Supplemental Digital Content is available in the text.
Keywords: arterial stiffness, blood pressure, hemodynamics, population, retina
Abstract
Arterial stiffness and wave reflection predict cardiovascular mortality and morbidity and are associated with renal microvascular disease. We hypothesized that the retinal microvascular traits might be associated with central hemodynamic properties. In 735 randomly recruited Flemish (mean age, 50.3 years; 47.1% women), we derived central pulse pressure and carotid-femoral pulse wave velocity by applanation tonometry and calculated forward (Pf) and backward (Pb) pulse waves, using an automated pressure-based wave separation algorithm. We measured central retinal arteriolar (CRAE) and venular equivalent and their ratio, using IVAN software (Vasculomatic ala Nicola, version 1.1). Mean values for pulse wave velocity (n=554), Pf and Pb were 7.50 m/s, 32.0 mm Hg, and 21.5 mm Hg, respectively. In multivariable-adjusted analyses, CRAE was 4.62 µm and 1.26 µm smaller (P≤0.034) for a 1-SD increment in central mean arterial pressure (+11.3 mm Hg) and central pulse pressure (+15.2 mm Hg); a 1-SD increment in the augmentation ratio (+7.0%), aortic pulse wave velocity (+1.66 m/s), Pf (+10.0 mm Hg), and Pb (+8.5 mm Hg), was associated with smaller CRAE; the association sizes were −1.91 µm, −1.59 µm, −1.45 µm, and −2.38 µm (P≤0.014), respectively. Associations of arteriole-to-venule diameter ratio with the central hemodynamic traits mirrored those of CRAE. None of the multivariable-adjusted associations of central retinal venular diameter with the central hemodynamic traits reached significance with the exception of central diastolic blood pressure (−1.62 µm; P=0.030). In conclusion, in the general population, higher central pulse pressure, pulse wave velocity, Pf, and Pb were associated with smaller CRAE.
The mechanical and functional properties of blood vessels, including arterial stiffness and wave reflection, predict cardiovascular complications over and beyond traditional risk factors.1–6 The arterial pressure wave consists of a forward component generated by the heart and reflected waves returning from peripheral branching sites to the central aorta.7–9 In stiff compared with elastic arteries, reflected waves return faster, reach the proximal aorta during systole, and augment late systolic blood pressure.10 Aortic pulse wave velocity (PWV) is the gold standard in the assessment of arterial stiffness.11
The observation that retinal microvascular diameters predict cardiovascular mortality,12 coronary heart disease,13 and lacunar stroke,14 extended research from the macrocirculation to the microcirculation. The brain is continually perfused at high volume flow throughout systole and diastole, so that pulsations are transmitted through the capillary network up to the venous efflux.15 The retina shares similar embryological origin, anatomic features, and physiological properties with cerebral microvasculature.16 Nonmydriatic retinal photography allows noninvasive visualization of the retinal microvasculature in population surveys.17–19 Few studies addressed the association between the retinal microcirculation and the properties of the large arteries20–24 but are difficult to interpret, because they cannot be generalized to the general population,21,23 because they involved brachial-ankle PWV and thereby encompassed both elastic and muscular arteries,22,24 or because they had a small sample size.21,23 Our literature review did not reveal any previous study that addressed the possible association of retinal microvascular traits with the forward and backward components of the aortic pulse wave.20–24 We addressed this knowledge gap by analyzing data from the FLEMENGHO (Flemish Study on Environment, Genes, and Health Outcomes).25
Methods
Study Population
FLEMENGHO complies with the Helsinki declaration for research in humans.26 The Ethics Committee of the University Hospitals Leuven approved the protocol. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure, because informed consent did not cover this option. FLEMENGHO is a longitudinal family based population study, which enrolled 3343 individuals from 1985 until 2004.25 Participants were repeatedly followed-up. At each contact, participants gave or renewed informed written consent. The participation rate was 78.0% at enrollment and 76.0% for retinal photography (2008−2015). Surviving participants, who still lived in the catchment area of the study (North Limburg, Belgium), who were not hospitalized or institutionalized, and who had undergone both retinal photography (2008−2015) and a tonometric assessment of their central hemodynamics (2005−2015) were eligible for the current analysis. Of 1027 eligible participants, we excluded 292 from analysis if retinal photographs were of too low quality to be reliably graded (n=187) or if the quality of the pulse wave analysis (n=60) or wave separation analysis (n=45) was substandard. Therefore, our current analysis included 735 participants.
Clinical and Biochemical Measurements
Participants were asked to refrain from heavy exercise, smoking, drinking alcohol, or caffeine-containing beverages for at least 3 hours before being examined. Study nurses administered validated questionnaires, inquiring into each participant’s medical history, smoking and alcohol intake, intake of medications, and lifestyle. Body mass index was calculated as body weight in kilograms divided by height in meters squared. Fasting blood samples were analyzed for plasma glucose and serum total cholesterol, using automated enzymatic methods in a single certified laboratory. Diabetes mellitus was defined as a fasting plasma glucose of 7.0 mmol/L (126 mg/dL) or higher or use of antidiabetic agents.
