Abstract
Two people with the same lifespan do not necessarily have the same healthspan. One person may retain locomotor and cognitive abilities until the end of life, while another person may lose them during adulthood. Unbiased searches for genes that are required to maintain locomotor ability during adulthood may uncover key regulators of locomotor healthspan. Here, we take advantage of the relatively short lifespan of the nematode Caenorhabditis elegans and develop a novel screening procedure to collect mutants with locomotor deficits that become apparent in adulthood. After ethyl methanesulfonate mutagenesis, we isolated five C. elegans mutant strains that progressively lose adult locomotor ability. In one of the mutant strains, a nonsense mutation in elpc-2, which encodes Elongator Complex Protein Component 2, causes a progressive decline in locomotor ability during adulthood. Mutants and mutations identified in the present screen may provide insights into mechanisms of age-related locomotor impairment and the maintenance of locomotor healthspan.
Keywords: age-related locomotor impairment, elpc-2, forward genetic screen
Locomotor ability indicates an animal’s healthspan across many species such as worms, flies, mice, and humans (Cesari et al. 2009; Grotewiel et al. 2005; Hahm et al. 2015; Justice et al. 2014). In these species, declines in locomotor abilities can be a feature of the normal aging process, or a symptom of an age-related disease. Currently, the genetic regulators that work to prevent age-related declines in locomotor ability are largely unknown.
Recent studies have suggested that the genetic bases of lifespan and healthspan may not completely overlap (Bansal et al. 2015; Iwasa et al. 2010; Tissenbaum 2012). From a candidate-based genetic screen, Iwasa et al. found that activation of the epidermal growth factor signaling pathway prolongs adult swimming ability in C. elegans without large effects on lifespan (Iwasa et al. 2010). More examples of genetic pathways that work to maintain locomotor healthspan may be discovered by carrying out unbiased searches for mutant animals that show progressive declines in locomotor capacity.
A forward genetic screen using C. elegans has previously been employed to identify genes that affect locomotor ability during development (Brenner 1974). However, unbiased screens that focus on locomotor deficits occurring later in life have not been carried out, in part due to the difficulty in distinguishing whether symptoms observed during adulthood were already present during development.
In the present study, we established the “Edge Assay” to measure locomotor ability of hundreds of adult worms at once. Using the Edge Assay, we developed a screening procedure to remove mutant worms with strong developmental locomotor defects on the first day of adulthood, and then isolated mutant worms that progressively lose their locomotor ability on the third or fifth days of adulthood. After ethyl methanesulfonate (EMS)-mutagenesis, we isolated five mutant strains that progressively lose their ability to complete the Edge Assay. In one mutant strain, we found that a mutation in the elpc-2 gene, encoding Elongator complex protein component 2, causes progressive loss of locomotor ability. elpc-2 works with other Elongator complex genes, elpc-1 and elpc-3, to maintain adult locomotor ability in C. elegans. Along with the Elongator complex mutants, isolated mutants from the present screen can be used as tools to explore mechanisms that work to maintain adult locomotor ability in C. elegans, and may provide insights into the evolutionarily conserved regulators of locomotor healthspan.
Materials and Methods
Strains
C. elegans Bristol N2 strain was used as wild type. Worms were cultivated on Nematode Growth Media (NGM) agar plates with Escherichia coli strain OP50 at 20° (Brenner 1974). Full details of strains used in the present study are listed in Table S6.
Edge Assay
Edge Assay plates were prepared by pouring 16 ml of NGM agar into a circular 9 cm plate. NGM plates were dried overnight with the lid on at 25°, then kept at 4° until use. On the day before the Edge Assay, a total of 100 µl of E. coli suspension was spotted on four spots near the edge of the NGM plate. The tip of a 50 ml serological pipette was briefly placed over a flame to smoothen the tip. The NGM plate was placed on an inoculating turntable and the smoothened pipette tip was held against the E. coli drop. The plate was slowly rotated while holding the pipette tip still. The plate was rotated 360° to spread the E. coli around the edge of the whole plate. Plates were incubated overnight at 25° and used the next day. Synchronized worms were collected and washed twice with M9 buffer containing 0.1% gelatin. Worms were placed on the center of an Edge Assay plate and excess M9 buffer was removed with the edge of a Kimwipe. The number of worms that reached or did not reach the edge were counted at various time points to measure the Edge Assay completion rate. Floxuridine (FUDR) was not used at any point for worms tested using the Edge Assay.
Isolation of mutants that show a progressive decline in locomotor ability
Wild-type N2 worms were mutagenized and cultured as previously described (Brenner 1974). Larval stage-4 worms were mutagenized by incubation in a 50 mM EMS solution for 4 h. EMS-mutagenized F2 adult day 1 worms were collected and washed twice with M9 buffer containing 0.1% aqueous gelatin. Worms were placed at the center of an Edge Assay plate and excess buffer was removed with the edge of a Kimwipe. After 15 min, worms that did not reach the edge were removed using an aspirator. Worms that reached the edge were maintained on the same plate until adult day 3. On adult day 3, worms were collected and washed with M9 buffer containing 0.1% gelatin and the Edge Assay was repeated on a new Edge Assay plate. Worms that were unable to reach the edge were collected as adult day 3 progressive locomotor deficit mutants. Worms that reached the edge were maintained on the same plate until adult day 5. On adult day 5, worms were collected and washed with M9 buffer containing 0.1% gelatin and the Edge Assay was repeated on a new Edge Assay plate. Worms that were unable to reach the edge were collected as adult day 5 progressive locomotor deficit mutants.
Measurements of maximum speed and travel distance
Worms were synchronized by placing five adult day 1 worms onto an NGM plate with food, and allowed to lay eggs for 3 h. When the offspring reached adult day 1, 15 worms were picked randomly onto a 6 cm NGM plate without bacteria. After the worms moved away from the initial location with residual food, worms were again moved onto a different NGM plate without bacteria. Movement of worms was recorded for 1.0 min with a charge-coupled device camera INFINITY3-6URM (Lumenera Corporation, Ottawa, Canada). Images were analyzed using ImageJ and wrMTrck software (www.phage.dk/plugins) to produce maximum speed and travel distance (Nussbaum-Krammer et al. 2015). Measurements were made with the lid on in a temperature-controlled room set at 20°. At least three biological replicate plates of 15 worms each were measured for each strain. Worms that were lost during the video recording were not included in the analysis. FUDR was not used at any point for worms subjected to maximum speed and travel distance measurements.
Lifespan measurements
The lifespan of a population of worms was measured on NGM plates with food at 20°. Worms that did not move after gentle prodding to the head and tail were counted as dead. Worms that were lost, died from an exploded vulva, or from the bag-of-worms phenotype were censored. For the ix243 strain, some worms died from the bag-of-worms phenotype. Therefore, we measured lifespan of ix243 and control worms on plates containing 25 µM FUDR, which is an inhibitor of germline proliferation. Worms were transferred from NGM plates to FUDR-containing plates after reaching the L4 stage. All other lifespan measurements were carried out in the absence of FUDR.
