Abstract
With steadily improving pregnancy and live birth rates, IVF over approximately the first two and a half decades evolved into a highly successful treatment for female and male infertility, reaching peak live birth rates by 2001–2002. Plateauing rates, thereafter, actually started declining in most regions of the world. We here report worldwide IVF live birth rates between 2004 and 2016, defined as live births per fresh IVF/ICSI cycle started, and how the introduction of certain practice add-ons in timing was associated with changes in these live birth rates. We also attempted to define how rapid worldwide ‘industrialization’ (transition from a private practice model to an investor-driven industry) and ‘commoditization’ in IVF practice (primary competitive emphasis on revenue rather than IVF outcomes) affected IVF outcomes. The data presented here are based on published regional registry data from governments and/or specialty societies, covering the USA, Canada, the UK, Australia/New Zealand (combined), Latin America (as a block) and Japan. Changes in live birth rates were associated with introduction of new IVF practices, including mild stimulation, elective single embryo transfer (eSET), PGS (now renamed preimplantation genetic testing for aneuploidy), all-freeze cycles and embryo banking. Profound negative associations were observed with mild stimulation, extended embryo culture to blastocyst and eSET in Japan, Australia/New Zealand and Canada but to milder degrees also elsewhere. Effects of ‘industrialization’ suggested rising utilization of add-ons (‘commoditization’), increased IVF costs, reduced live birth rates and poorer patient satisfaction. Over the past decade and a half, IVF, therefore, has increasingly disappointed outcome expectations. Remarkably, neither the profession nor the public have paid attention to this development which, therefore, also has gone unexplained. It now urgently calls for evidence-based explanations.
Keywords: IVF, live birth rate, elective single embryo transfer, PGS, preimplantation genetic testing for aneuploidy, embryo banking, blastocyst-stage embryo transfer, all-freeze cycles
Introduction
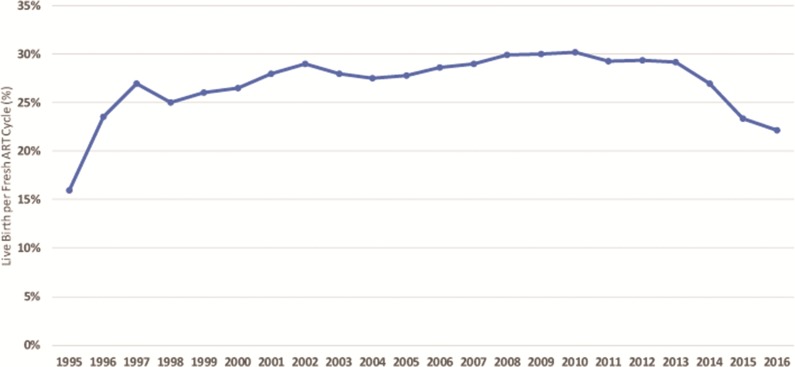
US live birth rates, defined as live birth rates per fresh cycle of IVF or ICSI started, almost steadily improved until 2001–2002, declined between 2003 and 2007, reached a new high in 2008, plateaued till 2010 and have since been steadily declining, especially profoundly between 2013 and 2016 (Fig. 1). Similar trends have also been observed in other regions of the globe, including Canada, Australia/New Zealand (reporting combined) and especially Japan (for detailed figures and analyses the reader is referred to Kushnir et al., 2017a). Quite surprisingly, these developments have, however, neither attracted attention from the medical profession nor public, even though live birth rates in the USA by 2016 had fallen to levels not seen since 1998 (Centers for Disease Control and Prevention, 2016).
Figure 1.
US annual live birth rates following fresh autologous IVF cycles from 1995 to 2016. The data for this figure are derived from annual CDC ART Success Rate Reports (www.cdc.gov/art/artdata/), accessed 10 August 2018 and again 7 March 2019. Live birth rates demonstrate almost steady improvements until 2002, a decline between 2003 and 2007, reaching a new peak similar to that in 2002 between 2008 and 2010, only to again decline by 2016 to rates not seen since 1998.
The declines in IVF live birth rates pointed out here represent a long-term reversal in a previously consistent trend of outcome improvements. They, therefore, also are a formal rebuttal of what patients’ primary desires are in IVF cycles, reported as timely conception and delivery (Scotland et al., 2007; Schaller et al., 2016). Exploring why these declines are happening, therefore, appears overdue. In presenting here the first results of such an investigation, we attempted to associate changes in IVF practice in different regions of the world with observed declines in live birth rates.