Hemodynamic Measurements
After the participants had rested for at least 5 minutes in the supine position, nurses measured blood pressures twice to the nearest 2 mm Hg on the right arm, using a standard mercury sphygmomanometer (Riester GmbH, Jungingen, Germany) fitted with the appropriate cuff size according to European guidelines.27 The second of the 2 measurements was analyzed and used for calibration of the pulse wave analysis. Hypertension was defined as a brachial blood pressure of at least 140 mm Hg systolic or 90 mm Hg diastolic or use of antihypertensive drugs. Pulse pressure was systolic minus diastolic blood pressure. Mean arterial pressure was diastolic pressure plus 40% of pulse pressure.28
Next, experienced observers (F.-F. Wei and Z.-Y. Zhang) recorded during an 8-second period, the radial arterial waveform at the dominant arm by applanation tonometry. They used a high-fidelity SPC-301 micromanometer (Millar Instruments Inc, Houston, TX) interfaced with a laptop computer running the SphygmoCor software (AtCor Medical Pty Ltd, West Ryde, New South Wales, Australia), version 9.0. Recordings were discarded when blood pressure variability of consecutive waveforms exceeded 5% or the amplitude of the pulse wave signal was <80 mV. From the radial signal, the SphygmoCor software calculates the aortic pulse wave by means of a validated generalized transfer function.29,30 The software returns the central systolic, diastolic and pulse pressure, and the pressure at the first and second peak (shoulder) of the central waveform (Figure S1 in the online-only Data Supplement). The augmentation ratio and index are quotients of the second over the first peak of the central blood pressure wave and of the absolute difference between the second and first peak over central pulse pressure, both expressed as a percentage. From the central waveform, a triangular-flow pressure-based wave separation algorithm,31 as implemented in the SphygmoCor software, allows computing the forward and backward pulse pressure amplitudes (Figure S1) and the timing of their peak height relative to the electrocardiographic QRS complex. The reflection index was the ratio of the backward to the forward pulse pressure amplitude expressed as percentage.
In 554 participants, aortic PWV was measured by sequential electrocardiographically gated recordings of the arterial pressure waveform at the carotid and femoral arteries. The observers measured the distance from the suprasternal notch to the carotid sampling site (distance A) and from the suprasternal notch to the femoral sampling site (distance B). Pulse wave travel distance was calculated as distance B minus distance A.32 Pulse transit time was the average of 10 consecutive beats.11 Carotid-femoral PWV is the ratio of the travel distance in meters to transit time in seconds. PWV was discarded if the SEM of 10 beats was >10%. We standardized the augmentation ratio and index, the aortic PWV, the forward and backward wave amplitudes and peak times to a heart rate of 65 beats per minute (approximately the mean in the study population).
Retinal Photography
We applied a nonmydriatic approach in a dimly lit room to acquire retinal photographs, one image per eye in each participant, with the Canon Cr-DGi retinal visualization system combined with the Canon D 50 digital camera (Canon Inc, Medical Equipment Group, Utsunomiya, Japan). After centering the image on the optic disk, we measured the central retinal arteriolar (CRAE) and venular (CRVE) equivalent, which represent the retinal arteriolar and venular diameter. We used the validated computer-assisted programme IVAN (Vasculomatic ala Nicola, version 1.1, Department of Ophthalmology and Visual Science, University of Wisconsin-Madison, Madison, WI) based on formulae published by Parr and Spears33 and Hubbard et al.34 The IVAN software returns average vessel diameters according to the revised Knudtson formula.35 The arteriole-to-venule diameter ratio (AVR) was CRAE divided by CRVE. For analysis, we averaged each participant’s measurements at both eyes. Intraobserver variability according to the Bland and Altman method36 was 11.7% for CRAE, 9.6% for CRVE, and 12.5% for AVR.18 The corresponding estimates for interobserver variability were 10.8%, 9.9%, and 14.6%, respectively.18
Statistical Analysis
For database management and statistical analysis, we used SAS software, version 9.4 (SAS Institute Inc, Cary, NC). We compared means and proportions by the large-sample Z test or ANOVA and by the χ2 statistic, respectively. Statistical significance was a 2-sided significance of 0.05.
In exploratory analyses, we plotted the retinal arteriolar diameter by quartiles of the distributions of forward and backward wave amplitudes and aortic PWV. In unadjusted and multivariable-adjusted analyses, we expressed association sizes of the retinal phenotypes for a 1-SD increment in the central hemodynamic measurements. As in previous publications,37 we adjusted for sex, age, body mass index, heart rate, serum total cholesterol, plasma glucose, current smoking (0, 1) and alcohol intake (0, 1), use of antihypertensive drugs (0, 1), and history of cardiovascular disease (0, 1), and additionally brachial diastolic blood pressure for the time-dependent central hemodynamic measurements, including the augmentation ratio and index, PWV, the forward and backward amplitudes, and the reflection magnitude. Sensitivity analyses excluding those on antihypertensive drug treatment were performed to assess the association between CRAE and all central hemodynamic measurements. In sensitivity analyses, the models relating CRAE to the central hemodynamic measurements were additionally adjusted for CRVE to provide unbiased results.38
Results
Characteristics of Participants
All 735 participants were white Europeans, of whom 346 (47.1%) were female. In all participants, mean values (±SD) were 50.3±15.0 years for age, 26.5±4.1 kg/m2 for body mass index, and 127.8±16.4/79.2±9.7 mm Hg for systolic/diastolic blood pressure. Of 303 participants with hypertension, 158 were on antihypertensive drug treatment (52.1%) with diuretics (n=54 [34.2%]), β-blockers (n=87 [55.1%]), inhibitors of the renin-angiotensin system (angiotensin-converting enzyme inhibitors or angiotensin II type-1 receptor blockers; n=56 [35.4%]) or vasodilators (calcium-channel blockers or α-blockers; n=49 [31.0%]), prescribed in varying combinations in 70 patients (44.3%). Thirty-four participants (4.6%) had diabetes mellitus.
