Abstract
Electrophysiology is a direct measure of neuronal processes, and it is uniquely sensitive to canonical neural operations that underlie emergent psychological operations. These qualities make it well suited for discovery of aberrant neural mechanisms that underlie complicated disease states. This technique is routinely utilized in vitro, in vivo, and in outpatient neurological clinics, offering a translatable bridge between animal models and human patients. The bench-to-bedside potential of this approach is unparalleled, yet it also remains undeveloped due to the slow inertia of legacy techniques and interpretations. In this review, I discuss these strengths of the method, and I detail compelling reasons why future advancements can have a direct and tangible influence over clinical practice. I hope to motivate a blurring of traditional boundaries between preclinical, computational, imaging, and clinical fields by advancing electrophysiology as a common hub for methodological integration and theoretical advancement.
Keywords: computational modeling, EEG, ERPs, neurology, psychiatry, translation
“Beware the man of one method or instrument … he tends to become method-oriented rather than problem-oriented … Strong inference redirects a man to problem-orientation, but it requires him to be willing repeatedly to put aside his last methods and teach himself new ones.”
“Whether you can observe a thing or not depends on the theory which you use. It is theory which decides what can be observed.”
Albert Einstein to Werner Heisenberg, 1926
1 |. INTRODUCTION
This review presents a case for increased utilization of a specific technique, with an altered interpretation of what it can mean. As Platt warns in the introductory quote, a focus on any one technique can lead to narrowed thinking. Hopefully, he would agree that sometimes the problem is in the theory and not the technique. Einstein’s perspective may offer a solution. Theory matters, even the theory about how your measurements can be used to draw conclusions.
The aim of this theoretical review is to motivate an increased utilization of electrophysiology in the understanding of neural coding, the translation of findings across species, and for diagnosis and prognosis in clinical practice. The basis for this motivation is predicated on the assertion that electrophysiological oscillations allow a unique measure of the neural computations that underlie cognition. This has particularly promising implications for advancing clinical practice. These qualities of oscillations, computations, and implications are described below.
1.1 |. Oscillations: Electrophysiology offers a direct measure of neuronal activity
It is important to move beyond the platitude that “electrophysiology has good temporal resolution” and appreciate why timing matters (Cohen, 2011; Nadasdy, 2010). Neuronal populations participating in a given frequency perturbation will be more (trough) or less (peak) likely to be excited as a function of the population oscillation, and thus will more likely to interact, exchange information, and modulate synaptic plasticity together (Fries, 2005). Electrophysiological recordings encompass multiple scales of measurement in a hierarchical, integrated manner. Coupling between single spikes and local fields provides a direct line of interpretation from the neural code (Rieke, Warland, de Ruyter van Stevenick, & Bialek, 1999), and local fields couple all the way up scalp-recorded EEG. Regardless of scale (depth, dura, scalp, etc.), one is always measuring field activity (Buzsáki, Anastassiou, & Koch, 2012). Common techniques for measuring human neural activities are either fundamentally related to electrophysiology (e.g., magnetoencephalogram [MEG]) or are epiphenomenal measures of information coding in the brain (blood-oxygen-level-dependent [BOLD], positron-emission tomography [PET], functional near-infrared spectroscopy [fNIRS]). This makes electrophysiology uniquely well suited for addressing questions about neural information processing.
1.2 |. Computations: Event-related electrophysiological responses reflect canonical neural computations
Canonical neural computations are stereotyped patterns of neural activity that sculpt information processing (Fries, 2009; Siegel, Donner, & Engel, 2012). Event-related EEG activities are stereotyped patterns that are modulated by changing demands in information processing (Luck, 2005). Here, the question of scale becomes critical: To what degree are electrophysiological signals representative of underlying neural mechanisms that compute cognition? As described later in this review, this inference can be informed by convergent cognitive process models, neural network simulations, or by more abstract theories of information processing in neural circuits.
1.3 |. Implications: EEG is highly sensitive to aberrant processes in neurological and psychiatric diseases
Identification of aberrant neural mechanisms underlying a disorder may be a more fruitful target for clinical translation than expanded phenotypic characterization (Insel et al., 2010; Montague, Dolan, Friston, & Dayan, 2012). EEG activities are highly sensitive to neural dysfunction in varied psychiatric and neurological disorders, and many have good reliability (Hämmerer, Li, Völkle, Müller, & Lindenberger, 2013; Leue, Klein, Lange, & Beauducel, 2013; Meyer, Bress, & Proudfit, 2014; Towers & Allen, 2009; Weinberg & Hajcak, 2011). If specificity can be improved, then EEG can offer an ideal biomarker for disease presence and trajectory. In this review, I discuss how existing clinical infrastructure can facilitate rapid application of new EEG-based biomarkers.
1.4 |. Three tenets: Oscillations, computations, and implications
These three qualities suggest that local fields are highly promising for advanced inference of neural function, translation of findings across species, and direct application to clinical practice. In subsequent sections, evidence is provided to support the aim of this review. Section 2 contrasts conventional interpretations of event-related EEG signals with alternative interpretations that have been proposed. A goal of objective, system-defined, quantitative interpretation of event-related EEG is advanced. Section 3 elucidates how computational modeling can help achieve this goal, with convergent evidence from other imaging methods and causal testing using cross-species models. Section 4 describes how current clinical infrastructure would allow a rapid and inexpensive adoption of promising electrophysiological biomarkers and concludes with a vision for future engagement across traditional fields of neural science.
2 |. IT IS THEORY WHICH DECIDES WHAT CAN BE OBSERVED
As a foundation of psychophysiology, Cacioppo and Tassinary’s (1990) treatise builds on the logic offered by Platt, Donders, James, and Popper to detail the inferential challenges in an inherently correlative technique. This approach affords a taxonomy of brain-behavior relationships, but it still doesn’t answer the question of what an EEG signal means (see Cohen, 2017). In the sections below, I argue that the status quo procedures for addressing this question need to be advanced and that the history of this field is ripe with promising suggestions.
2.1 |. Psychological inference of event-related EEG
Over fifty years of research on cognitive ERP components has advanced the understanding of numerous psychological events.1 While a description of the limitations of this method does not denigrate these foundational findings, it would be a mistake to consider a component-based focus as the sine qua non of event-related EEG. In principle, ERP components are inferred by indicators such as polarity, latency, and topography, yet their ultimate definition relies upon a presumed latent computational operation (Luck, 2005), which can complicate a straightforward identification by manifest indicators. This often requires an understanding of what the experimenter did to elicit them, which forces a definition based on external (experimenter) influence and not on intrinsic brain operations. In practice, components are often identified by peaks and troughs that tend to strongly correspond with components, yet do not necessarily define them. To summarize, ERP components have three inherent deficits when inferring psychological states: (a) they are not objectively definable, (b) they rely on the point of view of the observer and not the system, and (c) current practice uses idiosyncratic and simplified shortcuts, again forcing a qualitative definition.
The nebulous practice of defining EEG features as ERP components may hinder the ultimate aim of understanding common parsimonious mechanistic processes underlying varied neural events. A goal for continued advancement should rely on objective, system-defined, quantitative interpretation of our observations. By defining processes in mathematical terms, computational models address these desiderata. Notably, these interpretations are unlikely to be concepts common to cognitive psychology and ERP component interpretation (e.g., working memory, attention, categorization, evaluation, etc.). Theoretical frameworks for such a physiological perspective have been advanced, but they remain outside the status quo, as detailed below.
