict of interest with the information presented in this manuscript.
Other Disclosures
Presented at the Southern Society of Plastic and Reconstructive Surgery Annual meeting 2016.
References:
- 1.http://www.cdc.gov/nchs/data/nhsr/nhsr011.pdf National Health Statistics Reports Ambulatory Surgery in the United States, 2006. [PubMed]
- 2.Joshi GP, Ogunnaike BO. Consequences of inadequate postoperative pain relief and chronic persistent postoperative pain. Anesthesiol Clin North America 2005;23:21–26. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012. February;116(2):248–73. doi: 10.1097/ALN.0b013e31823c1030. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 4.Stanley SS, Hoppe IC, Ciminello FS. Pain control following breast augmentation: a qualitative systematic review. Aesthet Surg J. 2012. November;32(8):964–72. doi: 10.1177/1090820X12457014. Epub 2012 Aug 22. Review. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 5.Kelly DJ, Ahmad M, Brull SJ. Preemptive analgesia I: Physiological pathways and pharmacological modalities. Can J Anaesth 48: 1000, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Gutta R, Koehn CR, James LE. Does ketorolac have a preemptive analgesic effect? A randomized, double-blind, control study. J Oral Maxillofac Surg. 2013. December;71(12):2029–34. doi: 10.1016/j.joms.2013.06.220. Epub 2013 Aug 29. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 7.Ferreira SH, Nakamura M, Abreau-Castro MS. The hyperanalgesic effects of prostacyclin and PGE2. Prostaglandins 16:31, 1978 [DOI] [PubMed] [Google Scholar]
- 8.Rundshagen I, Kochs E, and Schulteam EJ Surgical stimulation increases median nerve somatosensory evoked responses during isoflurane-nitrous oxide anaesthesia. Br. J. Anaesth. 75: 598, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Melzack R, and Wall PD The Challenge of Pain, 2nd Ed. New York: Basic Books, 1988. [Google Scholar]
- 10.Mahabir RC, Peterson BD, Williamson JS, Valnicek SM, Williamson DG, East WE. Locally administered ketorolac and bupivacaine for control of postoperative pain in breast augmentation patients. Plast Reconstr Surg. 2004. December;114(7):1910–6. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 11.Katz J, Clairoux M, Redahan C, et al. High dose alfentanil pre-empts pain after abdominal hysterectomy. Pain 68: 109, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow M, and Jones R The duration of action of bupivacaine, prilocaine and lignocaine. Br. J. Anaesth. 42: 335, 1970. [DOI] [PubMed] [Google Scholar]
- 13.Magni G, La Rosa I, Melillo G, Abeni D, Hernandez H, Rosa G. Intracranial hemorrhage requiring surgery in neurosurgical patients given ketorolac: a case-control study within a cohort (2001–2010). Anesth Analg. 2013. February;116(2):443–7. doi: 10.1213/ANE.0b013e3182746eda. Epub 2013 Jan 9. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 14.Litvak KM, McEvoy GK. Ketorolac, an injectable nonnarcotic analgesic. Clin Pharm 1990;9:921–35 [PubMed] [Google Scholar]
- 15.DeAndrade JR, Maslanka M, Reines HD, Howe D, Rasmussen GL, Cardea J, Brown J, Bynum L, Shefrin A, Chang YL, Maneatis T. Ketorolac versus meperidine for pain relief after orthopaedic surgery. Clin Orthop Relat Res 1996;301–12 [PubMed] [Google Scholar]
- 16.O’Hanlon DM, Thambipillai T, Colbert ST, Keane PW, and Given HF Timing of pre-emptive tenoxicam is important for postoperative analgesia. Can. J. Anaesth. 48: 162, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Thorne AC Local anesthetics In Aston SJ, Beasley RW and Thorne CHM (Eds.), Grabb and Smith’s Plastic Surgery, 5th Ed. Philadelphia: Lippincott-Raven, 1997. [Google Scholar]
- 18.Baxter R, Bramlett K, Onel E, Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin Ther. 2013. March;35(3):312–320.e5. doi: 10.1016/j.clinthera.2013.02.005. Epub 2013 Mar 1. Review. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 19.Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended- release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28:776–788. [DOI] [PubMed] [Google Scholar]
- 20.Gorfine SR, Onel E, Patou G, et al. Bupivacaine extendedrelease liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum. 2011;54:1552–1559. [DOI] [PubMed] [Google Scholar]
- 21.Smoot JD, Bergese SD, Onel E, et al. The efficacy and safety of DepoFoam® bupivacaine in patients undergoing bilateral, cosmetic, submuscular augmentation mammoplasty: a randomized, double-blind, active-control study. Aesthet Surg J. 2012;32:69–76. [DOI] [PubMed] [Google Scholar]