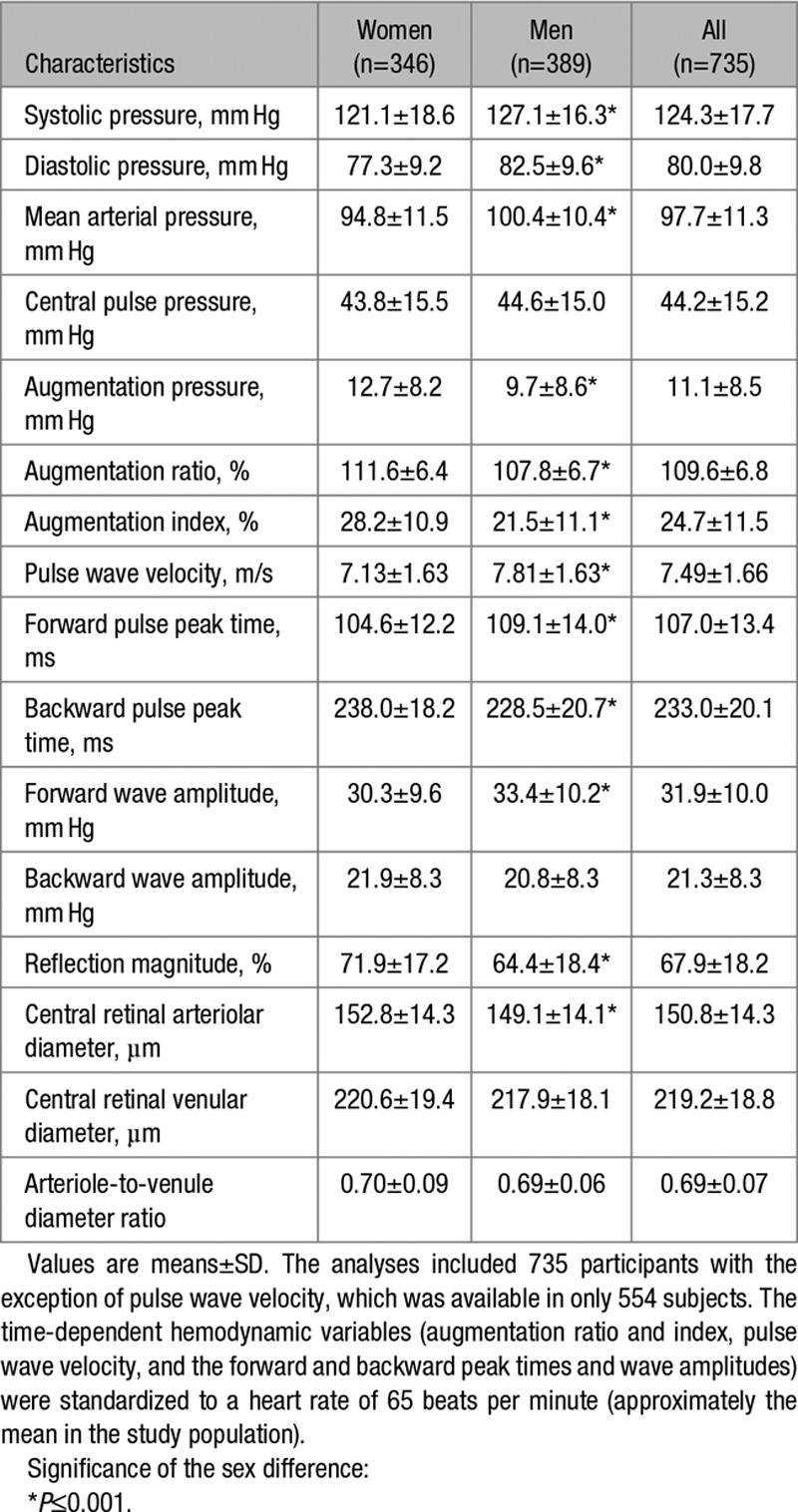
Tables 1 and 2 list the clinical, hemodynamic, and retinal microvascular characteristics of the participants by sex. Compared with women, men had a higher prevalence of smoking, alcohol intake, and hypertension and had significantly higher body mass index, blood pressure, and plasma glucose (P≤0.043; Table 1). Mean values for aortic PWV (n=554) and the forward and backward wave amplitudes were 7.50±1.66 m/s, 32.0±10.1 mm Hg, and 21.5±8.5 mm Hg, respectively. Compared with women, men had significantly (P<0.001) higher central systolic and diastolic blood pressure, aortic PWV and forward wave amplitude but lower augmentation pressure, augmentation ratio and index, reflection magnitude, and smaller CRAE (Table 2).
Table 1.
Characteristics of Participants
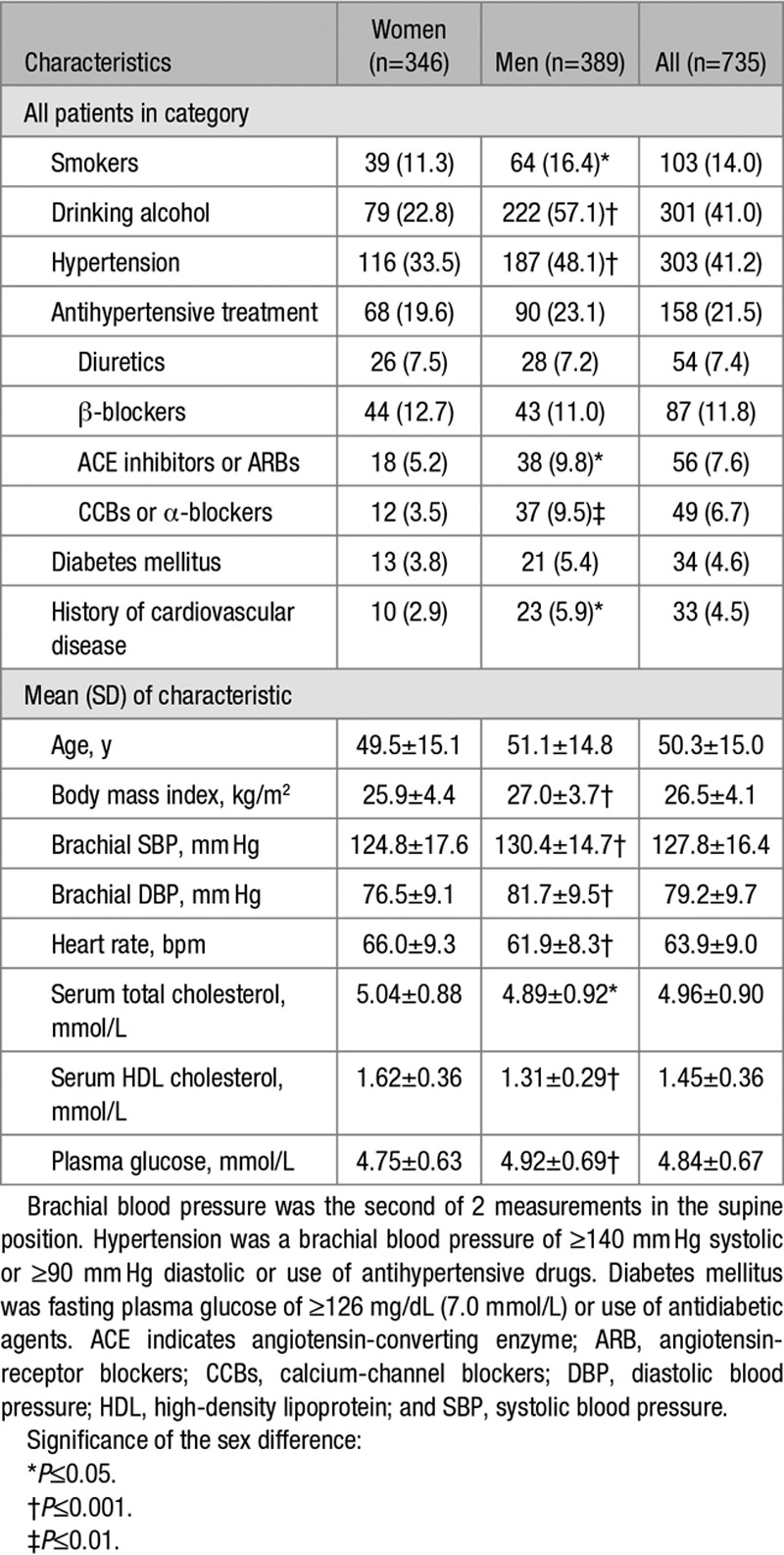
Table 2.
Central Hemodynamics and Retinal Microvascular Traits
Unadjusted Analyses
The Figure demonstrates that in unadjusted analyses, the CRAE decreased across quartiles of the distributions of the forward and backward wave amplitudes and aortic PWV. In unadjusted analyses (Table 3), the CRAE was inversely associated with all central hemodynamic measurements with