Abstract
Lobectomy is considered the standard strategy for early-stage non-small cell lung cancer (NSCLC). However, sublobar resection for NSCLC has recently received increased attention. The objective of this study was to compare 5-year survival, recurrence-free survival, postoperative mortality, and postoperative morbidities in patients who received segmentectomy versus those who received lobectomy through a meta-analysis. Sixteen studies were included and the combined hazard ratios or odds ratios were calculated. The results revealed that the 5-year survival rate after segmentectomy was comparable to that of lobectomy for stage IA NSCLC. However, segmentectomy for stage I NSCLC had lower rates of postoperative mortality and morbidities than lobectomy.
Keywords: Meta-analysis, Non-small-cell lung carcinoma, Lobectomy, Segmentectomy, Survival
Introduction
Lung cancer is the leading cause of malignancy-related deaths in many countries. Surgical resection is considered the standard treatment for early-stage non-small cell lung cancer (NSCLC) [1]. Surgical resection can achieve a 5-year survival rate as high as 70% in patients with stage IA NSCLC [2]. Therefore, early detection is a promising strategy for NSCLC. According to the National Lung Screening Trial, screening with low-dose computed tomography (CT) scans can reduce the mortality of lung cancer by increasing the detection of early-stage lung cancer, thereby enabling more patients to receive surgical treatment [3]. Therefore, it is expected that early-stage lung cancer will be detected more frequently as a result of the widespread application of screening chest CT scans. However, the optimal extent of surgical resection for early-stage lung cancer is still debatable. Although lobectomy has been the standard surgical strategy for NSCLC since the randomized controlled trial performed by the Lung Cancer Study Group in 1995 [4], the question has been repeatedly raised of whether sublobar resection could be an alternative surgical strategy to lobectomy. Large-scale randomized controlled studies comparing sublobar resection to lobectomy for NSCLC are now in progress [5,6].
The issues that should be clarified are whether segmentectomy is beneficial for early outcomes and whether its long-term outcomes are comparable to those of lobectomy. Several meta-analyses have addressed these issues with inconsistent results [7–12]. Therefore, the aim of this study was to compare early and late outcomes between segmentectomy and lobectomy. The 5-year survival, recurrence-free survival, postoperative mortality, and postoperative morbidities after segmentectomy and lobectomy were compared through a meta-analysis.
Methods
1) Study selection
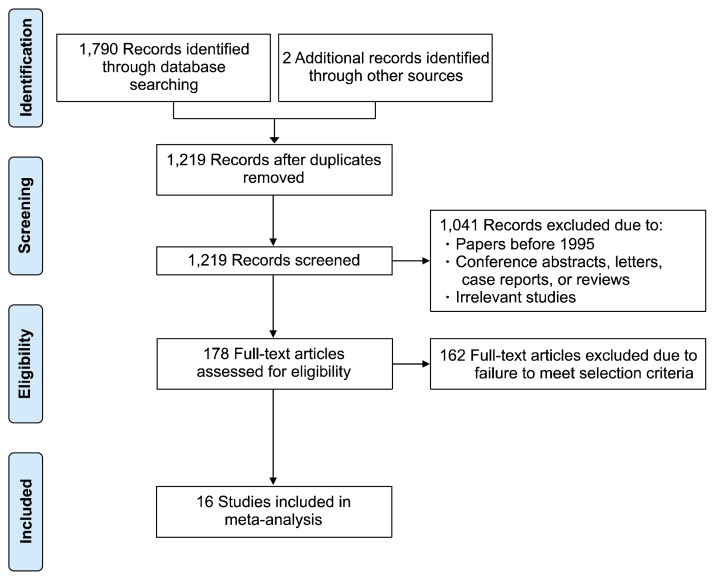
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram was used to conduct the meta-analysis [13]. Studies on lobectomy and segmentectomy for stage I NSCLC were searched through PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, and the Cochrane Library. To maximize the sensitivity of the search strategy and to identify all potentially relevant studies, we combined the search terms as follows: “lobectomy” AND “sublobar resection” OR “sublobectomy” OR “limited resection” OR “segmentectomy” AND “NSCLC” OR “lung neoplasm” OR “lung cancer” AND “survival” OR “recurrence.” In total, 1,790 articles were found. After eliminating duplicates, a total of 1,219 articles remained.