Whole-genome DNA sequencing
C. elegans DNA was sequenced using the MiSeq platform (Illumina, San Diego, CA). Libraries were prepared with an Illumina TruSeq Library Prep Kit. Mapping was conducted with BWA software (Li and Durbin 2009). Resulting files were converted to bam files, then to pileup format with Samtools (Li and Durbin, 2009a; Li et al. 2009b). Variant analysis was conducted using VarScan and SnpEff available on the Galaxy platform (Blankenberg et al. 2010; Cingolani et al. 2012; Giardine et al. 2005; Goecks et al. 2010; Koboldt et al. 2009). Mutation frequencies along the chromosome were calculated and visualized using CloudMap (Minevich et al. 2012).
Transcriptional reporter expression
A genomic fragment of 2090-bp immediately upstream of the start codon of the elpc-2 gene was PCR-amplified using “5’ elpc-2p overlap ppd95.79” and “3’ elpc-2p overlap ppd95.79” primers, which have 15-bp overhangs that anneal upstream of the GFP sequence in the pPD95.79 vector (Primer details are listed in Table S7). The pPD95.79 vector containing GFP was linearized by PCR using the “5’ ppd95.79” and “3’ ppd95.79” primers. The template vector was digested with restriction enzyme Dpn I (New England Biolabs, Ipswich, MA), and the linearized vector was purified by Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI). The pure linearized vector and the elpc-2 promoter were fused using an In-Fusion HD Cloning Kit (Takara, Kusatsu, Japan) to make the elpc-2p::GFP transcriptional reporter construct. The construct was microinjected into the gonads of wild-type worms at a concentration of 50 ng/µl. Worms that expressed the reporter construct were immobilized in 25 mM sodium azide and observed under a confocal microscope LSM710 (Carl Zeiss, Oberkochen, Germany). A z-stack image was created from images taken at 1 µm increments.
Creation of double mutants
Double mutant strains were created by crossing males of one strain with hermaphrodites of another. Double mutants were checked by extracting their DNA, amplifying a genomic fragment flanking the mutation site by PCR, and sequencing the PCR product by Sanger sequencing. See Supplementary Information for primer details.
Statistics
All results are expressed as means with a 95% confidence interval. Student’s t-test was used for pairwise comparisons with Excel 2010 (Microsoft). For multiple comparisons, one-way ANOVA was followed with Dunnett’s post hoc test or Tukey’s Honest Significant Difference test using R (Team 2015). Statistical significance was set at *P < 0.05; **P < 0.01; ***P < 0.001.
Data availability
All isolated strains and plasmids are available upon request. DNA sequencing data are available on NCBI Sequence Read Archive: PRJNA530333. Supplemental material available at FigShare: https://doi.org/10.25387/g3.8150759.
Results
The “Edge Assay” can test locomotor ability of hundreds of worms
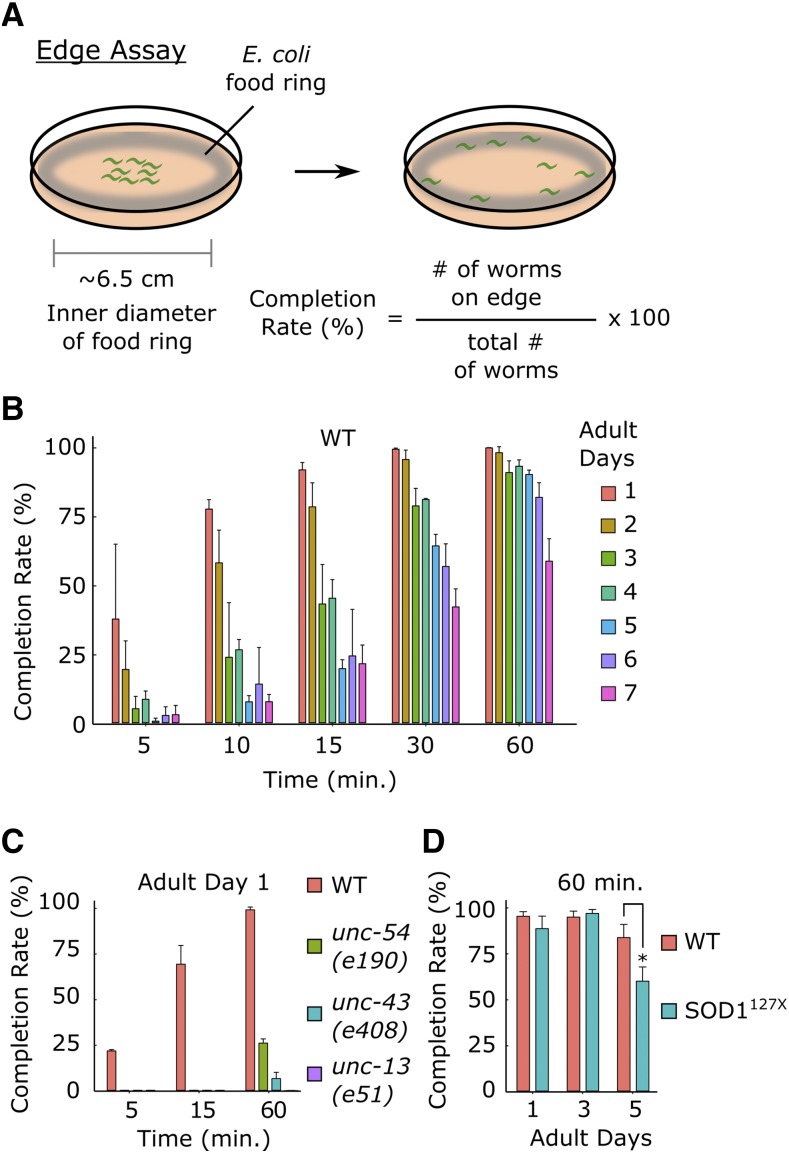
The present forward genetic screen isolates mutant worms that progressively lose locomotor ability. We established the Edge Assay to measure locomotor ability of hundreds of worms at once. The Edge Assay is carried out on a 9-cm agar plate with E. coli bacterial feed spread only on the outer edge of the plate. Up to a few hundred adult worms are placed on the center of the plate where there is no food (Figure 1A; Fig. S1). Motile worms reach the E. coli on the edge of the plate, while worms with defects in locomotion or chemotaxis remain in the center of the plate.
Figure 1.
“Edge Assay” can measure locomotor ability of worms (A) (Left) Schematic diagram of an Edge Assay plate immediately after placing worms at the center of the plate. (Right) Schematic diagram of Edge Assay plate after most worms reached the edge. (B) Edge Assay completion rates of wild-type worms from adult day 1 to 7 after 5, 10, 15, 30, and 60 min. (C) Completion rates for WT and developmental mutants deficient in locomotor function, unc-54(e190), unc-43(e408), and unc-13(e51). (D) Completion rates of WT and a previously reported C. elegans model of amyotrophic lateral sclerosis (SOD1127X). For Edge Assay experiments, n = 3 biological replicate plates with each plate starting with approximately 100 worms per plate on adult day 1. Error bars indicate 95% confidence intervals. *P < 0.05; Unpaired Student’s t-test.
On the first day of adulthood, 91.3% of wild-type worms reached the edge in 15 min and 99.6% reached the edge in 60 min (Figure 1B; Fig. S1). C. elegans mutant strains that are defective in the function of neurons, including unc-13(e51) and unc-43(e408) (Maruyama and Brenner 1991; Reiner et al. 1999) or muscles such as unc-54(e190) (MacLeod et al. 1981) could not reach the edge in 15 min on the first day of adulthood (Figure 1C). After 60 min, 26% of unc-54(e190) mutants, 6.4% of unc-43(e408) mutants, and 0% of unc-13(e51) mutants reached the edge (Figure 1C). Therefore, carrying out the Edge Assay for 15 min on the first day of adulthood can separate wild-type worms from worms with strong developmental locomotor defects.
