Abstract
Freshwater snails are obligate intermediate hosts for numerous parasitic trematodes, most notably schistosomes. Schistosomiasis is a devastating human and veterinary illness, which is primarily controlled by limiting the transmission of these parasites from their intermediate snail hosts. Understanding how this transmission occurs, as well as the basic immunobiology of these snails may be important for controlling this disease in the future. Allelic variation in the Guadeloupe resistance complex (GRC) of Biomphalaria glabrata partially determines their susceptibility to parasitic infection, and can influence the microbiome diversity and microbial defenses in the hemolymph of these snails. In the present study, we examine the most abundant proteins present in the hemolymph of snails that are resistant or susceptible to schistosomes, as determined by their GRC genotype. Using proteomic analysis, we found that snails with different GRC genotypes have differentially abundant hemolymph proteins that are not explained by differences in transcription. There are 13 revealed hemolymph proteins that differ significantly between resistant and susceptible genotypes, nearly 40% of which are involved in immune responses. These findings build on the mounting evidence that genes in the GRC region have multiple physiological roles, and likely contribute more extensively to the general immune response than previously believed. These data also raise the intriguing possibility that the GRC region controls resistance to schistosomes, not directly, but indirectly via its effects on the snail’s proteome and potentially its microbiome.
Keywords: Biomphalaria glabrata, hemolymph, allelic variation, proteomics
1. Introduction
Planorbid snails are obligate intermediate hosts for parasitic trematodes, many of which are responsible for numerous human and veterinary illnesses [1-3]. Characterizing the biology and immune responses of these intermediate hosts has been essential for the understanding of trematode immune evasion, or clearance before they can be transmitted to mammalian hosts such as humans [1]. Indeed, with the recent explosion of molecular tools for genetic modification, knowledge regarding the biology underlying molluscan immunity to parasitic infection may become essential to effectively controlling these diseases [4, 5].
The most detrimental human helminth parasite, Schistosoma mansoni, is transmitted to humans in the Americas via the intermediate host Biomphalaria glabrata (Bg) [2, 6, 7]. A genome-wide association study on replicate populations of outbred Guadeloupean B. glabrata (BgGUA) selected for high schistosome resistance revealed a single genomic region in which allelic variation at one, or more genes has a very strong effect on resistance to Guadeloupean S. mansoni [8]. The region of statistical association is approximately 1 Mb in size and contains 15 protein coding loci [8]. The causal gene/s in the region is/are not known with certainty, but substantial evidence points to genes for seven single-pass, transmembrane proteins that lie in the middle of the region [9-11]. For example, these genes code for putative proteins which show structural similarity to pathogen recognition proteins in other taxa, and knocking down the expression of one of these proteins increased shedding of the parasite in infected snails [8, 9]. There are three main haplotypes of the GRC, one of which (R) confers resistance to the parasite, acting dominantly to the two that confer susceptibility (S1 and S2). We will hereafter refer to these three haplotypes of the GRC region as alleles, and to diploid combinations of the haplotypes (RR, S1S1 and S2S2) as genotypes.
Allelic variation in the GRC region has also been associated with changes to the microbiome of BgGUA, independent of parasite infection, implicating this region in biological roles extending beyond schistosome defense [12]. Hemolymph transfer and hemocyte activity assays strongly suggest that its effects on parasite infection are likely mediated via cellular mechanisms in the hemolymph [10, 11]. The GRC’s additional biological roles may not share these cellular mechanisms and, given the GRC’s unexpected effects on the microbiome, it is important to consider if allelic variation in the GRC region could alter other biological properties of the hemolymph. Recent efforts have begun to characterise, using proteomic analyses, the complex proteomic interplay during hemolymph mediated host defense [13-16]. Schistosome primed snails or resistant strains of snail differ from more susceptible snails in a number of immune-related proteins, including fibrinogen-related proteins (FREPs) and C-type lectin-related proteins (CREPs) which are known to influence infection, further implicating the proteomic profile of hemolymph in gastropod host defense [14, 16].
Given the GRC’s important role in hemolymph mediated-immune clearance of schistosomes, the effects of this region on the microbiome, and the recent work on hemolymph derived proteins in schistosome defense in other Bg-Schistosome models, we were interested in determining if allelic variation at the GRC region could cause changes in the proteomic profile of snail hemolymph in the absence of schistosome infection. Here we examine the proteomic profile of BgGUA and show that allelic variation in the GRC region has a strong effect on the levels of numerous proteins detected in the hemolymph, none of which are coded for by genes linked to the GRC. Interestingly, the schistosome-resistant RR genotype exhibits a number of differentially abundant immune proteins when compared to both susceptible genotypes. Our findings build on the growing body of evidence suggesting that the GRC region may have more extensive immune roles, including roles related to schistosome defense, than previously suspected.
