Abstract
Objective
To identify and explore change processes explaining the effects of the Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) intervention taking account of reach, amount of intervention received, delivery fidelity and patient and caregiver perspectives.
Design
Mixed methods process evaluation parallel to a randomised controlled trial using data from the intervention group (REACH-HF plus usual care).
Setting
Four centres in the UK (Birmingham, Cornwall, Gwent and York).
Participants
People with heart failure with reduced ejection fraction (HFrEF) and their caregivers.
Methods
The REACH-HF intervention consisted of a self-help manual for patients with HFrEF and caregivers facilitated over 12 weeks by trained healthcare professionals. The process evaluation used multimodal mixed methods analysis. Data consisted of audio recorded intervention sessions; demographic data; intervention fidelity scores for intervention group participants (107 patients and 53 caregivers); qualitative interviews at 4 and 12 months with a sample of 19 patients and 17 caregivers.
Outcome measures
Quantitative data: intervention fidelity and number, frequency and duration of intervention sessions received. Qualitative data: experiences and perspectives of intervention participants and caregivers.
Results
Intervention session attendance with facilitators was high. Fidelity scores were indicative of adequate quality of REACH-HF intervention delivery, although indicating scope for improvement in several areas. Intervention effectiveness was contingent on matching the intervention implementation to the concerns, beliefs and goals of participants. Behaviour change was sustained when shared meaning was established. Respondents’ comorbidities, socio-economic circumstances and existing networks of support also affected changes in health-related quality of life.
Conclusions
By combining longitudinal mixed methods data, the essential ingredients of complex interventions can be better identified, interrogated and tested. This can maximise the clinical application of research findings and enhance the capacity of multidisciplinary and multisite teams to implement the intervention.
Trial registration number
ISRCTN25032672; Pre-results.
Keywords: heart failure, primary care, qualitative research, rehabilitation medicine, clinical trials
Strengths and limitations of this study.
A strength of this study is that it is underpinned by qualitative and quantitative systematic reviews conducted by a multidisciplinary and multiprofessional team and, supported by a patient and public involvement group including people with heart failure, builds on the existing evidence base for CR interventions.
A further strength of the study is the use of multiple sources of both qualitative and quantitative data to illustrate change processes during intervention and implementation practices relevant to effectiveness.
An additional strength is the longitudinal data collection over 12 months.
A limitation is that we did not collect data from the facilitator training or conduct interviews with facilitators.
An additional limitation is that there was a limited number of minority ethnic participants in both the trial and process evaluation.
Introduction
Heart failure (HF) is a long term condition associated with shortness of breath, fatigue and fluid retention which inhibits activities of daily living and leads to increased hospitalisations and premature mortality.1 2 The prevalence of HF is increasing due to an ageing population, improved survival of people with HF after myocardial infarction, and more effective treatments for HF.3 With improving prognosis, the focus in HF has shifted towards optimising health-related quality of life (HRQoL). Exercise based cardiac rehabilitation (CR) in patients with HF have shown improvements in HRQoL and a reduction in hospitalisations.1
While evidence for the added value of centre and group based cardiac rehabillitation (CR) for patients with HF is strong, questions remain about the effectiveness of alternative modes of delivery.3 The Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) multicentre Randomised controlled trial (RCT) assessed the clinical effectiveness and cost-effectiveness of the addition of the REACH-HF intervention to usual care compared with usual care alone in patients with HF with reduced ejection fraction (HFrEF) and their caregivers.4 5 The patient, caregiver and health economic outcome results of the REACH-HF trial are reported elsewhere.5–7
This paper reports on a process evaluation conducted in parallel to the REACH-HF trial. Recent reviews of RCTs for HF have identified a lack of process evaluations and associated qualitative studies assessing change processes.8 Indeed, prior research has failed to identify essential programme characteristics showing a consistent pattern in modifying the effects of self-management interventions for people with heart failure.9 Commentators have also noted a failure to explain how programme elements of CR interventions interact10; to adequately describe the real world complexity of living with HF and the burden of both self-management and adherence to intervention regimens.11 The work reported here constituted part of the REACH-HF process evaluation that assessed intervention fidelity, patients’ and caregivers’ experiences of trial participation and sought to identify change processes that may be responsible for change in HRQoL (the trial primary outcome).5–7
Methods
Design
Details of the REACH-HF multicentre trial have been published elsewhere.4 5 In brief, 216 patients and 97 caregivers were recruited to the trial from four geographical regions (Birmingham, Cornwall, Gwent and York) across the UK. One hundred and seven patients with 53 caregivers were randomised to REACH-HF intervention and usual care (intervention group), and 109 patients and 44 caregivers to usual care alone (control group).
A mixed methods process evaluation using linked data from multiple sources to build individual case studies was undertaken,12 paying particular attention to fidelity (the consistency of what was delivered with the planned intervention) and adaptations (alterations made to the intervention in order to achieve contextual fit)13 (figure 1). The intervention group formed the sampling frame for the process evaluation. Nineteen patients were sampled for maximum variation, based on their geographical region and their scores for the primary outcome measure of the trial, the Minnesota Living with Heart Failure Questionnaire (MLHFQ)14 and the Hospital Anxiety and Depression Scale.15 The four regions enabled adequate participant enrolment to achieve the required sample size and afforded the opportunity to explore local contexts. Sampling different scores at baseline allowed us to explore different longitudinal change within and across cases. Seventeen caregivers were also purposively sampled.7
Figure 1.
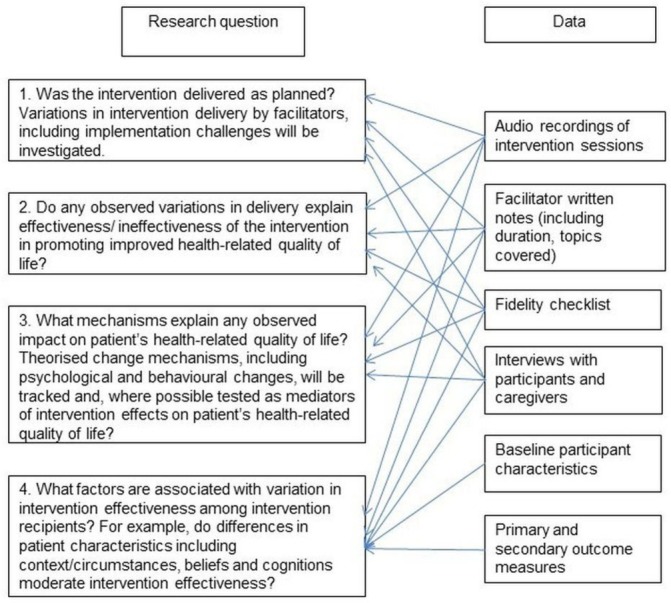
Sources of data used to address process evaluation questions.