2) Inclusion and exclusion criteria
The retrieved studies were then selected according to the following inclusion and exclusion criteria. The inclusion criteria were: (1) a study population comprising stage I NSCLC patients, with all histologic types and pathologic confirmation; (2) an analysis of lobectomy or limited resection (specifically segmentectomy); (3) study outcomes including 5-year survival, recurrence-free survival, postoperative mortality (in-hospital or 30-day mortality), and postoperative morbidities; and (4) any of the following study designs: randomized controlled trial, cohort, or case-control (retrospective or prospective). The exclusion criteria were: (1) a study population of patients who received wedge resection; (2) data that merged wedge resection and segmentectomy as ‘sublobar resection’; (3) study participants who received neo-adjuvant chemotherapy or induction treatment before surgery; (4) case reports, reviews, or expert opinions; (5) studies not written in English; (6) studies for which only the abstract was available; and (7) studies published before 1995.
3) Data extraction
Based on the inclusion and exclusion criteria, a total of 178 full-text articles were assessed for eligibility. Two investigators (T.Y.L. and S.P.) independently reviewed each retrieved article. All discrepancies between the 2 reviewers were resolved by discussion. As a result, 162 articles were excluded and 16 articles were included in the meta-analysis.
4) Statistical analysis
In this meta-analysis, we compared 5-year survival, recurrence-free survival, postoperative mortality, and postoperative morbidities. We used hazard ratios and standard error to interpret 5-year survival and recurrence-free survival. The hazard ratios and associated 95% confidence intervals were directly used if they were provided by the original studies. If they were not, they were indirectly obtained by using the Kaplan-Meier survival curve, the number of patients at risk, and the TechDig software. The details of these techniques were described by Tierney et al. [14] and Parmar et al. [15]. RevMan 5.3 was used to generate forest plots and funnel plots. The I2 statistic and p-values were used to evaluate the heterogeneity of the studies, with I2>50% or p>0.1 suggesting that the outcomes were heterogeneous. In the analyses of postoperative mortality and morbidities, odds ratios were used to measure the effect size.
Results
1) Literature search and general characteristics of the studies included
A total of 1,790 studies were identified from 4 electronic databases. Two studies were identified through other sources. After removing duplicates, studies before 1995, and irrelevant studies, 178 articles were assessed for eligibility, of which 162 were excluded due to failure to meet the inclusion criteria. Sixteen studies remained. The characteristics of these characteristics are summarized in Table 1 and Table 2 [16–31]. A summary of the search strategy and selection process used are presented in Fig. 1 following the PRISMA flow diagram.
Table 1.
Demographic characteristics of studies included in the meta-analysis of lobectomy versus segmentectomy for stage I non-small cell lung cancer
| Author (year) | Country | Institution | No. of patients | Study period | Stage confirmed | Type of operation | Industry involvement |
|---|---|---|---|---|---|---|---|
| Kodama et al. [16] (1997) | Japan | Osaka Medical Center for Cancer & Cardiovascular Diseases | 140 | Jan 1985–Sep 1996 | Mediastinal LND | Thoracotomy | NR |
| Okada et al. [17] (2001) | Japan | National Hyogo Central Hospital | 172 | Jun 1984–Dec 1998 | Mediastinal LND | NR | NR |
| Martin-Ucar et al. [18] (2005) | UK | Glenfield Hospital | 34 | Apr 1997–Apr 2004 | Systemic LND | Open & VATS | NR |
| Okumura et al. [19] (2007) | Japan | National Hospital Organization, Kinki Medical Center for Chest Diseases | 2,009 | 1980–2002 | Mediastinal LND | Thoracotomy | NR |
| Schuchert et al. [20] (2007) | USA | University of Pittsburgh | 428 | 2002–2006 | Mediastinal LND | Open & VATS | NR |
| Sienel et al. [21] (2007) | Germany | Albert-Ludwigs-University Freiburg | 199 | 1987–2002 | Systemic LND | NR | NR |
| Kilic et al. [22] (2009) | USA | University of Pittsburgh Medical Center | 184 | 2002–2007 | NR | Open & VATS | NR |
| Yamashita et al. [23] (2011) | Japan | Oita University Hospital | 109 | Sep 2003–Oct 2008 | Systemic LND | VATS | NR |
| Schuchert et al. [24] (2011) | USA | University of Pittsburgh | 72 | 1999–2008 | Lobectomy 100%, segmentectomy 83% | Open &VATS | NR |
| Whitson et al. [25] (2011) | USA | University of Minnesota | 14,473 | 1998–2007 | NR | NR | NR |
| Carr et al. [26] (2012) | USA | University of Pittsburgh Medical Center | 429 | 2002–2009 | Systemic LND | Open & VATS | NR |
| Schuchert et al. [27] (2012) | USA | University of Pittsburgh | 757 | 2002–2010 | NR | Open & VATS | NR |
| Schuchert et al. [28] (2012) | USA | University of Pittsburgh | 899 | 1999–2010 | NR | Open & VATS | NR |
| Yamashita et al. [29] (2012) | Japan | Oita University Hospital | 214 | Sep 2003–Jun 2011 | Systemic LND | VATS | NR |
| Zhong et al. [30] (2012) | China | Shanghai Chest Hospital | 120 | Mar 2006–Aug 2011 | Systemic LND | VATS | NR |
| Zhao et al. [31] (2013) | China | Shanghai Chest Hospital | 174 | Nov 2009–May 2012 | Systemic LND | VATS | NR |
LND, lymph node dissection; NR, not reported; VATS, video-assisted thoracoscopic surgery.