2. Materials and Methods
2.1. Animals.
The source BgGUA snail population was collected in 2005 from Dans Fond on the island of Guadeloupe in the West Indies [17]. These snails were maintained under standard conditions as previously described, and housed and fed identically [8, 17, 18]. Independent homozygous lines of BgGUA were created by genotyping at the GRC locus, segregating homozygous individuals (RR, S1S1, and S2S2), and allowing them to self for 1-3 generations [8]. These lines were maintained for a minimum of 3 years [9, 11]. Fifteen homozygous lines, five of each genotype (5 RR, 5 S1S1, and 5 S2S2), were used for the analysis of hemolymph, and all individual snails were size matched (7-9 mm). This protocol was approved by the Oregon State University Institutional Animal Care and Use Committee (Animal Care and Use Proposal 4360).
2.2. Hemolymph extraction.
BgGUA hemolymph was extracted from 20 snails from each of fifteen homozygous lines (5 RR, 5 S1S1, and 5 S2S2) by the headfoot retraction technique [10, 11, 19]. Hemolymph from the 20 snails of each individual line was pooled to yield one single hemolymph sample per homozygous line (15 samples total: 5 RR, 5 S1S1, and 5 S2S2); cell counts did not differ between pooled samples. Hemolymph samples were homogenized in protease-phosphatase-inhibitor cocktail according to the manufacturer’s instructions (Thermo-Fisher Scientific), via passage 20x through an 18 G needle, 20x through a 25 G needle, and 3 rounds of freeze thawing before storage at −80.
All subsequent hemolymph processing was conducted by the Oregon State Mass Spectrometry Center.
2.3. Trypsin digestion and Liquid Chromatography Mass Spectrometry (LC-MS)
Protein trypsin digestion was performed following the protocol provided by Promega (Madison, WI) with minor modifications. Briefly, 200 μL LC-MS grade cold acetone was added to 50 μL homogenized snail hemolymph samples which were centrifuged at 8000 g for 10 min at 4 °C. The supernatants were carefully discarded, and the pellets were dried under room temperature to remove any acetone residue. Then the pellets were resuspended in 50 μL of 50 mM ammonium bicarbonate with 0.1% RapiGest™ SF surfactant. Samples were vortexed, and dithiothreitol (DTT) was added to a final concentration of 10 mM before a 1 h incubation at 56 °C. This incubation was immediately followed by adding iodoacetamide (IAM) to a final concentration 5 mM before a second 1 h incubation in the dark at room temperature. Trypsin digestion was carried out overnight at 37 °C and terminated by the addition of 0.5% trifluoroacetic acid (TFA). Subsequently, digested samples were centrifuged at 15000 g for 10 min and the supernatants were transferred and diluted ten times using 3% acetonitrile (ACN) with 0.1% formic acid. All samples were kept at −80 °C until LC-MS analysis.
Trypsin-digested samples were analyzed by a Waters nanoAcquity UPLC system (Waters, Milford, MA) coupled to Thermo Orbitrap Fusion Lumos mass spectrometer with a Nano ESI source (Thermo Scientific, Waltham, MA). In brief, 1 μL peptide mixture was trapped onto a nanoAcquity UPLC 2 G Trap C18 Column (180 μm × 20 mm, 5 μm) and loaded onto a nanoAcquity UPLCPeptide BEH C18 column (100 μm × 100 mm, 1.7 μm). Peptides were eluted by a 120 min ACN gradient at flow rate 500 nL/min (mobile phase A: 0.1% formic acid in H2O; mobile phase B: 0.1% formic acid in ACN), where phase B was increased from 3% to 10% over 3 min, to 30% over 102 min, then to 90% over 3 min followed by a 4 min hold. Following the hold phase B dropped to 3% over 1 min and held for 7 min. The eluted peptides were directly electrosprayed into the mass spectrometer (MS) and all spectra data were acquired under positive ion mode. The spray voltage was set at 2400 V and the ion transfer tube temperature was held at 300 °C. MS and MS/MS spectra were respectively recorded in an orbitrap analyzer with 120 K resolution and linear trap quadrupole (LTQ) with 1.6 m/z isolation width. In addition, MS/MS scans were acquired by setting the normalized collision energy to 35% on precursor ion, under collision induced dissociation (CID) mode.
2.4. Data and Statistical analyses.
Analysis was performed by Thermo Scientific Proteome Discoverer 2.2 software for all raw LC-MS data files. Sequest HT search engine was applied to search protein IDs against the Biomphalaria glabrata protein database, downloaded from the UniProt Knowledgebase website (www.uniprot.org). As all precursor ion data were obtained in the orbitrap analyzer at high resolving power, the mass tolerances for precursor ions were set at 10 ppm. For fragmentation data obtained in LTQ, a tolerance on the mass measurement of 0.6 Da and a maximum of two missed cleavage sites were allowed. Carbamidomethylation of cysteine was specified as a static modification and oxidation of methionine and acetylation of N-terminal were specified as two dynamic modifications. Only proteins with overall false discovery rates (FDR) of less than 1% were assigned a high confidence indicator (a strict FDR is defined as < 1%, and relaxed FDR is defined as >1% but < 5%. A protein that passed the strict FDR filter was assigned as a protein with high confidence; a protein passed the relaxed FDR filter but not pass strict FDR was assigned as a protein with medium confidence; all other proteins were assigned as proteins with low confidence) and counted as one protein ID. Moreover, all statistical analyses, including Principal Component Analysis (PCA), were done by Proteome Discoverer 2.2 software. Fold change of a protein between groups was considered significantly different when the P value is less than 0.05 calculated by the Benjamini-Hochberg method.