REACH-HF intervention
The REACH-HF intervention is a comprehensive self-care support programme comprising the ‘REACH-HF Manual,’ which includes lay explanations of HF and how people can learn to live with the condition to maximise their quality of life.16 It also includes a choice of two exercise programmes (chair based and walking); information about managing medications, monitoring fluid build-up and other symptoms (to inform appropriate help-seeking), and managing stress, anxiety, and low mood (including a relaxation CD); a ‘Family and Friends Resource’ for caregivers; and a participant ‘Progress Tracker’ booklet to record daily physical activity, symptoms and other self-care-related actions. Facilitators visited patients at home to introduce the programme, and subsequently supported patients and caregivers in face-to-face or telephone sessions.
Participants were required to have a first face-to-face contact with facilitators and at least two facilitator contacts thereafter, with at least one face-to-face. It was expected that participants would typically receive 4 to 6 contacts each, although the facilitators could vary the number of contacts as they considered necessary around this guideline range.
Data collection
Qualitative data: For the 19 patients who constituted our sampling frame (two to three patients per facilitator across the four regions), facilitators were required to audio-record all face-to face meetings with the patient (typically one 90 min and two to three 45 min sessions), and subsequent telephone contacts (typically three to six per patient). Qualitative interviews were conducted in peoples’ homes (or by telephone if a visit was not possible), and field notes collated.
Quantitative data: quantitative data were collected at visits to the research centre. A 13-domain fidelity checklist was developed and piloted during the feasibility study, and used in the process evaluation to assess the facilitators’ delivery of the intervention. The checklist and descriptions of the intended content of the intervention are published elsewhere.16The checklist items represented key intervention components defined by the intervention designers, including use of a person-centred counselling style, addressing the emotional consequences of heart failure and involving caregivers in the intervention process. One of the intervention designers (JW) applied the checklist to the audio recorded intervention sessions and another (CG) scored a subsample (around one third of the sessions). The coders compared and discussed the scoring to facilitate consistency. Fidelity scoring attributed a numerical value (0–6) for each of the 13 domains on the fidelity checklist. Fidelity of intervention was considered to be adequate if scored ≥3 for each domain. The score of 3 was agreed as an anchor-point for scoring between the coders and was interpreted for each domain in relation to detailed coding instructions. On the basis of fidelity scores (to achieve maximum diversity within and between patients, and where dissonance was identified by JW and CG), a purposive sample of audio recordings were used to identify examples of optimal and suboptimal delivery fidelity.
Patients and caregivers were contacted by the interviewer and, with their consent, interviewed at 4 and 12 months after the baseline facilitator visit. Interviews were conducted by experienced qualitative researchers and, with participant consent, audio-recorded and transcribed. The interviewers followed a topic guide based on the objectives of both REACH-HF and the process evaluation (table 1). Interviews sought to capture change over time.17
Table 1.
Qualitative interview topic guide
| Topic | Questions |
| Opening question Engagement with the intervention First interview |
Before you started, what were your expectations of the REACH-HF programme? What did you want to know about? Once the programme started, How did you use the manual? |
| Second interview | Since we last spoke how have you been getting on? (Reflect on a topic recalled in that interview if little discussion starts—or something observed) |
| Relationship with REACH-HF facilitator First interview |
Can you describe how the facilitator worked with you? What did you like or dislike about how the facilitator worked with you? What did you feel about your (husband, wife, son etc) being present? Did the facilitator help you meet your needs? |
| Second interview | Since we last spoke have you had any further contact with the facilitator? (explore if yes) |
| Involvement of Family and Friends Both interviews |
Do you have any friends or family that help you manage your heart condition? If so: In what way did your family or friends get involved? How did you feel about it? What if any discussions did you have with your family or friends about what support you needed from them in managing your heart failure? |
| Using the manual First interview |
As a result of using the manual and working with the facilitator what has changed about how you manage your heart failure? What do you think about the medication section? What do you think about the physical activity programme? How do you plan to keep your fitness up in the future under your own steam? What other support might be useful? What did you think about the progress tracker? How did you use it? |
| Second Interview | Can you tell me about what physical activity or exercise do you do now? What are you doing to keep track of your progress? |
| Processes Both interviews |
Has taking part in the intervention affected your understanding of the condition/your situation? If so, probe further Has it made you feel any more or less confident about how you manage your heart failure? If so, probe further |
| Psychological adjustment to living with heart failure Both interviews |
Has this programme changed the way you feel about having heart failure? If so, in what way? Has this programme changed the way you manage these feelings? If so, in what way? What do you think about the sections of the manual that are about managing stress? Were these helpful? If so how? How did you monitor your progress? |
| Moderators and Mediators First interview |
Is there anything else that affects how you look after your heart? |
| Second interview | What do you do now to look after your heart? Does this differ from the first interview What other sources of information or support do you use? This is important if there are other heart failure management programmes being used |
Data analysis
Quantitative data: using Stata V.14.2,18 descriptive statistics were used to summarise the level of intervention received and fidelity of delivery.
Qualitative data: all available data were managed with Nvivo V.11 Pro.19 Individual cases were created for each respondent,12 including interview transcripts, contact sheets, audio recordings of facilitator-patient interactions and associated fidelity scores, summaries of the intervention sessions, field notes, clinical data such as case-report forms, and patient reported outcome measures. Interview transcripts were initially analysed using evaluative coding, which assigned a judgement about the merit or significance of the intervention from the participant’s perspective.20 This noted the presence or absence of a section of the intervention and the extent to which it was positively or negatively evaluated by the participant/caregiver. Second cycle coding20 extended the mapping of variance across the dataset, and enabled within-case analyses (between patients and caregivers, and between 4 and 12 months) and cross-case analyses (between facilitators and sites, and also on the basis of variables of interest identified in the first round, eg, gender, age and multimorbidities) and the further identification of directional processes.20 Fidelity was coded on the basis of the emphasis given to the core intended components of the intervention, while adaptations were coded in relation to the spirit of the intervention being adapted to the local context in order to achieve a ‘good ecological fit’.13 Data were anonymised and participants given pseudonyms.