Table 2.
Main characteristics and results of studies included in the meta-analysis of lobectomy versus segmentectomy for stage I non-small cell lung cancer
| Author | Age (mean, yr) | Tumor size (mean, mm) | Tumor stage | No. of S | No. of L | 5-Year survival | Recurrence-free survival | Postop mortality | Postop morbidity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
||||||||||
| S | L | S | L | HR | SD | HR | SE | S | L | S | L | ||||
| Kodama et al. [16] | 61M | 77M | 16.7±4.97 | 22.9±5.22 | IA | 46 | 77 | 0.96 | 0.62 | ||||||
|
| |||||||||||||||
| Okada et al. [17] | IA | 68 | 104 | 0.89 | 0.63 | ||||||||||
|
| |||||||||||||||
| Martin-Ucar et al. [18] | 70M | 69M | 32M | 34M | I | 17 | 17 | 0.83 | 1.43 | 1 | 1 | 3 | 3 | ||
|
| |||||||||||||||
| Okumura et al. [19] | 65.2 | 61.2 | 14.4 | 16.3 | IA | 55 | 187 | 2.01 | 0.74 | ||||||
|
| |||||||||||||||
| Schuchert et al. [20] | 70 | 68 | 23 | 31 | I | 182 | 246 | 2.08 | 0.47 | 2 | 8 | 59 | 83 | ||
|
| |||||||||||||||
| Sienel et al. [21] | IA | 49 | 150 | 3.50 | 0.52 | ||||||||||
|
| |||||||||||||||
| Kilic et al. [22] | 79 | 78.5 | 25 | 35 | I | 78 | 106 | 0.78 | 0.26 | 0.99 | 0.3 | 1 | 5 | 23 | 53 |
|
| |||||||||||||||
| Yamashita et al. [23] | 72M | 68M | 15M | 25M | I | 38 | 71 | 1.18 | 1.10 | 1.55 | 1.28 | ||||
|
| |||||||||||||||
| Schuchert et al. [24] | 68.5 | 66.1 | 8 | 8 | IA | 40 | 32 | 0.79 | 0.52 | 1.02 | 0.85 | 0 | 1 | 9 | 12 |
|
| |||||||||||||||
| Whitson et al. [25] | I | 581 | 13,892 | 1.37 | 0.08 | ||||||||||
|
| |||||||||||||||
| Carr et al. [26] | IA | 178 | 251 | 0.76 (T1a) 1.65 (T1b) |
1.27 (T1a) 1.37 (T1b) |
2 | 3 | ||||||||
|
| |||||||||||||||
| Schuchert et al. [27] | IA | 325 | 432 | 1.08 | 0.20 | ||||||||||
|
| |||||||||||||||
| Schuchert et al. [28] | 70.0±8.6 | 68.4±10.1 | 20M | 25M | I | 305 | 594 | 4 | 13 | 109 | 271 | ||||
|
| |||||||||||||||
| Yamashita et al. [29] | 69M | 68M | 15M | 20M | IA | 90 | 124 | 1.99 | 0.99 | 2.26 | 0.95 | ||||
|
| |||||||||||||||
| Zhong et al. [30] | 63.6±8.0 | 64.9±7.3 | 11±5 | 15±4 | IA | 39 | 81 | 0.47 | 0.61 | 0.89 | 0.49 | 5 | 10 | ||
|
| |||||||||||||||
| Zhao et al. [31] | 59.3±13.77 | 58.8±10.77 | 14.2±7.15 | 19.8±9.82 | I | 36 | 138 | 3 | 3 | ||||||
S, segmentectomy; L, lobectomy; HR, hazard ratio; SD, standard deviation; SE, standard error; Postop, postoperative;
median.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram presenting the search strategy used to compare the outcomes of segmentectomy versus lobectomy for stage I non-small cell lung cancer.
