Abstract
Cux1 is a homeodomain protein involved in cell cycle regulation and kidney development. Cux1 represses the cyclin kinase inhibitor p27 during early kidney development, promoting cell proliferation in the nephrogenic zone. Promoter reporter analysis of p27 revealed that Cux1 represses p27 in a concentration dependent manner, and immunoprecipitation showed that Cux1 interacts with the co-repressor Grg4 and the histone deacetylases HDAC1 and HDAC3. Chromatin immunoprecipitation (ChIP) identified the interaction of Cux1, Grg4, HDAC1, and HDAC3 at two different sites in the p27 promoter. To determine whether there was an interaction between these two loci in the developing kidney, we performed chromatin conformation capture (3C) assay. Analysis of newborn kidney tissue with 3C and ChIP-loop showed that the p27 promoter forms a loop intersecting at these two loci and that Cux1 bridges these two sites. To determine whether HDACs are required for Cux1 repression of p27 we analyzed p27 promoter activity in the presence of the HDAC inhibitor trichostatin A (TSA). TSA treatment completely relieved the repression of p27 by Cux1 and Grg4, demonstrating that Cux1 represses p27 in an HDAC dependent manner. To begin to test whether HDAC inhibitors could be used to target Cux1 repression of p27 for the treatment of PKD, we treated Pkd1 targeted pregnant mice with TSA or vehicle beginning at embryonic day 10.5 until embryonic day 18.5. Newborn Pkd1 mutant mice that received vehicle exhibited extensive collecting duct cysts, while newborn Pkd1 mutant mice that received TSA showed a significant reduction in cysts. Moreover, p27 expression was upregulated in TSA treated Pkd1 mice. Taken together, these results suggest that HDACs are required for cyst growth, and further support studies indicating that HDAC inhibitors may be an effective treatment for PKD.
Abbreviations: CCC, chromosome confirmation capture; 3C, chromosome confirmation capture; ChIP, chromatin immunoprecipitation; TSA, trichostatin A; HDAC, histone deacetylase; PKD, polycystic kidney disease
Keywords: Cux1, Polycystic kidney disease, p27 running title: regulation of p27 by Cux1
Highlights
-
•
Cux1 simultaneously interacts with two separate sites in the p27 promoter forming a loop in the p27 regulatory region.
-
•
Cux1 repression of p27 is dependent on HDAC activity.
-
•
Treatment of PKD mice with HDAC inhibitors attenuates Cux1 repression of p27 resulting in reduced cyst growth.
1. Introduction
Cux1 is the murine homologue of the Drosophila gene Cut (Nepveu, 2001; Vanden Heuvel et al., 1996). Mammalian homologues of Cut have also been identified in human, dog, and rat (Sansregret and Nepveu, 2008; Gupta et al., 2003). In Drosophila, Cut is required for the development of the Malpighian tubules, which serve as primitive kidneys and function as the excretory organs in these insects (Gupta et al., 2003). In the mouse kidney, Cux1 is highly expressed in the nephrogenic zone, which is a region characterized by actively proliferating cells (Vanden Heuvel et al., 1996). Cux1 downregulation is associated with cell cycle exit and terminal differentiation of nephron progenitor cells (Vanden Heuvel et al., 1996).
Cut proteins have five evolutionarily conserved domains. These include a homeodomain, three Cut repeats, and a coiled coil structure. The cut repeats, called CR1, CR2, and CR3, are composed of 70 amino acids, and, along with the homeodomain, are each capable of binding DNA. Mammalian cut proteins function as cell cycle dependent transcription factors that can function as activators or repressors (Lievens et al., 1995; Nirodi et al., 2001; Nishio, 2004; Zhu et al., 2004; Truscott et al., 2003; Moon et al., 2001; Cadieux et al., 2008; Truscott et al., 2008; Mailly et al., 1996). Cux1 can repress target genes by 1) competing for CCAAT or Sp1 binding site occupancy, preventing the binding of the corresponding transcriptional activators, or 2) active repression involving the recruitment of histone deacetylases (HDACs) (Li et al., 1999; Coqueret et al., 1998). Cux1 represses the cyclin kinase inhibitors p21 and p27 and promotes proliferation of nephron progenitor cells (Coqueret et al., 1998; Ledford et al., 2002; Sharma et al., 2009). Transgenic mice constitutively expressing Cux1 develop multiorgan hyperplasia, including renal hyperplasia, phenocopying p27 knockout mice (Ledford et al., 2002; Fero et al., 1996; Kiyokawa et al., 1996; Nakayama et al., 1996). In contrast, p21 knockout mice do not exhibit renal hyperplasia (Deng et al., 1995).