Patient involvement
A patient and public involvement (PPI) group of 9 patients and caregivers led by a lay chair cocreated the REACH-HF intervention, the recruitment process, and the associated research. Research questions and topic guides were developed with the group. Patient representatives shared their experiences of how their needs were often not considered as part of healthcare consultations, and advised on how to ask sensitive questions.
Results
Study participants
The flow of study participants is shown in figure 2. The characteristics of the intervention group patients who participated in the process evaluation are summarised in table 2.
Figure 2.
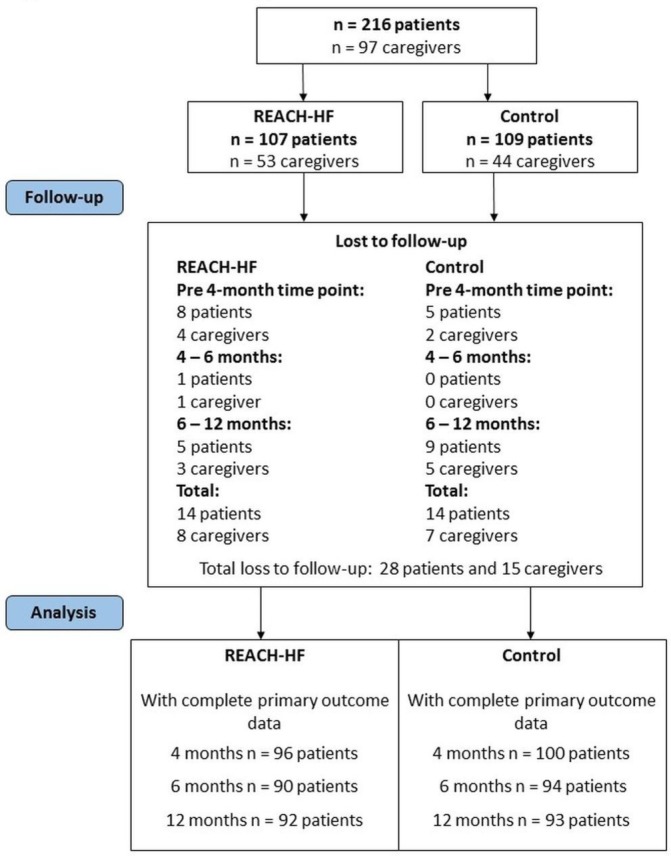
CONSORT diagram. CONSORT, consolidated standards of reporting trials; REACH-HF, Rehabilitation Enablement in Chronic Heart Failure.
Table 2.
Baseline characteristics of patients in the intervention arm
| Characteristic | REACH-HF n=107 (%) | Qualitative interview sample n=19 (%) |
| Age (years), mean (SD) | 69.7 (10.9) | 68.5 (9.8) |
| Female sex | 26 (24) | 7 (37) |
| Body Mass Index BMI (kg/m2); mean (SD) | 29.5 (6.6) | 31.5 (7.4) |
| Main activity | ||
| Retired | 81 (76) | 15 (79) |
| In employment or self-employment | 18 (17) | 2 (11) |
| Unemployed | 5 (5) | 1 (5) |
| Other | 3 (3) | 1 (5) |
| Ethnic origin | ||
| White | 100 (93) | 19 (100) |
| Other (Black, Asian, other) | 7 (7) | 0 (0) |
| NYHA status | ||
| Class I | 24 (22) | 0 (0) |
| Class II | 63 (59) | 13 (68) |
| Class III | 20 (19) | 6 (32) |
| Ischaemic aetiology of HF | 48 (45) | 9 (47) |
| Time since diagnosis of HF (years); mean (SD) | 3.6 (4.2) | 3.8 (3.9) |
| <1 | 35 (33) | 6 (32) |
| 1–2 | 18 (17) | 1 (5) |
| 2–5 | 28 (26) | 7 (37) |
| 5–10 | 14 (13) | 3 (16) |
| >10 | 12 (11) | 2 (11) |
| NTerminal-pro-BNP level (pg/mL); mean (SD) | 1460 (1928) | 1321 (1123) |
| Current smoker | 6 (6) | 1 (5) |
| Comorbidities (past or present) | ||
| Diabetes mellitus | 26 (24) | 5 (26) |
| Myocardial infarction | 29 (27) | 7 (37) |
| Hypertension | 45 (42) | 9 (47) |
| Chronic renal impairment | 14 (13) | 3 (16) |
| Arthritis (osteoarthritis or rheumatoid) | 45 (42) | 11 (58) |
| Atrial fibrillation or atrial flutter | 48 (45) | 9 (47) |
| COPD | 9 (8) | 2 (11) |
| Depression | 27 (25) | 7 (37) |
| Number of comorbidities | ||
| 0 | 63 (59) | 9 (47) |
| 1 | 32 (30) | 7 (37) |
| 2 | 7 (7) | 1 (5) |
| 3 or more | 5 (5) | 2 (11) |
| Baseline use of drugs | ||
| Beta-blocker | 90 (84) | 18 (5) |
| Angiotensin II receptor antagonist | 31 (29) | 8 (42) |
| ACE inhibitor | 68 (64) | 10 (53) |
| Baseline use of devices | ||
| Implantable cardiverter defibillator (ICD) | 10 (9) | 3 (16) |
| Cardiac Synchronization Therapy (CRT) | 10 (9) | 1 (5) |
| Combined CRT/ICD | 5 (5) | 2 (11) |
| Pacemaker | 11 (10) | 1 (5) |
| Location | ||
| Cornwall | 30 (28) | 7 (37) |
| Gwent | 23 (22) | 3 (16) |
| Birmingham | 27 (25) | 4 (21) |
| York | 27 (25) | 5 (26) |
| Caregiver present at randomisation | 53 (50) | 13 (68) |
COPD, chronic obstructive pulmonary disease; HF, heart failure; NYHA, New York Heart Association.
Patients were predominantly male and classed as II or III on the New York Heart Association classification, with an average age of 70 years and mean left ventricular ejection fraction of 33%. Caregivers were typically the partner or direct relative, were of a younger mean age (63 years) and predominantly female. The qualitative sample was representative of the wider intervention group.