2) Meta-analysis of 5-year survival
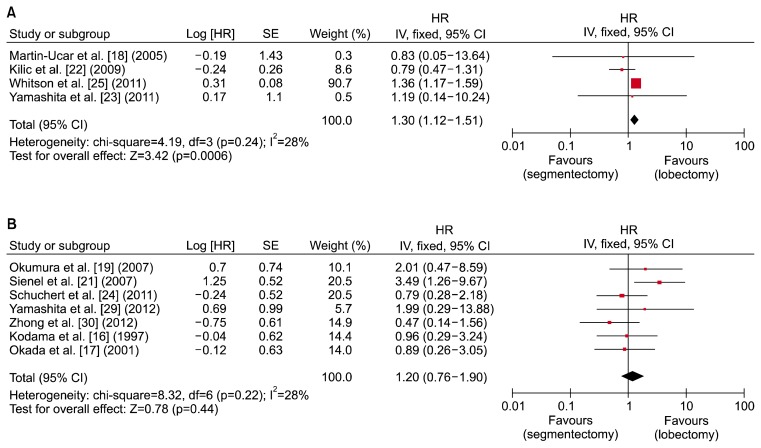
Eleven studies described 5-year survival outcomes using Kaplan-Meier survival curves, with either stage I NSCLC patients or stage IA NSCLC patients [16–19,21–25,29,30]. Two separate meta-analyses were therefore conducted to determine the 5-year survival of stage I and IA NSCLC. Four studies of stage I NSCLC and 7 studies of stage IA NSCLC were analyzed. The results are presented in Fig. 2. The combined hazard ratio for segmentectomy was 1.30, with a 95% confidence interval of 1.12–1.51 for stage I NSCLC, suggesting that patients who received segmentectomy had a 1.30-times lower 5-year survival rate than those who received lobectomy. Heterogeneity was negligible was present, as the I2 statistic was lower than 50% and the p-value was greater than 0.1. However, there was no significant (p>0.05) difference in the 5-year survival rate between the 2 surgical intervention groups for stage IA NSCLC. The combined hazard ratio for segmentectomy was 1.20 with a 95% confidence interval of 0.76–1.90. No heterogeneity was present.
Fig. 2.
Forest plot showing a comparison of 5-year survival between segmentectomy and lobectomy in (A) stage I and (B) stage IA non-small cell lung cancer. HR, hazard ratio; SE, standard error; CI, confidence interval; df, degrees of freedom.
3) Meta-analysis of recurrence-free survival
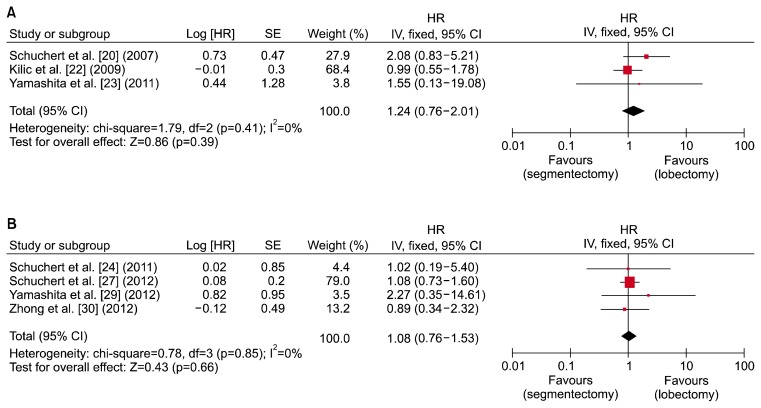
Seven studies compared recurrence-free survival in segmentectomy versus lobectomy for stage I and IA NSCLC [20,22–24,27,29,30]. All of them used Kaplan-Meier survival curves. The hazard ratios and standard error were calculated using the same technique as used for 5-year survival. In this analysis, stage I and stage IA patient groups were analyzed separately. Results are shown in forest plots in Fig. 3. Three studies were included in the forest plot for stage I NSCLC, while 4 studies were used for stage IA NSCLC. The combined hazard ratio was 1.24 (95% confidence interval, 0.76–2.01; p=0.39) for stage I NSCLC and 1.08 (95% confidence interval, 0.76–1.53, p=0.66) for stage IA NSCLC. As shown in Fig. 3, there was no statistically significant difference in recurrence-free survival between patients who received segmentectomy and those who received lobectomy for both stage I and IA NSCLC. Heterogeneity was not present in either group, because the p-value was larger than 0.1.