association sizes for a 1-SD increment in the explanatory hemodynamic variables (P≤0.009) ranging from −5.97 µm (mean arterial pressure) to −1.38 µm (forward wave amplitude). The CRVE significantly (P≤0.030) decreased with all central hemodynamic measurements (Table S1 and Figure S2) with the exception of the forward wave amplitude (P=0.72). The associations of the AVR with the central hemodynamic traits were directionally similar compared with the CRAE but were weaker for the augmentation ratio and index and the reflection magnitude (Table 4 and Figure S3).
Table 3.
Central Retinal Arteriolar Diameter in Relation to Central Hemodynamics
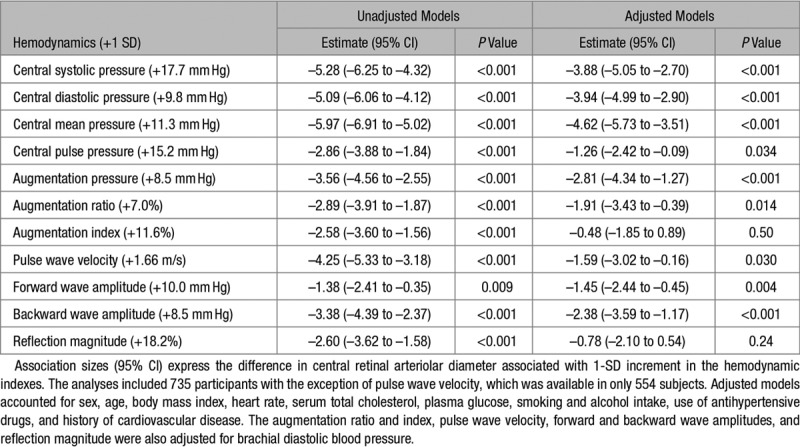
Table 4.
Arteriole-to-Venule Diameter Ratio in Relation to Hemodynamic Traits
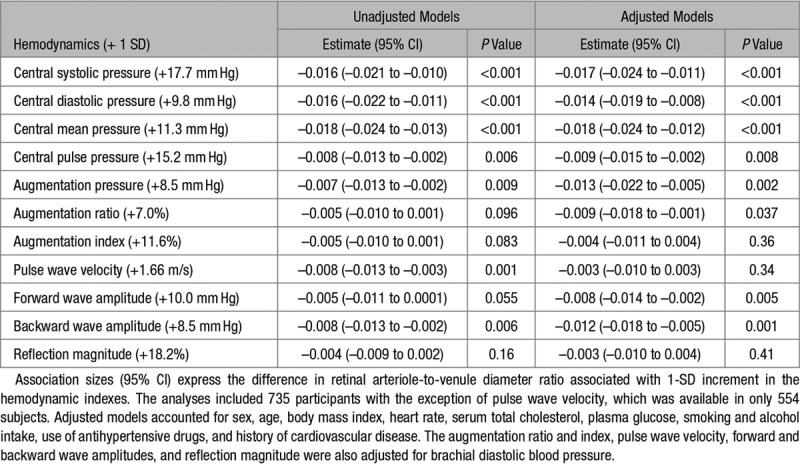
Figure.
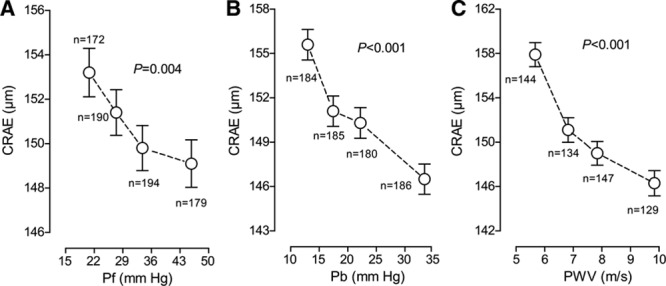
Central retinal arteriolar diameter (CRAE) by quartiles of the distribution of forward wave amplitude (Pf), backward wave amplitude (Pb), and aortic pulse wave velocity (PWV, n=554). Each plotted point represents the unstandardized and unadjusted mean. Vertical bars indicate the SE. P values are for linear trend across the quartiles by Pf (A), Pb (B), and PWV (C).