Level of intervention received
Across the intervention group, a mean 6.5 (SD 2.6) facilitated sessions were provided with 4.0 (SD 1.2) being face-to-face contacts. A similar number of facilitated intervention sessions were received by the subsample of process evaluation participants, (total: mean 7.0, SD 2.6, and mean face-to-face: 4.0, SD 1.8; telephone: 2.9, SD 2.6).
Face-to-face contacts lasted from 10 to 170 min, while telephone contacts ranged from 2 to 75 min. Participants in Birmingham received the lowest mean number of contacts (6.25, SD 1.2), and at Cornwall the highest mean contact (9.5, SD 3.1); the duration of contacts was comparable.
Fidelity of intervention delivery
Audio recordings of facilitator contacts were available for 110 out of 322 contacts (34%). Face-to-face contacts were more consistently recorded than telephone contacts, but there were no other obvious patterns of recording (eg, all of the contacts in York were recorded, but there was a smaller number).
Fidelity was considered to be adequate (score of ≥3 out of 6) in most domains (summarised in table 3).
Table 3.
Fidelity of intervention delivery summary scores for patients in the process evaluation
| Participant Involvement | Assessment of individual needs | Tailored treatment plan | Building under- standing of HF | Support progress monitoring | Review progress | Physical activity plan | Address patient emotion | Medication issues | Involve caregiver | Address caregiver emotion | Address caregiver well-being | Closure | |
| Mean | 3.3 | 4.1 | 2.9 | 3.3 | 2.9 | 3.2 | 4.2 | 3.4 | 3.3 | 1.6 | 2.2 | 1.1 | 3.4 |
| Min | 1.3 | 2.5 | 1.8 | 1.5 | 1.8 | 2.2 | 3.0 | 1.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 |
| Max | 4.9 | 5.0 | 5.1 | 5.0 | 5.0 | 4.8 | 5.5 | 5.0 | 5.0 | 3.1 | 4.0 | 3.3 | 5.0 |
| n | 20 | 15 | 20 | 17 | 20 | 18 | 15 | 17 | 17 | 16 | 14 | 16 | 14 |
HF, heart failure.
There was room for improvement, however, particularly with respect to involving the caregiver, addressing caregiver concerns and addressing caregiver well-being, where the mean scores were well below the agreed ‘adequate’ score (table 4).
Table 4.
Number and duration of REACH-HF sessions for patients in the intervention arm
| REACH-HF (n=107) |
Qualitative interview sample (n=19) |
|
| Number of sessions per participant1; mean (SD), n; [min, max]; | ||
| Total sessions | ||
| Overall | 5.6 (2.7), 107; [0, 13] | 6.5 (2.2), 19; [4, 11] |
| Cornwall | 6.4 (2.4), 30; [0, 12] | 8.0 (2.6), 7; [4, 11] |
| Gwent | 5.3 (3.0), 23, [0, 12] | 6.7 (2.1), 3; [5, 9] |
| Birmingham | 5.8 (3.3), 27; [0, 13] | 6.3 (1.3), 4; [5, 8] |
| York | 4.6 (1.5), 27 [2, 9] | 4.6 (0.5), 5; [4, 5] |
| Face-to-face sessions1 | ||
| Overall | 3.6 (1.4), 107; [0, 7] | 3.9 (1.1), 19; [2, 7] |
| Cornwall | 3.1 (1.0), 30; [0, 5] | 3.9 (0.7), 7; [3, 5] |
| Gwent | 3.9 (1.8), 23; [0, 7] | 4.7 (2.1), 3; [3, 7] |
| Birmingham | 3.6 (1.6), 27; [0, 7] | 4.0 (0.8), 4; [3, 5] |
| York | 3.8 (1.2), 27; [1, 7] | 3.6 (1.1), 5; [2, 5] |
| Telephone sessions1 | ||
| Overall | 2.0 (2.1), 107; [0, 9] | 2.6 (2.1), 19; [0, 7] |
| Cornwall | 3.4 (2.0), 30; [0, 8] | 4.1 (2.4), 7; [0, 7] |
| Gwent | 1.4 (1.8), 23; [0, 6] | 2.0 (0.0), 3; [2, 2] |
| Birmingham | 2.1 (2.4), 27; [0, 9] | 2.3 (1.7), 4; [0, 4] |
| York | 0.9 (0.8), 27; [0, 3] | 1.0 (0.7), 5; [0, 2] |
| Duration of sessions (minutes; combined across participants); mean (SD) n, [min, max] | ||
| Face-to-face sessions1 | ||
| Overall | 70 (28), 380; [15, 170] | 72 (32), 75; [20, 170] |
| Cornwall | 75 (25), 92; [15, 145] | 83 (27), 27; [30, 135] |
| Gwent | 69 (26), 89; [20, 160] | 59 (31), 14; [20, 140] |
| Birmingham | 69 (33), 97; [15, 170] | 76 (38), 16; [30, 170] |
| York | 66 (26), 102; [20, 135] | 62 (28), 18; [20, 120] |
| Telephone sessions1 | ||
| Overall | 20 (9), 215; [2, 50] | 21 (12), 50; [2, 50] |
| Cornwall | 21 (10), 102; [5, 50] | 23 (12), 30; [5, 50] |
| Gwent | 14 (8), 32; [2, 33] | 14 (12), 6; [2, 33] |
| Birmingham | 20 (9), 58; [2, 45] | 17 (8), 9; [4, 28] |
| York | 19 (7), 23; [5, 35] | 21 (10), 5; [8, 33] |
REACH-HF, Rehabilitation Enablement in Chronic Heart Failure.
Furthermore, there was variation in fidelity scores across participants and facilitators. There was no discernible ‘fidelity style’ for each facilitator/site (one to three facilitators per site) and all had participated in the 3-day training for the study. Six of the seven facilitators had nursing backgrounds (various hospital/community, rehabilitation/cardiac/cardiology configurations). The one physiotherapist had the highest fidelity scores, although this was too small a number of patients to be statistically significant.
Fidelity scores for participants in Birmingham were consistently higher (>3) than those for participants in Cornwall (<3). For all process evaluation participants, fidelity scores were lower in telephone contacts than with face-to-face consultations. Assessment of their content identified that they were typically more didactic in nature, and generally used to remind participants of key messages rather than to elicit or discuss any new concerns. This may reflect the briefer time spent in telephone sessions and their use as a method for briefly checking-in rather than raising or dealing with any more complex issues.