2.2 |. Physiological inference of event-related EEG
Many EEG researchers have been well trained on the best techniques (Cohen, 2014a; Luck, 2005) and proper psychological interpretations (Cacioppo & Tassinary, 1990). Yet, the field lacks a prevalent influence of approaches that have aimed to answer mechanistic questions of neural function. In the tripartite logic of Marr and Poggio (1976), neural systems have a particular computational goal, executed via algorithms that are instantiated with some physiological mechanism. If you can identify the goal of a system (e.g., line orientation in V1), then you can derive how this goal is achieved through information processing and observe what the physical implementation of this process looks like. In contrast to the informal orthodoxy advanced by Marr, I propose to work backward in this logic. By interpreting event-related fields mechanistically and using experimental demands to evoke a specific algorithmic demand, we can infer the computational goal of the generative system.
2.3 |. Mechanism
Event-related local field oscillations are closely linked to any neuronal mechanism that implements neural computations (Bastos et al., 2012; Friston, 2005; Siegel et al., 2012; Womelsdorf, Valiante, Sahin, Miller, & Tiesinga, 2014). Any definition of the necessary unit of neural computation often becomes a question of scale: Is it the dendritic field, cortical microcircuit, cortical column, or network assembly? Regardless of one’s preference for an answer, local fields are present at all these levels. As a consequence of summed postsynaptic potentials and membrane voltage change, local fields can create temporal windows for network formation. Whether this quality at any scale is facilitating (necessary for the computation) or epiphenomenal (unnecessary for the computation) is often debated. Until these questions are resolved, the most conservative interpretation—epiphenomena—still offers a closer measure of neural coding than any other measure of human imaging (e.g., blood flow) and may be interpreted as a marker of mechanistic processes.
The idea of a field-based marker or a “spectral finger print” from canonical circuits has been advanced within systems neuroscience, but this approach is rarely discussed at the level of scalp recording. Groups of neurons (circuits) can implement a generic computational function (algorithms) that is reflected by a frequency-specific fingerprint (motif; Siegel et al., 2012; Womelsdorf & Everling, 2015; Womelsdorf et al., 2014). There is good reason to think that stereotyped circuit motif fingerprints and the computations therein scale up to systems observable on the scalp (Bastos et al., 2012; Buzsáki et al., 2012; Cohen, 2014b; Womelsdorf et al., 2014). Event-related EEG responses are stereotyped patterns evolving from specific, low-level, sensory responses toward generic, high-level, modality-independent phenomena. These responses appear to represent overlapping circuit motifs that facilitate canonical neural computations (Fries, 2009; Siegel et al., 2012).
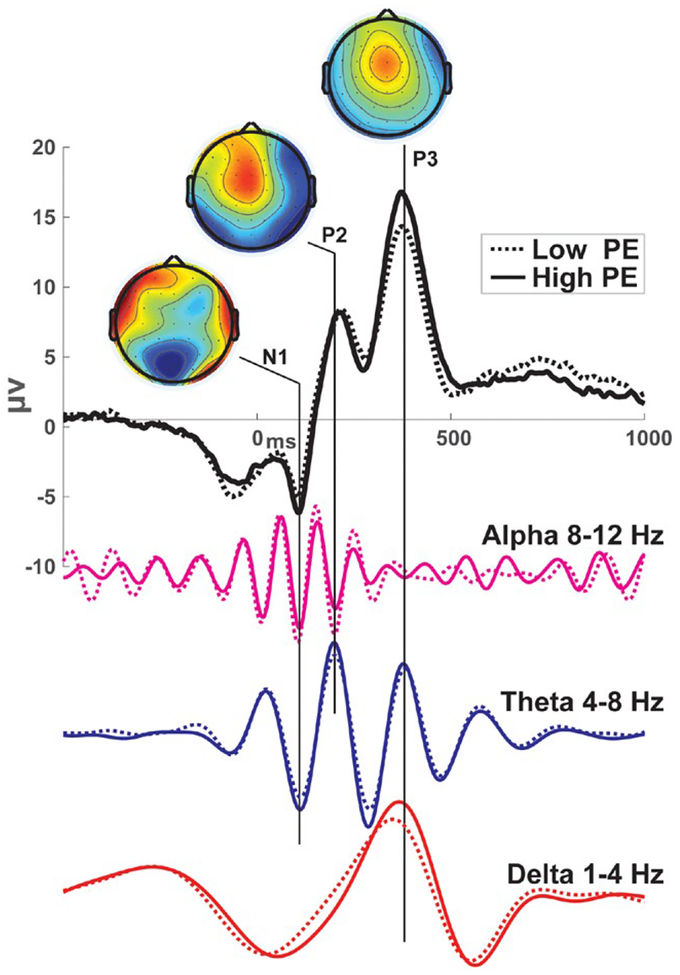
Basar (1998a) theorized that ERPs are a compound superposition of temporally coupled damped oscillations from different neural systems or, to rephrase, different phase- and frequency-specific phase patterns (i.e., motifs). He described these processes as self-sustained attractor states with predictable decay, reflecting a transition of unrelated brain processes into an ordered state. The superposition of activities evolving over time begins with faster frequency events reflecting low-information sensory reactions, culminating into slower frequency events that integrate increasingly varied cognitive interpretations. We observe the summation of the resonant parallel systems in the ERP (see Gruber, Klimesch, Sauseng, & Doppelmayr, 2005) and in component variations that often reflect one-quarter or one-half cycle alterations of existing frequency-specific phase patterns (see Figure 1). If we could understand the information content of these underlying motifs, we may achieve a physiology-level understanding of the signal with our interpretation reflecting the system operations and not the experimenter’s choice of interpretative terminology.
FIGURE 1.
Example ERPs and constituent frequency motifs. These ERPs were taken from the FCz electrode following punishing feedback in a reinforcement learning task. Data were filtered in different frequency bands (arbitrarily scaled) to demonstrate the compound superposition of multiple temporally evolving motifs underlying the ERP. While alpha and theta troughs combine at the N1, the P2 and N2 are largely theta-specific with global modulation by low-frequency activity. The P3 is a culmination of both delta and theta peaks. Surprise was quantified as the absolute value of the negative reward prediction error (PE). Higher information content (high PE) is associated with theta band power increase and a steeper delta band slope culminating in a higher peak. Only phase-locked responses are detailed here, providing a partial snapshot of EEG activities to this event. Data and scripts to recreate this figure can be downloaded from www.predictsite.com Accession #d006
2.4 |. Algorithm
The information content of many neural computations can be algorithmically represented as surprise signals within select areas of the neural hierarchy (Friston, 2010; Marr, 1970). Friston (2003, 2005, 2010) has shown how event-related EEG is specifically sensitive to the temporal and hierarchical evolution of surprise, which is the same basic information quantity that underlies abstract models like reinforcement learning (Sutton & Barto, 1998). This may be a disappointing outcome for asking what an event-related EEG signal means, since the answer is almost always “surprise.”2 But, surprise is a common neurocomputational currency that has specific representational content depending on the generative neural system (den Ouden, Kok, & de Lange, 2012) and temporal order (Kolossa, Kopp, & Fingscheidt, 2015). In other words, even if algorithm and implementation are common, different spatiotemporal-frequency aspects of a signal can reflect different computational goals in a temporally evolving network.
As an example of motifs underlying a temporally evolving network, consider how feedforward and feedback surprise predictions may be mechanistically communicated with gamma versus beta/alpha cycles (Antzoulatos & Miller, 2016; Arnal & Giraud, 2012; Michalareas et al., 2016). Slower oscillations such as theta may be long-term readers of fast activities (Buzsáki, 2010; Jensen & Colgin, 2007; Womelsdorf, Vinck, Leung, & Everling, 2010), and later low-frequency bursts may be low-entropy attractor states of integrated processes (Womelsdorf & Everling, 2015). Together, these examples provide a mechanistic explanation of the observation that fast activities underlie earlier sensory processes and slower activities underlie later cognitive and decision processes (Basar, 1998b).
Joint understanding of algorithm and implementation afford an understanding of the computational goal of a system—one that is based on the point of view of the system and not the experimenter’s terminology. In Section 3 below, I detail how, using electrophysiology as a hub between disciplines, we can address interpretative dilemmas by identifying distinct computational goals, testing circuit mechanisms in animal models, and ultimately translate novel findings toward direct clinical utility.