On average, over 90% of wild-type worms could complete the Edge Assay in 60 min during the first five days of adulthood (Figure 1B). A C. elegans model of amyotrophic lateral sclerosis (SOD1127X) (Gidalevitz et al. 2009) showed a significant reduction in Edge Assay completion rate compared to that of wild-type worms on the fifth day of adulthood (Figure 1D). Therefore, carrying out the Edge Assay for 60 min on the fifth day of adulthood can separate wild-type worms from worms that progressively lose their locomotor ability.
Isolation of mutants that progressively lose locomotor ability during adulthood
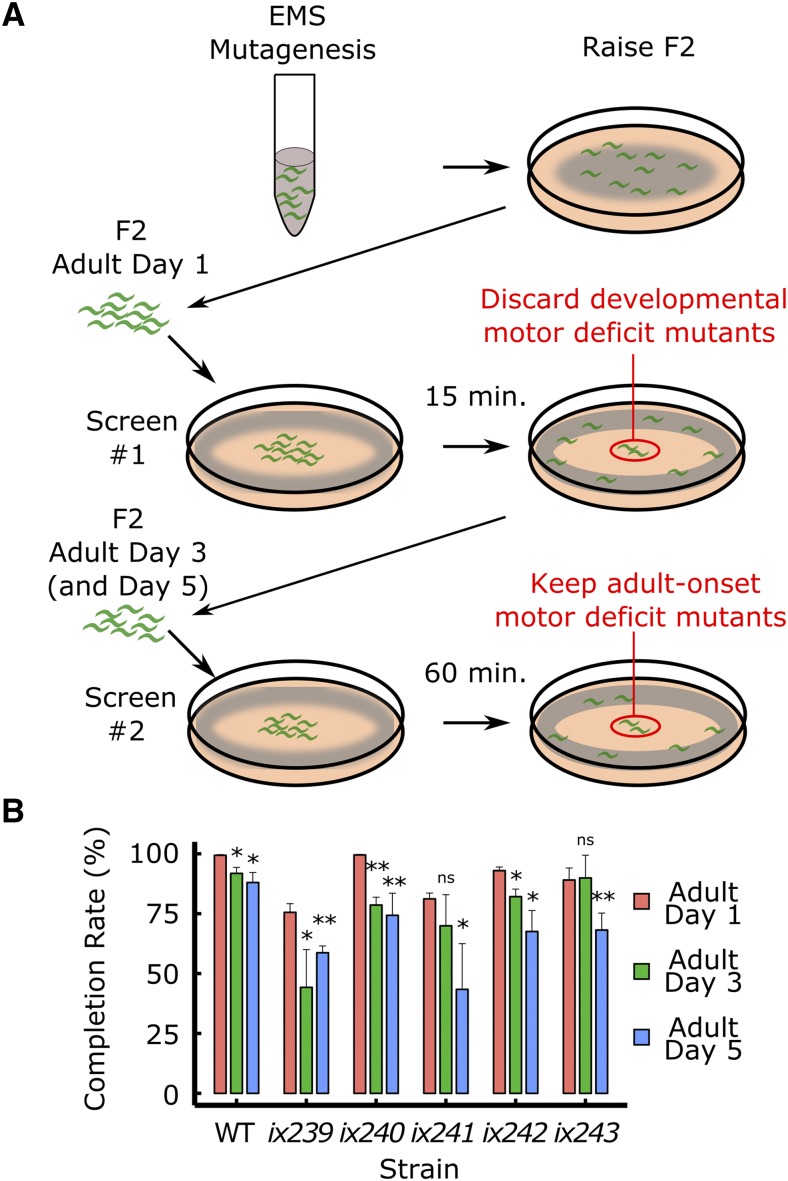
We mutagenized wild-type N2 worms using EMS, and screened 3352 F2 offspring from 500 F1 worms (1000 genomes) (Table S1). We carried out the Edge Assay for the mutagenized F2 offspring on the first day of adulthood (Figure 2A). To remove worms with developmental defects, worms that could not complete the Edge Assay in 15 min were aspirated away (Figure 2A). Only worms that completed the Edge Assay on the first day of adulthood were kept for further screening. On the third and fifth days of adulthood, we tested the worms again with the Edge Assay and collected slow or uncoordinated mutants that remained near the center of the Edge Assay plate after 60 min (Figure 2A). By removing worms with strong developmental defects on the first day of adulthood, we were able to isolate worms that progressively lost locomotor ability during adulthood. We isolated 22 viable mutants, and created individual strains from those mutants (Table S1). Five of those mutant strains reproducibly showed progressive deficits in completing the Edge Assay during adulthood (Figure 2B).
Figure 2.
Isolation of mutant strains that progressively lose locomotor ability (A) Schematic description of a forward genetic screen to isolate mutants that progressively lose locomotor ability. (B) Edge Assay completion rates of mutants identified from the screen. Error bars indicate 95% confidence intervals. n = 3 biological replicate plates, with each plate starting with approximately one hundred worms per plate on adult day 1. *P < 0.05; **P < 0.01; ns, not significant; Paired Student’s t-test vs. adult day 1 completion rate.
To determine whether isolated mutant strains have deficits in locomotor ability and not sensory function or search behavior, we measured locomotor ability of worms on an agar plate without food. We recorded one-minute videos of 15 worms freely moving on a plate, and measured the maximum velocities and total travel distances for each worm. For each strain, we recorded three plates of 15 worms on the first, third, and fifth days of adulthood. All isolated mutant strains showed significantly greater reductions in maximum velocity and travel distance from the first to fifth days of adulthood compared to wild type except for ix240 worms (Figure 3A, 3B, 3D; Fig. S2A–S2D, S3A–S3D). In the ix240 worms, progressive deficits other than locomotor ability, such as sensory function or search behavior, may cause the reduction in Edge Assay completion rate.
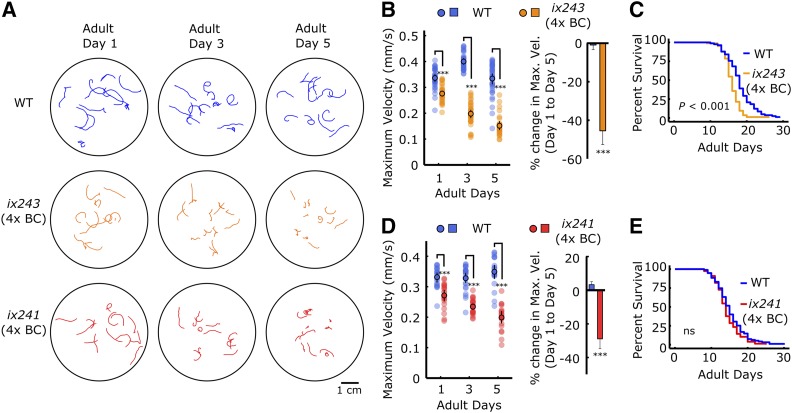
Figure 3.