The quality of delivery at the initial session with the facilitator set the tone for future sessions and was associated with perceptions of intervention effectiveness, as two examples illustrate. Mary received one of the lowest amounts of REACH-HF (a total of 293 min of contact time, over three face-to-face and three telephone-based intervention sessions), but her fidelity scores were high (scoring ≥5 in seven of 13 domains) at the initial intervention session and subsequently. The audio-recording indicated that the facilitator listened to her concerns, elicited her goals and beliefs, and employed a range of behaviour change techniques, before explaining the purpose of the manual and resources. At 4 months Mary explained how the support of the facilitator combined with the walking programme had re-orientated her understanding of HF:
I’ve learnt a lot and I think it is a very good… manual, I think it’s excellent and I think, … given to people – I wished I’d had it right at the beginning. Because it has changed my attitude… (Mary: 4 months).
Her family were strongly engaged in supporting the intervention and created a simplified one-page progress tracker, which Mary used postintervention. At 12 months she suggested that by ‘sticking to the walking programme’ she was ‘feeling better in myself’ and ‘doing things which I couldn’t have done a year ago’. At 12 months her HRQoL score as assessed in terms of MLHFQ total score had reduced from 50 at baseline to 18, indicating a considerable improvement.
In contrast, Dorothy received a larger amount of REACH-HF (440 min over four face-to-face and two telephone sessions), but fidelity scores were low at the initial intervention and afterwards (<2.5). Audio-recordings detail how the facilitator adopted a didactic approach with no individualised assessment. In subsequent sessions the facilitator failed to answer clinical questions and was unable to signpost Dorothy to appropriate resources. Dorothy’s HRQoL was good (total MLHFQ score 16) initially suggesting a good HRQoL, despite her having one of the poorest ejection fractions in the sample (21%, indicative of severe decompensation). Interview data suggests, however, her good HRQoL were due to her lack of insight, which was repeatedly reinforced by the poor facilitation of the intervention, and at 12 months there was no evidence of change in either understanding or behaviour:
I can’t seem to get the seriousness of it… [exercise] it is recreation, isn’t it? Mainly….it keeps, you know, things going…I can’t explain it really now…I did have a diary at first ‘cos they said keep a diary, but even that I don’t tend to…No, have got a bit complacent… (Dorothy: 12 months)
Key processes
Analysing linked data over time12 17 enabled us to identify key processes that explained the effects of the REACH-HF intervention: adaptation, competence, comorbidities and social context.
Adaptation
Change was sustained when the facilitator adapted the intervention to participant needs. In one example, Helen had underlying anxiety and misconceptions about the need for rest, and the facilitator literally took ‘small steps’, walking with the participant to build capacity:
[Facilitator] said it’ll, you know, it’ll build your heart muscle up. You know, which I wasn’t thinking that way. I was thinking: if I rest my heart and don’t do anything, you know, it would - you know, keep me going a bit longer, you know…And the more I thought about walking, and my heart muscle and all, the more, well, I thought, “Well, I’ll walk, and try to get it stronger. (Helen: 4 months)
In contrast, where participants suggested that they had already acquired expertise, or had been living with HF for years, some facilitators struggled to engage them or tailor the programme effectively:
I didn’t want to do [exercise]…I couldn’t see the point in pushing myself to do it when I personally couldn’t see I were going to get any extra benefit from it, as such… [Facilitator] were just doing her job and that were it… She weren’t trying to interfere with anything. I think she, she knew she were wasting her time, not being rude to her. (Michael: 4mth)
Key to both fidelity and adaptation were the competences of the facilitator, the participant and their caregiver, the existence of comorbidities and wider contextual factors such as material or social deprivation.
Competence
The facilitators’ competence was key to participants’ skills acquisition and behaviour change. Rachel, who has postpartum cardiomyopathy and was swimming to achieve the required pretransplant weight loss, explained how the facilitator told her that swimming was dangerous, although it took her ‘a few weeks to convince me though’. The facilitator’s challenging approach was appreciated, and Rachel subsequently attributed both her positive mental attitude and weight loss to the difficult conversations with her facilitator:
[I]t helped to have someone just there ‘cos your GPs busy… [GP] actually said: ‘I don’t quite know what to do and you’re a very rare case, and you probably know more about it than what I do’. You don’t want to hear that from a GP…Whereas [Facilitator] would go: ‘I’ll go back and find that out’, but she knew most of the answers anyway. She was very with it.(Rachel: 12mth)
Having a friendlier and less challenging facilitator could, in contrast, have inhibited learning in this case. Post intervention, several participants without strong social support described how they had come to depend on the facilitator in a way that inhibited the development of self-efficacy; with some described feeling abandoned or isolated:
I was supposed to go in for the pacemaker and then they decided that I didn’t need it…I was looking forward to an operation…I felt it was gonna be a bit of a safety net…[I feel] abandoned! [Cries]…There’s so many things that…turn up, just from day to day. And it’s usually something daft and it’s usually something you think: I can’t ring the doctor for that. I don’t want to bother them for that. So I suppose it would just be useful to have that contact…with someone who definitely knows what they are talking about… (Esther: 12 month)
Where facilitators were able to use participants’ existing networks of health professionals and family members, participants expressed confidence in their newly acquired ability to self-manage:
I got the manual here, the big manual here. But they’re explaining it to me, and seeing it in black and white it was like ‘Oh yeah’, ‘Cos I know when you go down to see [Cardiac Nurse] I know they, you know, that she would answer questions if I asked but you’re always conscious of the fact that there’s a queue outside waiting to see her. (Mary: 4 month)
However, referrals to unfamiliar health and social care professionals did not always materialise, were not always followed up by the facilitator, and could leave participants feeling as though they no longer had an advocate:
Maybe I got the wrong impression…like with (Facilitator) and the nurses… they’ve got the title of heart nurses…I don’t know whether I’m allowed to ask them questions and whether they would get involved on my behalf…I’m almost looking for the advocate that says: ‘I know what you want. I’ll get it sorted.’ And you know…I don’t even know who to talk to…What it really boils down to: I don’t know where…to go to get the information. (John:12 month)
Comorbidities
Most participants had multiple comorbidities, and some valued the simplicity of the REACH-HF programme, and specifically the accessibility of the information provided. Mary has arthritis, chronic back pain and breast cancer, and engaged with ‘simple explanations’ about medicines in the manual:
P: Yeah, medication, information on that, which was brilliant. What they are is simple explanations, there’s no, you know, high falutin medical jargon in there, it’s basically straightforward, you read it, you understand it… yes, you don’t remember the whole book, obviously not, but you can go back to it at any time that you want to, anything that crops up that you’re not sure of, I just go in the other room where I’ve got it on the table, and I open it up and read it. I don’t even put it away, I keep it out so that I got it there… (Mary: 4 months)
In contrast, Stephen, who has arthritis, diabetes and chronic obstructive pulmonary disease (COPD), wanted to ‘be a little bit fitter and a bit more active’, but his need to manage multiple conditions inhibited his ability to fully engage with the intervention:
[I’m] a little bit more active, a little bit less tired… But, that still remains the same, that still remains the major problem for me…. And I’ve got knee problems as well so… that stops me doing a lot really. I mean that was the biggest thing on the walk test really, that’s what…curtailed things for me really…Oh the cough, yeah, I’ve got a cough which is… drive me around the bend, yeah… Most of the time, like now, it’s…. it’s just an annoyance, but if I get a bad cold …. it’s horrendous. It always ends up here, at the top of the chest straightaway and I’ve got about four or five weeks of purgatory, you know, it’s really bad. (Stephen: 4mth)
Social context
Sustaining change was easier for participants with favourable material or social circumstances. For example, Peter is retired, owns his own house in a rural setting and regularly plays golf as a form of gentle exercise and relaxation:
In the last 12 months I’ve been fine…we have been away, we went to South Africa for five weeks…we had a wonderful time…I am relatively fit because I go and play golf most days…(Peter:12 mth)
However, sustaining change was much harder for those with limited material or social means. Simon has COPD and requires oxygen therapy, but his wife smoked in the house and neither of them engaged with REACH-HF:
I’m one of them typical blokes: ‘Oh, it’ll be alright’… ‘You know, I know what I’ve got, I know what’s gonna happen, sort of thing. But… I don’t wanna talk about, like, you know?’ (Simon:12 mth).
Discussion
This process evaluation increases understanding of how the REACH-HF intervention operated in practice, in terms of reach, level of intervention delivered, fidelity and patient perspectives. We observed that intervention attendance was high, with most participants receiving the minimum intended amount of REACH-HF. However, an appropriate ‘dose’ of REACH-HF is contingent on fidelity of intervention delivery and skilled facilitation, rather than the duration or frequency of contact alone. Importantly, the overall fidelity score at the initial ‘assessment’ session was indicative of the overall fidelity score (across all sessions) for an individual participant. This highlights the importance of the first intervention session in setting the tone for subsequent sessions. Intervention effectiveness was dependent on the facilitator’s style of engagement, and the extent to which the trial materials were matched to or adapted to the existing beliefs and goals of participants.21 Behaviour change was sustained when facilitators established shared meaning with participants. Respondents’ comorbidities, existing networks of support and socio-economic circumstances were also key to any changes in quality of life but, importantly in terms of measuring effectiveness, this may not always be reflected in the patient reported quantitative outcome measures. There was room for improvement with respect to involving the caregiver, and refinement of the REACH-HF intervention may be now be required.7
This study has a number of strengths. First, is the ability to examine the implementation in detail through the use of a wide range of data sources, including qualitative data from patient interviews and facilitated contacts; observational field notes; and quantitative data about key trial processes and practice-level effectiveness. By combining longitudinal mixed methods data, the essential ingredients of complex interventions can be better identified, interrogated and tested.12 This can maximise the clinical application of research findings and enhance the capacity of multidisciplinary and multisite teams to implement the intervention.
Second, this research is underpinned by qualitative and quantitative systematic reviews conducted by a multidisciplinary and multiprofessional team,1 2 supported by a PPI group including people with HF, and builds on the existing evidence base for CR interventions. Research has suggested that comprehensive CR interventions may offer added benefits over single component interventions.10 11 We propose this multicomponent home-based CR intervention can be optimised for the patient when both fidelity and adaption are maximised using an initial rigorous personalised assessment, skilled facilitation and the appropriate use of programme elements.
Third, this process evaluation was conducted in parallel with the REACH-HF trial and has been able to investigate process elements and their associations with outcomes. Existing studies of self-management interventions for patients with HF (using composite or clinical endpoint scores) have shown limited effectiveness (ie, they could not identify effective programme characteristics),9 22–24 and a criticism is that few have conducted parallel mixed methods process evaluations, which have the potential to illuminate important contextual factors, such as patient needs, comborbidities and social context.8 Where process evaluations have been conducted in relation to CR,23 and other chronic conditions,24–27 qualitative findings have helped to explain the quantitative outcomes and suggest future directions for research. As with previous research,28 29 a key finding here is the dissonance between researchers’, physicians’ and patients’ understanding of HRQoL. This may account for the outcome measures used in trials of interventions for the self-management of HF not always reflecting the effectiveness of the intervention at the level of the patient’s experience (as seen with Dorothy). Specifically, if trials of self-management interventions for heart failure are premised on filling the knowledge gaps of often elderly patients with poor health literacy and inhibited cognitive function, then more needs to be done to enable patients to express their own goals and values and interpretations of HRQoL in ways that are clinically meaningful and measurable.30–33 More generally, high quality process evaluations conducted in parallel with clinical trials have the potential to identify which interventions or elements are effective, and improve or eradicate those that are not.13
This study also has limitations. We did not collect data from the facilitator training or conduct interviews with facilitators. Furthermore, the facilitators did not record all intervention sessions or their duration as instructed, thus the estimated fidelity of delivery may be an over-estimation of the actual fidelity. This limits our insight into the interaction between a practitioner’s existing professional repertoire and their engagement with and delivery of REACH-HF.6 A further limitation is the small number of minority ethnic participants in both the trial and process evaluation.