To test whether differences among GRC genotypes in patterns of protein expression result simply from constitutive differences in gene expression, we re-analyzed published RNA-Seq data from BgGUA (NCBI BioProject Accession PRJNA264063) [8]. These data consisted of whole-body RNA Illumina reads extracted from 36 outbred snails of known genotype (18 RR, 9 S1S1, and 9 S2S2) that were sampled from an outbred stock population of BgGUA (and that had not been challenged with schistosome miracidia). We aligned reads to B. glabrata reference transcripts, as identified in UniProt, corresponding to proteins showing differential protein expression between resistant and susceptible genotypes. We used BWA v. 0.7.12 [20] to generate separate sequence alignment/map files (SAM; a generic format for storing and working on large nucleotide sequence alignments) for each sample, and we then counted the number of reads aligned to each transcript in each file. We performed one-tailed t-tests comparing RR read depth with S1S1 and S2S2 read depth, considering the alternate hypothesis that gene expression was different in the same direction as observed in the proteomic data (e.g. higher in RR at both the RNA and protein level). In order to infer the genomic locations of these loci, we used BLAST within VectorBase (https://www.vectorbase.org/blast) to assign these transcripts to scaffolds on the reference genome, and identified the linkage groups containing these scaffolds [21].
3. Results
Allelic variation in the GRC region can alter the hemolymph proteome of BgGUA.
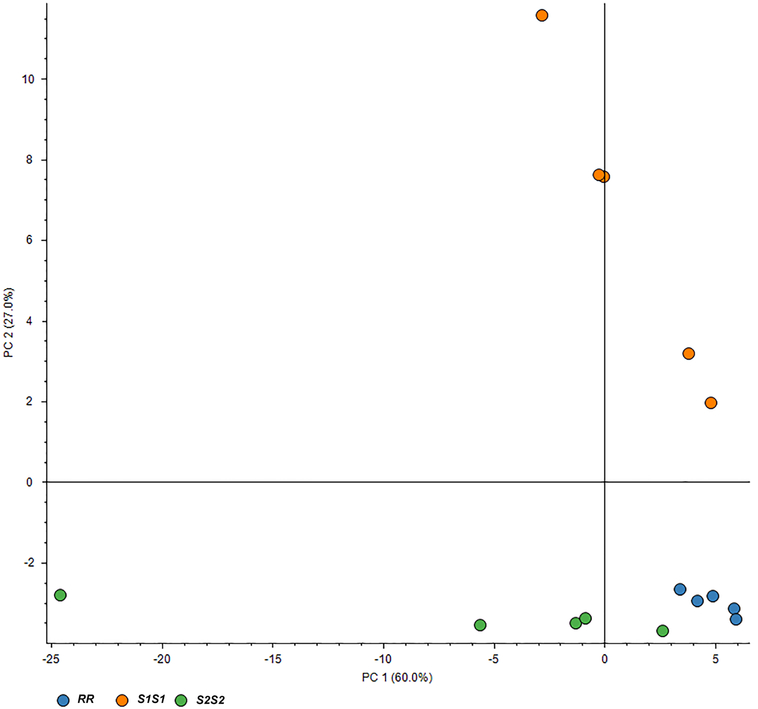
We were able to identify 111 unique proteins from whole BgGUA hemolymph (Table S1). Of these, there were 88 proteins revealed by proteomic analysis while the rest remain uncharacterized and have unknown functions (Table S1). A PCA analysis shows that proteomic profiles cluster by genotype, and of the 111 proteins nearly ~60% (65/111) significantly differed between at least two genotypes (Figure 1). The abundances of many proteins appear to vary extensively (some showing a very large fold change) by genotype (Table S1). Thirteen revealed proteins have significantly different fold changes in protein levels between the resistant genotype (RR) and both susceptible genotypes (S1S1 and S2S2) (Table 1). Of these 13 proteins, ~40% (5/13) come from classes of proteins that have been implicated in snail immunity and/or schistosome defense, the others being proteins with diverse functions. RR individuals tend to have lower levels of these immune proteins, despite many homeostatic proteins showing no change in protein levels (Table S1), with the exception of a massive 44 fold increase in a fibrinogen-related protein (FREP) (Table 1). This exception is notable because allelic variation in the GRC region has been previously implicated in both hemolymph-dependent immune responses and immune recognition, both of which are known functions of some FREPs [10, 11].