Cux1 is highly and ectopically expressed in several mouse models of polycystic kidney disease (PKD), including cpk, Pkd1 null, and Pkd1CD mice, which carry a collecting duct specific deletion of Pkd1 (Vanden Heuvel et al., 1996; Sharma et al., 2005; Paul et al., 2011), and is upregulated in human kidney cells isolated from the cysts of ADPKD patients (Alcalay et al., 2008). However, Cux1 transgenic mice do not develop cystic kidneys, indicating that overexpression of Cux1 alone is insufficient to develop PKD in mice (Ledford et al., 2002). However, sustained expression of a shorter isoform of Cux1 was shown to induce cysts in transgenic mice after 12 months (Cadieux et al., 2008). This is similar to the protracted cyst development that occurs when the Pkd1 gene is deleted in mice after a developmental switch (Piontek et al., 2007). A number of studies have shown that renal injury induces rapid cyst growth in mice when Pkd1 or cilia genes are disrupted after a developmental switch (Piontek et al., 2007; Davenport et al., 2007; Lantinga-van Leeuwen et al., 2007; Patel et al., 2008). However, when cilia were disrupted in adult Cux1 transgenic mice, rapid cyst development did not occur, even though cell proliferation remained high (Sharma et al., 2013). Rather, mice carrying both a collecting duct deletion of Pkd1 and a targeted deletion of Cux1 showed that Cux1 is required for cyst growth (Porath et al., 2017). Reduced Cux1 expression in PKD mice suppresses cyst growth, and tubules in which both Cux1 and Pkd1 were deleted showed increased expression of p27 and diminished cell proliferation. Cux1 interacts with the co-repressor Grg4 to repress p27 (Sharma et al., 2009), and along with the histone deacetylases HDAC1 and HDAC3, all four proteins bind to two different sites on the p27 promoter (Sharma et al., 2009). Thus, to determine whether inhibition of Cux1 repression of p27 could be a therapeutic approach for the treatment of PKD, we characterized the role of HDACs in p27 repression and evaluated Cux1 and p27 expression following treatment of Pkd1CD mice with the HDAC inhibitor trichostain A (TSA).
2. Materials and methods
2.1. Chromosome conformation capture (3C) assay
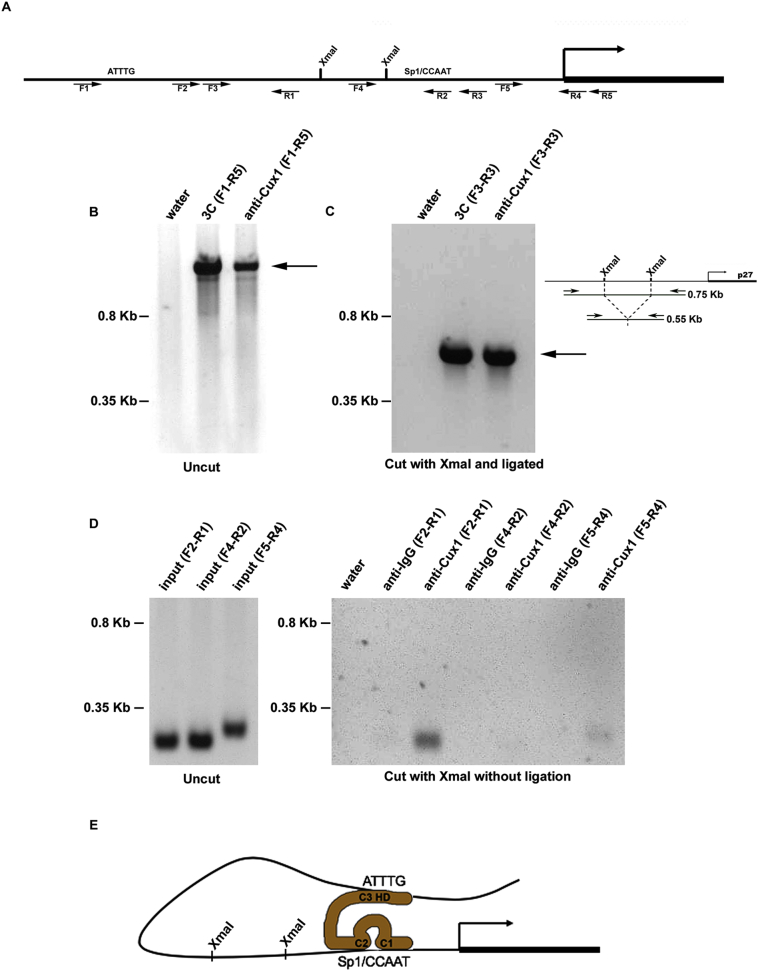
The 3C assay was performed as described with the following modifications (Horike et al., 2005). Isolated metanephroi were minced and passed through a 70 μm strainer into a chilled petri dish in cold D-MEM with 10% FBS. Kidney tissue was fixed in room temperature medium containing 2% formaldehyde for 5 min, followed by quenching by addition of 5 ml of 1 M glycine. The kidney tissue was resuspended in ice-cold lysis buffer containing protease inhibitors and sonicated on ice. DNA fragments from cross-linked chromatin were digested with XmaI. T4 DNA Ligase was added to the reaction with Ligase buffer and, following overnight incubation at 16C, cross-linking was reversed. The DNA was purified by phenol/chloroform and ethanol precipitated. To increase the sensitivity and specificity of the PCR analysis for detecting the ligated products from the 3C assay, we performed a preamplification step using primers F1 and R5 (Fig. 1A, B). This step was performed with 200–300 ng of DNA sample from the 3C assay, 0.5 U Taq polymerase, 10× Taq PCR buffer with 15 mM MgCl2, 2.5 μl dNTP mixture, and 1 μl each of primer F1 and R2. PCR conditions were: 94 °C, 30 s; followed by 35 cycles of 94 °C, 30 s; 53 °C, 30 s; 72 °C, 30 s and a final 7 min extension at 72 °C. The products were diluted 1:100 with TE buffer, and 2 μl was used as a template for a PCR reaction using primers F2 and R3. These primers amplify a 0.75 kb product flanking the XmaI sites in uncut DNA, but amplify a 0.55 kb product in DNA cut with XmaI and ligated with T4 DNA ligase. Amplified DNA fragments were analyzed on a 1% agarose gel by electrophoresis. Primers used for 3C assays are shown in Table 1.