3 |. ELECTROPHYSIOLOGY AS A HUB FOR THE NEURAL SCIENCES
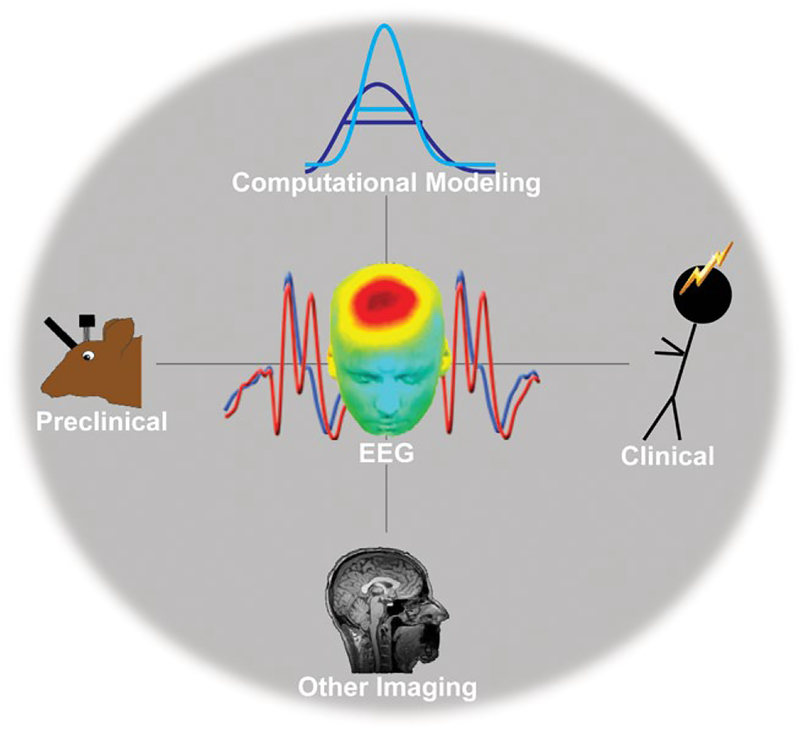
Advanced inference will require convergent evidence across many subfields of neuroscience. Fortunately, electrophysiology is uniquely positioned at the crossroads of many of these subfields. Figure 2 details this vision for electrophysiology as a common hub for methodological integration and theoretical advancement across preclinical, computational, other imaging, and clinical fields. Event-related frontal midline theta band activities are used as a common illustrative thread between each major point below.3
FIGURE 2.
Electrophysiology as a theoretical and methodological hub. Electrophysiological responses are particularly amenable to interpretation with computational perspectives (Section 3.1). Since they can be recorded in both animals and humans, it offers strong translational potential (Section 3.2). The current infrastructure of clinical neurophysiology in hospitals suggests that viable biomarkers could be directly applied to clinical practice (Section 3.3)
3.1 |. Computational goals
Cognitive process models, neural network models, and simulations have all led to better understanding of the nature of event-related EEG. Cognitive process models offer a way to objectively define a specific algorithmic feature that may be expected to be reflected within neural circuits. As an example of the evolution of definitions, consider the P3b (also called P300). Theoretical accounts of P3b (Donchin, 1981; Johnson, 1986; Nieuwenhuis, Aston-Jones, & Cohen, 2005; Polich, 2007) suggest that it reflects the meaning- and surprise- driven updating of cognitive schema. However, descriptors in these influential reports vary among investigator-defined, experiment-specific psychological terms like context, strategy, meaning, attention, and working memory. A recent objective, system-defined, quantitative interpretation of P3b suggests that it represents the updating of predictive surprise during Bayesian estimation (Kolossa et al., 2015), which could be considered a quantitative definition of context updating. Admittedly, predictive surprise summarizes the total update process, and it does not dissociate the influence of meaning from subjective probability, but this is an empirically testable goal. Predictive surprise can also be defined as the evolution of evidence during a decision process (Kolossa et al., 2015), also known as the drift rate in drift diffusion modeling (Ratcliff, 1978). The drift rate corresponds very closely to the slope of the P3b (O’Connell, Dockree, & Kelly, 2012; Philiastides, Heekeren, & Sajda, 2014; Twomey, Murphy, Kelly, & O’Connell, 2015). This drift rate-based modeling of P3b slope was directly motivated by highly influential work from nonhuman primates detailing how neurons can integrate firing rates from lower-level visual stream areas in order to build evidence toward a decision (Gold & Shadlen, 2007). This physiological and computational interpretation of P3b thus allows translation across multiple fields of the neural sciences (for a recent review, see O’Connell, Shadlen, Wong-Lin, & Kelly, 2018). While these studies focus on the generative slope of the P3b, the oft-overlooked downslope may reflect the decay of this attractor state (Basar, 1998b) and may simply be a physiological consequence of facilitating the algorithmic process of decision formation.
Cognitive process models also offer a way to objectively define how a signal does not reflect a specific type of information content. Reinforcement learning defines a special form of surprise as a reward prediction error that includes a valence term to motivate behavior (after reward) or inhibit it (after punishment). Like the neural responses shown in Figure 1, frontal midline theta band power is sensitive to negative reward prediction error (surprising punishments: Cavanagh, Frank, Klein, & Allen, 2010). Yet, neither frontal theta nor the N2 are specific to negative prediction error, as they are both elicited by a variety of surprising perceptual or decision events (Folstein & Van Petten, 2008; Holroyd, 2002; Wessel, Danielmeier, Morton, & Ullsperger, 2012). Most importantly, frontal theta and N2 can be enhanced by highly surprising rewards, particularly if they inform strategic adjustments (Cavanagh, Figueroa, Cohen, & Frank, 2012; Oliveira, McDonald, & Goodman, 2007; Talmi, Atkinson, & El-Deredy, 2013). This finding demonstrates that this neural response is sensitive to other forms of surprise besides negative prediction error. In sum, while frontal theta reflects information content that can be highly similar to negative prediction error, it does not faithfully represent this construct. Since phasic pauses in midbrain dopamine underlie formal negative prediction errors and cortical responses do not reflect this information quantity, the theory that these systems work in tandem can be falsified (Holroyd & Coles, 2002). We have suggested that the computational goal of this generative system is thus not specific to learning and is instead a more generic orienting system involved in signaling the need for cognitive control (Cavanagh & Frank, 2014). A generic orienting response can then invoke adaptive control processes (see Wessel, 2018), possibly due to theta band phase-based coupling between brain regions (Cavanagh, Cohen, & Allen, 2009; see Figure 3a). It is fair to call out the irony within this definition, since cognitive control is a psychological term. However, computational models have a solution for that, too.