ix241 and ix243 worms show progressive locomotor decline after four backcrosses (A) Representative locomotor tracks from 1-min video recordings of WT, ix243 backcrossed four times (4x BC), and ix241(4x BC) on adult days 1, 3, and 5 on plates with no food. n = 10–15 tracks per plate (some worms were unable to be tracked for a full minute, and were removed from analysis) (B) (Left) Maximum velocities of WT and ix243(4x BC) worms. (Right) Percent change in maximum velocity of WT and ix243(4x BC) worms on adult day 5 compared to adult day 1. (C) Survival curve of WT (n = 56) worms and ix243(4x BC) (n = 89) worms. (D) (Left) Maximum velocities of WT and ix241(4x BC) worms. (Right) Change in maximum velocity of WT and ix241(4x BC) worms. (E) Survival curve of WT (n = 94) and ix241(4x BC) (n = 77) worms. Error bars indicate 95% confidence intervals. For maximum velocity experiments, n = 30–45 worms per strain for each day (10–15 worms from 3 biological replicate plates). For percent change in maximum velocity graphs, n = 3 biological replicate plates. ***P < 0.001; ns, not significant; Unpaired Student’s t-test for maximum velocity comparisons; Log-rank test for lifespan comparisons.
ix241 and ix243 mutant strains show progressive decline in locomotor ability
ix241 and ix243 worms were backcrossed with the parental N2 strain to reduce the number of mutation sites that do not affect locomotor ability. After each backcross, we checked for individual lines that still showed a progressive decline in locomotor ability. We measured the maximum velocity and travel distance of individual worms on an agar plate without food on the first, third, and fifth days of adulthood. ix241 and ix243 worms still showed significant reductions in both maximum velocity and travel distance after the fourth backcross (Figure 3A, 3B, 3D; Fig. S3A–S3D).
To check whether ix241 and ix243 worms were simply aging faster than wild-type worms, we measured lifespans of the two strains. The lifespan of ix241 worms was not significantly shortened compared to that of wild type (Figure 3E; Table S2). The median lifespan of the ix243 worms was shortened by two days (Figure 3C; Table S2). To compare relative reductions in lifespan and locomotor healthspan, we measured the maximum velocities of wild type and ix243 worms for 10 days (Fig. S4A). We quantified the percent decrease in lifespan by comparing the areas under the survival curves of wild type and ix243 worms (Fig. S4B). We quantified the percent decrease in locomotor healthspan by comparing the areas under the decline in maximum velocity curves of wild type and ix243 worms (Fig. S4C). For ix243 worms, there is an average 11.5% reduction in lifespan, while there is a significantly greater 18.5% reduction in locomotor healthspan (Fig. S4D).
ix243 worms take a 13.9% longer time to reach adulthood (Table S3). The developmental delay was taken into account for locomotor and lifespan measurements by allowing ix243 worms an extra 10 h to develop, and starting locomotor and lifespan measurements from the first day of adulthood. ix243 worms show a 17.7% decrease in maximum locomotor ability on the first day of adulthood compared to wild-type worms (Figure 3B). The deficit in locomotor ability compared to wild-type worms increases to 54.8% on the fifth day of adulthood (Figure 3B). These results suggest that the ix243 mutant allele has modest negative effects on development and lifespan, with relatively stronger negative effects on locomotor healthspan.
ix241 worms take 4.0% longer to reach adulthood (Table S3) and show an 18.1% decrease in maximum locomotor ability on the first day of adulthood compared to wild-type worms (Figure 3D). The deficit in locomotor ability compared to wild-type worms increases to 43.0% on the fifth day of adulthood (Figure 3D). The ix241 mutant allele has no negative effect on lifespan, a modest negative effect on development, and a relatively stronger negative effect on locomotor healthspan.
Nonsense mutation in elpc-2 causes progressive loss of adult locomotor ability in ix243 worms
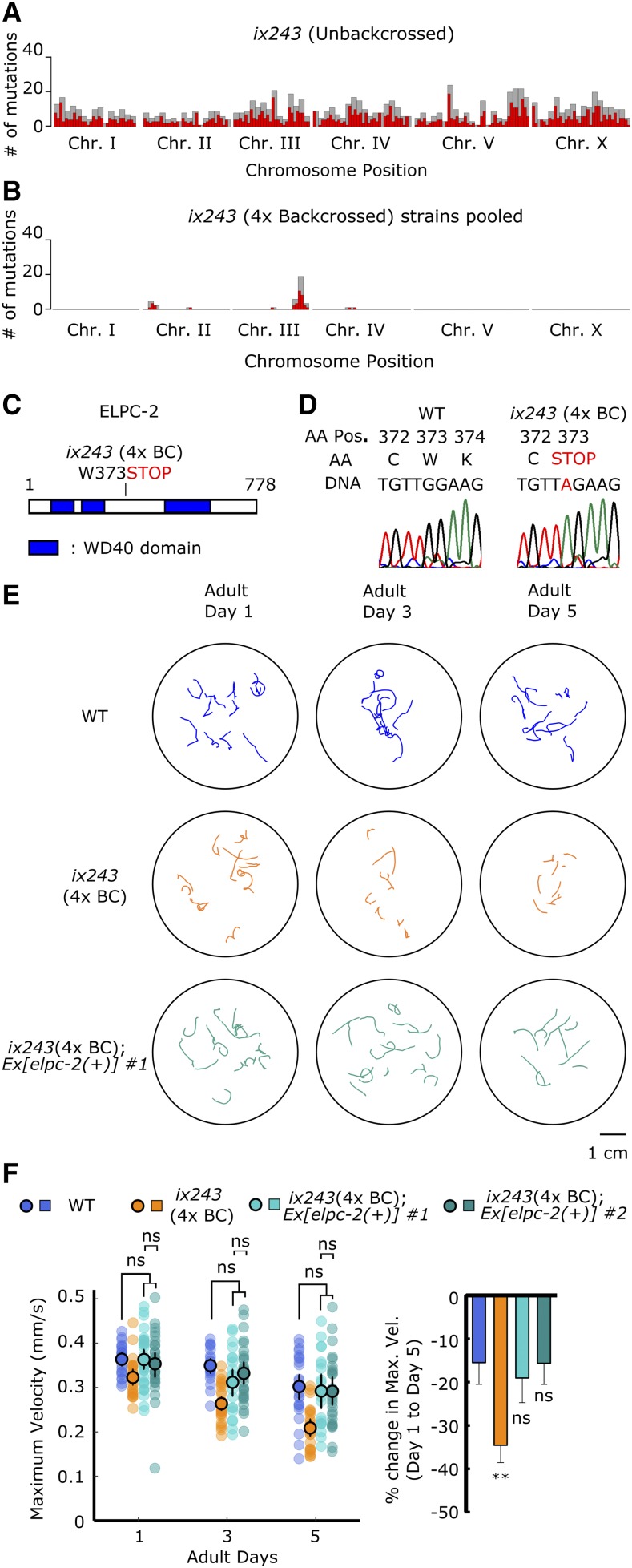
We used whole genome sequencing and a modified version of the sibling subtraction method to identify the causative mutation site in the ix243 strain (Fig. S5) (Joseph et al. 2018). Mutations were evenly induced on all chromosomes in the ix243 mutant strain before backcrossing (Figure 4A). Many mutations remained on Chromosome III after comparing mutations in backcrossed strains that show a progressive loss of adult locomotor ability and subtracting mutations in backcrossed strains that do not show progressive loss of adult locomotor ability (Figure 4B; Table S4). A nonsense mutation from TGG to TAG within the protein coding region of elpc-2 was predicted to disrupt protein function (Figure 4C; Table S4). Presence of the elpc-2 mutation site was confirmed by Sanger sequencing (Figure 4D).