More research is now required to optimise the ‘workability’ of the REACH-HF intervention and integration of the intervention components into the facilitators’ clinical practice. This will ensure that fidelity and adaptation are maximised in the complex context of HF.30 Other studies that have involved facilitated interventions for HF have augmented facilitator training via the provision of regular multidisciplinary feedback,34 and the use of videoconferencing and peer discussion of difficult cases.35 The uptake of CR is lower among people from minority ethnic backgrounds,36 which has been attributed to: confusion about the aim, structure and content of the rehabilitation programmes; language barriers; poor mobility; prohibitive illness; distance from the rehabilitation centre; need for permission to attend; and lack of follow-up if a session was missed.37–39 More research is required to understand the specific support needs of people from more diverse ethnic backgrounds and the extent to which cultural beliefs may moderate or mediate the effectiveness of REACH-HF.
Conclusions
The findings of this process evaluation indicate that the REACH-HF intervention for patients with HFrEF and caregivers was adequately delivered, well received and could be implemented more widely. However, there was considerable scope for improvement, as indicated by the fidelity scores and in-depth qualitative analysis. Behaviour change appeared to be sustained when shared meaning between the participant and facilitator was established, although patients’ comorbidities, socio-economic circumstances and existing networks of support were key to any changes in HRQoL, which may not always be reflected in quantitative outcome measures. For example, where a facilitator was able to elicit a participant perspective, and tailor the intervention accordingly, this was often contingent on the nature of the wider contextual issues: if a ‘problem’ was within the scope of the intervention or facilitator (ie, anxiety) then tailoring was appropriate although fidelity could be compromised. However, if the problem was not within scope (ie, socio-economic disadvantage), then tailoring was more difficult. More now needs to be done to ensure fidelity and identify those patients for whom effectiveness may be more difficult to achieve.
Supplementary Material
Acknowledgments
We thank the patients and caregivers who took part in the REACH-HF trial and process evaluation. We thank Kevin Paul for his contribution as patient and public involvement representative. We acknowledge the contribution of the Heart Manual Office at NHS Lothian who contributed to the design of the intervention and facilitator training and the Peninsula Clinical trials Unit for study management.
This report is independent research funded by the UK National Institute for Health Research (NIHR) Programme Grants for Applied Research. The views expressed in this publication are those of the authors and not necessarily of the NHS, the NIHR, or UK Department of Health and Social Care. We would like to thank all facilitators and clinicians involved in the study and acknowledge the vital contributions of study researchers and administrators in Birmingham, Cornwall, Gwent, the Heart Manual Office (Dr Carolyn Deighan and Jenny Edwards for the facilitator training), the Peninsula Clinical Trials Unit, the Royal Cornwall Hospitals Trust (Research, Development and Innovation and Clinical Chemistry departments), the Programme Steering Committee (Martin Cowie, Graham Dunn, Suzanna Hardman, Roger Boyle, and Liz Clark), the Data Monitoring Committee (Alan Montgomery, Ann Dorthe-Zwisler and Gill Furze) and independent adjudicators (Iain Squire, Sern Lim and Paco Leyva). Lucy Moore conducted a small number of interviews.
Footnotes
Contributors: HD, RT, PJD, KJ, JM, JW, NB, CG, CA, and SJS designed the trial, were responsible for its conduct, and obtained trial funding. CG led the design of the intervention, with strong contributions from JW, PJD, the Heart Manual Department (Edinburgh), and the REACH-HF service user advisory group. JF, JW, NB, CG and CA designed the qualitative study and conducted the data analysis. FCW undertook the statistical analysis. JF led the mixed methods analysis. KP represented patient and public involvement on the Programme Management Group and liaised with the patient involvement group throughout the study. He commented upon the manuscript. All authors contributed to writing and editing the manuscript, with the lead taken by JF. All authors commented on the manuscript and agreed the final version. JF and RT are the guarantors.
Funding: REACH-HF was funded by a Programme Grant for Applied Research (RP-PG-1210-12004) awarded by the National Institute for Health Research (NIHR). Nicky Britten and Charles Abraham are part-funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula. Kate Jolly is part-funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) West Midlands.
Competing interests: None declared.
Ethics approval: Ethical approval was granted by the North West— Lancaster Research Ethics Committee (ref 14/NW/1351). Trial registration number ISRCTN86234930.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Transcripts will not be shared in their entirety to protect the anonymity of participants and workers delivering the interventions. However, requests for excerpts of the data will be considered on an individual basis. Please contact the corresponding author.
Patient consent for publication: Obtained.
References
- 1. Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014;7 10.1002/14651858.CD003331.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wingham J, Harding G, Britten N, et al. Heart failure patients' attitudes, beliefs, expectations and experiences of self-management strategies: a qualitative synthesis. Chronic Illn 2014;10:135–54. 10.1177/1742395313502993 [DOI] [PubMed] [Google Scholar]
- 3. NICE. Chronic heart failure: management of chronic heart failure in adults in primary and secondary care Chronic heart failure: National Institute for Health and Care Excellence, 2010. [Google Scholar]
- 4. Taylor RS, Hayward C, Eyre V, et al. Clinical effectiveness and cost-effectiveness of the Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) facilitated self-care rehabilitation intervention in heart failure patients and caregivers: rationale and protocol for a multicentre randomised controlled trial. BMJ Open 2015;5:e009994 10.1136/bmjopen-2015-009994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dalal HM, Taylor RS, Jolly K, et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: The REACH-HF multicentre randomized controlled trial. Eur J Prev Cardiol 2019;26:262–72. 10.1177/2047487318806358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greaves C. on behalf of the REACH-HF investigators. Rehabilitation Enablement in CHronic Heart Failure (REACH-HF): findings and delivery quality issues in a multicentre randomised controlled trial of a facilitated, exercise based rehabilitation intervention in heart failure. Wanchai, Hong Kong: International Society of Behavioural Nutrition and Physical Activity (ISBNPA), 2018. [Google Scholar]