- 22.Haas E, Onel E, Miller H, et al. A double-blind, randomized, active-controlled study for post-hemorrhoidectomy pain management with liposome bupivacaine, a novel local analgesic formulation. AmSurg. 2012;78:574–581. [DOI] [PubMed] [Google Scholar]
- 23.Pavy TJ, Paech MJ, Evans SF. The effect of intravenous ketorolac on opioid requirement and pain after cesarean delivery. Anesth Analg. 2001. April;92(4):1010–4. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 24.Gin T, Kan AF, Lam KK, O’Meara ME. Analgesia after caesarean section with intramuscular ketorolac or pethidine. Anaesth Intensive Care 1993;21:420–3. [DOI] [PubMed] [Google Scholar]
- 25.Tzeng JI, Mok MS. Combination of intramuscular ketorolac and low dose epidural morphine for the relief of post-caesarean pain. Ann Acad Med Singapore 1994;23:10–3. [PubMed] [Google Scholar]
- 26.Gan TJ, Daniels SE, Singla N, Hamilton DA, Carr DB. A novel injectable formulation of diclofenac compared with intravenous ketorolac or placebo for acute moderate-to-severe pain after abdominal or pelvic surgery: a multicenter, double-blind, randomized, multiple-dose study. Anesth Analg. 2012. November;115(5):1212–20. doi: 10.1213/ANE.0b013e3182691bf9. Epub 2012 Aug 10. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 27.Elia N, Lysakowski C, Tramer MR. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology 2005;103:1296–304 [DOI] [PubMed] [Google Scholar]
- 28.Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia mor- phine side effects: meta-analysis of randomized controlled trials. Anesthesiology 2005;102:1249–60 [DOI] [PubMed] [Google Scholar]
- 29.Le Roux PD, Samudrala S. Postoperative pain after lumbar disc surgery: a comparison between parenteral ketorolac and narcotics. Acta Neurochir (Wien) 1999;141:261–7 [DOI] [PubMed] [Google Scholar]
- 30.Resman-Targoff BH. Ketorolac: a parenteral nonsteroidal anti-inflammatory drug. DICP 1990;24:1098–104 [DOI] [PubMed] [Google Scholar]
- 31.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012. February;116(2):248–73. doi: 10.1097/ALN.0b013e31823c1030. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 32.Acute Pain Management: Scientific Evidence. 3rd ed. Mel- bourne, Australia: Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine, 2010 [Google Scholar]
- 33.Ashburn MA, Caplan RA, Carr DB, Connis RT, Ginsberg B, Green CR, Lema MJ, Nickinovich DG, Rice LJ. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2004;100:1573–81 [DOI] [PubMed] [Google Scholar]
- 34.Carr DB, Jacox AK, Chapman CR, Ferrell B, Fields HL, Heidrich G, Hester NK, Hill CS, Lipman AG, McGarvey CL, Miaskowski C, Mulder DS, Payne R, Schecter N, Shapiro BS, Smith RS, Tsou CV, Vecchiarelli L. Acute Pain Management: Operative or Medical Procedures and Trauma. Clinical Practice Guideline Number 1 AHCPR Publication No. 92 0032. Rockville, MD: Agency for Healthcare Research and Quality, Public Health Service, US Department of Health and Human Services, 1992 [Google Scholar]
- 35.Apfelbaum JL. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. [DOI] [PubMed] [Google Scholar]
- 36.McGuire L,Heffner K,Glaser R, et al. Pain and wound healing in surgical patients. Ann Behav Med.2006;31:165172. [DOI] [PubMed] [Google Scholar]
- 37.Jin F, Chung F. Multimodal analgesia for postoperative pain control. J Clin Anesth. 2001;13:524–539. [DOI] [PubMed] [Google Scholar]
- 38.Ziemann-Gimmel P, Hensel P, Koppman J, Marema R. Multimodal analgesia reduces narcotic requirements and antiemetic rescue medication in laparoscopic Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2013. Nov-December;9(6):975–80. doi: 10.1016/j.soard.2013.02.003. Epub 2013 Feb 13. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 39.Noviasky J, Pierce DP, Whalen K, Guharoy R, Hildreth K. Bupivacaine liposomal versus bupivacaine: comparative review. Hosp Pharm. 2014. June;49(6):539–43. doi: 10.1310/hpj4906-539. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woolf CJ, Chong MS. Preemptive analgesia—Treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 77:362, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Savage MG, Henry MA. Preoperative nonsteroidal anti-inflammatory agents: Review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 98:146, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Katz J, Kavanagh BP, Sandler A, et al. Preemptive analgesia: Clinical evidence of neuroplasticity contributing to postoperative pain. Anesthesiology 77: 439, 1992. [DOI] [PubMed] [Google Scholar]