Adjusted Analyses
With adjustments applied for sex, age, body mass index, heart rate, serum total cholesterol, plasma glucose, current smoking and alcohol intake, antihypertensive drug treatment, and history of cardiovascular disease, a 1-SD increment in central mean pressure (+11.3 mm Hg), pulse pressure (+15.2 mm Hg), and augmentation pressure (+8.5 mm Hg) was associated with smaller CRAE (P≤0.034; Table 3) with association sizes amounting to −4.62, −1.26, and −2.81 µm, respectively. Additionally adjusted for brachial diastolic blood pressure, for a 1-SD increment in the augmentation ratio (+7.0 %), aortic PWV (+1.66 m/s), and the forward (+10.0 mm Hg) and backward (+8.5 mm Hg) wave amplitudes, the association sizes with CRAE were −1.91, −1.59, −1.45, and −2.38 µm (P≤0.030), respectively (Table 3). None of the multivariable-adjusted associations of the CRVE with the central hemodynamic traits reached significance (P≥0.22; Table S1) with the exception of central diastolic blood pressure (P=0.030). The multivariable-adjusted associations of AVR with the central hemodynamic variables mirrored those of the CRAE (Table 4). They were nonsignificant for the augmentation index, aortic PWV, and the reflection magnitude (Table 4; P≥0.34). The association sizes of the AVR with the other central hemodynamic traits were significant (P≤0.037; Table 4) with association sizes ranging from −0.018 (central mean arterial pressure) to −0.008 (forward wave amplitude).
Sensitivity Analyses
Multivariable-adjusted analyses stratified by median age (≤50.8 versus >50.8 years; Table S2) generated results similar to those reported in Table 3. Among 577 untreated participants, 432 had normal blood pressure. Sensitivity analyses of retinal microvascular traits in relation to the central hemodynamics in participants not on antihypertensive drug treatment (Tables S3 and S4) and in those with normal blood pressure (Table S3) were confirmatory. Similarly, excluding 34 participants with diabetes mellitus produced confirmatory results (Table S5).
Next, we ran an analysis of the central hemodynamics calibrated by diastolic blood pressure and mean arterial pressure, defined as diastolic blood pressure plus one-third of pulse pressure (Table S6). Correlation coefficients of the central hemodynamic measurements calibrated in this alternative way with those derived by the recommended calibration were all higher than 0.986. Consequently, there were no material differences between the association sizes in Table S6 and Table 3. The same was true when models relating CRAE to the central hemodynamic measurements were additionally adjusted for CRVE (Table S7), although with a marginally trend of inverse association between CRAE and aortic PWV (P=0.072) and with a significant association with the augmentation index (P<0.001).
Discussion
To our knowledge, our study is the first population survey assessing the association of retinal microvascular traits with central hemodynamic measurements. The key finding of our study was that the CRAE was smaller with higher values of aortic PWV, central pulse pressure, augmentation ratio, and the forward and backward wave amplitudes. There is close anatomic proximity between the central arteries and the retinal arterioles. The ophthalmic artery branches directly from the internal carotid artery. The inverse association of the retinal arteriolar diameter with central hemodynamic indexes reflecting arterial stiffness is, therefore, coherent and physiologically plausible. Indeed, in a stiffer arterial tree, the backward wave returns during systole, thus augmenting central systolic blood pressure and widening central pulse pressure. The pulsatile stress on the wall of the large arteries leads to further degradation of the elastin fibers, so that extremely stiff collagen increasingly bears the pulsatile load.39
Several studies investigated the association between the central retinal microvascular diameter and aortic stiffness in hypertensive patients.21,23 In 3425 participants (46.4% women; mean age, 62.0 years) enrolled in the Multi-Ethnic Study of Atherosclerosis, aortic distensibility was determined from chest magnetic resonance imaging.20 With adjustments applied for demographic variables and cardiovascular risk factors, participants in the lowest quartile of the distribution of aortic distensibility, compared with those in top quartile, had an increased risk of retinal arteriolar narrowing (odds ratio, 1.72; 95% CI, 1.15–2.58).20 In multivariable-adjusted analyses of 137 never-treated early stage hypertensive patients and 86 normotensive individuals (36.3% women; mean age, 43.8 years),21 0.098 m/s higher PWV and a 0.113 higher augmentation index were associated with a 1-µm decrease in the CRAE diameter (P≤0.049).21 In 181 hypertensive patients (44.8% women; mean age, 53.9 years; treated for hypertension, 60.2%), the CRAE was significantly and inversely associated with the tonometrically assessed aortic PWV in an unadjusted model (β=−0.228; P<0.001). This association remained significant in multivariable-adjusted analyses (β=−0.233; P<0.001).23 Aortic PWV was also measured over 24 hours, using Mobil-O-Graph 24-hour PWA monitors (IEM GmbH, Stolberg, Germany).40 The correlations of the CRAE with PWV measured over 24 hours and during wakefulness and sleep ranged from −0.241 to −0.204 in unadjusted analyses and from −0.292 to −0.222 in multivariable-adjusted models.23
Recent studies reported associations of the renal41 or cerebral42,43 microvasculature with PWV. The Age, Gene/Environment Susceptibility-Reykjavik Study41 enrolled 629 randomly selected community-dwelling elderly 629 (45.2% women; mean age 74.8 years), which were followed-up for 5.3 years (mean). In multivariable-adjusted models, a 1-SD increment in the baseline carotid-femoral PWV and carotid pulse pressure, predicted a decline in the estimated glomerular filtration rate at follow-up, amounting to 0.23 mL/min per 1.73 m2 per year and 0.25 mL/min per 1.73 m2 per year, respectively.41 With adjustments applied, higher PWV was also associated with greater volume of white matter hyperintensity (β=0.108 mL; P=0.018) and lower memory scores (β=−0.095; P=0.028) in multivariable-adjusted model.42 A meta-analysis of summary statistics extracted from 3 cross-sectional and 4 longitudinal studies and one study with mixed design addressed the association between intelligence quotient, a potential marker of the cerebral microcirculation,44 and PWV in participants older than 30 years without comorbidities.43 Cognitive impairment was associated with higher PWV.43 Previous studies demonstrated that retinal venular diameter predicts the risk of stroke.45 However, among 8794 participants (54.6% women; mean age, 60.0 years) of the Atherosclerosis Risk in Communities study, the CRAE and CRVE were differentially associated with cardiovascular risk factors.46 The venular calibers were correlated with cigarette smoking, blood pressure, biomarkers of systemic inflammation, and body mass index, whereas the main correlate of the arteriolar diameters was blood pressure.46 Thus, association sizes of the venular diameters with cardiovascular risk factors are bound to differ between population studies based on their prevalence, thereby explaining the null association of venular diameters with the central hemodynamics in our multivariable-adjusted models (Table S1).