Fig. 1.
Cux1 cross links two sites in the p27 promoter during kidney development. (A) Map of p27 promoter showing the XmaI sites and forward (F) and reverse (R) primers used for 3C and ChIP-Loop experiments used for PCR. The location of sites where Cux1 binds to p27 are indicated by ATTTG and Sp1/CCAAT. Primer sequences are shown in Table 1. (B) Chromatin conformation capture (3C) and ChIP-loop (anti-Cux1) assays were done on genomic DNA containing the p27 promoter region without restriction enzyme digestion. The predicted 1.45 kb fragment of the murine p27 promoter from −1307 to 142 is indicated by the arrow. (C) Chromatin conformation capture (3C) assay amplified a shorter region of DNA looping structure after restriction enzyme digestion and ligation. The 0.55 kb PCR product, indicated by the arrow, is 200 bp shorter than the predicted uncut 0.75 kb DNA, indicating amplification across ligated XmaI sites, shown in the adjacent diagram. Immunoprecipitation with Cux1 antibody (anti-Cux1) after restriction enzyme digestion with XmaI and ligation, followed by reverse cross-linking and amplification, showed the identical 0.55 kb product (arrow), indicating that Cux1 binds the two sites in the p27 promoter simultaneously. (D) Chromatin immunoprecipitation (ChIP) analysis of genomic DNA containing the p27 promoter region following restriction digestion with XmaI shows amplification of a 224 bp fragment of the p27 promoter spanning −940 to −716 (F2-R1) and a 272 bp fragment of the p27 promoter spanning −204 to +68 (F5-R4) relative to the transcription start site and flanking the XmaI sites. In contrast, amplification of a 221 bp fragment of the p27 promoter spanning −483 to −262 (F4-R2), which includes an XmaI site at −383, was observed in the input DNA, but not after immunoprecipitation of XmaI digested DNA with Cux1 antibody. (E) A model of the interaction between Cux1 and the p27 promoter. Cut repeats 1 and 2 compete for CCAAT or Sp1 binding site occupancy, preventing binding by the corresponding transcriptional activators, while the homeodomain binds to AT rich regions. Adapted from (Nepveu, 2001).
Table 1.
Primers used in CCC and ChIP-loop assays.
| Primer | Sequence (relative position to transcription start site) |
|---|---|
| F1 | 5′-AGTGTTTTCGCCCTTTAACA-3′ (−1307) |
| F2 | 5′-GAAACACCCTGATAAGAGCGGTC-3′ (−957) |
| F3 | 5′-CGGTCAGTCCTGGCTTCTTT-3′ (−939) |
| F4 | 5′-TGTTGGCAGTCGTACACCTC-3′ (−483) |
| F5 | 5′-TCATCCCTTGTCCCGACTCA-3′ (−204) |
| R1 | 5′-TAGGAGCTTCGGTGGCTAGT-3′ (-716) |
| R2 | 5′-AGGGAGGCTGACGAAGAAGA-3′ (−262) |
| R3 | 5′-AAAACACCCCAAAAGCACGAG-3′ (−208) |
| R4 | 5′-TCAAATGGAGCAGAGCCAGG-3′ (+68) |
| R5 | 5′-AACCGAACAAAAGCGAAACG-3′ (+141) |
2.2. ChIP loop assay
ChIP-loop was performed as described for the 3C assay, except that following ligation with T4 DNA ligase, ligated chromatin fragments were immunoprecipitated with anti-Cux1 antibody with protein-G agarose beads at 4 °C overnight, as previously described (Sharma et al., 2009). The cross-links were reversed and the DNA was purified. The DNA was then used as a PCR template as described above using primers F1 and R5 (Fig. 1A, B) followed by nested PCR using primers F2 and R3, as described above. Amplified DNA fragments were analyzed on a 1% agarose gel by electrophoresis. Primers used for ChIP-loop assays are shown in Table 1.
2.3. Transient reporter assay
Transient luciferase assay was performed as previously described (Ledford et al., 2002). Briefly, human embryonic kidney (293 T) cells were cultured in DMEM supplemented with 10% FBS and 100 U/ml penicillin and 100 mg/ml streptomycin in 5% CO2//95% air at 37 °C. Cells were plated at a concentration of 6 × 105 cells per well in 12 well plates 18 h prior to transfection. Transient transfections were performed using Fugene transfection reagent (Roche Applied Science), using 0.5 μg of luciferase reporter plasmid containing p27 promoter sequence (−1609 to +178), along with 0.1 μg renilla-expressing plasmid (to correct for transfection efficiencies), pCMV/CUX1 and pKW/Grg4 at the concentrations indicated, and pcDNA3.1 (to control for non-specific vector effects). After 20 h the transfected cells were trypsinized, separated into two groups, and reseeded in the absence of serum and antibiotics. Trichostatin A was added to one of the groups at a final concentration of 100 ng/ml. After 48 h, cells were lysed, and luciferase and renilla activities were determined by enzyme assay kits. Luciferase activity was normalized to renilla activity as an internal transfection control. Comparisons between luciferase activities were made using one-way ANOVA and Tukey post hoc analyses. P < 0.05 was considered statistically significant.