Figure 4.
elpc-2 mutation causes locomotor deficits in the ix243 mutant strain (A) Mutation frequency along each chromosome of ix243 mutant strain before backcrossing. Red bars indicate 0.5-Mb bins and gray bars indicate 1.0-Mb bins. (B) Mutation frequency along each chromosome for pooled ix243(4x BC) worms. (C) Schematic diagram of ELPC-2 protein and location of mutation site in ix243 allele. (D) ix243 mutation site on ELPC-2 amino acid (AA) sequence and elpc-2 DNA sequence. (E) Representative locomotor tracks of WT, ix243(4x BC), and ix243(4x BC);Ex[elpc-2(+)] #1 worms. (F) (Left) Maximum velocities of WT, ix243(4x BC), ix243(4x BC);Ex[elpc-2(+)] #1, and ix243(4x BC);Ex[elpc-2(+)] #2 worms. n = 30–45 worms per strain for each day (10–15 worms from 3 biological replicate plates). (Right) Percent change in maximum velocity of worms from left panel. n = 3 biological replicate plates. Error bars indicate 95% confidence intervals. **P < 0.01; ns, not significant; One-way ANOVA with Dunnett’s post hoc test vs. WT.
To test whether loss of elpc-2 causes a progressive decline in locomotor ability, we injected a genomic fragment of elpc-2 including 2090-base pairs (bp) upstream of the start codon and 851-bp downstream of the stop codon in the ix243 mutant strain. The wild-type elpc-2 fragment rescued the progressive loss of adult locomotor ability (Figure 4E, 4F; Fig. S6A, B). These results suggest that elpc-2 is required for maintenance of adult locomotor ability in C. elegans. The ix243 mutant strain is the first reported mutant of the elpc-2 gene in C. elegans.
The Elongator complex is required to maintain locomotor ability
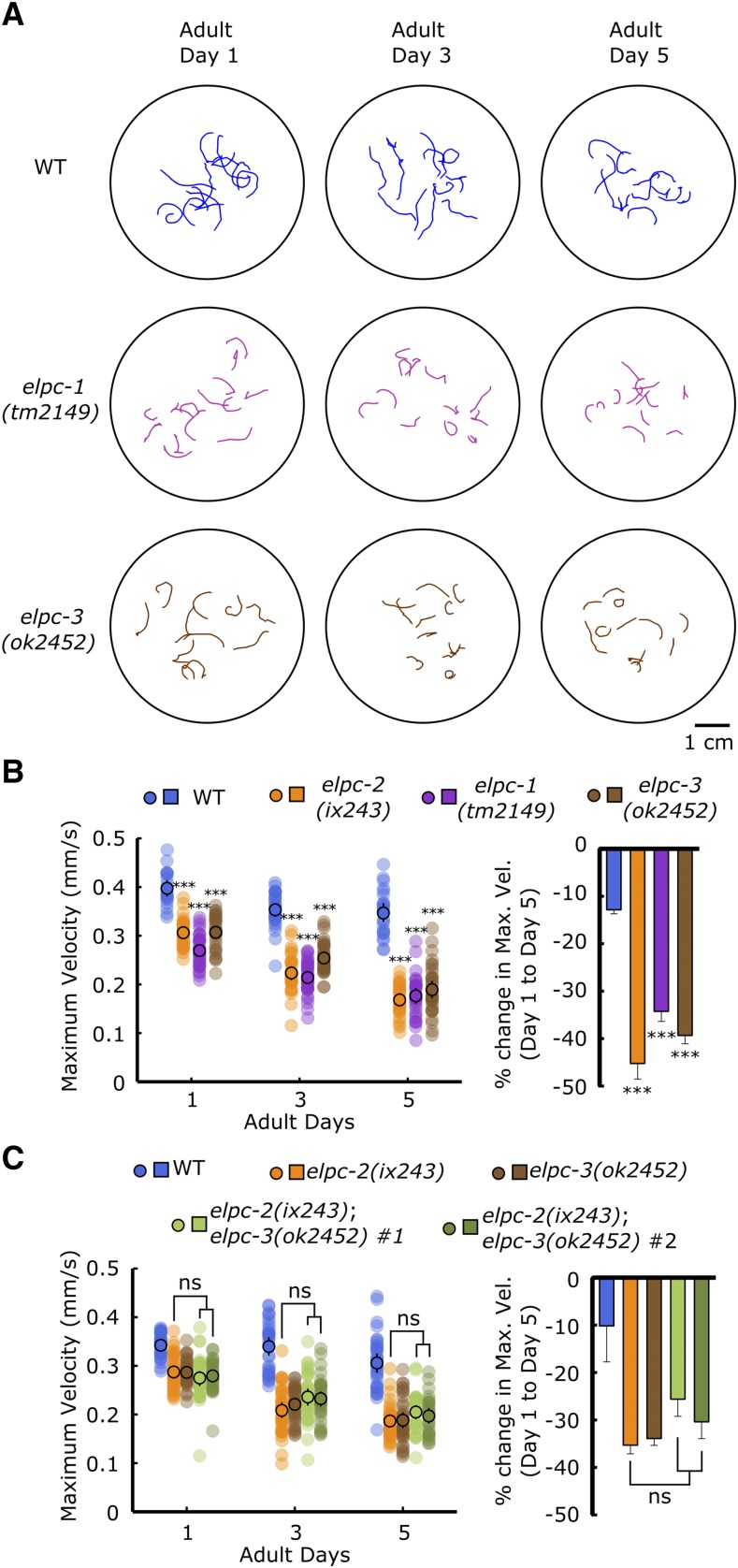
ELPC-2 is a component of the Elongator complex. In C. elegans, there are four predicted components of the Elongator complex (ELPC1–4) (Solinger et al. 2010). To test whether functional loss of elpc-2 causes the locomotor defect independently or as part of the Elongator complex, we measured locomotor ability of strains carrying deletions in elpc-1 and elpc-3. We found that elpc-1(tm2149) and elpc-3(ok2452) mutant strains also cannot maintain locomotor ability during adulthood (Figures 5A, 5B). elpc-1(tm2149);elpc-2(ix243) and elpc-2(ix243);elpc-3(ok2452) double mutants did not show additive deficiencies in locomotor ability (Figure 5C; Fig. S7A–H). These results suggest that proper functioning of the entire Elongator complex is necessary to maintain locomotor healthspan. We assessed the expression pattern of elpc-2 by creating an elpc-2p::GFP transcriptional reporter that expresses GFP under control of the elpc-2 promoter. The transcriptional reporter was broadly expressed in many tissues including head and body wall muscles, head neurons, pharynx, canal cell, coelomocytes, intestine, and tail (Fig. S8A–C). The expression pattern of elpc-2 overlaps with previously reported expression of elpc-1 in the pharynx, head neurons, and body wall muscles (Chen et al. 2009a).
Figure 5.