Background
On 25 July 2018, the world celebrated the 40th anniversary of the 1978 birth of the first ‘test-tube baby’ (Steptoe and Edwards, 1978). Initially an experimental procedure with very limited outcomes, IVF quickly developed into a clinically and economically highly successful medical sub-specialty area. In the USA, IVF primarily evolved in private practice settings outside of academia because the procedure was by Congress implicitly excluded from most government-funded research. Economic competition between centers based on pregnancy and live birth outcomes, therefore, became a principal reason for quick outcome improvements. Except for a short plateau in live birth rates in the mid-1990s, IVF outcomes, therefore, steadily improved until approximately 2001–2002 (Fig. 1).
By the mid-1990s, some US states (MA, IL, VA, CA) had mandated varying levels of IVF insurance coverage for IVF. Insurance carriers in those states, therefore, started contracting with preferred medical providers—in the process creating a first generation of mega-IVF centers. Controlling large local market shares, these IVF centers were largely relieved from outcome-driven competition but now faced significant cost-based competition. As Fig. 1 demonstrates, this led to first ever observed evidence that ‘industrialization’ of IVF may lead to negative outcome consequences, as national live birth data between 1997 and 1999 for the first time ever flattened. On an interesting side note, US live birth rates in 2016, the last year of available national data, fell to exactly the same low levels as seen during those 2 years (see also later discussion).
Outcome improvements, however, resumed, reaching a new peak around 2001–2002. As Fig. 1 further demonstrates, US live birth rates then again dipped between 2002 and 2008, recovered between 2008 and 2010 and have, ever since, fallen precipitously to the lowest levels since 1998. Especially after 2010, rates truly plummeted, strongly suggesting that significant changes in national US IVF practice must have occurred to cause such significant changes in national live birth rates.
Fresh autologous IVF cycles in the USA, nevertheless, continued to outperform other regions of the world (Kushnir et al., 2017a), with greater outcome-dependent competition between US centers, possibly, a contributing factor. US society apparently values individual pregnancy success very highly, while, for example, many European countries favor more a social construct of national health care priorities (Gleicher et al., 2007).
Looking at the world over decades, consistency of observed geographic variations in fresh autologous IVF cycle outcomes between regions is, indeed, remarkable (Kushnir et al., 2017a). One, therefore, must assume that observed differences in macro-health are caused by how local health care systems provide services. When, despite such varying health care systems, however, similar declines in live birth rates are observed at approximately similar time periods, it is reasonable to assume that those declines must be associated with new practice changes that affected IVF worldwide. We, therefore, attempted to identify practice changes that may be causally associated with universally observed declines in live birth rates in different regions of the world.
Methods
The here-discussed autologous IVF outcome data, mostly representing live birth rates for the 12 years between 2004 and 2016 and defined as live births per fresh IVF/ICSI cycle start, reflect distinctively different racial and ethnic patient populations in the USA, Canada, the UK, Australia/New Zealand (combined), Latin America (as a block) and Japan, either based on national data sets or outcome data reported by regional fertility societies. Data sources used were retrieved from publicly available local registries of either government agencies and/or regional professional societies (Kushnir et al., 2017a) and, where applicable, are referenced. Though the original intent was also to include annual data sets published by ESHRE, those data, ultimately, had to be removed since the year-to-year variations in reporting countries varied too much to allow for reliable conclusions.
Add-ons to IVF practice that were investigated included extended embryo culture to blastocyst stage (BSC), other BSC-dependent new procedures, including mild stimulation, elective single embryo transfer (eSET), PGS (preimplantation genetic testing for aneuploidy: PGT-A), all-freeze cycles and embryo banking. We, furthermore, investigated time associations between IVF results and ‘industrialization’, defined as transition from a private practice model into an investor-driven industry, and for ‘commoditization’, defined as primary emphasis on revenue rather than IVF outcomes. Effects of ‘industrialization’ and ‘commoditization’ were in detail only investigated in Australia/New Zealand, where these developments have been most advanced, with only three companies controlling a lion share of the national IVF market. Since the medical literature until very recently did not comment on this subject, our sources for these associations were primarily media reports, and they are referenced.
Time associations are hypothesis generating and not proof of causation. Persistently observed associations with identical treatment changes in different regions of the world, however, do suggest potential causality. They, however, still require independent verification to prove cause and effect.
Results
Why IVF outcomes, likely, have declined
Kushnir et al. (2017a) recently reported live birth rates for various regions till 2014, including the USA, Canada, the UK, Latin America, Australia and New Zealand (combined) and Japan. We here, when available, added the years 2015–2016 for some regions (the USA, Japan and Australia/New Zealand). European data, other than the UK’s, were not included. Kushnir and colleagues reported impressive gains in Canadian live birth rates until 2009–2010. In subsequent years, the Canadian live birth rate, however, remarkably declined. US rates by 2016, indeed, fell to levels not seen since 1998 (Fig. 1).