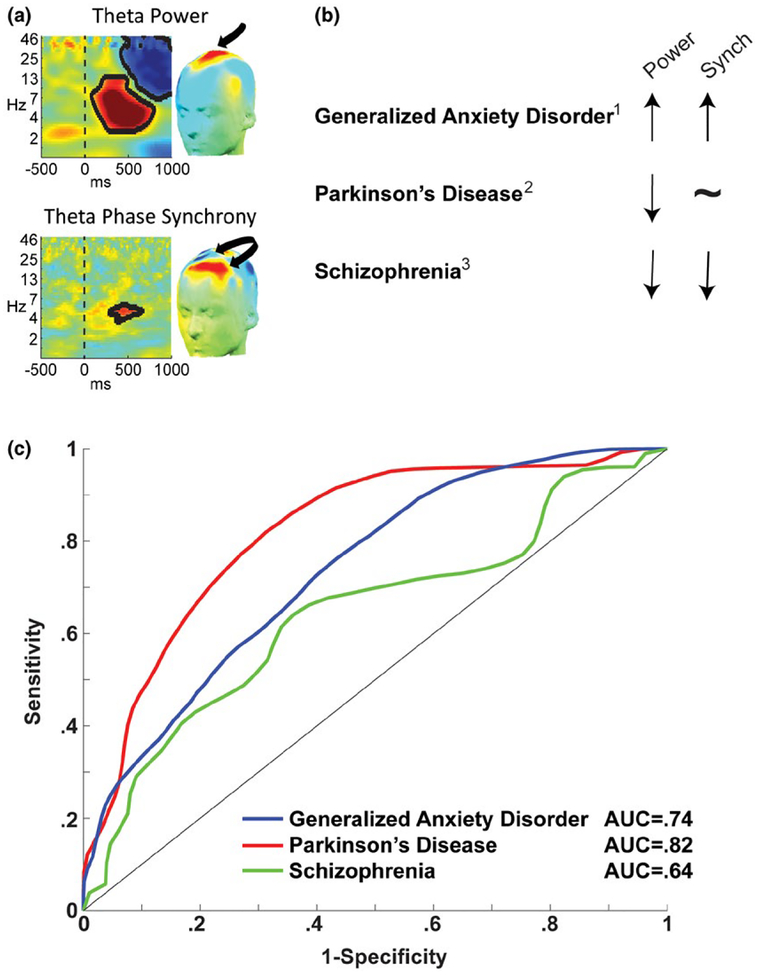
FIGURE 3.
Frontal theta, a candidate mechanism for cognitive control, is not uniformly affected by disease states. (a) Frontal midline theta power is enhanced during the need for control, whereas mediolateral theta phase synchrony is proposed to reflect the communication of this need to lateral frontal areas (Cavanagh & Frank, 2014). (b) Different psychiatric and neurological diseases characterized by frontal dysfunction have different patterns of theta band dynamics; 1Cavanagh, Meyer, et al. (2017); 2Singh, Pirio Richardson, Narayanan, and Cavanagh (2018); 3Ryman et al. (2018). (c) Using features identified in the studies in (b), receiver operating characteristic (isosensitivity) plots can be created to determine the classification success against a control group. This proof-of-principle plot motivates two caveats: First, discriminability can scale with the number of unique predictors. Whereas the paper on Parkinson’s disease identified five unique predictors, generalized anxiety disorder had only three, and schizophrenia had one. Second, sample sizes in the dozens are not adequate for generalizable discrimination; only through collective action and data sharing are we likely to integrate the best set of predictors in large enough data sets to ensure reliable biomarkers. Data and scripts to recreate panel (c) can be downloaded from www.predictsite.com Accession #t003. AUC = area under the curve
Neural network models have been very influential for defining the information content and computational role of frontal control signals (e.g., error-related negativity [ERN], N2, etc.), as well as generating testable hypotheses (Holroyd & Coles, 2002; Yeung, Botvinick, & Cohen, 2004). Network models allow definitions of high-level latent psychological constructs, such as defining “conflict” as Hopfield energy between competing units (Hopfield, 1982) and defining “control” as synchronous activity between detector, goal, and effector representations (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Yeung et al., 2004). More recent models have focused on biological plausibility by demonstrating how frontal theta oscillations can emerge from reentrant projections and facilitate phase-amplitude gamma coupling during events indicating the need for control (Gratton, 2018; Verguts, 2017). Theory and data can be contrasted with simulations of different event-related phase- and power-based perturbations, allowing empirical comparisons of controversial issues like oscillatory phase reset and potential influence of filter ringing in the ERN (Trujillo & Allen, 2007; Yeung, Bogacz, Holroyd, Nieuwenhuis, & Cohen, 2007). Together, these cognitive process, network, and simulation models suggest that frontal theta is a well-characterized candidate mechanism underlying the ability to realize and communicate the need for cognitive control.
3.2 |. Translation
Neural mechanisms are often addressed at molecular and cellular levels, but the extension to complex behavior may be best suited by a neural circuit-level perspective. Electrophysiology is a promising tool for this extended vision, as it is a viable method for animal-to-human translation of complex behaviors. There is good reason to theorize that some scales of electrophysiological activities remain preserved across species. There is a marked preservation of temporal scales across vertebrate brains, likely due to architectural adjustments that evolved to prioritize the retention of temporal coding schemes (Buzsáki, Logothetis, & Singer, 2013).
A direct correspondence between a signal in humans and a similar spatiotemporal frequency signal in another species would provide ideal evidence of preserved neural processes. Nonhuman primates have similar error, conflict, and feedback field responses at the scalp (Godlove et al., 2011), dura (Vezoli & Procyk, 2009), and within cingulate cortex (Emeric, Leslie, Pouget, & Schall, 2010), although the spectral representation of these signals has not been defined. Rats have a frontal midline theta-dominant control network that is transiently instantiated following an imperative tone, affording a chance to causally manipulate this network and draw parallel conclusions to humans (Narayanan, Cavanagh, Frank, & Laubach, 2013). This common theta-band electrophysiological response is diminished in Parkinson’s patients as well as in a dopamine depletion rodent model (Parker, Chen, Kingyon, Cavanagh, & Narayanan, 2015), allowing a novel model of cognitive dysfunction in Parkinsonism.
In the case of an indirect correspondence between a signal in humans and a different spatiotemporal frequency signal in rat, a computational approach affords a chance for translational logic. Some neural processes may be comparable in their computational goal even if they differ in spectral fingerprint; regression with model parameters like reward prediction error or drift rate offer a way to objectively define such coding. Sometimes, concerns about correspondence between species can be ignored, as there is a lot to be learned in preclinical studies that are necessary to inform human-level inferences. Computationally informed theories about layer-specific circuit motifs contributing to human scalp activity (Cohen, 2014b) may require animal models for testing. A better understanding of the role of interneurons in oscillatory sculpting (see Markram et al., 2004) would be very helpful, particularly in creating different frequency bands (Chen et al., 2017; Dumitriu, Cossart, Huang, & Yuste, 2007), differentiating computational motifs (Womelsdorf et al., 2014), or enabling long-distance synchrony (Medalla & Barbas, 2009, 2010).
3.3 |. Clinical biomarkers
As a preserved measure of primitive information processing, event-related EEG activities are promising biomarkers of dysfunction in psychiatric and neurological disease. Frontal theta is reliably altered in groups with frontal distress, but this alteration is not isomorphic across disease states. Figure 3b shows varied patterns of frontal midline theta power and mediolateral phase synchrony in different patient groups (compared to matched controls). These findings suggest different deficits in the realization versus the signaling of the need for control, which can generate novel hypotheses. For example, current work in my lab is investigating the influence of disturbed white matter tracts on mediolateral phase synchrony following acute traumatic brain injury.
A disease biomarker requires sensitive and specific discrimination to an out-of-sample participant (Atkinson et al., 2001), which can be assessed through pattern classification techniques. Figure 3c shows a proof-of-principle plot of the discrimination of patients from controls using frontal theta features. These findings are presented as only a preliminary example of this broader idea, since two major methodological issues need to be resolved before the discriminability and generalizability of these measures can be assessed. First, each data set included only a small number of discriminating features (each detailed in the relevant publication), which limits discriminability but enhances generalizability. The opposite approach can also be tried—a very large amount of data followed by iterative reduction to identify maximally discriminating features (see Cavanagh et al., 2018). Classification success will ultimately depend on identification of the ideal balance between number and type of neural feature. Second, each of these studies shown in Figure 3c included modest numbers of participants. Unfortunately, the scale of data required for robust and reliable identification of a clinical biomarker transcends the ability of any single laboratory. To surmount these logistical hurdles hindering this clinical goal, we have created the open-source Patient Repository for EEG Data + Computational Tools (PRED+CT: www.predictsite.com; Cavanagh, Napolitano, Wu, & Mueen, 2017). This site is a fully open resource where researchers can test novel approaches to feature selection and pattern classification on real patient data. It is hoped that this online data repository will encourage data sharing for the purpose of large-scale testing of candidate biomarkers. There are good reasons to think that these advancements in EEG techniques can directly improve clinical practice and clinical trial outcome, as described below.