Fig. 3.
Forest plot showing a comparison of recurrence-free survival between segmentectomy and lobectomy in (A) stage I and (B) stage IA non-small cell lung cancer. HR, hazard ratio; SE, standard error; CI, confidence interval; df, degrees of freedom.
4) Meta-analysis of postoperative mortality
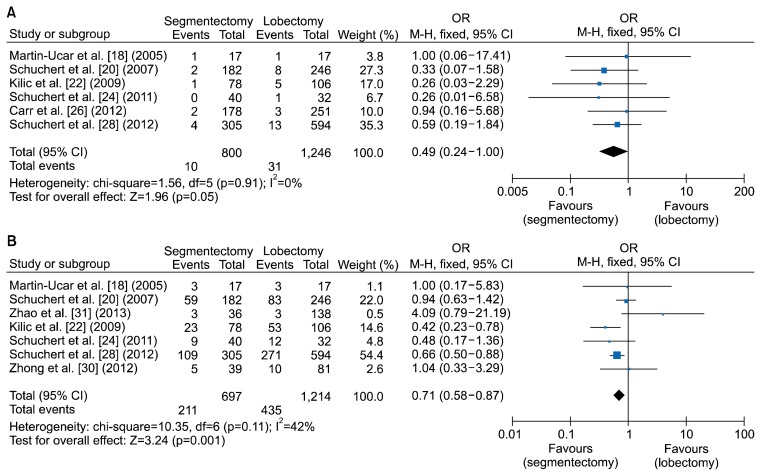
In this study, postoperative mortality was defined as including 30-day mortality and in-hospital mortality. Odds ratios were used to compare the mortality rate of segmentectomy versus lobectomy for stage I NSCLC. The corresponding forest plot is shown in Fig. 4 [18,20,22,24,26,28,30,31]. A total of 800 patients who underwent segmentectomy were compared to 1,246 patients who underwent lobectomy. The combined odds ratio for segmentectomy was 0.49, with a 95% confidence interval of 0.24–1.00. Heterogeneity was not present (p=0.91). The p-value for the overall effect was 0.05. From the perspective of postoperative mortality, segmentectomy might be more beneficial for stage I NSCLC patients than lobectomy.
Fig. 4.
Forest plot showing a comparison of postoperative (A) mortality and (B) morbidities between segmentectomy and lobectomy in stage I non-small cell lung cancer. OR, odds ratio; M-H, Mantel-Haenszel test; CI, confidence interval; df, degrees of freedom.
5) Meta-analysis of postoperative morbidities
Postoperative morbidities between segmentectomy and lobectomy were compared in 7 studies. In most of these studies, postoperative morbidities included cardiac (myocardial infarction and cardiac arrest), pulmonary (pneumonia, empyema, bronchopleural fistula, respiratory failure requiring re-intubation, and tracheostomy), and others (septicemia, pulmonary embolism, and stroke). All intraoperative and postoperative complications were included in the category of postoperative morbidities. Although some studies classified morbidities as major or minor, all adverse events were combined in this analysis. As shown in Fig. 4 [18,20,22,24,26,28,30,31], the total odds ratio for segmentectomy was 0.71 (p=0.001), with a 95% confidence interval of 0.58–0.87. In the heterogeneity analysis, the p-value was 0.11 and I2 was 42%. Therefore, heterogeneity was not present. Because the p-value for the overall effect was less than 0.05, the total odds ratio of 0.71 was considered to be significant. This suggests that segmentectomy can lead to fewer postoperative morbidities than lobectomy.