Fig 1. The hemolymph proteome clusters by GRC genotype in BgGUA.
A 2D scores plot of the principal component analysis (PCA) for the proteomic profile of pooled hemolymph. Each point represents pooled hemolymph from 20 snails from each of 15 independent inbred lines: RR, S1S1, S2S2 (n =5 lines/genotype).
Table 1. The levels of thirteen identified hemolymph proteins that differ significantly between RR and SS GRC genotypes.
The fold change in hemolymph protein levels for pooled hemolymph samples from independent inbred lines of RR, S1S1, S2S2 (n = 5 lines/genotype). Only resolved proteins that significantly differ between RR and both S1S1 and S2S2, but not between S1S1 and S2S2, are shown.
| Accession | Protein Description | Function | Fold Change (RR/S1S1) |
Adj. P-Value | Fold Change (RR / S2S2) |
Adj. P-Value |
|---|---|---|---|---|---|---|
| W5VR79 | tubulin beta chain | cell organization | 0.24 | 0.020479055 | 0.144 | 0.000393795 |
| W5VJQ8 | tubulin alpha chain | cell organization | 0.171 | 0.017865714 | 0.111 | 0.000393795 |
| P92179 | actin, cytoplasmic | cytoskeleton | 0.284 | 0.029289267 | 0.184 | 0.000393795 |
| Q45RT4 | histone H4 | DNA binding | 0.087 | 0.001191739 | 0.083 | 0.000237885 |
| B5L013 | schistosomin | immune | 0.236 | 0.035546154 | 0.151 | 0.000549489 |
| Q95UT6 | BgMFREP5 | immune | 0.169 | 0.023140182 | 0.207 | 0.007891621 |
| A0A1S6XX83 | fibrinogen-related protein J3 | immune | 44.025 | 9.96975E-05 | N/A | N/A |
| H8XYG5 | granulin-like protein | immune/growth | 0.17 | 0.028492759 | 0.143 | 0.004611146 |
| O18474 | heat shock protein 70 | immune/stress | 0.145 | 0.022534849 | 0.117 | 0.004611146 |
| Q45RT8 | fructose-bisphosphate aldolase | metabolic process | 0.259 | 0.017865714 | 0.171 | 0.000428649 |
| W5VJG5 | elongation factor 1-alpha-like isoform X1 | metabolic process | 0.172 | 0.003244166 | 0.146 | 0.000393795 |
| W5VJE1 | malate dehydrogenase | metabolic process | 0.311 | 0.042830772 | 0.186 | 0.000976712 |
| Q9BH32 | Ubiquitin | metabolic process | 0.112 | 0.042830772 | 0.093 | 0.004473035 |
Proteomic differences between RR and SS hemolymph are not explained by transcriptional changes to the mRNA of these proteins in BgGUA.
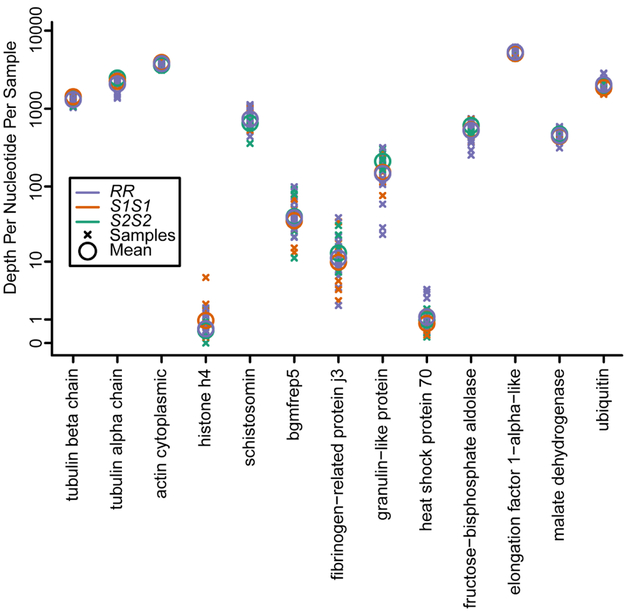
Previous reports on mRNA transcript abundance comparing RR, S1S1, and S2S2 snails have shown no differences in gene expression aside from genes coded for in the GRC region [8]. Using this previously compiled data set, we examined the mRNA levels of the 13 revealed proteins (from Table 1) that exhibited statistically different abundances between RR and SS hemolymph (Figure 2). As expected from Tennessen et al. (2015), there were no significant differences in transcript levels of any of these proteins when RR is compared to both SS genotypes (Figure 2) [8]. Consistent with the proteomic data (Table 1), there is a trend for alpha tubulin mRNA (p=0.014, not significant with Bonferroni correction) to be 12% lower in RR snails (adjusted mean sequencing depth = 2075) compared to susceptible snails (adjusted mean sequencing depth = 2246 for S1S1, 2470 for S2S2) (Figure 2). No other directional trends in mRNA of these 13 genes were observed.