4 |. CLINICAL PRACTICE
EEG has been used as a biomedical diagnostic tool for nearly a century. Clinical applications followed shortly after the widespread communication of scalp EEG in humans in 1929, largely due to the utility for directly observing epileptiform activity. Current practice of clinical EEG has changed very little since then.
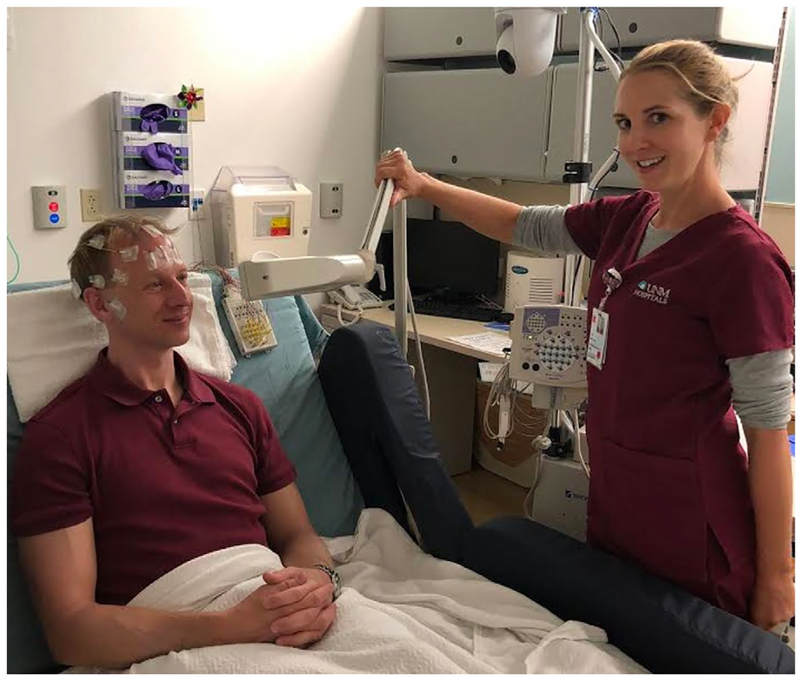
Clinical EEG assessment includes regular outpatient procedures (<2 hr) where a patient is assessed by a 21-channel array of scalp electrodes and undergoes rest and evocation procedures like photic stimulation and hyperventilation (Sinha et al., 2016); see Figure 4. In other words, patients complete tasks while their EEG is recorded in order to inform their treatment. The clinical bedside is closer to the lab bench than many researchers realize.
FIGURE 4.
Current practice of clinical EEG for outpatient procedures. EEG is routinely assessed in clinical inpatient, outpatient, and ambulatory procedures. While it is largely utilized for epilepsy and seizure diagnosis, many different patient groups are assessed (largely to rule out epilepsy). Outpatient procedures utilize an array of 21 scalp electrodes, and the EEG technologist assesses resting activity, photic stimulation, hyperventilation, and light sleep in less than 2 hr. The photic stimulation arm is shown here. Video recording is commonly embedded with the signal (white sphere with black circle at top). These features demonstrate that the infrastructure already exists to capitalize upon any novel task based assessment of an EEG feature that contributes to diagnosis or prognosis for neurological or psychiatric disease
Outpatient procedures are used for seizure detection and medication management, but also as a first-line assessment of patients experiencing a change in mental status (sleep problems, headaches, syncope, confusion, etc.). Although this procedure is largely completed in order to rule out seizures, the breadth of patients who may receive a task-based clinical EEG is wide and includes incidental assessment of many disorders such as stroke, attention-deficit hyperactivity disorder, or dementia. It doesn’t take a lot of imagination to consider that a task added to this short battery could help rule in criteria for these or other diseases. The base rate for any specific neurological or psychiatric disease is low enough to dismiss the plausibility of a new EEG-based diagnostic test with global sensitivity and specificity. Yet, if a patient is already being treated for a disease, as is common in current clinical practice, then this obviates some base rate problems.
4.1 |. Mechanisms to biomarkers
The best chance for success may depend on the ability to identify the right task to probe the aberrant mechanism underlying a specific disorder, particularly with a task that is clinically translatable. While a biomarker does not need to be based on a mechanism, this basis helps to reduce the uncertainty of the role a biomarker plays in mediating a treatment effect on a clinical end point (Atkinson et al., 2001; Fleming & DeMets, 1996). Both biomarker and mechanism differ from an endophenotype, which describes an intermediate expression of genetic risk, usually in psychiatric populations (Iacono, 2014; Insel & Cuthbert, 2009). Fortuitously, frontal midline theta and related ERP features appear to be viable neural mechanisms (Section 3.1), endophenotypes (Kang et al., 2012; Zlojutro et al., 2011), diagnostic tools (Figure 3c), and predictors of treatment response (Burkhouse et al., 2016; Gorka et al., 2018) for patient groups defined by frontal dysfunction. These signatures can be up- and downregulated with pharmacology (Jocham & Ullsperger, 2009) and transcranical electric current (Reinhart & Woodman, 2014; Reinhart, Zhu, Park, & Woodman, 2015), demonstrating causal control over this signal and downstream effects on network instantiation and behavior in humans.
Frontal midline theta can be elicited in simple tasks that are viable within a clinical environment. Aberrant auditory orienting responses have already been advanced as candidate biomarkers, like diminished mismatch negativity (MMN) in schizophrenia (Javitt, Lee, Kantrowitz, & Martinez, 2018; Light et al., 2015) or diminished novelty habituation in Parkinson’s disease (Cavanagh et al., 2018). The MMN is a theta-dominant response with separable frontal and temporal processes (Fuentemilla, Marco-Pallarés, Münte, & Grau, 2008; Ko et al., 2012) that may interact with theta-band phase synchrony (Choi et al., 2013). The neural systems underlying auditory novelty detection are well detailed in rodent models (Escera & Malmierca, 2014; Featherstone, Melnychenko, & Siegel, 2018; Lee et al., 2018), facilitating further cross-species translation. Auditory evoked responses are already routinely used in brainstem auditory testing for hearing acuity in newborns, again demonstrating that clinical infrastructure and expertise exists for applying relevant tasks to patient groups when using EEG as a diagnostic tool.
I submit that if there is even one disease state not in the current repertoire of clinical EEG that may be sensitively and specifically identified with a novel EEG signal, then it is worth tremendous effort to weed through a multitude of candidates in order to advance a single success. Advancement to clinical practice is a worthwhile but lofty goal. In the nearer term, a candidate EEG-based biomarker may be initially used as a surrogate end point in Phase I and Phase II clinical trials, where it could be rapidly applied and efficiently used to screen the impact of novel treatments (Fleming & DeMets, 1996; Strimbu & Tavel, 2010).
4.2 |. Conclusion
The identity thesis suggests that, if the brain underlies the mind, then for every psychological feature there must be a corresponding neural feature (Cacioppo & Tassinary, 1990). Cognitive neuroscience relies on the tacit assumption that some of these relationships are quantifiable and that they are reliable and valid. Electrophysiology is a uniquely sensitive quantification of neural mechanisms and computations, thus it is a uniquely powerful way of addressing the identity thesis. We can gain significant traction on these questions by using electrophysiology as a theoretical and methodological hub for inference, combining strengths across other imaging, computational, and translational fields.