BSC and eSET
Looking for associated events that could explain Canada’s sudden drop in birth rates, Canada’s most populous province, Québec, in 2010 introduced legislation that initiated funding of IVF, while restricting the number of embryos that could be transferred, strongly promoting the use of eSET. By 2011, the programme was hailed as a success for significantly reducing twin pregnancy rates (Bissonnette et al., 2011), while others noted that pregnancy rates had precipitously dropped and that 33.1% fewer IVF infants had been born following a directed practice change to greater utilization of eSET (Gleicher, 2011).
Kushnir et al. (2017a) also explored eSET utilization around the world and demonstrated uniform increases in eSET utilization during the study period. Australia/New Zealand and Japan were the two world regions demonstrating the largest increases in eSET utilization, while, starting in 2007, Japan, the UK and Canada demonstrated the most rapid increases.
Recently extensively reviewed (Adashi and Gleicher, 2017), we here do not intend to address controversies surrounding eSET. That eSET, in comparison to double embryo transfer, reduces clinical pregnancy rates is well established (McLernon et al., 2010). Observed associations between declining live birth rates and compensatory increases in IVF cycles started also support a likely association between growing eSET utilization and declines in live birth rates (Kushnir et al., 2017a).
Evidence of such an association is nowhere more obvious than in Japan. Already in 2004 demonstrating the lowest fresh cycle live birth rates in the world, the country’s rates further plummeted between 2004 and 2008, only to experience further small declines in 2009–2016, reaching and maintaining over these years an astonishing live birth rate with reference to cycles started of only ~5.0%. Japan, over the here presented study period, thus lost two-thirds of its already very low 2004 live birth rate (Kushnir et al., 2017a). This decline coincided with introduction of the so-called ‘Kato protocol’, first published in 2007 (Teramoto and Kato, 2007) but widely known, and copied in the Japanese IVF community since 2002–2003. The protocol was defined by mild ovarian stimulation, BSC and eSET and quickly became the dominant IVF protocol in all of Japan.
Demonstrating a likely association between declining live birth rates and increasing IVF cycle starts remarkably well, Japan compensated for the loss of two-thirds of her live births between 2004 and 2013 (from ca. 15% to ca. 5%), by tripling the cycles started (Kushnir et al., 2017a).
A quantitatively less radical but similar association is offered by Australia/New Zealand. These two countries doubled their utilization of BSC and eSET between 2004 and 2013 to a world-leading 80% of fresh IVF cycles (only Japan reached similar eSET utilization). Only outperformed by Japan, Australia/New Zealand since 2004 also persistently demonstrated the second lowest IVF live birth rates among regions of the world here investigated. Australia/New Zealand also demonstrated the same mirror image between falling live birth rates and increasing cycle starts (Kushnir et al., 2017a).
Proponents of eSET argue that lower live birth rates after eSET are largely compensated by improving outcomes with, and increasing birth rates of, frozen-thawed second cycles (Pandian et al., 2013). This argument is based on two reported observations: cumulative pregnancy and live birth rates from fresh plus one thaw cycle match, or even slightly exceed, rates from only a fresh IVF cycle (Pandian et al., 2013) and elective transfers of frozen embryos in a later cycle produce improved pregnancy and live birth rates in comparison to fresh transfers in stimulated cycles (Wei et al., 2019).
Both arguments are, however, incorrect: first, no patient can ever be guaranteed an opportunity for a subsequent frozen-thawed cycle. Moreover, second cycles increase cost and length to conception; second, arguments that pregnancy and live birth rates are improved by avoiding fresh transfers in stimulated cycles and delaying them into subsequent frozen-thawed cycles are based on inappropriate patient selection and, therefore, at best, only apply to good prognosis patients. This was recently well demonstrated by two mega-studies from China, where one reported improved IVF outcomes with frozen transfers (Wei et al., 2019) and the other demonstrated absolutely no outcome differences (Shi et al., 2018). Both studies were multi-center studies, conducted in China on a predominantly Han population. The only difference was that the first study compared blastocyst-stage transfers, where the study design excluded poorer prognosis patients whose embryos cannot reach blastocyst-stage in culture, while the second study performed transfers at cleavage stage. In unselected patient populations, deferred embryo transfer after embryo freezing, therefore, does ‘not’ improve IVF outcomes. Indeed, it is reasonable to assume that this practice in poor prognosis patients, whose embryos survive freezing and thawing poorly, may actually adversely affect IVF outcomes. In addition, here too, a second cycle is needed, increasing costs and potentially delaying time to pregnancy.