The Elongator complex is required to maintain locomotor ability (A) Representative locomotor tracks of WT, elpc-1(tm2149) and elpc-3(ok2452) worms. n = 10–15 tracks per plate. (B) (Left) Maximum velocities of WT, elpc-1(tm2149), and elpc-3(ok2452) worms. (Right) Percent change in maximum velocity of worms from left panel. (C) (Left) Maximum velocities of WT, elpc-2(ix243), elpc-3(ok2452), and elpc-2(ix243);elpc-3(ok2452) worms. (Right) Percent change in maximum velocity of worms from left panel. Error bars indicate 95% confidence intervals. For maximum velocity experiments, n = 30–45 worms per strain for each day (10–15 worms from 3 biological replicate plates). For percent change in maximum velocity graphs, n = 3 biological replicate plates. ***P < 0.001; ns, not significant; One-way ANOVA with Dunnett’s post hoc test vs. WT for B; One-way ANOVA with Tukey’s post hoc test for C.
Loss-of-function mutation in tut-1 also causes progressive decline in locomotor ability
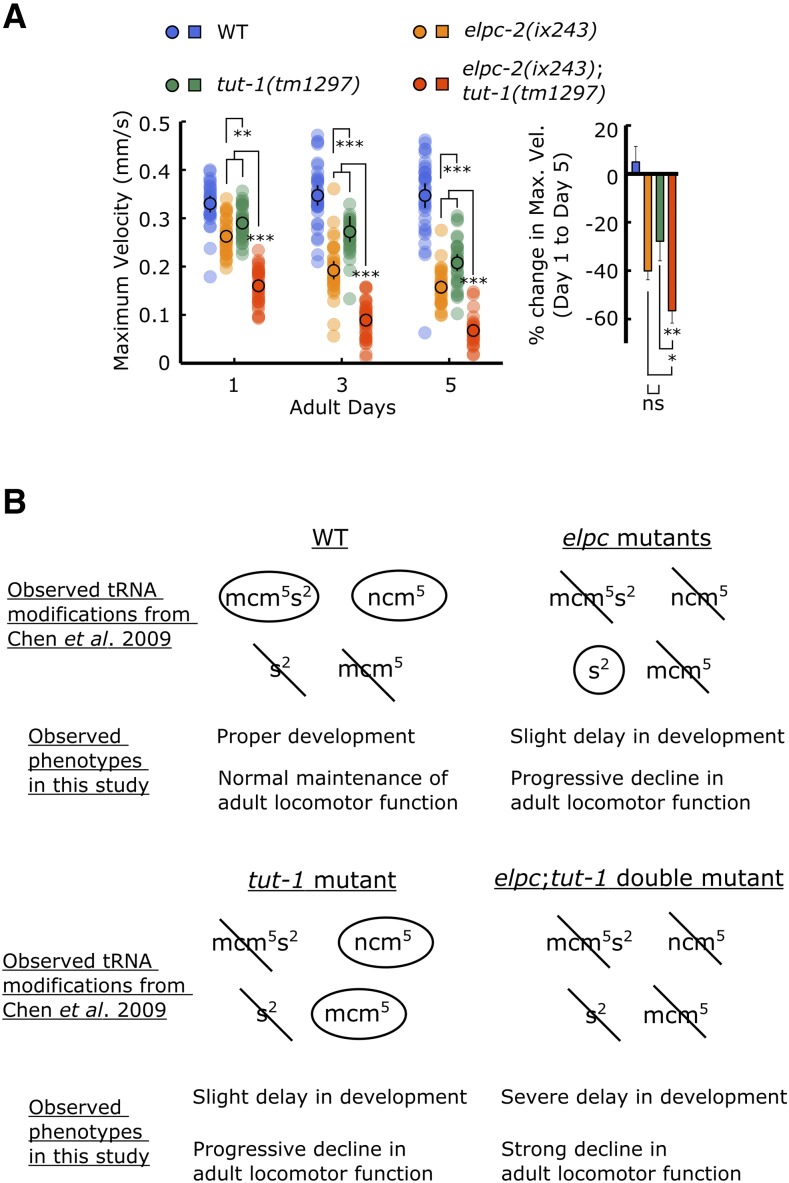
Mutants for elpc-1 and elpc-3 have previously been reported to modify the wobble uridine (U34) of tRNA by adding carbamoylmethyl (ncm) and methoxycarbonylmethyl (mcm) side chains to the 5′carbon of U34 (Chen et al. 2009a; Nedialkova and Leidel 2015). Wobble uridines with the mcm5 modification are further modified by TUT-1 to add a thio-group at the 2’ carbon to create mcm5s2U (Chen et al. 2009a). In wild-type worms, only ncm5 and mcm5s2 modifications are present (Chen et al. 2009a). In tut-1(tm1297) mutants, an mcm5 modification was observed, which is not normally present in wild-type worms (Chen et al. 2009a). In elpc mutants, an s2 modification was observed, which is not normally present in wild-type worms (Chen et al. 2009a).
To check whether loss of tRNA thiolation could cause a progressive decline in locomotor function, we measured the locomotor ability of tut-1(tm1297) mutant worms. tut-1(tm1297) mutant worms showed a significantly greater decline in locomotor ability during adulthood compared to wild-type worms, indicating that tRNA modifications may be a general mechanism involved in maintenance of locomotor healthspan in C. elegans (Figure 6A; Fig. S9A, B).
Figure 6.
tut-1(tm1297) mutant shows progressive decline in locomotor ability (A) (Left) Maximum velocities of WT, elpc-2(ix243), tut-1(tm1297), and elpc-2(ix243);tut-1(tm1297) worms. (Right) Percent change in maximum velocity of worms from left panel. Error bars indicate 95% confidence intervals. n = 30–45 worms per strain for each day (10–15 worms from 3 biological replicate plates). For percent change in maximum velocity graphs, n = 3 biological replicate plates. *P < 0.05; ***P < 0.001; ns, not significant; One-way ANOVA with Tukey’s post hoc test. (B) Summary of observed tRNA modifications in elpc and tut-1 mutants from Chen et al. (2009b), and summary of observed phenotypes in elpc and tut-1 mutants from this study.
The elpc-2(ix243);tut-1(tm1297) double mutant showed synthetic effects for locomotor ability and for developmental maturation. elpc-2(ix243);tut-1(tm1297) double mutant worms showed a strong defect in locomotor ability on the first day of adulthood and a significantly greater reduction in maximum velocity and travel distance during adulthood relative to either of the single mutants (Figure 6A; Fig. S9A, B). In addition, elpc-2(ix243);tut-1(tm1297) double mutant worms took almost twice as long to reach adulthood (145.4 h) compared to elpc-2(ix243) worms (80.2 h) or tut-1(tm1297) worms (82.0 h) (Tables S3 and S5). The synthetic effects may be explained by the complete absence of U34 modifications in the elpc-2(ix243);tut-1(tm1297) double mutant strain. The presence of the s2 modification in the elpc mutants, and the presence of the mcm5 and ncm5 modifications in the tut-1 mutant may enable partial tRNA functionality and allow relatively proper development and partial capacities to maintain locomotor ability (Figure 6B).