2.4. Mouse strains and treatment
Animal studies were conducted at the University of Kansas Medical Center, the University of Illinois at Chicago, and at Western Michigan University Homer Stryker MD School of Medicine. Animal protocols were approved by the Institutional Animal Care and Use Committees at these institutions. Pkd1CD mice were generated by crossing Pkd1cond mice with Hoxb7/cre mice, as previously described (Paul et al., 2011). Pkd1cond mice have loxP sites flanking exons 2–4 and crossing with Hoxb7/cre results in the deletion of Pkd1 gene in the collecting ducts (Pkd1CD) (Piontek et al., 2004). For TSA treatment experiments, Pkd1CD+/− females were put together with Pkd1CD+/− males. Pregnant Pkd1CD+/− mice from this pairing were injected daily, from 10.5 dpc to 18.5 dpc with 0.5 μg TSA per gram mouse body weight or with an equal volume of dimethyl sulfoxide (DMSO). To collect TSA treated kidneys, kidneys were harvested from P0 mice born from the TSA treated pregnant females and fixed in 4% paraformaldehyde. Genomic DNA was obtained from the newborn mice and genotyped as previously described (Piontek et al., 2004).
2.5. Morphology
Midsagittal paraffin sections (5 μM) of cystic kidneys treated with TSA or DMSO were stained with H&E and utilized to determine the effects of TSA on cyst growth. The cystic index (ratio of cystic area in the kidney to the total kidney area) was determined from H&E stained cystic kidney sections using ImageJ (National Institutes of Health) software.
2.6. Immunofluorescence
Five micron-thick kidney sections were de-paraffinized with xylene and hydrated with graded ethanols. The antigen unmasking and labeling was performed as previously described (Sharma et al., 2005). Briefly, kidney sections were incubated with 1 M ammonium chloride for 30 min to quench autofluorescence, followed by washing in PBST, and then blocked in 10% normal goat serum (NGS) or 10% normal horse serum (NHS) for 1 h at room temperature. Sections were treated with rabbit anti-Cux1 (1:50, Santa Cruz, sc-13024), mouse anti-PCNA (1:3000, Sigma, P8825), or rabbit anti-p27 (1:100, AbCam, ab7961) primary antibodies overnight at 4 °C. Biotinylated goat anti-rabbit (1:400, Vector) secondary antibody was used to detect Cux1 and p27. The sections were then subsequently probed with FITC-avidin (5 μg/ml, Vector). PCNA antibodies were detected using a horse anti-mouse Texas Red conjugated antibody (Vector, 1:400). To identify collecting ducts, kidney sections were labeled with anti-cytokeratin (1:400, Sigma, C2562). Sections were washed in PBST after the antibody treatments, mounted with Vectashield medium with DAPI (Vector) and slides were viewed on a Leica DM5000 fluorescence microscope and images captured with a Leica DFC365 digital camera.
3. Results
We previously showed that Cux1 represses p27 promoter activity in a concentration dependent manner and that repression is significantly enhanced when Cux1 is associated with the co-repressor Grg4 (Sharma et al., 2009). Further studies showed that, in addition to Grg4, Cux1 interacts with HDAC1 and HDAC3 and that all four proteins interact with two separate sites on the p27 promoter in RKE cells, a kidney epithelial cell line (Sharma et al., 2009). DNAse footprinting showed that these sites correspond to an AT rich sequence located approximately −1.3 kb from the transcription start site, and a CCAAT box and two GC rich sequences approximately −0.35 kb from the transcription start site. Both the AT rich sequence and the CCAAT and GC rich sequences have been previously described as binding sites for Cux1 (Mailly et al., 1996). The AT rich sequence is recognized by the homeodomain of Cux1, while the CCAAT box and GC rich sequences are recognized by cut repeats 1 and 2.
3.1. DNA looping in the p27 promoter is bridged by Cux1
These results suggested two different models of p27 repression by Cux1 in which, 1) two separate repressor complexes are formed at each of these two sites, or 2) a single repressor complex is formed that interacts with these two sites simultaneously. To determine between these possibilities, we performed the chromosome conformation capture (3C) combined with the ChIP assay (ChIP-Loop) on kidney tissue isolated from newborn mice, which express high levels of Cux1, HDACs, and Grg4. The 3C assay is used to determine whether two genomic sequences that are distantly separated interact in the nucleus, and the ChIP-loop assay is used to determine whether the interaction is mediated by a specific protein. To determine whether a loop is formed between these two sites we performed the 3C assay using restriction site XmaI at position −589 and XmaI at position −385. In this assay, if remote sequences are located near each other as a result of chromatin folding in vivo, they may be cross linked in the same conformation following digestion with restriction enzymes. Thus, the DNA fragments resulting from digestion with XmaI will be in the same location and a novel ligation will occur which can be detected by PCR. In contrast, if two separate complexes are formed at the two sites, no ligation will occur. We used primers F1 and R5 in a preamplification step, followed by PCR with primers F2 and R3. Using primers F2 and R3, a 0.75 kb fragment would be predicted without XmaI digestion, while a 0.55 kb fragment is seen if a novel ligation occurs. As shown in Fig. 1, a DNA loop was observed by gel electrophoresis using primers flanking the XmaI sites after formaldehyde induced cross-linking, digestion with XmaI, and ligation (Fig. 1C). To confirm that Cux1 protein was directly involved in the DNA loop complex, we performed ChIP loop assays by immunoprecipitating Cux1, following formaldehyde induced cross-linking, digestion with XmaI, and ligation. In this assay, if Cux1 interacts with both sites simultaneously, the DNA fragments resulting from digestion with XmaI will be in the same location and a novel ligation will occur which can be detected by PCR, following immunoprecipitation using anti-Cux1 antibody. Gel electrophoresis showed that the p27 promoter formed a looping complex that co-precipitated with Cux1 (Fig. 1C). We have previously shown that Cux1 interacts with the two different sites on the p27 promoter by ChIP analysis (Sharma et al., 2009), but does not interact with sequences between these sites. To verify that the interaction between Cux1 and the two sites on the p27 promoter were specific, we performed chromatin immunoprecipitation (ChIP) analysis of genomic DNA containing the p27 promoter region following restriction digestion with XmaI. Our results show that Cux1 bound two sites flanking the XmaI sites in the p27 promoter in its native chromatin configuration (Fig. 1D).