Fig 2. Expression of the genes coding for the 13 proteins revealed by proteomic analysis to differ significantly between RR and SS genotypes.
The relative expression of mRNA for 13 genes corresponding to 13 revealed hemolymph proteins from outbred RR (n = 18), S1S1 (n = 9), S2S2 (n = 9). Each sample point represents expression data from an individual snail sampled from our outbred population. Per-sample/site depth expression is shown for all three genotypes (log-scale). No significant differences were observed (ANOVA, Bonferroni adjusted p>0.003).
None of the 13 transcripts matched the same reference genome scaffolds as any other, and these scaffolds occurred on multiple separate linkage groups [21]. Therefore, the differentially abundant proteins do not originate from a suite of closely linked genes.
4. Discussion
Understanding the basic physiology of intermediate hosts and vectors of zoonotic parasites may be essential for improving human and veterinary health [4, 22, 23]. Indeed, mitigating schistosomiasis has hinged heavily on controlling snail populations that are capable of releasing infective parasitic larvae [4]. Understanding how snails evade infection by schistosomes, and why some are refractory to infection, may allow for the development of intermediate host control measures that are less ecologically damaging than current measures [9, 23]. Therefore, to better understand the mechanistic basis of genetic variation for resistance to schistosomes, we examined the most abundant proteins in the hemolymph, an essential immune component, from schistosome resistant and susceptible BgGUA. Our findings show that allelic variation in one or more than one of the genes in the GRC, a genomic region vital for BgGUA defense against schistosomes [9, 11], can modify the proteomic profile of hemolymph but that these changes are unlikely to stem from general transcriptional modifications to those genes. Intriguingly, schistosome-resistant genotypes primarily differ from susceptible genotypes in the amount of immune proteins present in the hemolymph (one immune protein is higher while others are lower in RR hemolymph), though all three genotypes exhibit a distinctive proteomic profile.
We found that 13 revealed hemolymph proteins had significantly lower abundances in RR hemolymph compared with S1S1 and S2S2 (SS) hemolymph and that one protein, a fibrinogen-related protein (FREP), was drastically elevated in RR compared to SS (it was not even detectable in S2S2). Furthermore, the genes for these 13 proteins are not linked to each or to the GRC region. Nearly 40% of these proteins have putative or defined immune functions, indicating that RR individuals may have a different homeostatic baseline of immune proteins when compared to SS individuals. Given that the GRC region appears to be directly involved in immune defense during schistosome infection via Grctm6 and carbohydrate binding, these findings broaden the ways in which the GRC region may be involved in defense systems [9, 10]. These results are also interesting given the GRC region can affect the snail microbiome, which also has roles in immunity [12, 24]. Given the expansive differences amongst genotypes, we will limit our biological speculations to the 5 immune/schistosomiasis related proteins that differ between RR and SS hemolymph: FREPs, granulin-like protein (GRP), schistosomin, and heat shock protein 70 (HSP-70).
FREPs can bind and agglutinate pathogens, and are known to be important components of snail hemolymph, in addition to being directly involved in schistosome defense [25-27]. Specifically, mRNA expression of FREP 5 is altered after a trematode infection in B. glabrata [28]. Alterations in the expression of FREPs can be deterministic during trematode infection, so the idea that GRC genotype can alter FREP abundance, on top of the direct effects GRC coded proteins have on the immune response, is both complicated and fascinating [9, 27]. RR hemolymph has less than 20% of the FREP 5 abundance, but 44 fold more FREP J3 segment than susceptible genotypes, indicating that the relative FREP constituents of resistant hemolymph likely differ substantially from those of S1S1 or S2S2 individuals. Given notable differences in the microbiome of different GRC genotypes, it possible that these distinctive FREP protein levels are part of a response to the differing microbes between RR and the two SS GRC genotypes, potentially as a result of differential ligand binding of GRC coded proteins in BgGUA [9, 10, 12]. GRPs are believed to be involved in immune cell growth [29, 30]. Indeed a similar GRP has been shown to directly influence hemocyte growth and schistosome infection in snails [30]. It is interesting that a number of proteins, including FREP 5 and GRP, that have been associated with defense against schistosomes have lower abundance in RR individuals in this study. It is possible that RR snails do not require as many other molecules associated with schistosome defense because they are more effective at clearing schistosomes via Grctm6 dependent mechanisms [9]. These potential differences in immune preparedness against an infection, via these Grctm6 dependent mechanisms and differential circulating immune protein levels, could mean that individuals of different GRC genotypes can differentially respond to a diverse array of pathogens, including schistosomes.