I hope to see the use of electrophysiology as a lingua franca to translate from animal models through large-scale human validation studies. This approach may begin with parallel testing of human and animal models of cognitive processes using electrophysiology as the outcome marker. Computational models will be used for interpretation of the information content of these outcome markers. The animal model will facilitate causal testing of the mechanism reflected by this signal. Multisite studies in human patients would follow to define the sensitivity and specificity of these markers, particularly in comparison or conjunction with other imaging markers. Algorithmic performance and generalizability will be boosted by having community input on openly available data sets. Successful biomarkers would then be directly implemented into clinical trials and clinical practice through existing hospitals and neurology clinics, capitalizing on existing infrastructure for a rapid and low-cost advancement in the diagnosis, prognosis, and treatment of mental health.
ACKNOWLEDGMENTS
The author thanks his formal and informal mentors John J. B. Allen, Michael Frank, and Mike X. Cohen, as well as Kumar Narayanan and Jonathan Brigman for shared enthusiasm of translational studies, Kate Cavanagh for insight into clinical neurology, and Jan Wessel and Jeremy Hogeveen for a review of this manuscript.
Funding information
National Institute of General Medical Sciences, Grant/Award Number: 1P20GM109089–01A1; National Institute of Mental Health, Grant/Award Number: 1UH2MH109168–01
Footnotes
These sources would be too numerous to cite individually, so the reader is referred to chapter 1 of Luck (2005).
Motor system responses may be a minor exception to this idea.
The use of frontal midline theta as an example throughout is primarily motivated by the author’s familiarity with this measure. This is hopefully only one of many suitable signals that can address these topics.
REFERENCES
- Antzoulatos EG, & Miller EK (2016). Synchronous beta rhythms of frontoparietal networks support only behaviorally relevant representations. eLife, 5(Nov), 1–22. 10.7554/eLife.17822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal LH, & Giraud A-L (2012). Cortical oscillations and sensory predictions. Trends in Cognitive Sciences, 16(7), 390–398. 10.1016/j.tics.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, … Zeger SL (2001). Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clinical Pharmacology and Therapeutics, 69(3), 89–95. 10.1067/mcp.2001.113989 [DOI] [PubMed] [Google Scholar]
- Basar E (1998a). Brain function and oscillations (Vol. I). Heidelberg, Germany: Springer; 10.1007/978-3-642-72192-2 [DOI] [Google Scholar]
- Basar E (1998b). Brain function and oscillations (Vol. II). Heidelberg, Germany: Springer; 10.1007/978-3-642-59893-7 [DOI] [Google Scholar]
- Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, & Friston KJ (2012). Canonical microcircuits for predictive coding. Neuron, 76(4), 695–711. 10.1016/j.neuron.2012.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&listuids=11488380 [DOI] [PubMed] [Google Scholar]
- Burkhouse KL, Kujawa A, Kennedy AE, Shankman SA, Langenecker SA, Phan KL, & Klumpp H (2016). Neural reactivity to reward as a predictor of cognitive behavioral therapy response in anxiety and depression. Depression and Anxiety, 33(4), 281–288. 10.1002/da.22482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G (2010). Neural syntax: Cell assemblies, synapsembles, and readers. Neuron, 68(3), 362–385. 10.1016/j.neuron.2010.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Anastassiou CA, & Koch C (2012). The origin of extracellular fields and currents—EEG, ECoG, LFP and Spikes. Nature Reviews Neuroscience, 13(6), 407–420. 10.1038/nrn3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Logothetis N, & Singer W (2013). Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms. Neuron, 80(3), 751–764. 10.1016/j.neuron.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, & Tassinary LG (1990). Inferring psychological significance from physiological signals. American Psychologist, 45(1), 16–28. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2297166 [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, & Allen JJB (2009). Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. Journal of Neuroscience, 29(1), 98–105. 10.1523/JNEUR0SCI.4137-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Figueroa CM, Cohen MX, & Frank MJ (2012). Frontal theta reflects uncertainty and unexpectedness during exploration and exploitation. Cerebral Cortex, 22(11), 2575–2586. 10.1093/cercor/bhr332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, & Frank MJ (2014). Frontal theta as a mechanism for cognitive control. Trends in Cognitive Sciences, 18(8), 1–8. 10.1016/j.tics.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, & Allen JJB (2010). Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage, 49(4), 3198–3209. 10.1016/j.neuroimage.2009.11.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Kumar P, Mueller AA, Pirio Richardson S, Mueen A, & Richardson SP (2018). Diminished EEG habituation to novel events effectively classifies Parkinson’s patients. Clinical Neurophysiology, 129(2), 10.1016/j.clinph.2017.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Meyer A, Hajcak G, Schroder HS, Larson MJ, Jonides J, & Smith EE (2017). Error-specific cognitive control alterations in generalized anxiety disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 53, 21–29. 10.1016/j.bpsc.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Napolitano A, Wu C, & Mueen A (2017). The Patient Repository for EEG Data + Computational Tools (PRED+CT). Frontiers in Neuroinformatics, 11 10.3389/fninf.2017.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zhang Y, Li X, Zhao X, Ye Q, Lin Y, … Zhang X(2017). Distinct inhibitory circuits orchestrate cortical beta and gamma band oscillations. Neuron, 96(6), 1403–1418.e6. 10.1016/j.neuron.2017.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JW, Lee JK, Ko D, Lee GT, Jung KY, & Kim KH (2013). Fronto-temporal interactions in the theta-band during auditory deviant processing. Neuroscience Letters, 548, 120–125. 10.1016/j.neulet.2013.05.079 [DOI] [PubMed] [Google Scholar]
- Cohen MX (2011). It’s about time. Frontiers in Human Neuroscience, 5, 2 10.3389/fnhum.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX (2014a). Analyzing neural time series data: Theory and practice. Cambridge, MA: MIT Press. [Google Scholar]
- Cohen MX (2014b). A neural microcircuit for cognitive conflict detection and signaling. Trends in Neurosciences, 37(9), 480–490. 10.1016/j.tins.2014.06.004 [DOI] [PubMed] [Google Scholar]
- Cohen MX (2017). Where does EEG come from and what does it mean? Trends in Neurosciences, 40(4), 208–218. 10.1016/j.tins.2017.02.004 [DOI] [PubMed] [Google Scholar]
- den Ouden HEM, Kok P, & de Lange FP (2012). How prediction errors shape perception, attention, and motivation. Frontiers in Psychology, 3(Dec), 548 10.3389/fpsyg.2012.00548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E (1981). Surprise! … Surprise? Psychophysiology, 18(5), 493–513. 10.1111/j.1469-8986.1981.tb01815.x [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Cossart R, Huang J, & Yuste R (2007). Correlation between axonal morphologies and synaptic input kinetics of interneurons from mouse visual cortex. Cerebral Cortex, 17(1), 81–91. 10.1093/cercor/bhj126 [DOI] [PubMed] [Google Scholar]
- Emeric EE, Leslie MW, Pouget P, & Schall JD (2010). Performance monitoring local field potentials in the medial frontal cortex of primates: Supplementary eye field. Journal of Neurophysiology, 104, 1523–1537. 10.1152/jn.01001.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escera C, & Malmierca MS (2014). The auditory novelty system: An attempt to integrate human and animal research. Psychophysiology, 51(2), 111–123. 10.1111/psyp.12156 [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Melnychenko O, & Siegel SJ (2018). Mismatch negativity in preclinical models of schizophrenia. Schizophrenia Research, 191, 35–42. https://doi.org/10.1016Zj.schres.2017.07.039 [DOI] [PubMed] [Google Scholar]