The current study must be interpreted within the context of its strength and potential limitations. Strengths of our study include its relatively large-sample size, population-based design, robust measurements for aortic stiffness, and a validated computer-based technique to quantify retinal vascular caliber. Our observations are in line with the literature and provide an external validation of our study. Among 4926 participants (56% women; age range, 43–84 years) enrolled in the Beaver Dam Eye Study, retinal arteriolar diameters were in multivariable-adjusted analyses 2.1 µm (95% CI, 1.5–2.7) lower for each decade increase in age, and 4.4 µm (95% CI, 3.8–5.0) lower for each 10 mm Hg increase in mean arterial blood pressure.47 In our study, the corresponding estimates were 1.5 µm (95% CI, 0.74–2.25) and 4.3 µm (95% CI, 3.3–5.3), respectively. Furthermore, in line with common knowledge, the central pressure augmentation was higher in women than men as a result of shorter stature,7 differences in the ventricular contraction pattern,48 and morphology of the forward and backward wave.49 Among the potential limitations of our current study is its cross-sectional design, which precludes direct causal inferences. Furthermore, our current findings in white Flemish cannot be extrapolated to other ethnicities. Finally, 292 of 1027 eligible participants (28.4%) were excluded from the current analysis. However, participants analyzed and not analyzed had similar body mass index, brachial diastolic blood pressure, and plasma glucose (P≥0.13) but were on average 6.7 years older and, therefore, had a slightly higher systolic blood pressure and serum total cholesterol (P≤0.013; Table S8).
The kidney and brain share unique susceptibilities to vascular injury, because they are continually perfused at high volume flow throughout systole and diastole, so that pulsations are transmitted through the capillary network up to the venous efflux.15 The cerebral microvasculature and the retinal microcirculation also share similar anatomic features and physiological properties.16 The retina offers a unique window to the brain.16 The clinical perspective of our current study should be gauged from this point of view. Our study thereby provides further insight on the crosstalk between microcirculation and macrocirculation in health and disease, as mechanistically described in the seminal article published by O’Rourke and Safar.15
Perspectives
For long clinicians focused on the macrocirculation because over the past 30 years invasive therapies were developed allowing effectively treating patients with acute coronary syndrome, myocardial infarction, ischemic or hemorrhagic stroke, or peripheral arterial disease. These invasive approaches preserve vital organs and save lives. Only recently, the insight grew that microcirculatory dysfunction is a main driver of age-related chronic disease, including worsening diastolic left ventricular function,50 chronic kidney disease,41 and cognitive decline.42,43 Our current observations highlight that dysfunction of the macrocirculation and microcirculation go hand in hand and suggest that the timely prevention of both macrovascular and microvascular alterations could have relevant clinical impact.
Acknowledgments
We gratefully acknowledge the clerical assistance of Vera De Leebeeck and Renilde Wolfs.
Sources of Funding
The European Union (HEALTH-F7-305507-HOMAGE), the European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET), the European Research Area Net for Cardiovascular Diseases (JTC2017-046-PROACT), and the Research Foundation Flanders, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13) supported the Studies Coordinating Centre in Leuven.
Disclosures
None.
Supplementary Material
Footnotes
This paper was sent to David Calhoun, Guest Editor, for review by expert referees, editorial decision, and final disposition.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.119.13255.
Novelty and Significance
What Is New?
Stiffening of the large arteries is forerunner of cardiovascular complications and is associated with microvascular disease in kidney and brain. We hypothesized that the retinal microvascular traits might be associated with central hemodynamic properties. In 735 Flemish, we derived central pulse pressure and carotid-femoral pulse wave velocity from applanation tonometry and calculated forward (Pf) and backward (Pb) pulse waves and measured retinal arteriolar and venular caliber and their ratio.
What Is Relevant?
In unadjusted analyses, smaller central retinal arteriolar caliber was significantly associated with higher central pulse pressure, pulse wave velocity, Pb, and Pf.
With adjustments applied for sex, age, body mass index, brachial diastolic blood pressure, heart rate, serum total cholesterol, plasma glucose, current smoking and alcohol intake, antihypertensive drug treatment, and history of cardiovascular disease, as appropriate, these associations remained significant. Associations of arteriole-to-venule diameter ratio with the central hemodynamic variables mirrored those of central retinal arteriolar diameter.
In adjusted models, central retinal venular diameter was only associated with central diastolic blood pressure.
Summary
In the general population, narrower retinal arterial diameter was associated with central hemodynamic indexes reflecting arterial stiffness and wave reflections. Our current observations highlight that dysfunction of the macrocirculation and microcirculation go hand in hand and suggest that the timely prevention of both macrovascular and microvascular alterations could have relevant clinical impact.