Schistosomins are neuropeptides that are believed to be involved with schistosome infection, though the extent and nature of this involvement is contested [31]. It has been suggested that increased expression of this protein could be correlated with some of the severe effects of shistosome infection including castration, although recent evidence does not support these speculations [31]. Resistant hemolymph contains less than a quarter the schistosomin of susceptible hemolymph, which may be important for the protection of RR individuals from schistosomes if indeed schistosomin is important for the pathogenicity of schistosomes in BgGUA. Similarly, resistant hemolymph contains less than 15% of the HSP-70 of SS BgGUA and elevated HSP-70 has been shown to reduce the resistance of refractory BS-90 snails [32]. It is possible that having the RR genotype at the GRC region reduces the likelihood of producing some proteins which are permissive for schistosome infection.
Given that the abundance of numerous innate immune proteins is correlated with GRC genotype, it is possible that snails with different GRC genotypes could be differentially prepared for encounters with both schistosomes as well as other infectious agents. Coupled with the observations regarding the role of Grtm6 (a GRC protein) in schistosome infection, and the recent findings linking the GRC region with the microbiome of these snails, these findings support a broader immune role for the GRC region during homeostasis [9, 12]. Like the effects of the GRC on the microbiome of BgGUA, we can only speculate as to the mechanism and role of the complex interaction of this region with the relative levels of hemolymph proteins [12]. It is possible that proteins coded in the GRC region are altering the microbiome, and that the microbiome has effects on a number of systems including protein levels in the hemolymph. Indeed, S1S1 snails have both the most distinctive microbiome community composition and the most unique proteomic profile [12]. Perhaps the immune-related proteins in the GRC region, such as Grctm6, help to determine the microbiome community [12], and that these different microbial communities contribute to the differences we observe in hemolymph protein abundance, such as FREPs. If so, this might be a fascinating example of host genetic variation mediating parasite resistance, not directly, but indirectly via its effects on the microbiome.
Another possibility, given that GRC genotypes differ in their response to microbial-associated monosaccharides [10], is that snails with certain GRC genotypes are detecting a benign molecule in their environment and mounting a modest immune response. Alternatively, it is possible that these proteins coded in the GRC region could have a number of broad effects on some unidentified signalling pathways involved in the post-transcriptional control of numerous proteins, although we have no evidence of this alternative explanation. Regardless, snails with different GRC genotypes appear to differ in their immune preparedness on multiple levels. These findings support the notion that genes in the GRC region have additional roles in schistosome defense and important roles outside schistosome defense [8-12]. The GRC region has been shown to be under balancing selection and, because schistosomes are not native parasites of BgGUA, it is very likely that the three alleles of the GRC region evolved in response to non-schistosome mediated evolutionary pressures [8]. We believe that these findings are outlining some of the natural roles for the GRC region in BgGUA.
Our findings indicate that the levels of certain proteins that are found in the hemolymph are linked to the GRC genotype in BgGUA. However, we were unable to determine if changes to specific protein networks underlie this pattern because of the limited number of total proteins identified. Given that protein annotation for BgGUA is in its infancy when compared to mammalian systems, we were pleasantly surprised we were able to identify this many proteins from whole snail hemolymph. We were able to show that the differences among genotypes in protein levels are not obviously the result of changes in corresponding gene transcription, as transcript levels of the revealed proteins were generally unaffected by GRC genotype. Given that transcript levels and protein concentration are poorly correlated (Pearson correlation coefficient ~.40) in general, it is unsurprising that the transcript levels of these proteins do not explain our observations [33]. One caveat to these findings is that RNAseq was performed on whole snail tissue collected for an alternative project [8], rather than the hemolymph used for protein abundance analysis. Therefore, we cannot definitively rule out the possibility that the differences in the original samples used for these two analyses could have hidden a relationship between the mRNA transcript levels and protein levels of some of the differentially abundant proteins. We were also technically limited because we had to use GRC inbred lines, rather than individual, outbred homozygous individuals, in order to pool enough hemolymph for protein analysis. Though these technical and experimental caveats exist, it seems clear that GRC genotype has a significant effect on the proteomic profile of hemolymph, and that this effect is probably not mediated by mRNA levels.
A handful of recent publications have begun to characterize the proteomic profile of the serum of B. glabrata, as well as the interactome between B. glabrata and S. mansoni [13-16]. This study represents an initial examination of one level of potential genetic control of the proteomic profile of whole hemolymph in BgGUA in a homeostatic state. Fully characterizing the genetic control of the proteome of Bg, and determining how this could affect snail immunity and defense against human parasites will require much more extensive experimentation. Future work should examine both plasma, and the cellular components of hemolymph in the context of schistosome infection and the genetics of resistance to schistosomes. These studies should also confirm the most interesting findings via western blot with specific antibodies as they become available. It is likely that some low abundance proteins, those that would have been excluded in this study because they did not meet the stringencies required for quantification, are also under the influence of regions like the GRC. It may be possible to examine some of these proteins if hemolymph is separated into multiple components and run on 2D gels prior to proteomic analysis, in a similar manner to what has been previously done to serum alone [14].