Being pioneering countries in basic reproductive biology research, the poor clinical performance of clinical IVF in countries like Japan, Australia/New Zealand and Canada is puzzling but emblematic of how difficult it can be to convert excellent basic science research into equally excellent clinical practice. That these clinical data were produced in scientifically highly developed countries, however, allows for the conclusion that the observed relatively poor IVF outcomes were not a consequence of technical weaknesses in local laboratories. These outcomes, therefore, with great likelihood must be the consequence of specific changes in clinical practice patterns. Interestingly, in the USA extended routine BSC and eSETs also started to increase in popularity around 2010 (Kushnir et al., 2017a), exactly when live birth rates initiated their precipitous decline (Fig. 1).
Other methods of embryo selection
BSC is also an essential component of other embryo selection methods, such as PGS/PGT-A, closed embryo incubation with time lapse imaging, embryo banking and all-freeze protocols. They all represent relatively new add-ons to IVF, characterized by questionable clinical utility (Harper et al., 2017). They also all share potentially negative effects on IVF outcomes.
Adverse effects will be even more obvious if add-ons are used indiscriminately and, especially, if they are used on patients of advanced age and/or with low functional ovarian reserve. This is, however, exactly what has been happening. As there is no evidence that BSC improves cumulative pregnancy rates (De Vos et al., 2016; Glujovsky et al., 2016) or improves implantation rates in first transfers in any patient population but good prognosis patients, BSC, developed as an embryo selection method in good prognosis patients, for no obvious reasons evolved in many IVF laboratories into a routine embryo culture practice for almost everybody. In the USA, 2016 Centers for Disease Control and Prevention (CDC) data demonstrate that approximately two-thirds of fresh autologous IVF cycles utilized BSC (Centers for Disease Control and Prevention, 2016). Routine BSC was recently also questioned based on biological concerns (Maheshwari et al., 2016).
The most controversial embryo selection method is currently, however, PGS/PGT-A. After version 1.0 was found ineffective (Practice Committee of the ASRM, 2008), currently used versions, 2.0 and 3.0, result in high numbers of false-positive diagnoses (Paulson, 2017). PGS/PGT-A was recently also, once again, declared ineffective in improving IVF outcomes in a combined statement from the Practice Committees of the American Society for Reproductive Medicine and Society for Assisted Reproductive Technology (2018). Because of the high false-positivity rate, a large number of perfectly normal embryos are now routinely discarded which, if transferred, in surprisingly high percentages still would result in normal births (Gleicher et al., 2015, 2016a; Greco et al., 2015; Morales et al., 2016; Munné et al., 2017; Victor et al., 2019). Especially in poorer prognosis patients, PGS/PGT-A affects IVF pregnancy chances negatively. Recent national US outcome data also suggest that in unselected patient populations, PGS/PGT-A actually reduces live birth rates (Kushnir et al., 2016a).
Mild ovarian stimulation
This IVF modification was already addressed within the context of the ‘Kato protocol’, which by design produces greatly reduced egg numbers in comparison to standard ovarian stimulations (Kushnir et al., 2016b, 2017a). After a woman’s age, transferrable embryo numbers are, however, the best predictors of IVF success (Gleicher et al., 2016b). Mild stimulations, therefore, appears counterintuitive, even if used separate from the ‘Kato protocol’ and eSET.
IVF cycle interruptions: embryo banking
The hypothesis of embryo banking is that, especially in poor prognosis patients (who usually produce few oocytes and embryos), sequential freeze-all cycles offer clinical and/or financial benefits by delaying embryo transfer or PGS/PGT-A until sufficient eggs/embryos have been accumulated. In the USA, embryo banking has led to major distortions in national outcome reporting (Kushnir et al., 2016b). A recent study of 10 outlier centers with especially high percentages of embryo banking cycles demonstrated corrected live birth rates for this group of centers that were below the median of all other remaining US centers (Kushnir et al., 2016b, 2017b).
Embryo banking has grown exponentially since 2007 but the fastest in the 2010–2011 period (Kushnir et al., 2017a,b), corresponding to first declines in US live birth rates (Fig. 1). Since the CDC registry did not consider cycles that did not reach embryo transfer, declines in US live births after 2010 are, therefore, likely actually underreported, even further increasing already highly disturbing declines in US live birth rates in recent years (Kushnir et al., 2017a and Fig. 1).
Industrialization and commoditization of IVF
The investment community has over the past 10–15 years come to view infertility, and especially the practice of IVF, as a potential growth industry. The sector, therefore, has been attracting significant outside equity, with IVF centers, and companies serving those centers, being bought up by corporate interests at an increasingly rapid pace—a process we here describe as the ‘industrialization’ of IVF.