3.2. HDACs are required for Cux1 repression of p27
We have previously demonstrated that Cux1 interacts directly with Grg4, a mammalian homologue of the co-repressor groucho (Sharma et al., 2009). Cux1 can be immunoprecipitated with HDAC1, and Grg4 immunoprecipitated with HDAC3, and all four proteins bind the two sites in the p27 promoter in vivo, suggesting a model in which these four proteins interact to repress p27. To evaluate the functional importance of HDAC1 and HDAC3, we compared the ability of Cux1 to repress p27 promoter activity with or without the HDAC inhibitor trichostatin A (TSA). Unsynchronized 293 T cells were co-transfected with a p27/luciferase reporter construct and either the empty CMV vector, or the CMV/Cux1 expression construct, with or without a CMV/Grg4 expression construct, and with or without the HDAC inhibitor Trichostatin A (TSA). In three separate experiments, the luciferase activity was reduced in the presence of Cux1 and Grg4, however, this repression was significantly relieved in the presence of TSA, demonstrating that Cux1 repression of p27 is dependent on HDAC activity (Fig. 2).
Fig. 2.
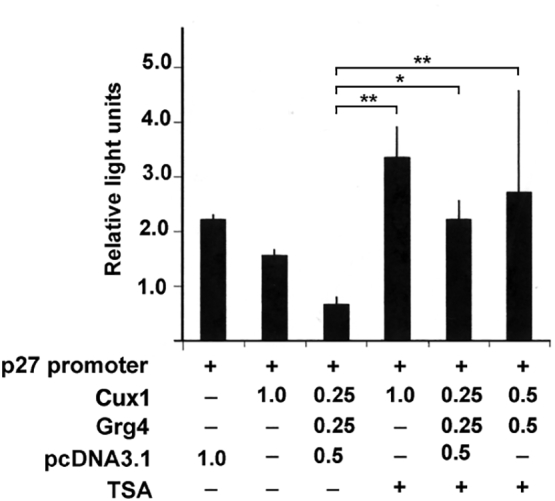
Repression of p27 by Cux1 is relieved by HDAC inhibitors. 293 T cells were transiently transfected with 1 μg of reporter construct containing p27 upstream sequences from −1609 to +178 fused to the luciferase reporter gene (+), together with different concentrations of the Cux1 and Grg4 expression vectors (amounts shown are in μg) or with empty pcDNA3.1 expression vector (amounts shown are in μg). While increasing concentrations of Cux1 and Grg4 enhance repression of p27, treatment with 1.0 mg/ml of the HDAC inhibitor trichostatin A (TSA) relieves the repression of p27 promoter activity. Activity is expressed as the mean of three separate experiments performed in triplicate. Error bars indicate standard deviation. For statistical analysis, one-way ANOVA and Tukey post hoc analyses were used (*P < 0.05, **P < 0.01).
3.3. HDAC inhibition reduces cyst growth in PKD1CD kidneys
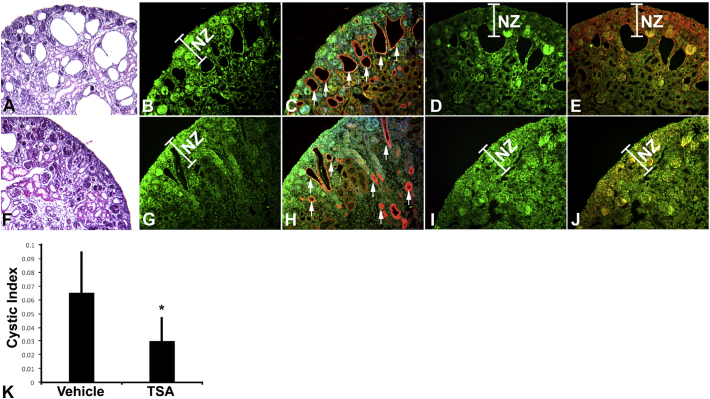
Cux1 is highly and ectopically expressed in the cyst lining epithelium of several mouse models of polycystic kidney disease and in cells isolated from ADPKD patients (Vanden Heuvel et al., 1996; Sharma et al., 2005; Paul et al., 2011; Alcalay et al., 2008). Associated with the ectopic expression of Cux1, p27 expression is reduced in kidneys from ADPKD mouse models and in human ADPKD cells. To determine whether Cux1 is required for PKD we crossed mice carrying a targeted deletion of Cux1 with a conditional Pkd1 mutation. These studies showed that reduced expression of Cux1 in the Pkd1 null mice slows cyst growth (Patel et al., 2008). The mechanism of the reduced cyst growth appears to be the increased expression of p27 in the cells of the collecting duct that lack both Pkd1 and Cux1, suggesting an important role for Cux1 in the progression of PKD. To determine whether interfering with the ability of Cux1 to repress p27 has an effect on PKD progression, we injected pregnant Pkd1CD+/− mice with TSA daily from 10.5 dpc to 18.5 dpc (days post coital), and analyzed the kidneys of Pkd1CD−/− mice at P0. Kidneys from newborn Pkd1CD5−/− mice showed a reduced cystic index compared to kidneys isolated from DMSO treated Pkd1CD+/− mice (Fig. 3A, F, K). Cell proliferation was reduced in the nephrogenic zone of the TSA treated kidneys (Fig. 3J), and this was associated with ectopic expression of p27 (Fig. 3I). Interestingly, the expression of Cux1 was not different between the treated and untreated kidneys (Fig. 3B, G). This suggests that although Cux1 is expressed, it requires HDACs to repress p27.