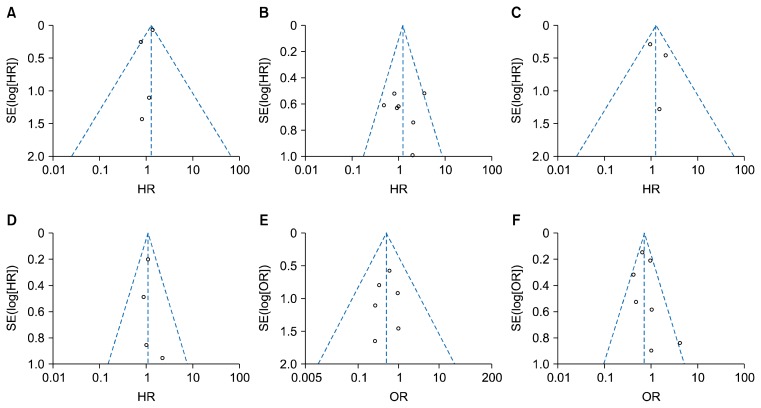
6) Publication bias
Funnel plots for 5-year survival (stage I, IA), recurrence-free survival (stage I, IA), postoperative mortality, and postoperative morbidities did not reveal any evidence of obvious asymmetry (Fig. 5). Therefore, publication bias was not a problem for this analysis.
Fig. 5.
Funnel plots for 5-year survival for stage I NSCLC (A), 5-year survival for stage IA NSCLC (B), recurrence-free survival for stage I NSCLC (C), recurrence-free survival for stage IA NSCLC (D), postoperative mortality (E), and postoperative morbidities (F). NSCLC, non-small cell lung cancer; SE, standard error; HR, hazard ratio; OR, odds ratio.
Discussion
Although lobectomy is considered to be the standard surgical treatment for patients with stage I NSCLC, the issue of sublobar resection versus lobectomy remains controversial. In this study, we analyzed 4 outcomes to compare lobectomy versus segmentectomy for stage I and IA NSCLC. Lobectomy showed better 5-year survival for stage I NSCLC. However, segmentectomy was comparable to lobectomy in terms of 5-year survival for stage IA NSCLC. Recurrence-free survival showed no statistically significant difference between lobectomy and segmentectomy. Segmentectomy demonstrated better early postoperative outcomes than lobectomy, resulting in superior postoperative mortality and morbidity.
Several meta-analyses have compared lobectomy to segmentectomy [7–11]. Many studies have concluded that segmentectomy can achieve comparable overall survival to lobectomy for stage IA NSCLC [8–10]. However, it has also been reported that the overall survival of patients with stage I NSCLC who underwent lobectomy was better than the overall survival of those who underwent segmentectomy [9,10]. Our results for 5-year survival outcomes are in agreement with previously published studies. However, in other studies [7,8], segmentectomy was associated with poorer overall survival than lobectomy for stage IA NSCLC. Therefore, controversy remains regarding this issue. We sought to clarify this controversial issue and to produce more objective results through this meta-analysis, which has 2 notable methodological differences from previously published meta-analyses. First, in our study, we compared 5-year survival rates, not arbitrary survival with an undefined follow-up period. Many other meta-analyses used overall survival with an undefined period, resulting in various follow-up times in their survival analysis. Instead, we only used 5-year survival and excluded many papers that used overall survival without a defined period. By doing so, we were able to control the follow-up period. The second difference was that we analyzed stage I and IA disease separately. This methodological choice may have reduced the heterogeneity of patients included in this meta-analysis.
Recurrence-free survival was not significantly different between the 2 surgical strategies for either stage I or stage IA NSCLC. For recurrence-free survival, our results are in agreement with previously published studies showing that there was no significant difference between lobectomy and segmentectomy [7,8]. However, considering the lower 5-year survival rate in stage I NSCLC in the segmentectomy group, it was unclear whether recurrence after segmentectomy was related to poor survival. One possible explanation may be selection bias in the segmentectomy group, according to which the reduced 5-year survival following segmentectomy might be related to other causes rather than cancer. Limited resection has been commonly performed in patients with poor pulmonary function and multiple comorbidities. For example, Kodama and colleagues reported that, among 63 patients who underwent segmentectomy, 17 had poor pulmonary reserve or other limiting factors making them unable to tolerate lobectomy [16]. Therefore, a randomized controlled clinical trial would be necessary to resolve the discrepancy between survival and recurrence-free survival.