Such attempts started in the USA in the 1990s, when the first mega-IVF centers were established, and Wall Street promoted as market leaders in medical specialty areas the concept of so-called physician practice management companies. Disappointing earnings, however, ended quickly what later by economists was called the ‘physician practice management bubble’ (https://www.beckershospitalreview.com/hospital-physician-relationships/physician-practice-management-a-new-chapter.html, 10 January 2019, date last accessed) and a large majority of so-created companies in many different medical specialties, including infertility and IVF, disappeared as quickly as they had initially appeared on the scene.
Aggregations of fertility centers into large provider networks started again in Australia and Europe and regained popularity in the USA only more recently. Over the past 5–6 years, investor-driven companies and entrepreneurs, however, invested in the USA hundreds of millions of dollars into the sector. The women’s health care unit of CooperSurgical (Trumbull, CT, USA), alone, spent close to $500 million, building during 2015–2016 an IVF-driven laboratory and supply conglomerate, by purchasing leading national PGS laboratories (and their offspring in the UK), the Danish company K-System Kivex Biotech A/S (Denmark), the world’s dominant equipment supplier to IVF clinics for closed incubation systems with time lapse imaging, and Wallace (Denmark), the IVF segment of Smith Medical, a major supply company for IVF laboratories.
Consolidation is, however, most advanced in the Australia/New Zealand market, where only three IVF companies control 80% of IVF cycles (IBIS World, 2015). Increasing complaints about rising IVF costs evoked media scrutiny, by 2013 suggesting ‘the new business model, driven largely by profit targets, could undermine the integrity of fertility medicine’ (Medew and Baker, 2013). The industry’s defense that rising costs for research and outcome improvements mandated those increases was contradicted by disappointingly low live birth rates of only ca. 15% in those two countries (Kushnir et al., 2017a). Australia’s IVF industry recently also found itself severely admonished by the ‘Australian Competition and Consumer Commission’ over misleading success rate claims by some of the country’s major IVF clinics (Morris, 2016).
In Australia and New Zealand private equity investments started flowing in 2006–2007, leading to quick consolidation and ‘industrialization’. From that point on, Australia/New Zealand’s fresh live birth rates have been steadily declining (Kushnir et al., 2017a). This region’s developments, therefore, likely offer the best example of what a future of consolidation and ‘industrialization’ may look like in the IVF field. Especially considering Australia’s and New Zealand’s longstanding reputations as early cradles of successful IVF research, rising cycle costs, declining live birth rates and increasing ethical concerns do not suggest an evolution that warrants copying.
‘Industrialization’ in the UK, Spain, Germany/Austria and, of course, the USA is speeding up as well: in the USA, a leading private IVF center recently merged with Spain’s ‘Instituto Valenciano de Infertilidad’, likely the world’s largest IVF company, which also owns centers in other European countries, Latin America, the Middle East and India. Via this merger, the company became the first foreign company to obtain a major foothold in the US IVF market. Its goal is continuous US expansion (IVI Press Release, 2017). Since then, a number of mainland Chinese and Korean financial interests have also entered the US IVF market.
As ‘Forbes’ reported (Helft, 2016), serial entrepreneur Martin Varsavsky, with a $200 million budget, launched ‘Prelude’, described as a ‘comprehensive fertility company focused on proactive fertility care to improve people’s chances of having healthy babies when they’re ready’ in a four-step process: fertility preservation through freezing of eggs and sperm at peak fertility (ages 20s to early 30s); embryo creation in the laboratory when clients are ready to start a family (in other words, what is proposed is the end of conception via intercourse), and eggs and sperm are unfrozen and embryos are created; genetic screening, where embryos are tested for chromosomal abnormalities and genetic mutations before transfer; and eSET.
Prelude is, thus, a remarkable accumulation of recent add-ons to IVF which, in this communication and elsewhere (Harper et al., 2017), have been described as ineffective and, likely, in selected patients even as detrimental to outcomes of IVF cycles. To offer these invasive and costly treatments to perfectly healthy young women and men, therefore, raises serious ethical questions since none of these individual steps has so far been validated as clinically effective and/or cost-effective. Currently available outcome data on social oocyte cryopreservation are, indeed, still considered inadequate (Hammarberg et al., 2017). Yet, ‘Prelude’ has become the largest accumulator of fertility clinics in the USA and, therefore, offers yet another example of why rapid ‘industrialization’ and ‘commoditization’ of IVF must be viewed with serious concern.