Fig. 3.
Treatment with TSA reduces cyst progression in Pkd1CD mice. (A, F) Representative histological sections of P0 Pkd1CD−/− kidneys from pregnant mothers that had been subcutaneously injected daily, from E10.5 to E18.5 with 0.5 mg TSA/g mouse body weight (F) or DMSO (A) in PBS. (K) Aggregate data from vehicle treated (n = 3) and TSA treated (n = 6) newborn mouse kidneys showing significant differences in cystic index (*P < 0.05). (B, C, G. H) P0 Pkd1CD−/− kidneys that had been treated in utero with TSA (G, H) or DMSO (B, C) were labeled with antibodies against cytokeratin (red) and Cux1 (green). While TSA treated kidneys showed reduced cyst progression, Cux1 expression was not changed in the nephrogenic zone (NZ), indicating that HDAC inhibition does not alter Cux1 expression. Cytokeratin labeling showed reduced cysts in the collecting ducts of TSA treated mice (arrows in H) compared to DMSO treated mice. (D, E, I, J) P0 Pkd1CD−/− kidneys that had been treated in utero with TSA (I, J) or DMSO (D, E) were labeled with antibodies against PCNA (red) and p27 (green). While p27 expression is normally restricted to maturing glomeruli and tubules (D), p27 was ectopically expressed in the nephrogenic zone in the kidneys from TSA treated mice (I), and this was associated with reduced PCNA expression (J) in the nephrogenic zone (NZ), compared to DMSO treated mice (E). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Cux1 is a cell cycle dependent transcription repressor that is aberrantly expressed in the Pkd1 null, Pkd1CD, and cpk mouse models of PKD, as well as in human ADPKD cells (Vanden Heuvel et al., 1996; Sharma et al., 2005; Paul et al., 2011; Alcalay et al., 2008). In Pkd1 null mice and in human ADPKD cells there is a reduction in the levels of a nuclear isoform of the cysteine endopeptidase cathepsin-l (Alcalay et al., 2008). This enzyme proteolytically processes Cux1 in S phase of the cell cycle, thus the reduction of cathepsin-l results in the accumulation of full length Cux1 protein in PKD (Alcalay et al., 2008; Goulet et al., 2004). Deletion of the cathepsin-L processing site in Cux1 in Cys1cpk mice similarly resulted in the accumulation of Cux1 (Alcalay et al., 2008). The increased expression of Cux1 in PKD is associated with increased cell proliferation and disease progression resulting from the down regulation of the cyclin kinase inhibitor p27 (Alcalay et al., 2008; Porath et al., 2017). However, forced expression of Cux1 in transgenic mice results in multiorgan hyperplasia, but does not result in cystic kidney disease (Ledford et al., 2002). Moreover, sustained overexpression of Cux1 failed to induce rapid cyst formation after the disruption of cilia in the kidneys of adult mice (Sharma et al., 2013). To determine whether Cux1 is required for PKD progression, we crossed mice carrying a targeted deletion of Cux1 with Pkd1CD mice, which have Pkd1 deleted in the collecting ducts (Porath et al., 2017). Mice that were homozygous mutant for both Cux1 and Pkd1 showed no cystic disease, and mice that were heterozygous for Cux1 and homozygous for Pkd1 showed significantly reduced cystic disease (Porath et al., 2017). These studies demonstrated that Cux1, while not sufficient to cause polycystic kidney disease when overexpressed, is required for cystic disease progression. These results suggest that targeting Cux1 regulation of the cell cycle may be an effective treatment for ADPKD. We have previously shown that Cux1 interacts with the co-repressor Grg4 and HDAC1 and HDAC3 in the context of the p27 promoter, and that this complex binds to two regions of the p27 promoter in its native configuration. Analysis of the two sites bound by Cux1 showed sequences that are recognized by different DNA binding domains in Cux1, suggesting that Cux1 could bind both sites simultaneously forming a loop in the p27 regulatory region. Our results support this model showing that a loop is formed between an AT rich region located a1–1.3 kb from the transcription start site and a GC rich region and a CCAAT box located at −0.35 kb from the transcription start site. Interaction with the GC rich region and CCAAT sequences located at −0.35 kb are associated with passive repression and involve the first two cut repeats of Cux1, which compete with transcriptional activators for binding (Mailly et al., 1996). Interaction with the AT rich sequences located at −1.3 are associated with active repression and involves the third cut repeat and the homeodomain (Mailly et al., 1996). Binding of this domain to target sequences is thought to involve the recruitment of HDACs. We have previously shown that HDAC1 and HDAC3 can be immunoprecipitated, along with Cux1 and Grg4, at both of these sites in vivo (Sharma et al., 2009). Moreover, the present results demonstrate that HDAC activity is necessary for Cux1 repression of p27. Recently, a number of studies have determined that treatment of Pkd1 and Pkd2 animal models of polycystic kidney disease with HDAC inhibitors reduces polycystic kidney disease (Cao et al., 2009; Xia et al., 2010; Fan et al., 2012). Similarly, our results show that treatment of Pkd1CD mouse embryos with TSA reduced cyst growth. Moreover, p27 expression is increased in the TSA treated mice. Thus, the mechanism of cyst reduction appears to be increased expression of p27 reducing cell proliferation. Interestingly, Cux1 expression was not changed in the TSA treated embryonic kidneys compared to the control kidneys, further supporting the requirement of HDAC activity for Cux1 repression of p27. Our results provide further support for the use of HDAC inhibitors as therapeutic treatments for PKD, and provide a potential mechanism of action for their effectiveness in treating PKD.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Rosetta Barkley for expert technical assistance. We thank Robin Maser, James Calvet, Darren Wallace, and Jovanka Koo for many helpful discussions and expert technical advice. This work was supported by National Institutes of Health, United States grant R15-DK100972 (G.B. Vanden Heuvel).