- 7. Taylor RS, Wingham J, Frost J, et al. Caregiver outcomes of the REACH-HF multicenter randomized controlled trial of homebased rehabilitation for heart failure with reduced ejection fraction (submitted to European Journal of Cardiovascular Nursing). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albano MG, Jourdain P, De Andrade V, et al. Therapeutic patient education in heart failure: do studies provide sufficient information about the educational programme? Arch Cardiovasc Dis 2014;107:328–39. 10.1016/j.acvd.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 9. Jonkman NH, Westland H, Groenwold RH, et al. What are effective program characteristics of self-management interventions in patients with heart failure? An individual patient data meta-analysis. J Card Fail 2016;22:861–71. 10.1016/j.cardfail.2016.06.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark AM. What are the components of complex interventions in healthcare? Theorizing approaches to parts, powers and the whole intervention. Soc Sci Med 2013;93:185–93. 10.1016/j.socscimed.2012.03.035 [DOI] [PubMed] [Google Scholar]
- 11. Greenhalgh T, A’Court C, Shaw S. Understanding heart failure; explaining telehealth – a hermeneutic systematic review. BMC Cardiovasc Disord 2017;17:1–16. 10.1186/s12872-017-0594-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bazeley P. Integrating analysis in mixed methods research. London: Sage, 2018. [Google Scholar]
- 13. Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350:h1258 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rector T, Kubo S, Cohn J. Patients’ self-assessment of their congestive heart failure. Part 2: content, reliability and validity of a new measure, the Minnesota Living with Heart Failure questionnaire. Heart Failure 1987;3:198–209. [Google Scholar]
- 15. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 16. Greaves CJ, Wingham J, Deighan C, et al. Optimising self-care support for people with heart failure and their caregivers: development of the Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) intervention using intervention mapping. Pilot Feasibility Stud 2016;2:37 10.1186/s40814-016-0075-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saldana J. Longitudinal qualitative research: analysing change through time. California: AltaMira, 2003. [Google Scholar]
- 18. StataCorp. Stata statistical software: release 14. College Station, TX: StataCorp LP, 2015. [Google Scholar]
- 19. QSR International Pty Ltd. NVivo qualitative data analysis software, version 10, 2012. [Google Scholar]
- 20. Miles M, Huberman A, Saldana J. Qualitative data analysis: a methods sourcebook. Los Angeles: Sage, 2014. [Google Scholar]
- 21. Hanson S, Cross J, Jones A. Promoting physical activity interventions in communities with poor health and socio-economic profiles: A process evaluation of the implementation of a new walking group scheme. Soc Sci Med 2016;169:77–85. 10.1016/j.socscimed.2016.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Edelmann F, Bobenko A, Gelbrich G, et al. Exercise training in Diastolic Heart Failure (Ex-DHF): rationale and design of a multicentre, prospective, randomized, controlled, parallel group trial. Eur J Heart Fail 2017;19:1067–74. 10.1002/ejhf.862 [DOI] [PubMed] [Google Scholar]
- 24. Haynes A, Brennan S, Redman S, et al. Figuring out fidelity: a worked example of the methods used to identify, critique and revise the essential elements of a contextualised intervention in health policy agencies. Implement Sci 2016;11:1–18. 10.1186/s13012-016-0378-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berg SK, Moons P, Christensen AV, et al. Clinical effects and implications of cardiac rehabilitation for implantable cardioverter defibrillator patients: a mixed-methods approach embedding data from the copenhagen outpatient programme-implantable cardioverter defibrillator randomized clinical trial with qualitative data. J Cardiovasc Nurs 2015;30:420–7. 10.1097/JCN.0000000000000179 [DOI] [PubMed] [Google Scholar]
- 26. Dyas JV, Togher F, Siriwardena AN. Intervention fidelity in primary care complex intervention trials: qualitative study using telephone interviews of patients and practitioners. Qual Prim Care 2014;22:25–34. [PubMed] [Google Scholar]
- 27. Arends RY, Bode C, Taal E, et al. A mixed-methods process evaluation of a goal management intervention for patients with polyarthritis. Psychol Health 2017;32:38–60. 10.1080/08870446.2016.1240173 [DOI] [PubMed] [Google Scholar]
- 28. Feldman AM, Bristow MR, Parmley WW, et al. Effects of vesnarinone on morbidity and mortality in patients with heart failure. Vesnarinone Study Group. N Engl J Med 1993;329:149–55. 10.1056/NEJM199307153290301 [DOI] [PubMed] [Google Scholar]
- 29. Bosworth HB, Steinhauser KE, Orr M, et al. Congestive heart failure patients' perceptions of quality of life: the integration of physical and psychosocial factors. Aging Ment Health 2004;8:83–91. 10.1080/13607860310001613374 [DOI] [PubMed] [Google Scholar]
- 30. May CR, Mair FS, Dowrick CF, et al. Process evaluation for complex interventions in primary care: understanding trials using the normalization process model. BMC Fam Pract 2007;8:42.1–9. 10.1186/1471-2296-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frazer MS, Mobley P. A mixed methods analysis of quality of life among late-life patients diagnosed with chronic illnesses. Health Qual Life Outcomes 2017;15:1–9. 10.1186/s12955-017-0797-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hunt KJ, May CR. Managing expectations: cognitive authority and experienced control in complex healthcare processes. BMC Health Serv Res 2017;17:1–13. 10.1186/s12913-017-2366-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kraai IH, Vermeulen KM, Hillege HL, et al. "Not getting worse" a qualitative study of patients perceptions of treatment goals in patients with heart failure. Appl Nurs Res 2018;39:41–5. 10.1016/j.apnr.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 34. McCarthy MM, Dickson VV, Katz SD, et al. Process evaluation of an exercise counseling intervention using motivational interviewing. Appl Nurs Res 2015;28:156–62. 10.1016/j.apnr.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lowery J, Hopp F, Subramanian U, et al. Evaluation of a nurse practitioner disease management model for chronic heart failure: a multi-site implementation study. Congest Heart Fail 2012;18:64–71. 10.1111/j.1751-7133.2011.00228.x [DOI] [PubMed] [Google Scholar]
- 36. Dalal HM, Doherty P, Taylor RS. Cardiac rehabilitation. BMJ 2015;351:h5000 10.1136/bmj.h5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jolly K, Greenfield SM, Hare R. Attendance of ethnic minority patients in cardiac rehabilitation. J Cardiopulm Rehabil 2004;24:308–12. 10.1097/00008483-200409000-00004 [DOI] [PubMed] [Google Scholar]
- 38. Chauhan U, Baker D, Lester H, et al. Exploring uptake of cardiac rehabilitation in a minority ethnic population in England: a qualitative study. Eur J Cardiovasc Nurs 2010;9:68–74. 10.1016/j.ejcnurse.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 39. Siabani S, Leeder SR, Davidson PM. Barriers and facilitators to self-care in chronic heart failure: a meta-synthesis of qualitative studies. Springerplus 2013;2:1–14. 10.1186/2193-1801-2-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