Concerns like this do not only exist in the IVF arena. Similar expressions of concern have, indeed, recently also come up with entry of private equity investments into dermatology (Konda et al., 2018) and for US clinical medicine in general (Gondi and Song, 2019).
Discussion
The here presented time associations between changes in practice patterns and outcome changes in multiple different regions of the world appear surprisingly robust. They, therefore, are convincing in suggesting that ineffective and/or even harmful additions to IVF practice have been added in recent years. Add-ons qualifying for such consideration are summarized in Table I. Additional potential causes for declining live birth rates, not named here, are likely. For example, ages of infertile women in IVF centers have been rapidly increasing (Gleicher et al., 2016c), suggesting that the proportion of good prognosis patients may be declining, while poor prognosis patients are increasing. Aging trends, however, only further emphasizes the importance of the here-reported associations, since the here-proposed causes for worldwide declining live birth rates are especially harmful for poorer prognosis patients, which includes older women.
Table I.
Newly introduced additions that may have adversely affected IVF outcomes.
| Newly identified additions |
|---|
| Embryo selection |
| Extended embryo culture to blastocyst |
| Elective single embryo transfer |
| Closed embryo incubation systems with time lapse imaging |
| PGS* |
| Mild ovarian stimulations |
| ‘Mini-IVF’ |
| Natural cycle IVF |
| Patient-friendly IVF |
| Cycle interruptions |
| All-freeze cycles with frozen-thawed cycle to follow |
| Embryo banking with combined frozen-thawed cycle to follow |
*Now also called PGT-A (preimplantation genetic testing for aneuploidy).
Our study also offers a warning about ‘industrialization’ and ‘commoditization’ of medical services for other economically successful private practice areas in medicine. ‘The New York Times’, for example, recently reported that the rapid ‘industrialization’ of dermatology practices in the USA, apparently, also has resulted in significant ‘commoditization’ in that respective practice arena (Hafner, 2018). As previously noted, the dermatology literature has also taken notice of this fact (Konda et al., 2018). As especially Australia’s experience suggests, such developments may lead to higher prices, poorer clinical outcomes and decreasing patient satisfaction. During current national discussions to rebuild the US health care system, these observations deserve special consideration.
Limitations and strength of the study
This study has a number of limitations, the most obvious being that associations do not establish causations. Another limitation is the absence of European data except for UK data. Finally, here-reported associations with ‘industrialization’ and ‘commoditization’ are limited to one world region, Australia/New Zealand, though some here discussed more peripheral developments in the USA also suggest adverse outcomes on practice from commercial developments. With practically complete absence of scientific investigations in the medical literature on these subjects, this analysis had to largely rely on data from the lay media. Two very recent publications, however, demonstrate awakening interest in the medical community regarding this subject (Konda et al., 2018; Gondi and Song, 2019). This manuscript, hopefully, will provide an incentive to conduct follow-up studies in the IVF arena.
The obvious primary strength of this study is the parallel observation of IVF outcomes in many regions of the world, thereby eliminating potential genetic/ethnic contributions to possible observations.
Key recommendations
Recommendations from this study include a need to:
-
(i)
more carefully consider changes to routine IVF practice before clinical implementation;
-
(ii)
conduct prospectively randomized studies of the effects of recent add-ons to IVF practice on IVF cycle outcomes in order to confirm or refute the observed associations in the present study; and
-
(iii)
urgently investigate the effects of ‘industrialization’ and ‘commoditization’ on IVF outcomes, as both are rapidly accelerating in most regions of the world.
Authors’ roles
This manuscript was conceived by N.G. who also wrote the first manuscript draft. Some regional fresh cycle live birth rates discussed in this manuscript were previously published by Kushnir et al. (2014) and modified with permission for this manuscript by S.K.D. and V.A.K. All authors contributed significant revisions to later versions of the manuscript and approved the final version before submission.
Funding
Center for Human Reproduction; Foundation for Reproductive Medicine.
Conflict of interest
N.G., D.H.B. and V.A.K. are listed as co-owners of a number of already awarded and still pending US patents, none related to the topic of this manuscript. N.G. is a shareholder in Fertility Nutraceuticals, LLC and owner of the CHR. N.G. and D.H.B. receive patent royalties from Fertility Nutraceuticals, LLC. N.G., D.F.A. and D.H.B. received research support, travel funding and lecture fees from various Pharma and medical device companies, none, in any way related to this manuscript. This study was funded by intramural funds from the Center for Human Reproduction (CHR) and by grants from the Foundation for Reproductive Medicine. V.A.K. previously served as a consultant to the Centers for Disease Control and Prevention (CDC). The CHR annually routinely reports IVF outcome data to CDC and Society for Assisted Reproductive Technology.