References
- Alcalay N.I., Sharma M., Vassmer D., Chapman B., Paul B., Zhou J., Brantley J.G., Wallace D.P., Maser R.L., Vanden Heuvel G.B. Acceleration of polycystic kidney disease progression in cpk mice carrying a deletion in the homeodomain protein Cux1. Am. J. Physiol. Ren. Physiol. 2008;295:F1725–F1734. doi: 10.1152/ajprenal.90420.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadieux C., Harada R., Paquet M., Côté O., Trudel M., Nepveu A., Bouchard M. Polycystic kidneys caused by sustained expression of Cux1 isoform p75. J. Biol. Chem. 2008;283:13817–13824. doi: 10.1074/jbc.M709332200. [DOI] [PubMed] [Google Scholar]
- Cao Y., Semanchik N., Lee S.H., Somlo S., Barbano P.E., Coifman R., Sun Z. Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21819–21824. doi: 10.1073/pnas.0911987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coqueret O., Berube G., Nepveu A. The mammalian cut homeodomain protein functions as a cell-cycle-dependent transcriptional repressor which downmodulates p21WAF1/CIP1/SDI1 in S phase. EMBO J. 1998;17:4680–4694. doi: 10.1093/emboj/17.16.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport J.R., Watts A.J., Roper V.C., Croyle M.J., van Groen T., Wyss J.M., Nagy T.R., Kesterson R.A., Yoder B.K. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J.W., Elledge S.J., Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Fan L.X., Li X., Magenheimer B., Calvet J.P., Li X. Inhibition of histone deacetylases targets the transcription regulator Id2 to attenuate cystic epithelial cell proliferation. Kidney Int. 2012;81:76–85. doi: 10.1038/ki.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fero M.L., Rivkin M., Tasch M., Porter P., Carow C.E., Firpo E., Polyak K., Tsai L.H., Broudy V., Perlmutter R.M., Kaushansky K., Roberts J.M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Goulet B., Baruch A., Moon N.S., Poirier M., Sansregret L.L., Erickson A., Bogyo M., Nepveu A. A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Mol. Cell. 2004;14:207–219. doi: 10.1016/s1097-2765(04)00209-6. [DOI] [PubMed] [Google Scholar]
- Gupta S., Luong M.X., Bleuming S.A., Miele A., Luong M., Young D., Knudsen E.S., Van Wijnen A.J., Stein J.L., Stein G.S. Tumor suppressor pRB functions as a co-repressor of the CCAAT displacement protein (CDP/cut) to regulate cell cycle controlled histone H4 transcription. J. Cell. Physiol. 2003;196:541–556. doi: 10.1002/jcp.10335. [DOI] [PubMed] [Google Scholar]
- Horike S., Cai S., Miyano M., Cheng J.F., Kohwi-Shigematsu T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat. Genet. 2005;37:31–40. doi: 10.1038/ng1491. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H., Kineman R.D., Manova-Todorova K.O., Soares V.C., Hoffman E.S., Ono M., Khanam D., Hayday A.C., Frohman L.A., Koff A. Enhanced growth of mice lacking the cyclin- dependent kinase inhibitor function of p27(Kip1) Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Lantinga-van Leeuwen I.S., Leonhard W.N., van der Wal A., Breuning M.H., de Heer E., Peters D.J. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum. Mol. Genet. 2007;16:3188–3196. doi: 10.1093/hmg/ddm299. [DOI] [PubMed] [Google Scholar]
- Ledford A.W., Brantley J.G., Kemeny G., Foreman T.L., Quaggin S.E., Igarashi P., Oberhaus S.M., Rodova M., Calvet J.P., Vanden Heuvel G.B. Deregulated expression of the homeobox gene Cux-1 in transgenic mice results in downregulation of p27(kip1) expression during nephrogenesis, glomerular abnormalities, and multiorgan hyperplasia. Dev. Biol. 2002;245:157–171. doi: 10.1006/dbio.2002.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Moy L., Pittman N., Shue G., Aufiero B., Neufeld E.J., LeLeiko N.S., Walsh M.J. Transcriptional repression of the cystic fibrosis transmembrane conductance regulator gene, mediated by CCAAT displacement protein/cut homolog, is associated with histone deacetylation. J. Biol. Chem. 1999;274:7803–7815. doi: 10.1074/jbc.274.12.7803. [DOI] [PubMed] [Google Scholar]
- Lievens P.M., Donady J.J., Tufarelli C., Neufeld E.J. Repressor activity of CCAAT displacement protein in HL-60 myeloid leukemia cells. J. Biol. Chem. 1995;270:12745–12750. doi: 10.1074/jbc.270.21.12745. [DOI] [PubMed] [Google Scholar]