References
- 1.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, Chou P, Chen CH. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension. 2010;55:799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamani P, Jacobs DR, Jr, Segers P, Duprez DA, Brumback L, Kronmal RA, Lilly SM, Townsend RR, Budoff M, Lima JA, Hannan P, Chirinos JA. Reflection magnitude as a predictor of mortality: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2014;64:958–964. doi: 10.1161/HYPERTENSIONAHA.114.03855. doi: 10.1161/HYPERTENSIONAHA.114.03855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamani P, Lilly SM, Segers P, Jacobs DR, Jr, Bluemke DA, Duprez DA, Chirinos JA. Pulsatile load components, resistive load and incident heart failure: the Multi-Ethnic Study of Atherosclerosis (MESA). J Card Fail. 2016;22:988–995. doi: 10.1016/j.cardfail.2016.04.011. doi: 10.1016/j.cardfail.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 8.Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR Asklepios Investigators. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007;49:1248–1255. doi: 10.1161/HYPERTENSIONAHA.106.085480. doi: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 9.Vasan RS. Pathogenesis of elevated peripheral pulse pressure: some reflections and thinking forward. Hypertension. 2008;51:33–36. doi: 10.1161/HYPERTENSIONAHA.107.101196. doi: 10.1161/HYPERTENSIONAHA.107.101196. [DOI] [PubMed] [Google Scholar]
- 10.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 11.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 12.Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, Hubbard LD, Tielsch JM. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110:933–940. doi: 10.1016/S0161-6420(03)00084-8. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 13.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 14.Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BE, Sharrett AR ARIC Study Investigators. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke. 2010;41:1349–1355. doi: 10.1161/STROKEAHA.110.580837. doi: 10.1161/STROKEAHA.110.580837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 16.Cheung CY, Ikram MK, Chen C, Wong TY. Imaging retina to study dementia and stroke. Prog Retin Eye Res. 2017;57:89–107. doi: 10.1016/j.preteyeres.2017.01.001. doi: 10.1016/j.preteyeres.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Klein BE, Hubbard LD, Nieto FJ Atherosclerosis Risk in Communities Study. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140:248–255. doi: 10.7326/0003-4819-140-4-200402170-00006. doi: 10.7326/0003-4819-140-4-200402170-00006. [DOI] [PubMed] [Google Scholar]
- 18.Liu YP, Richart T, Jin Y, Struijker-Boudier HA, Staessen JA. Retinal arteriolar and venular phenotypes in a Flemish population: reproducibility and correlates. Artery Res. 2011;5:72–79. [Google Scholar]
- 19.Cheung CY, Ikram MK, Sabanayagam C, Wong TY. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension. 2012;60:1094–1103. doi: 10.1161/HYPERTENSIONAHA.111.189142. doi: 10.1161/HYPERTENSIONAHA.111.189142. [DOI] [PubMed] [Google Scholar]
- 20.Cheung N, Sharrett AR, Klein R, Criqui MH, Islam FM, Macura KJ, Cotch MF, Klein BE, Wong TY. Aortic distensibility and retinal arteriolar narrowing: the multi-ethnic study of atherosclerosis. Hypertension. 2007;50:617–622. doi: 10.1161/HYPERTENSIONAHA.107.091926. doi: 10.1161/HYPERTENSIONAHA.107.091926. [DOI] [PubMed] [Google Scholar]
- 21.Triantafyllou A, Anyfanti P, Gavriilaki E, Zabulis X, Gkaliagkousi E, Petidis K, Triantafyllou G, Gkolias V, Pyrpasopoulou A, Douma S. Association between retinal vessel caliber and arterial stiffness in a population comprised of normotensive to early-stage hypertensive individuals. Am J Hypertens. 2014;27:1472–1478. doi: 10.1093/ajh/hpu074. doi: 10.1093/ajh/hpu074. [DOI] [PubMed] [Google Scholar]
- 22.Lin F, Zhu P, Huang F, Li Q, Yuan Y, Gao Z, Yu P, Lin J, Chen F. Aortic stiffness is associated with the central retinal arteriolar equivalent and retinal vascular fractal dimension in a population along the southeastern coast of China. Hypertens Res. 2015;38:342–348. doi: 10.1038/hr.2015.11. doi: 10.1038/hr.2015.11. [DOI] [PubMed] [Google Scholar]
- 23.Aissopou EK, Argyris AA, Nasothimiou EG, Konstantonis GD, Tampakis K, Tentolouris N, Papathanassiou M, Theodossiadis PG, Papaioannou TG, Stehouwer CD, Sfikakis PP, Protogerou AD. Ambulatory aortic stiffness is associated with narrow retinal arteriolar caliber in hypertensives: the SAFAR Study. Am J Hypertens. 2016;29:626–633. doi: 10.1093/ajh/hpv145. doi: 10.1093/ajh/hpv145. [DOI] [PubMed] [Google Scholar]
- 24.Kawashima-Kumagai K, Tabara Y, Yamashiro K, Setoh K, Yoshikawa M, Kawaguchi T, Miyake M, Nakanishi H, Muraoka Y, Takahashi Y, Kosugi S, Nakayama T, Yoshimura N, Matsuda F, Tsujikawa A Nagahama Study Group. Association of retinal vessel calibers and longitudinal changes in arterial stiffness: the Nagahama study. J Hypertens. 2018;36:587–593. doi: 10.1097/HJH.0000000000001602. doi: 10.1097/HJH.0000000000001602. [DOI] [PubMed] [Google Scholar]
- 25.Wei FF, Zhang ZY, Thijs L, Yang WY, Jacobs L, Cauwenberghs N, Gu YM, Kuznetsova T, Allegaert K, Verhamme P, Li Y, Struijker-Boudier HA, Staessen JA. Conventional and ambulatory blood pressure as predictors of retinal arteriolar narrowing. Hypertension. 2016;68:511–520. doi: 10.1161/HYPERTENSIONAHA.116.07523. doi: 10.1161/HYPERTENSIONAHA.116.07523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–848. doi: 10.1097/00004872-200305000-00001. doi: 10.1097/01.hjh.0000059016.82022.ca. [DOI] [PubMed] [Google Scholar]