Analyses of postoperative mortality and morbidities have not been commonly performed in previous studies, because most previous research has focused on survival and recurrence, rather than on early postoperative outcomes. However, we think that the most critical benefit of sublobar resection is the preservation of lung function with a reduced operative risk. If equal oncologic outcomes are expected, the safer technique with a greater preservation of function would be the preferable surgical option. Therefore, we also analyzed postoperative mortality and morbidities after segmentectomy in this study. Tan et al. [12] suggested that the incidence of postoperative morbidities in patients undergoing segmentectomy was higher than in those undergoing lobectomy, contrary to the result of our meta-analysis. However, they only included data from 95 patients from 2 papers, whereas our study included 1,246 patients from 6 papers for the mortality analysis and 1,214 patients from 7 papers for the morbidity analysis. Therefore, our results have more statistical power than the previous results. This is the first meta-analysis to find that segmentectomy is better than lobectomy in terms of early postoperative outcomes.
In the selection procedure for this study, we included studies that described pathologic stage I or IA NSCLC and 5-year survival. Strict inclusion criteria reduced bias due to confounding and heterogeneity. However, our study has some limitations of its own. First, the calculations of hazard ratios using Kaplan-Meier survival curves and the TechDig software could have involved errors due to potential inaccuracies of the curve-tracing procedure and assumptions about the censoring pattern [14,15]. This phenomenon is an inevitable methodological limitation caused by the assumption procedure used by the software. Secondly, we controlled for the follow-up period, pathologic stage, and study design. However, we could not control for differences among studies in patient characteristics such as age, sex, tumor size, and histological type. An ideal meta-analysis should use individual patient data (not recruited in our study). Therefore, we used summary data from the included studies. Thirdly, all studies included in our meta-analysis were aggregations of data obtained from retrospective studies. Those data need to be confirmed by prospective studies or randomized controlled trials. Finally, unpublished studies and studies not written in English were excluded from our study, potentially leading to publication bias, which is a major concern for all forms of meta-analysis. Although our study showed no finding indicative of publication bias, it should be noted that our meta-analysis could not completely be free from such bias.
Conclusion
In summary, this study showed that segmentectomy for stage IA NSCLC was comparable to lobectomy in terms of 5-year survival, although lobectomy had better survival for stage I NSCLC. However, recurrence-free survival was not significantly different between the 2 surgical strategies for either stage I or stage IA NSCLC. Based on the results for postoperative mortality and morbidities, segmentectomy is superior to lobectomy. However, randomized controlled trials are needed to compare segmentectomy to lobectomy, particular for stage IA NSCLC patients, to validate the results of our meta-analysis.
Acknowledgments
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund no. KTCS04-124).
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 2.Strand TE, Rostad H, Moller B, Norstein J. Survival after resection for primary lung cancer: a population based study of 3211 resected patients. Thorax. 2006;61:710–5. doi: 10.1136/thx.2005.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Lung Screening Trial Research Team. Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–91. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer: Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22. doi: 10.1016/0003-4975(95)00537-U. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L) Jpn J Clin Oncol. 2010;40:271–4. doi: 10.1093/jjco/hyp156. [DOI] [PubMed] [Google Scholar]
- 6.Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol. 2010;5:1583–93. doi: 10.1097/JTO.0b013e3181e77604. [DOI] [PubMed] [Google Scholar]
- 7.Cao C, Gupta S, Chandrakumar D, Tian DH, Black D, Yan TD. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg. 2014;3:134–41. doi: 10.3978/j.issn.2225-319X.2014.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Li M, Yin R, Zhang Q, Xu L. Comparison of the oncologic outcomes of anatomic segmentectomy and lobectomy for early-stage non-small cell lung cancer. Ann Thorac Surg. 2015;99:728–37. doi: 10.1016/j.athoracsur.2014.08.080. [DOI] [PubMed] [Google Scholar]