- Fleming TR, & DeMets DL (1996). Surrogate end points in clinical trials: Are we being misled? Annals o f Internal Medicine, 125(7), 605–613. 10.7326/0003-4819-125-7-199610010-00011 [DOI] [PubMed] [Google Scholar]
- Folstein JR, & Van Petten C (2008). Influence of cognitive control and mismatch on the N2 component oftheERP:A review. Psychophysiology, 45(1), 152–170. 10.1111/j.1469-8986.2007.00602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P (2005). A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends in Cognitive Science, 9(10), 474–480. 10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Fries P (2009). Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annual Review o f Neuroscience, 32, 209–224. 10.1146/annurev.neuro.051508.135603 [DOI] [PubMed] [Google Scholar]
- Friston K (2003). Learning and inference in the brain. Neural Networks, 16(9), 1325–1352. 10.1016/j.neunet.2003.06.005 [DOI] [PubMed] [Google Scholar]
- Friston K (2005). A theory of cortical responses. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 360(1456), 815–836. 10.1098/rstb.2005.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (2010). The free-energy principle: A unified brain theory? Nature Reviews Neuroscience, 11(2), 127–138. 10.1038/nrn2787 [DOI] [PubMed] [Google Scholar]
- Fuentemilla L, Marco-Pallarés J, Münte TF, & Grau C (2008). Theta EEG oscillatory activity and auditory change detection. Brain Research, 1220, 93–101. 10.1016/j.brainres.2007.07.079 [DOI] [PubMed] [Google Scholar]
- Godlove DC, Emeric EE, Segovis CM, Young MS, Schall JD, & Woodman GF (2011). Event-related potentials elicited by errors during the stop-signal task. I. Macaque Monkeys. Journal o f Neuroscience, 31(44), 15640–15649. 10.1523/JNEUR0SCI.3349-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, & Shadlen MN (2007). The neural basis of decision making. Annual Review of Neuroscience, 30, 535–574. 10.1146/annurev.neuro.29.051605.113038 [DOI] [PubMed] [Google Scholar]
- Gorka SM, Burkhouse KL, Klumpp H, Kennedy AE, Afshar K, Francis J, … Phan KL (2018). Error-related brain activity as a treatment moderator and index of symptom change during cognitive-behavioral therapy or selective serotonin reuptake inhibitors. Neuropsychopharmacology, 43(6), 1355–1363. 10.1038/npp.2017.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G (2018). Brain reflections: A circuit-based framework for understanding information processing and cognitive control. Psychophysiology, 55(3), 1–26. 10.1111/psyp.13038 [DOI] [PubMed] [Google Scholar]
- Gruber WR, Klimesch W, Sauseng P, & Doppelmayr M (2005). Alpha phase synchronization predicts P1 and N1 latency and amplitude size. Cerebral Cortex, 15(4), 371–377. 10.1093/cercor/bhh139 [DOI] [PubMed] [Google Scholar]
- Hämmerer D, Li S-C, Völkle M, Müller V, & Lindenberger U (2013). A lifespan comparison of the reliability, test-retest stability, and signal-to-noise ratio of event-related potentials assessed during performance monitoring. Psychophysiology, 50, 111–123. 10.1111/j.1469-8986.2012.01476.x [DOI] [PubMed] [Google Scholar]
- Holroyd CB (2002). A note on the oddball N200 and the feedback ERN In Ullsperger IM & Falkenstein M (Eds.), Errors, conflicts and the brain. Current opinions on performance monitoring (pp. 211–218). Dortmund, Germany: MPI of Cognitive Nueroscience. [Google Scholar]
- Holroyd CB, & Coles MG (2002). The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&d-b=PubMed&dopt=Citation&list_uids=12374324 [DOI] [PubMed] [Google Scholar]
- Hopfield JJ (1982). Neural networks and physical systems with emergent collective computational abilities. Proceedings o f the National Academy o f Sciences United States o f America, 79(8), 2554–2558. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cm-d=Retrieve&db=PubMed&dopt=Citation&list_uids=6953413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG (2014). Genome-wide scans of genetic variants for psychophysiological endophenotypes: Introduction to this special issue of Psychophysiology. Psychophysiology, 51, 1201–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, & Cuthbert BN (2009). Endophenotypes: Bridging genomic complexity and disorder heterogeneity. Biological Psychiatry, 66(11), 988–989. 10.1016/j.biopsych.2009.10.008 [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal o f Psychiatry, 167(7), 748–751. 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Javitt DC, Lee M, Kantrowitz JT, & Martinez A (2018). Mismatch negativity as a biomarker of theta band oscillatory dysfunction in schizophrenia. Schizophrenia Research, 191, 51–60. 10.1016/j.schres.2017.06.023 [DOI] [PubMed] [Google Scholar]
- Jensen O, & Colgin LL (2007). Cross-frequency coupling between neuronal oscillations. Trends in Cognitive Sciences, 11(7), 267–269. https://doi.org/10.1016Zj.tics.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Jocham G, & Ullsperger M (2009). Neuropharmacology of performance monitoring. Neuroscience Biobehavioral Review, 33(1), 48–60. 10.1016/j.neubiorev.2008.08.011 [DOI] [PubMed] [Google Scholar]
- Johnson R (1986). A triarchic model ofP300amplitude.Psychophysiology, 23(4), 367–384. 10.1111/j.1469-8986.1986.tb00649.x [DOI] [PubMed] [Google Scholar]
- Kang SJ, Rangaswamy M, Manz N, Wang JC, Wetherill L, Hinrichs T, … Porjesz B (2012). Family-based genome-wide association study of frontal theta oscillations identifies potassium channel gene KCNJ6. Genes, Brain and Behavior, 11(6), 712–719. 10.1111/j.1601-183X.2012.00803.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko D, Kwon S, Lee GT, Im CH, Kim KH, & Jung KY (2012). Theta oscillation related to the auditory discrimination process in mismatch negativity: Oddball versus control paradigm.Journal o f Clinical Neurology, 5(1), 35–42. 10.3988/jcn.2012.8.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolossa A, Kopp B, & Fingscheidt T (2015). A computational analysis of the neural bases of Bayesian inference. Neuroimage, 106, 222–237. 10.1016/j.neuroimage.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Lee M, Balla A, Sershen H, Sehatpour P, Lakatos P, & Javitt DC (2018). Rodent mismatch negativity/theta neuro-oscillatory response as a translational neurophysiological biomarker for N-methyl-D-aspartate receptor-based new treatment development in schizophrenia. Neuropsychopharmacology, 43(3), 571–582. 10.1038/npp.2017.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leue A, Klein C, Lange S, & Beauducel A (2013). Inter-individual and intra-individual variability of the N2 component: On reliability and signal-to-noise ratio. Brain and Cognition, 83, 61–71. 10.1016/j.bandc.2013.06.009 [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Thomas ML, Calkins ME, Green MF, Greenwood TA, … Turetsky BI (2015). Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: Characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophrenia Research, 163(1–3), 63–72. 10.1016/j.schres.2014.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S (2005). An introduction to the event-related potential technique (Cognitive neuroscience) (1st ed.). Retrieved from http://www.citeulike.org/group/214/article/472845 [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, & Wu C (2004). Interneurons of the neocortical inhibitory system. Nature Reviews Neuroscience, 5(10), 793–807. 10.1038/nrn1519 [DOI] [PubMed] [Google Scholar]
- Marr D (1970). A theory for cerebral neocortex. Proceedings of the Royal Society o f London B: Biological Sciences, 176(43), 161–234. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=4394740 [DOI] [PubMed] [Google Scholar]
- Marr D, & Poggio T (1976). From understanding computation to understanding neural circuitry. AI Memos, 1959–2004(357), 1–22. [Google Scholar]