- 28.Bos WJ, Verrij E, Vincent HH, Westerhof BE, Parati G, van Montfrans GA. How to assess mean blood pressure properly at the brachial artery level. J Hypertens. 2007;25:751–755. doi: 10.1097/HJH.0b013e32803fb621. doi: 10.1097/HJH.0b013e32803fb621. [DOI] [PubMed] [Google Scholar]
- 29.Karamanoglu M, O’Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–167. doi: 10.1093/eurheartj/14.2.160. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- 30.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 31.Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A. Quantification of wave reflection in the human aorta from pressure alone: a proof of principle. Hypertension. 2006;48:595–601. doi: 10.1161/01.HYP.0000238330.08894.17. doi: 10.1161/01.HYP.0000238330.08894.17. [DOI] [PubMed] [Google Scholar]
- 32.Weber T, Ammer M, Rammer M, Adji A, O’Rourke MF, Wassertheurer S, Rosenkranz S, Eber B. Noninvasive determination of carotid-femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J Hypertens. 2009;27:1624–1630. doi: 10.1097/HJH.0b013e32832cb04e. doi: 10.1097/HJH.0b013e32832cb04e. [DOI] [PubMed] [Google Scholar]
- 33.Parr JC, Spears GF. General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol. 1974;77:472–477. doi: 10.1016/0002-9394(74)90457-7. [DOI] [PubMed] [Google Scholar]
- 34.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD, Cai J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 35.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 36.Dewitte K, Fierens C, Stöckl D, Thienpont LM. Application of the Bland-Altman plot for interpretation of method-comparison studies: a critical investigation of its practice. Clin Chem. 2002;48:799–801.. [PubMed] [Google Scholar]
- 37.Wei FF, Huang QF, Zhang ZY, Van Keer K, Thijs L, Trenson S, Yang WY, Cauwenberghs N, Mujaj B, Kuznetsova T, Allegaert K, Struijker-Boudier HAJ, Verhamme P, Vermeer C, Staessen JA. Inactive matrix Gla protein is a novel circulating biomarker predicting retinal arteriolar narrowing in humans. Sci Rep. 2018;8:15088. doi: 10.1038/s41598-018-33257-6. doi: 10.1038/s41598-018-33257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liew G, Sharrett AR, Kronmal R, Klein R, Wong TY, Mitchell P, Kifley A, Wang JJ. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci. 2007;48:52–57. doi: 10.1167/iovs.06-0672. doi: 10.1167/iovs.06-0672. [DOI] [PubMed] [Google Scholar]
- 39.Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010;74:2257–2262. doi: 10.1253/circj.cj-10-0910. [DOI] [PubMed] [Google Scholar]
- 40.Boggia J, Luzardo L, Lujambio I, Sottolano M, Robaina S, Thijs L, Olascoaga A, Noboa O, Struijker-Boudier HA, Safar ME, Staessen JA. The diurnal profile of central hemodynamics in a general Uruguayan population. Am J Hypertens. 2016;29:737–746. doi: 10.1093/ajh/hpv169. doi: 10.1093/ajh/hpv169. [DOI] [PubMed] [Google Scholar]
- 41.Huang N, Foster MC, Mitchell GF, Andresdottir MB, Eiriksdottir G, Gudmundsdottir H, Harris TB, Launer LJ, Palsson R, Gudnason V, Levey AS, Inker LA. Aortic stiffness and change in glomerular filtration rate and albuminuria in older people. Nephrol Dial Transplant. 2017;32:677–684. doi: 10.1093/ndt/gfw050. doi: 10.1093/ndt/gfw050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility–Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Lyu P, Ren Y, An J, Dong Y. Arterial stiffness and cognitive impairment. J Neurol Sci. 2017;380:1–10. doi: 10.1016/j.jns.2017.06.018. doi: 10.1016/j.jns.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Wei FF, Raaijmakers A, Zhang ZY, van Tienoven TP, Huang QF, Yang WY, Thijs L, Struijker-Boudier HAJ, Verhamme P, Allegaert K, Staessen JA. Association between cognition and the retinal microvasculature in 11-year old children born preterm or at term. Early Hum Dev. 2018;118:1–7. doi: 10.1016/j.earlhumdev.2018.01.018. doi: 10.1016/j.earlhumdev.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, de Jong PT, Witteman JC, Breteler MM, Shaw J, Zimmet P, Wong TY. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol. 2009;170:1323–1332. doi: 10.1093/aje/kwp306. doi: 10.1093/aje/kwp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liew G, Sharrett AR, Wang JJ, Klein R, Klein BE, Mitchell P, Wong TY. Relative importance of systemic determinants of retinal arteriolar and venular caliber: the atherosclerosis risk in communities study. Arch Ophthalmol. 2008;126:1404–1410. doi: 10.1001/archopht.126.10.1404. doi: 10.1001/archopht.126.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44:4644–4650. doi: 10.1167/iovs.03-0079. [DOI] [PubMed] [Google Scholar]
- 48.Celentano A, Palmieri V, Arezzi E, Mureddu GF, Sabatella M, Di Minno G, De Simone G. Gender differences in left ventricular chamber and midwall systolic function in normotensive and hypertensive adults. J Hypertens. 2003;21:1415–1423. doi: 10.1097/00004872-200307000-00033. doi: 10.1097/01.hjh.0000059088.43904.ba. [DOI] [PubMed] [Google Scholar]
- 49.Torjesen AA, Wang N, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Forward and backward wave morphology and central pressure augmentation in men and women in the Framingham Heart Study. Hypertension. 2014;64:259–265. doi: 10.1161/HYPERTENSIONAHA.114.03371. doi: 10.1161/HYPERTENSIONAHA.114.03371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]