Data availability statement
The referenced source data are available from the referenced data sources in this manuscript and from our center’s research data bank by contacting Ms Jolanta Tapper (jtapper@thechr.com).
References
- Adashi EY, Gleicher N. Is a blanket elective single embryo transfer policy defensible? Rambam Maimonides Med J 2017;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette F, Phillips SJ, Gunby J, Holzer H, Mahutte N, St-Michel P, Kadoch IJ. Working to eliminate multiple pregnancies: a success story in Québec. Reprod Biomed Online 2011;23:500–504. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Assisted Reproductive Technology National Summary Report. https://www.cdc.gov/art/reports/2016/national-summary-figures.html. (4 March 2019, date last accessed)2016.
- De Vos A, Van Landuyt L, Santos-Ribeiro S, Camus M, Van de Velde H, Tournaye H, Verheven G. Cumulative live birth rates after fresh and vitrified cleavage-stage versus blastocsyst-stage embryo transfer in the first treatment cycle. Hum Reprod 2016;31:2442–2449. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Barad D. Update on the comparison of assisted reproduction outcomes between Europe and the USA: the 2002 data. Fertil Steril 2007;87:1301–1305. [DOI] [PubMed] [Google Scholar]
- Gleicher N. Eliminating multiple pregnancies: an appropriate target for government intervention? Reprod Biomed Online 2011;23:403–406. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Vidali A, Braverman J, Kushnir VA, Albertini DF, Barad DH. Further evidence against use of PGS in poor prognosis patients: report of normal births after transfer of embryos reported as aneuploid. Fertil Steril 2015;104:e9.26211885 [Google Scholar]
- Gleicher N, Vidali A, Braverman J, Kushnir VA, Barad DH, Hudson C, Wu YG, Wang Q, Zhang L, Albertini DF et al. Accuracy of preimplantation genetic screening (PGS) is compromised by degree of mosaicism of human embryos. Reprod Biol Endocrinol 2016a;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Kushnir VA, Sen A, Darmon SK, Weghofer A, Wu YG, Wang Q, Zhang L, Albertini DF, Barad DH. Definition by FSH, AMH and embryo numbers of good-, intermediate- and poor-prognosis patients suggests previously unknown IVF outcome-determining factor associated with AMH. J Transl Med 2016b;10:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Kushnir VA, Albertini DF, Barad DH. Improvements in IVF in women of advanced age. J Endocrinol 2016c;230:F1–F6. [DOI] [PubMed] [Google Scholar]
- Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev 2016;30:CD002118. [DOI] [PubMed] [Google Scholar]
- Gondi S, Song Z. Potential implications of private equity investments in health care delivery. JAMA 2019;321:1047–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco E, Giulia Minasi M, Florentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med 2015;373:2989–2090. [DOI] [PubMed] [Google Scholar]
- Hafner K. Why Private Equity Is Furious over a Paper in a Dermatology Journal. https://www.nytimes.com/2018/10/26/health/private-equity-dermatology.html (10 January 2019, date last accessed)2018.
- Hammarberg K, Kirkman M, Pritchard N, Hickey M, Peate M, McBain J, Agresta F, Bayly C, Fisher J. Reproductive experience of women who cryopreserved oocytes for non-medical reasons. Hum Reprod 2017;32:575–581. [DOI] [PubMed] [Google Scholar]
- Harper J, Jackson E, Sermon K, Aitken RJ, Harbottle S, Mocanu E, Hardarson T, Mathur R, Viville S, Vail A et al. Adjuncts in the IVF laboratory: where is the evidence for ‘add-on’ interventions? Hum Reprod 2017;31:1–7. [DOI] [PubMed] [Google Scholar]
- Helft M. End of the Biological Clock. Forbes, 2016, 84–91. New York, NY, 10011-8868. [Google Scholar]
- IBIS World Fertility Service Providers Adopt Differing Strategies to Nurture Growth. www.ibisworld.com.au (19 March 2017, date last accessed)2015.
- IVI Press Release IVI Arrives in America Hand-In-Hand with RMANJ and Consolidates as the Largest Group of Assisted Reproduction in the World. wwwivi-fertility.com (19 March 2017, date last accessed)2017.