The present study identifies some of the most abundant proteins found in the whole hemolymph of BgGUA, while also exploring the effects of allelic variation in the GRC region on the abundance of these proteins. Our findings suggest that allelic variation in this genomic region can alter hemolymph protein levels, and that schistosome-resistant hemolymph has a particular propensity to have lower levels of immune-related proteins (with one exception) when compared to susceptible hemolymph. This study also adds to the mounting evidence that at least one protein coded for in the GRC region has extensive physiological roles beyond schistosome defense, and that it may be important for understanding the biology of the intermediate hosts of schistosomes. To date, the effects of allelic variation in the GRC region have only been studied in Guadeloupean B. glabrata. However, we suspect that genes in this region are important in B. glabrata immune function in general. Exploration beyond this initial study is warranted given the complexity of these relationships and the importance of this species to human and agricultural health.
Supplementary Material
Table S1. Allelic variation in the GRC region significantly influences the levels of proteins identified in hemolymph extracted from BgGUA. The fold change in protein levels for all identified hemolymph proteins. Pooled hemolymph samples from independent inbred lines of RR, S1S1, S2S2 (n = 5 lines/genotype). Fold changes that significantly differ are bolded.
Highlights:
The Guadeloupe Resistance complex significantly alters the proteomic profile of hemolymph
The Guadeloupe Resistance complex does not alter the mRNA levels of these proteins
40% of altered proteins are immune related
Acknowledgements
We would like to thank Stephanie Bollmann and Ekaterina Peremyslova for their technical support. Funding: This work was supported by the National Institutes of Health [AI109134 and AI111201]. The procurement of the Thermo Scientific Orbitrap Fusion Lumos was made possible by a NIH grant S10 OD020111 to Oregon State University’s Mass Spectrometry Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Loker ES, Gastropod immunobiology, Adv Exp Med Biol 708 (2010) 17–43. [DOI] [PubMed] [Google Scholar]
- [2].Pearce EJ, MacDonald AS, The immunobiology of schistosomiasis, Nat Rev Immunol 2(7) (2002) 499–511. [DOI] [PubMed] [Google Scholar]
- [3].Giannelli A, Cantacessi C, Colella V, Dantas-Torres F, Otranto D, Gastropod-Borne Helminths: A Look at the Snail-Parasite Interplay, Trends Parasitol 32(3) (2016) 255–64. [DOI] [PubMed] [Google Scholar]
- [4].Sokolow SH, Wood CL, Jones IJ, Lafferty KD, Kuris AM, Hsieh MH, De Leo GA, To Reduce the Global Burden of Human Schistosomiasis, Use 'Old Fashioned' Snail Control, Trends Parasitol 34(1) (2018) 23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sokolow SH, Wood CL, Jones IJ, Swartz SJ, Lopez M, Hsieh MH, Lafferty KD, Kuris AM, Rickards C, De Leo GA, Global Assessment of Schistosomiasis Control Over the Past Century Shows Targeting the Snail Intermediate Host Works Best, PLoS Negl Trop Dis 10(7) (2016) e0004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colley DG, Bustinduy AL, Secor WE, King CH, Human schistosomiasis, Lancet 383(9936) (2014) 2253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Allan ERO, Blouin MS, The behavioral effects of antibiotic treatment on the snail Biomphalaria glabrata, PeerJ 5 (2017) e4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tennessen JA, Theron A, Marine M, Yeh JY, Rognon A, Blouin MS, Hyperdiverse gene cluster in snail host conveys resistance to human schistosome parasites, PLoS Genet 11(3) (2015) e1005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Allan ER, Tennessen JA, Bollmann SR, Hanington PC, Bayne CJ, Blouin MS, Schistosome infectivity in the snail, Biomphalaria glabrata, is partially dependent on the expression of Grctm6, a Guadeloupe Resistance Complex protein, PLoS Negl Trop Dis 11(2) (2017) e0005362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Allan ERO, Blouin MS, Allelic variation partially regulates galactose-dependent hydrogen peroxide release from circulating hemocytes of the snail Biomphalaria glabrata, Fish Shellfish Immunol 72 (2018) 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Allan ERO, Gourbal B, Dores CB, Portet A, Bayne CJ, Blouin MS, Clearance of schistosome parasites by resistant genotypes at a single genomic region in Biomphalaria glabrata snails involves cellular components of the hemolymph, Int J Parasitol (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Allan ERO, Tennessen JA, Sharpton TJ, Blouin MS, Allelic variation in a single genomic region alters the microbiome of the snail Biomphalaria glabrata, J Hered (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mone Y, Gourbal B, Duval D, Du Pasquier L, Kieffer-Jaquinod S, Mitta G, A large repertoire of parasite epitopes matched by a large repertoire of host immune receptors in an invertebrate host/parasite model, PLoS Negl Trop Dis 4(9) (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pinaud