- 9.Fan J, Wang L, Jiang GN, Gao W. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol. 2012;19:661–8. doi: 10.1245/s10434-011-1931-9. [DOI] [PubMed] [Google Scholar]
- 10.Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg. 2014;46:1–7. doi: 10.1093/ejcts/ezt554. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Huang C, Liu H, Chen Y, Li S. Sublobectomy versus lobectomy for stage IA (T1a) non-small-cell lung cancer: a meta-analysis study. World J Surg Oncol. 2014;12:138. doi: 10.1186/1477-7819-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan Q, Huang J, Ding Z, Lin H, Lu S, Luo Q. Meta-analysis for curative effect of lobectomy and segmentectomy on non-small cell lung cancer. Int J Clin Exp Med. 2014;7:2599–604. [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Kodama K, Doi O, Higashiyama M, Yokouchi H. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg. 1997;114:347–53. doi: 10.1016/S0022-5223(97)70179-X. [DOI] [PubMed] [Google Scholar]
- 17.Okada M, Yoshikawa K, Hatta T, Tsubota N. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg. 2001;71:956–60. doi: 10.1016/S0003-4975(00)02223-2. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Ucar AE, Nakas A, Pilling JE, West KJ, Waller DA. A case-matched study of anatomical segmentectomy versus lobectomy for stage I lung cancer in high-risk patients. Eur J Cardiothorac Surg. 2005;27:675–9. doi: 10.1016/j.ejcts.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Okumura M, Goto M, Ideguchi K, et al. Factors associated with outcome of segmentectomy for non-small cell lung cancer: long-term follow-up study at a single institution in Japan. Lung Cancer. 2007;58:231–7. doi: 10.1016/j.lungcan.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg. 2007;84:926–32. doi: 10.1016/j.athoracsur.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Sienel W, Stremmel C, Kirschbaum A, et al. Frequency of local recurrence following segmentectomy of stage IA non-small cell lung cancer is influenced by segment localisation and width of resection margins: implications for patient selection for segmentectomy. Eur J Cardiothorac Surg. 2007;31:522–7. doi: 10.1016/j.ejcts.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg. 2009;87:1662–6. doi: 10.1016/j.athoracsur.2009.02.097. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita S, Chujo M, Kawano Y, et al. Clinical impact of segmentectomy compared with lobectomy under complete video-assisted thoracic surgery in the treatment of stage I non-small cell lung cancer. J Surg Res. 2011;166:46–51. doi: 10.1016/j.jss.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Schuchert MJ, Kilic A, Pennathur A, et al. Oncologic outcomes after surgical resection of subcentimeter non-small cell lung cancer. Ann Thorac Surg. 2011;91:1681–7. doi: 10.1016/j.athoracsur.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Whitson BA, Groth SS, Andrade RS, Maddaus MA, Habermann EB, D’Cunha J. Survival after lobectomy versus segmentectomy for stage I non-small cell lung cancer: a population-based analysis. Ann Thorac Surg. 2011;92:1943–50. doi: 10.1016/j.athoracsur.2011.05.091. [DOI] [PubMed] [Google Scholar]
- 26.Carr SR, Schuchert MJ, Pennathur A, et al. Impact of tumor size on outcomes after anatomic lung resection for stage 1A non-small cell lung cancer based on the current staging system. J Thorac Cardiovasc Surg. 2012;143:390–7. doi: 10.1016/j.jtcvs.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Schuchert MJ, Abbas G, Awais O, et al. Anatomic segmentectomy for the solitary pulmonary nodule and early-stage lung cancer. Ann Thorac Surg. 2012;93:1780–5. doi: 10.1016/j.athoracsur.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 28.Schuchert MJ, Awais O, Abbas G, et al. Influence of age and IB status after resection of node-negative non-small cell lung cancer. Ann Thorac Surg. 2012;93:929–35. doi: 10.1016/j.athoracsur.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita S, Tokuishi K, Anami K, et al. Thoracoscopic segmentectomy for T1 classification of non-small cell lung cancer: a single center experience. Eur J Cardiothorac Surg. 2012;42:83–8. doi: 10.1093/ejcts/ezr254. [DOI] [PubMed] [Google Scholar]
- 30.Zhong C, Fang W, Mao T, Yao F, Chen W, Hu D. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small-sized stage IA lung cancer. Ann Thorac Surg. 2012;94:362–7. doi: 10.1016/j.athoracsur.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 31.Zhao X, Qian L, Luo Q, Huang J. Segmentectomy as a safe and equally effective surgical option under complete video-assisted thoracic surgery for patients of stage I non-small cell lung cancer. J Cardiothorac Surg. 2013;8:116. doi: 10.1186/1749-8090-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]