Discussion
In this study, we established the Edge Assay to simultaneously measure locomotor ability of up to a few hundred adult worms. For the present forward genetic screen, we used the Edge Assay to remove worms with strong developmental locomotor defects, and isolated worms with locomotor deficits that become apparent in adulthood. By carrying out the Edge Assay on the first day of adulthood, we were able to remove worms with strong developmental locomotor defects and overcome the difficulty of distinguishing developmental and progressive locomotor deficit mutants.
The Edge Assay may be used for a variety of applications involving locomotor ability. For example, the Edge Assay can be used in suppressor screens to search for mutant worms that show improvements in locomotor ability of previously characterized C. elegans models of neurodegenerative disease. It may also be possible to use the Edge Assay to screen for other types of progressive declines in functional capacity such as sensory or cognitive deficits by replacing the food ring with specific chemicals or learned cues.
The ix241 and ix243 mutant strains show similar declines in locomotor ability, but have different phenotypes in regard to lifespan. This suggests that genes that regulate lifespan and locomotor healthspan may not completely overlap. In terms of improving quality of life, genetic regulators of healthspan may be better therapeutic targets than regulators of lifespan. Further studies and genetic screens that focus on healthspan-related phenotypes may provide novel insights into mechanisms that regulate healthspan and quality of life across many species.
In the ix243 mutant strain, we found that elpc-2 is required to maintain locomotor healthspan, and works as part of the Elongator complex. The Elongator complex is an evolutionarily conserved protein complex that consists of six subunits in Saccharomyces cerevisiae, Arabidopsis thaliana, Mus musculus, and humans (Creppe and Buschbeck 2011; Dauden et al. 2017). ELP1–ELP3 form the core complex, and ELP4–ELP6 form a sub-complex (Creppe and Buschbeck 2011). In C. elegans, there are currently only four predicted homologs of the Elongator complex (elpc-1–4). From the present study, loss-of-function mutations in elpc-1, elpc-2, and elpc-3 caused a shortened locomotor healthspan. Proper functioning of the Elongator complex may require multiple or all components of the complex (Dauden et al. 2017). The present work suggests that the Elongator complex is essential in maintenance of locomotor healthspan.
Allelic variants of ELP3, the catalytic subunit of the Elongator complex, were found to be associated with amyotrophic lateral sclerosis (ALS) in three human populations (Simpson et al. 2009). Risk-associated alleles have lower levels of ELP3 in the cerebellum and motor cortex of ALS patients, and protection-associated alleles have higher levels of ELP3 (Simpson et al. 2009). Overexpression of ELP3 reduced levels of axonopathy in the SOD1A4V zebrafish model of ALS and SOD1G93A mouse model of ALS (Bento-Abreu et al. 2018). The present work complements studies that have been performed in the context of ALS, and suggests that loss of the Elongator complex alone can cause locomotor deficits during adulthood in C. elegans. Future therapies that target multiple subunits of the Elongator complex may provide more robust effects than therapies that target only the catalytic ELP3 subunit.
ELP2 mutations were reported as the causative mutations in a familial form of neurodevelopmental disability (Cohen et al. 2015). Patients who are compound heterozygotes for two different ELP2 missense mutations demonstrate a lack of motor control starting in early development, severe intellectual disability, and progressive loss of locomotor function (Cohen et al. 2015). In our newly isolated elpc-2(ix243) strain, we also see deficits in locomotor function on the first day of adulthood and a delay in development (Figure 3A, 3B; Fig. S3A, S3B; Table S3). Since the amino acid sequences of C. elegans ELPC-2 and human ELP2 are highly conserved (Fig. S10) (Sievers et al. 2011), some aspects of the neurodevelopmental dysfunctions that result from the human ELP2 mutation may be modeled in our elpc-2(ix243) mutant strain.
Mutations in ELP1 and ELP4 are also implicated with neuronal disorders. ELP1 is associated with familial dysautonomia, a neurodevelopmental disorder that affects the sensory and autonomic nervous system (Rubin and Anderson 2017). ELP4 is implicated in rolandic epilepsy, which is characterized by seizures that affect the vocal tract (Strug et al. 2009). Mutations in ELP1–4 all affect nervous system function, suggesting the importance of the Elongator complex in the development and maintenance of neurons. However, mutations in each ELP subunit cause different symptoms, suggesting that ELP subunits may have additional roles specific to neuronal subtype. Mice with complete loss of ELP1 or ELP3 are not viable (Chen et al. 2009b; Yoo et al. 2016), so it may be difficult to study the roles of each ELP subunit in mice. Since C. elegans ELP mutants are viable, it may provide an opportunity to explore the different functions of each ELP subunit.
The Elongator complex was originally identified as a transcriptional regulator associated with RNA polymerase II (Otero et al. 1999). However, follow-up studies have found that the main functions of the Elongator complex may involve tRNA modification (Chen et al. 2009a; Huang et al. 2005), and tubulin acetylation (Solinger et al. 2010). The tRNA thiolation mutant, tut-1(tm1297), also showed a progressive decline in locomotor function. Since tRNA modifications are important for proper translation and folding of proteins in yeast (Nedialkova and Leidel 2015), it may affect locomotor healthspan by regulating translation efficiency and protein folding in other organisms including C. elegans and humans.
C. elegans loses production of molecular chaperones at an early stage of adulthood (Ben-Zvi et al. 2009). Therefore, misfolded proteins that are produced by inefficient tRNA modifications may begin to accumulate during early adulthood and cause a progressive decline in locomotor ability. Regulation of proper tRNA modifications during aging may represent a new avenue to promote proteostasis and locomotor healthspan.
Starting from an unbiased forward genetic screen using C. elegans, we found that mutations in Elongator complex and tut-1 cause progressive declines in locomotor ability during adulthood. Future screening procedures that utilize the Edge Assay, and further analysis of the isolated mutants from the present screen may provide insights into how locomotor ability is maintained during adulthood.
Acknowledgments
We thank H. Goto, M. Kanda, M. Kawamitsu, S. Yamasaki and other DNA sequencing section members for technical assistance with whole genome sequencing. We are grateful to T. Murayama and E. Saita for helpful advice and support. We thank D. Van Vactor, B. Kuhn and members of the Maruyama unit for helpful discussions and comments. We are grateful to H. Ohtaki for administrative support. We thank the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), for providing worm strains. We also thank the National Bioresource Project (Japan) for providing worm strains. K. K. was supported by Japan Society for the Promotion of Science KAKENHI (Grant 16J06404).
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25387/g3.8150759.