- Konda S, Francis J, Motaparthi K, Grant-Kels JM, Group for Research of Corporization and private Equity in Dermatology . Future considerations for clinical dermatology in the setting of 21st century American policy reform: corporization and the rise of private equity in dermatology. J Am Acad Dermatol 2018;pii:S0190–9622(18)32667–7 doi:10.1016/j.jaad.2018.09.052 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Kushnir VA, Darmon SK, Albertini DF, Barad DH, Gleicher N. Effectiveness of in vitro fertilization with preimplantation genetic screening: a reanalysis of United States assisted reproductive technology data 2011–2012. Fertil Steril 2016a;106:75–79. [DOI] [PubMed] [Google Scholar]
- Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Effect of embryo banking on U.S. National Assisted Reproductive Technology Live Birth Rates. PLoS One 2016b;11:e0154620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproduction 2014–2013. Reprod Biol Endocrinol 2017a;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir VA, Choi J, Darmon SK, Albertini DF, Barad DA, Gleicher N. CDC reported assisted reproductive technology live birth rates may mislead the public. Reprod Biomed Online 2017b;35:161–164. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Hamilton M, Bhattacharya S. Should we be promoting embryo transfer at blastocyst stage. Reprod Biomed Online 2016;32:142–146. [DOI] [PubMed] [Google Scholar]
- McLernon DJ, Harrild K, Bergh C, Davies MJ, de Neubourg D, Dumoulin JC, Gerris J, Kremer JA, Martikainen H, Mol BW et al. Clinical effectiveness of elective single embryo transfer versus double embryo transfer-meta analysis of individual patient data from randomized trials. BMJ 2010;341:c6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medew J, Baker M. Making Babies. www.smh.com.au (19 March 2017, date last accessed) The Sunday Morning Harald, 2013. [Google Scholar]
- Morales R, Lledó B, Ortiz JA, Ten J, Lláce J, Bernabeu R. Embryos showing mosaicism in trophectoderm cells can achieve good pregnancy rates. Hum Reprod 2016;31: i14, O–030. [Google Scholar]
- Morris M. ACC Puts IVF Clinics ‘On Notice’ Over Misleading Success Rate Claims wwwabc.net.au (19 March 2017, date last accessed) ABC News, 2016. [Google Scholar]
- Munné S, Blazek J, Large M, Martinez-Ortiz PA, Nisson H, Liu E, Tarozzi N, Borini A, Becker A, Zhang J et al. Detailed investigation into the cytogenic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil Steril 2017;108:62–71. [DOI] [PubMed] [Google Scholar]
- Pandian Z, Marjoribanks J, Ozturk O, Serour G, Bhattacharya S. Number of embryos for transfer following in vitro fertilisation or intra-cytoplasmic sperm injection. Cochrane Database Syst Rev 2013;29:CD003416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson RJ. Preimplantation genetic screening: what is the clinical efficiency? Fertil Steril 2017;108:228–230. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine Preimplantation genetic testing: a practice committee opinion. Fertil Steril 2008;90:S136–S143. [DOI] [PubMed] [Google Scholar]
- Practice Committees of the American Society for Reproductive Medicine, Society for Assisted Reproductive Technology . The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril 2018;109:429–436. [DOI] [PubMed] [Google Scholar]
- Schaller MA, Griesinger G, Benz-Jansen C. Women show a higher level of anxiety during IVF treatment than men and hold different concerns: a cohort study. Arch Gynecol Obstet 2016;293:1137–1145. [DOI] [PubMed] [Google Scholar]
- Scotland GS, McNamee P, Peddie PL, Bhattacharya S. Safety versus success in elective single embryo transfer: women’s preferences for outcomes of in vitro fertilization. BJOG 2007;114:977–983. [DOI] [PubMed] [Google Scholar]
- Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, Zhu Y, Deng X, Qi X, Li H et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med 2018;378:126–136. [DOI] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet 1978;2:366. [DOI] [PubMed] [Google Scholar]
- Teramoto S, Kato O. Minimal ovarian stimulation with clomiphene citrate: a large-scale retrospective study. Reprod Biomed Online 2007;15:134–148. [DOI] [PubMed] [Google Scholar]
- Victor AR, Tyndall JC, Brake AJ, Lepkowsky LT, Murphy AE, Griffin DK, McCoy RC, Barnes FL, Zouves CG, Viotti M. One hundred mosaic embryos transferred prospectively in a single clinic: exploring when and why they result in healthy pregnancies. Feril Steril 2019;111:280–293. [DOI] [PubMed] [Google Scholar]
- Wei D, Liu J-Y, Sun Y, Shi Y, Zhang B, Liu J-O, Tan J, Liang X, Cao Y, Wang Z et al. Frozen versus fresh single blastocyst transfer in ovulating women: a multicenter, randomized controlled trial. Lancet 2019;393:1310–1318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The referenced source data are available from the referenced data sources in this manuscript and from our center’s research data bank by contacting Ms Jolanta Tapper (jtapper@thechr.com).