- 43.Ejlersen E, Andersen HB, Eliasen K, and Mogensen T A comparison between preincisional and postincisional lidocaine infiltration and postoperative pain. Anesth. Analg. 74: 495, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Mahabir RC, Peterson BD, Williamson JS, Valnicek SM, Williamson DG, East WE. Locally administered ketorolac and bupivacaine for control of postoperative pain in breast augmentation patients: part II. 10-day follow-up. Plast Reconstr Surg. 2008. February;121(2):638–43. doi: 10.1097/01.prs.0000298112.52389.e1. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 45.Palmer JD, Sparrow OC, Iannotti F. Postoperative hematoma: a 5-year survey and identification of avoidable risk factors. Neurosurgery 1994;35:1061–4. [DOI] [PubMed] [Google Scholar]
- 46.De Oliveira GS Jr, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesth Analg 2012;114:424–33. [DOI] [PubMed] [Google Scholar]
- 47.White PF, Raeder J, Kehlet H. Ketorolac: its role as part of a multimodal analgesic regimen. Anesth Analg 2012;114:250–4. [DOI] [PubMed] [Google Scholar]
- 48.Magni G, La Rosa I, Gimignani S, Melillo G, Imperiale C, Rosa G. Early postoperative complications after intracranial surgery: comparison between total intravenous and balanced anesthesia. J Neurosurg Anesthesiol 2007;19:229–34. [DOI] [PubMed] [Google Scholar]
- 49.Singer AJ, Mynster CJ, McMahon BJ. The effect of IM ketorolac tromethamine on bleeding time: a prospective, interventional, controlled study. Am J Emerg Med 2003;21:441–3 [DOI] [PubMed] [Google Scholar]
- 50.Cassinelli EH, Dean CL, Garcia RM, Furey CG, Bohlman HH. Ketorolac use for postoperative pain management following lumbar decompression surgery: a prospective, randomized, double-blinded, placebo-controlled trial. Spine 2008;33:1313–7. [DOI] [PubMed] [Google Scholar]
- 51.Lieh-Lai MW, Kauffman RE, Uy HG, Danjin M, Simpson PM. A randomized comparison of ketorolac tromethamine and morphine for postoperative analgesia in critically ill children. Crit Care Med 1999;27:2786–91. [DOI] [PubMed] [Google Scholar]
- 52.Forrest JB, Camu F, Greer IA, Kehlet H, Abdalla M, Bonnet F, Ebrahim S, Escolar G, Jage J, Pocock S, Velo G, Langman MJ, Bianchi PG, Samama MM, Heitlinger E. POINT Investigators. Ketorolac, diclofenac, and ketoprofen are equally safe for pain relief after major surgery. Br J Anaesth 2002;88:227–33. [DOI] [PubMed] [Google Scholar]
- 53.Gillis JC, Brogden RN. Ketorolac. A reappraisal of its pharmacodynamics and pharmacokinetic properties and therapeutic use in pain management. Drugs 1997;53:139–88. [DOI] [PubMed] [Google Scholar]
- 54.Parker RK, Holtmann B, Smith I, White PF. Use of ketorolac after lower abdominal surgery. Anesthesiology 1994;80:6–12. [DOI] [PubMed] [Google Scholar]
- 55.Myles PS, Power I. Does ketorolac cause postoperative renal failure: how do we assess the evidence? Br J Anaesth 1998;80: 4201. [DOI] [PubMed] [Google Scholar]
- 56.Pavy TJ, Paech MJ, Evans SF. The effect of intravenous ketorolac on opioid requirement and pain after cesarean delivery. Anesth Analg. 2001. April;92(4):1010–4. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 57.Lee A, Cooper MC, Craig JC, Knight JF, Keneally JP. Effects of nonsteroidal anti-inflammatory drugs on post-operative renal function in normal adults. Cochrane Database Syst Rev. 2001;(2):CD002765. Review. Update in: Cochrane Database Syst Rev. 2004;(2):CD002765. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 58.Chang CH, Chen HC, Lin JW, Kuo CW, Shau WY, Lai MS. Risk of hospitalization for upper gastrointestinal adverse events associated with nonsteroidal anti-inflammatory drugs: a nationwide case-crossover study in Taiwan. Pharmacoepidemiol Drug Saf. 2011. July;20(7):763–71. doi: 10.1002/pds.2140. Epub 2011 May 25. PubMed PMID: . [DOI] [PubMed] [Google Scholar]
- 59.Rainer TH, Jacobs P, Ng YC, Cheung NK, Tam M, Lam PK, Wong R, Cocks RA. Cost effectiveness analysis of intravenous ketorolac and morphine for treating pain after limb injury: double blind randomised controlled trial. BMJ. 2000. November 18;321(7271):1247–51. PubMed PMID: ; [DOI] [PMC free article] [PubMed] [Google Scholar]