Communicating editor: S. Lee
Literature Cited
- Bansal A., Zhu L. J., Yen K., and Tissenbaum H. A., 2015. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc. Natl. Acad. Sci. USA 112: E277–E286. 10.1073/pnas.1412192112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A., Miller E. A., and Morimoto R. I., 2009. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc. Natl. Acad. Sci. USA 106: 14914–14919. 10.1073/pnas.0902882106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento-Abreu A., Jager G., Swinnen B., Rué L., Hendrickx S. et al. , 2018. Elongator subunit 3 (ELP3) modifies ALS through tRNA modification. Hum. Mol. Genet. 27: 1276–1289. 10.1093/hmg/ddy043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenberg D., Von Kuster G., Coraor N., Ananda G., Lazarus R. et al. , 2010. Galaxy: A web-based genome analysis tool for experimentalists. Curr. Protoc. Mol. Biol. Chapter 19: Unit 19.10.1–Unit 19.10.21. 10.1002/0471142727.mb1910s89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M., Pahor M., Lauretani F., Zamboni V., Bandinelli S. et al. , 2009. Skeletal muscle and mortality results from the InCHIANTI study. Journals Gerontol. - Ser. A Biol. Sci. Med. Sci. 64: 377–384. 10.1093/gerona/gln031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-T., Hims M. M., Shetty R. S., Mull J., Liu L. et al. , 2009a Loss of Mouse Ikbkap, a Subunit of Elongator, Leads to Transcriptional Deficits and Embryonic Lethality That Can Be Rescued by Human IKBKAP. Mol. Cell. Biol. 29: 736–744. 10.1128/MCB.01313-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Tuck S., and Byström A. S., 2009b Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genet. 5: e1000561 10.1371/journal.pgen.1000561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P., Platts A., Wang L. L., Coon M., Nguyen T. et al. , 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w 1118; iso-2; iso-3. Fly (Austin) 6: 80–92. 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. S., Srivastava S., Farwell K. D., Lu H.-M., Zeng W. et al. , 2015. ELP2 is a novel gene implicated in neurodevelopmental disabilities. Am. J. Med. Genet. A. 167: 1391–1395. 10.1002/ajmg.a.36935 [DOI] [PubMed] [Google Scholar]
- Creppe C., and Buschbeck M., 2011. Elongator: an ancestral complex driving transcription and migration through protein acetylation. J. Biomed. Biotechnol. 2011: 924898 10.1155/2011/924898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauden M. I., Kosinski J., Kolaj‐Robin O., Desfosses A., Ori A. et al. , 2017. Architecture of the yeast Elongator complex. EMBO Rep. 18: 264–279. 10.15252/embr.201643353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardine B., Riemer C., Hardison R. C., Burhans R., Elnitski L. et al. , 2005. Galaxy: A platform for interactive large-scale genome analysis. Genome Res. 15: 1451–1455. 10.1101/gr.4086505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidalevitz T., Krupinski T., Garcia S., and Morimoto R. I., 2009. Destabilizing protein polymorphisms in the genetic background direct phenotypic expression of mutant SOD1 toxicity. PLoS Genet. 5: e1000399 10.1371/journal.pgen.1000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goecks J., Nekrutenko A., and Taylor J., 2010. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 11: R86 10.1186/gb-2010-11-8-r86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewiel M. S., Martin I., Bhandari P., and Cook-Wiens E., 2005. Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 4: 372–397. 10.1016/j.arr.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Hahm J.-H., Kim S., DiLoreto R., Shi C., Lee S.-J. V. et al. , 2015. C. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Nat. Commun. 6: 8919 10.1038/ncomms9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Johansson M. J. O., and Byström A. S., 2005. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 11: 424–436. 10.1261/rna.7247705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa H., Yu S., Xue J., and Driscoll M., 2010. Novel EGF pathway regulators modulate C. elegans healthspan and lifespan via EGF receptor, PLC-γ, and IP3R activation. Aging Cell 9: 490–505. 10.1111/j.1474-9726.2010.00575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B. B., Blouin N. A., and Fay D. S., 2018. Use of a Sibling Subtraction Method for Identifying Causal Mutations in Caenorhabditis elegans by Whole-Genome Sequencing. G3 (Bethesda) 8: 669–678. 10.1534/g3.117.300135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice J. N., Carter C. S., Beck H. J., Gioscia-Ryan R. A., McQueen M. et al. , 2014. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Omaha) 36: 583–595. 10.1007/s11357-013-9589-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koboldt D. C., Chen K., Wylie T., Larson D. E., McLellan M. D. et al. , 2009. VarScan: Variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics 25: 2283–2285. 10.1093/bioinformatics/btp373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., and Durbin R., 2009a. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J. et al. , 2009b. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod A. R., Karn J., and Brenner S., 1981. Molecular analysis of the unc-54 myosin heavy-chain gene of Caenorhabditis elegans. Nature 291: 386–390. 10.1038/291386a0 [DOI] [PubMed] [Google Scholar]
- Maruyama I. N., and Brenner S., 1991. A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 88: 5729–5733. 10.1073/pnas.88.13.5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minevich G., Park D. S., Blankenberg D., Poole R. J., and Hobert O., 2012. CloudMap: a cloud-based pipeline for analysis of mutant genome sequences. Genetics 192: 1249–1269. 10.1534/genetics.112.144204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedialkova D. D., and Leidel S. A., 2015. Optimization of Codon Translation Rates via tRNA Modifications Maintains Proteome Integrity. Cell 161: 1606–1618. 10.1016/j.cell.2015.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum-Krammer C. I., Neto M. F., Brielmann R. M., Pedersen J. S., and Morimoto R. I., 2015. Investigating the Spreading and Toxicity of Prion-like Proteins Using the Metazoan Model Organism C. elegans. J. Vis. Exp. 95: 52321 10.3791/52321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero G., Fellows J., Li Y., Bizemont De, Dirac A. M. et al. , 1999. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol. Cell 3: 109–118. 10.1016/S1097-2765(00)80179-3 [DOI] [PubMed] [Google Scholar]
- Reiner D. J., Newton E. M., Tian H., and Thomas J. H., 1999. Diverse behavioural defects caused by mutations in Caenorhabditis elegans unc-43 CaM kinase II. Nature 402: 199–203. 10.1038/46072 [DOI] [PubMed] [Google Scholar]
- Rubin B. Y., and Anderson S. L., 2017. IKBKAP/ELP1 gene mutations: Mechanisms of familial dysautonomia and gene-targeting therapies. Appl. Clin. Genet. 10: 95–103. 10.2147/TACG.S129638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F., Wilm A., Dineen D., Gibson T. J., Karplus K. et al. , 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7: 539 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C. L., Lemmens R., Miskiewicz K., Broom W. J., Hansen V. K. et al. , 2009. Variants of the elongator protein 3 (ELP3) gene are associated with motor neuron degeneration. Hum. Mol. Genet. 18: 472–481. 10.1093/hmg/ddn375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinger J. A., Paolinelli R., Klöß H., Scorza F. B., Marchesi S. et al. , 2010. The Caeborhabditis elegans elongator complex regulates neuronal alpha-tubulin acetylation. PLoS Genet. 6: e1000820 10.1371/journal.pgen.1000820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strug L. J., Clarke T., Chiang T., Chien M., Baskurt Z. et al. , 2009. Centrotemporal sharp wave EEG trait in rolandic epilepsy maps to Elongator Protein Complex 4 (ELP4). Eur. J. Hum. Genet. 17: 1171–1181. 10.1038/ejhg.2008.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team R. C., 2015. 2015 R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria: [Internet]. [Google Scholar]
- Tissenbaum H. A., 2012. Genetics, Life span, Health Span, and the Aging Process in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 67: 503–510. 10.1093/gerona/gls088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H., Son D., Jang Y.-J., and Hong K., 2016. Indispensable role for mouse ELP3 in embryonic stem cell maintenance and early development. Biochem. Biophys. Res. Commun. 478: 631–636. 10.1016/j.bbrc.2016.07.120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All isolated strains and plasmids are available upon request. DNA sequencing data are available on NCBI Sequence Read Archive: PRJNA530333. Supplemental material available at FigShare: https://doi.org/10.25387/g3.8150759.