- Mailly F., Berube G., Harada R., Mao P.L., Phillips S., Nepveu A. The human cut homeodomain protein can repress gene expression by two distinct mechanisms: active repression and competition for binding site occupancy. Mol. Cell. Biol. 1996;16:5346–5357. doi: 10.1128/mcb.16.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon N.S., Premdas P., Truscott M., Leduy L., Bérubé G., Nepveu A. S phase-specific proteolytic cleavage is required to activate stable DNA binding by the CDP/cut homeodomain protein. Mol. Cell. Biol. 2001;21:6332–6345. doi: 10.1128/MCB.21.18.6332-6345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Ishida N., Shirane M., Inomata A., Inoue T., Shishido N., Horii I., Loh D.Y., Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Nepveu A. Role of the multifunctional CDP/cut/Cux homeodomain transcription factor in regulating differentiation, cell growth and development. Gene. 2001;270:1–15. doi: 10.1016/s0378-1119(01)00485-1. [DOI] [PubMed] [Google Scholar]
- Nirodi C., Hart J., Dhawan P., Moon N.S., Nepveu A., Richmond A. The role of CDP in the negative regulation of CXCL1 gene expression. J. Biol. Chem. 2001;276:26122–26131. doi: 10.1074/jbc.M102872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio H. Walsh, M.J. CCAAT displacement protein/cut homolog recruits G9a histone lysine methyltransferase to repress transcription. EMBO J. 2004;101:11257–11262. doi: 10.1073/pnas.0401343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V., Li L., Cobo-Stark P., Shao X., Somlo S., Lin F., Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B.M., Vassmer D., Taylor A., Magenheimer L., Carlton C.G., Piontek K.B., Germino G.G., Vanden Heuvel G.B. Ectopic expression of Cux1 is associated with reduced p27 expression and increased apoptosis during late stage cyst progression upon inactivation of Pkd1 in collecting ducts. Dev. Dyn. 2011;240:1493–1501. doi: 10.1002/dvdy.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontek K.B., Huso D.L., Grinberg A., Liu L., Bedja D., Zhao H., Gabrielson K., Qian F., Mei C., Westphal H., Germino G.G. A functional floxed allele of Pkd1 that can be conditionally inactivated in vivo. J. Am. Soc. Nephrol. 2004;15:3035–3043. doi: 10.1097/01.ASN.0000144204.01352.86. [DOI] [PubMed] [Google Scholar]
- Piontek K., Menezes L.F., Garcia-Gonzalez M.A., Huso D.L., Germino G.G. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath B., Livingston S., Andres E.L., Petrie A.M., Wright J.C., Woo A.E., Carlton C.G., Baybutt R., Vanden Heuvel G.B. Cux1 promotes cell proliferation and polycystic kidney disease progression in an ADPKD mouse model. Am. J. Physiol. Ren. Physiol. 2017;313:F1050–F1059. doi: 10.1152/ajprenal.00380.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansregret L., Nepveu A. The multiple roles of CUX1: insights from mouse models and cell- based assays. Gene. 2008;412:84–94. doi: 10.1016/j.gene.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Sharma M., Brantley J.G., Alcalay N.I., Zhou J., Heystek E., Maser R.L., Vanden Heuvel G.B. Differential expression of Cux-1 and p21 in polycystic kidneys from Pkd1 null and cpk mice. Kidney Int. 2005;67:432–442. doi: 10.1111/j.1523-1755.2005.67099.x. [DOI] [PubMed] [Google Scholar]
- Sharma M., Brantley J.G., Vassmer D., Chaturvedi G., Baas J., Vanden Heuvel G.B. The homeodomain protein Cux1 interacts with Grg4 to repress p27 kip1 expression during kidney development. Gene. 2009;439:87–94. doi: 10.1016/j.gene.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Malarkey E.B., Berbari N.F., O'Connor A.K., Vanden Heuvel G.B., Mrug M., Yoder B.K. Proximal tubule proliferation is insufficient to induce rapid cyst formation after cilia disruption. J. Am. Soc. Nephrol. 2013;24:456–464. doi: 10.1681/ASN.2012020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott M., Raynal L., Premdas P., Goulet B., Leduy L., Bérubé G., Nepveu A. CDP/Cux stimulates transcription from the DNA polymerase alpha gene promoter. Mol. Cell. Biol. 2003;23:3013–3028. doi: 10.1128/MCB.23.8.3013-3028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott M., Harada R., Vadnais C., Robert F., Nepveu A. p110 CUX1 cooperates with E2F transcription factors in the transcriptional activation of cell cycle-regulated genes. Mol. Cell. Biol. 2008;28:3127–3138. doi: 10.1128/MCB.02089-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Heuvel G.B., Bodmer R., McConnell K.R., Nagami G.T., Igarashi P. Expression of a cut-related homeobox gene in developing and polycystic mouse kidney. Kidney Int. 1996;50:453–461. doi: 10.1038/ki.1996.336. [DOI] [PubMed] [Google Scholar]
- Xia S., Li X., Johnson T., Seidel C., Wallace D.P., Li R. Polycystin-dependent fluid flow sensing targets histone deacetylase 5 to prevent the development of renal cysts. Development. 2010;137:1075–1084. doi: 10.1242/dev.049437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Maitra U., Johnston D., Lozano M., Dudley J.P. The homeodomain protein CDP regulates mammary-specific gene transcription and tumorigenesis. Mol. Cell. Biol. 2004;24:4810–4823. doi: 10.1128/MCB.24.11.4810-4823.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]