S, Portela J, Duval D, Nowacki FC, Olive MA, Allienne JF, Galinier R, Dheilly NM, Kieffer-Jaquinod S, Mitta G, Theron A, Gourbal B, A Shift from Cellular to Humoral Responses Contributes to Innate Immune Memory in the Vector Snail Biomphalaria glabrata, PLoS Pathog 12(1) (2016) e1005361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tetreau G, Pinaud S, Portet A, Galinier R, Gourbal B, Duval D, Specific Pathogen Recognition by Multiple Innate Immune Sensors in an Invertebrate, Front Immunol 8 (2017) 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu XJ, Dinguirard N, Sabat G, Lui HD, Gonzalez L, Gehring M, Bickham-Wright U, Yoshino TP, Proteomic analysis of Biomphalaria glabrata plasma proteins with binding affinity to those expressed by early developing larval Schistosoma mansoni, PLoS Pathog 13(5) (2017) e1006081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Theron A, Coustau C, Rognon A, Gourbiere S, Blouin MS, Effects of laboratory culture on compatibility between snails and schistosomes, Parasitology 135(10) (2008) 1179–88. [DOI] [PubMed] [Google Scholar]
- [18].Theron A, Rognon A, Gourbal B, Mitta G, Multi-parasite host susceptibility and multi-host parasite infectivity: a new approach of the Biomphalaria glabrata/Schistosoma mansoni compatibility polymorphism, Infect Genet Evol 26 (2014) 80–8. [DOI] [PubMed] [Google Scholar]
- [19].Hahn UK, Bender RC, Bayne CJ, Production of reactive oxygen species by hemocytes of Biomphalaria glabrata: carbohydrate-specific stimulation, Dev Comp Immunol 24(6–7) (2000) 531–41. [DOI] [PubMed] [Google Scholar]
- [20].Li H, Durbin R, Fast and accurate short read alignment with Burrows-Wheeler transform, Bioinformatics 25(14) (2009) 1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tennessen JA, Bollmann SR, Blouin MS, A Targeted Capture Linkage Map Anchors the Genome of the Schistosomiasis Vector Snail, Biomphalaria glabrata, G3 (Bethesda) 7(7) (2017) 2353–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Coustau C, Gourbal B, Duval D, Yoshino TP, Adema CM, Mitta G, Advances in gastropod immunity from the study of the interaction between the snail Biomphalaria glabrata and its parasites: A review of research progress over the last decade, Fish Shellfish Immunol 46(1) (2015) 5–16. [DOI] [PubMed] [Google Scholar]
- [23].Reardon S, Welcome to the CRISPR zoo, Nature 531(7593) (2016) 160–3. [DOI] [PubMed] [Google Scholar]
- [24].Thaiss CA, Zmora N, Levy M, Elinav E, The microbiome and innate immunity, Nature 535(7610) (2016) 65–74. [DOI] [PubMed] [Google Scholar]
- [25].Jiang Y, Loker ES, Zhang SM, In vivo and in vitro knockdown of FREP2 gene expression in the snail Biomphalaria glabrata using RNA interference, Dev Comp Immunol 30(10) (2006) 855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hanington PC, Forys MA, Dragoo JW, Zhang SM, Adema CM, Loker ES, Role for a somatically diversified lectin in resistance of an invertebrate to parasite infection, Proc Natl Acad Sci U S A 107(49) (2010) 21087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hanington PC, Forys MA, Loker ES, A somatically diversified defense factor, FREP3, is a determinant of snail resistance to schistosome infection, PLoS Negl Trop Dis 6(3) (2012) e1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hanington PC, Lun CM, Adema CM, Loker ES, Time series analysis of the transcriptional responses of Biomphalaria glabrata throughout the course of intramolluscan development of Schistosoma mansoni and Echinostoma paraensei, Int J Parasitol 40(7) (2010) 819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morita T, Structure-function relationships of C-type lectin-related proteins, Pathophysiol Haemost Thromb 34(4–5) (2005) 156–9. [DOI] [PubMed] [Google Scholar]
- [30].Pila EA, Gordy MA, Phillips VK, Kabore AL, Rudko SP, Hanington PC, Endogenous growth factor stimulation of hemocyte proliferation induces resistance to Schistosoma mansoni challenge in the snail host, Proc Natl Acad Sci U S A 113(19) (2016) 5305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang SM, Nian H, Wang B, Loker ES, Adema CM, Schistosomin from the snail Biomphalaria glabrata: expression studies suggest no involvement in trematode-mediated castration, Mol Biochem Parasitol 165(1) (2009) 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ittiprasert W, Knight M, Reversing the resistance phenotype of the Biomphalaria glabrata snail host Schistosoma mansoni infection by temperature modulation, PLoS Pathog 8(4) (2012) e1002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vogel C, Marcotte EM, Insights into the regulation of protein abundance from proteomic and transcriptomic analyses, Nat Rev Genet 13(4) (2012) 227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Allelic variation in the GRC region significantly influences the levels of proteins identified in hemolymph extracted from BgGUA. The fold change in protein levels for all identified hemolymph proteins. Pooled hemolymph samples from independent inbred lines of RR, S1S1, S2S2 (n = 5 lines/genotype). Fold changes that significantly differ are bolded.