- Medalla M, & Barbas H (2009). Synapses with inhibitory neurons differentiate anterior cingulate from dorsolateral prefrontal pathways associated with cognitive control. Neuron, 61(4), 609–620. 10.1016/j.neuron.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, & Barbas H (2010). Anterior cingulate synapses in prefrontal areas 10 and 46 suggest differential influence in cognitive control. Journal of Neuroscience, 30(48), 16068–16081. 10.1523/JNEUROSCI.1773-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Bress JN, & Proudfit GH (2014). Psychometric properties of the error-related negativity in children and adolescents. Psychophysiology, 51(7), 602–610. 10.1111/psyp.12208 [DOI] [PubMed] [Google Scholar]
- Michalareas G, Vezoli J, van Pelt S, Schoffelen JM, Kennedy H, & Fries P (2016). Alpha-beta and gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron, 89(2), 384–397. 10.1016/j.neuron.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Dolan RJ, Friston KJ, & Dayan P (2012). Computational psychiatry. Trends in Cognitive Sciences, 16(1), 72–80. 10.1016/j.tics.2011.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadasdy Z (2010). Binding by asynchrony: The neuronal phase code. Frontiers in Neuroscience, 4(Sept), 1–11. 10.3389/fnins.2010.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Cavanagh JF, Frank MJ, & Laubach M (2013). Common medial frontal mechanisms of adaptive control in humans and rodents. Nature Neuroscience, 16(Oct), 1–10. 10.1038/nn.3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, & Cohen JD (2005). Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological Bulletin, 131(4), 510–532. 10.1037/0033-2909.131.4510 [DOI] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, & Kelly SP (2012). A supramodal accumulation-to-bound signal that determines perceptual decisions in humans. Nature Neuroscience, 15(12), 1729–1735. 10.1038/nn.3248 [DOI] [PubMed] [Google Scholar]
- O’Connell RG, Shadlen MN, Wong-Lin KF, & Kelly SP (2018). Bridging neural and computational viewpoints on perceptual decision-making. Trends in Neurosciences, 1(11), 367–384. 10.1016/j.tins.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira FT, McDonald JJ, & Goodman D (2007). Performance monitoring in the anterior cingulate is not all error related: Expectancy deviation and the representation of action-outcome associations. Journal o f Cognitive Neuroscience, 19(12), 1994–2004. 10.1162/jocn.2007.19.12.1994 [DOI] [PubMed] [Google Scholar]
- Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, & Narayanan NS (2015). Medial frontal ~4-Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. Journal o f Neurophysiology, 114(2), 1310–1320. 10.1152/jn.00412.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philiastides MG, Heekeren HR, & Sajda P (2014). Human scalp potentials reflect a mixture of decision-related signals during perceptual choices. Journal o f Neuroscience, 34(50), 16877–16889. 10.1523/JNEUROSCI.3012-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt JR (1964). Strong inference. Science, 146(3642), 347–353. [DOI] [PubMed] [Google Scholar]
- Polich J (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118(10), 2128–2148. 10.1016/j.clinph.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R (1978). A theory of memory retrieval. Psychological Review, 85(2), 59–108. 10.1037/0033-295X.85.2.59 [DOI] [Google Scholar]
- Reinhart RMG, & Woodman GF (2014). Causal control of medial-frontal cortex governs electrophysiological and behavioral indices of performance monitoring and learning. Journal o f Neuroscience, 34(12), 4214–4227. 10.1523/JNEUROSCI.5421-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RMG, Zhu J, Park S, & Woodman GF (2015). Medial-frontal stimulation enhances learning in schizophrenia by restoring prediction error signaling. Journal of Neuroscience, 35(35), 12232–12240. 10.1523/JNEUROSCI.1717-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Warland D, de Ruyter van Stevenick R, & Bialek W (1999). Spikes: Exploring the neural code. Cambridge, MA: MIT Press [Google Scholar]
- Ryman SG, Cavanagh JF, Wertz CJ, Shaff NA, Dodd AB, Stevens B, … Mayer AR (2018). Impaired midline theta power and connectivity during proactive cognitive control in schizophrenia. Biological Psychiatry, 84(9), 675–683. 10.1016/j.biopsych.2018.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, & Engel AK (2012). Spectral fingerprints of large-scale neuronal interactions. Nature Reviews. Neuroscience, 13(2), 121–134. 10.1038/nrn3137 [DOI] [PubMed] [Google Scholar]
- Singh A, Pirio Richardson S, Narayanan N, & Cavanagh JF (2018). Mid-frontal theta activity is diminished during cognitive control in Parkinson’s disease. Neuropsychologia, 117, 113–122. 10.1016/J.NEUROPSYCHOLOGIA.2018.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha SR, Sullivan L, Sabau D, San-Juan D, Dombrowski KE, Halford JJ, … Stecker MM (2016). American Clinical Neurophysiology Society Guideline 1: Minimum technical requirements for performing clinical electroencephalography. Journal of Clinical Neurophysiology, 33(4), 303–307. 10.1097/WNP.0000000000000308 [DOI] [PubMed] [Google Scholar]
- Strimbu K, & Tavel JA (2010). What are biomarkers? Current Opinion in HIV and AIDS, 5(6), 463–466. 10.1097/COH.0b013e32833ed177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, & Barto AG (1998). Reinforcement learning: An introduction. Adaptive computation and machine learning. Cambridge, MA: MIT Press. [Google Scholar]
- Talmi D, Atkinson R, & El-Deredy W (2013). The feedback-related negativity signals salience prediction errors, not reward prediction errors. Journal o f Neuroscience, 33(19), 8264–8269. 10.1523/JNEUROSCI.5695-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers DN, & Allen JJB (2009). A better estimate of the internal consistency reliability of frontal EEG asymmetry scores. Psychophysiology, 46(1), 132–142. 10.1111/j.1469-8986.2008.00759.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo LT, & Allen JJ (2007). Theta EEG dynamics of the errorrelated negativity. Clinical Neurophysiology, 118(3), 645–668. 10.1016/jxlinph.2006.11.009 [DOI] [PubMed] [Google Scholar]
- Twomey DM, Murphy PR, Kelly SP, & O ‘Connell RG (2015). The classic P300 encodes a build-to-threshold decision variable. European Journal o f Neuroscience, 42(1), 1636–1643. 10.1111/ejn.12936 [DOI] [PubMed] [Google Scholar]
- Verguts T (2017). Binding by random bursts: A computational model of cognitive control. Journal of Cognitive Neuroscience, 29(6), 1103–1118. 10.1162/jocn_a_01117 [DOI] [PubMed] [Google Scholar]
- Vezoli J, & Procyk E (2009). Frontal feedback-related potentials in nonhuman primates: Modulation during learning and under haloperidol. Journal o f Neuroscience, 29(50), 15675–15683. 10.1523/JNEUROSCI.4943-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, & Hajcak G (2011). Longer term test-retest reliability of error-related brain activity. Psychophysiology, 48, 1420–1425. 10.1111/j.1469-8986.2011.01206.x [DOI] [PubMed] [Google Scholar]
- Wessel JR (2018). An adaptive orienting theory of error processing.Psychophysiology, 55(3), 1–21. 10.1111/psyp.13041 [DOI] [PubMed] [Google Scholar]
- Wessel JR, Danielmeier C, Morton JB, & Ullsperger M (2012). Surprise and error: Common neuronal architecture for the processing of errors and novelty. Journal o f Neuroscience, 32(22), 7528–7537. 10.1523/JNEUROSCI.6352-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, & Everling S (2015). Long-range attention networks: Circuit motifs underlying endogenously controlled stimulus selection. Trends in Neurosciences, 38(11), 682–700. 10.1016/j.tins.2015.08.009 [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Valiante TA, Sahin NT, Miller KJ, & Tiesinga P (2014). Dynamic circuit motifs underlying rhythmic gain control, gating and integration. Nature Neuroscience, 17(8), 1031–1039. 10.1038/nn.3764 [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Vinck M, Leung LS, & Everling S (2010). Selective theta-synchronization of choice-relevant information subserves goal-directed behavior. Frontiers in Human Neuroscience, 4, 210 10.3389/fnhum.2010.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Bogacz R, Holroyd CB, Nieuwenhuis S, & Cohen JD (2007). Theta phase resetting and the error-related negativity. Psychophysiology, 44(1), 39–49. 10.1111/j.1469-8986.2006.00482.x [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, & Cohen JD (2004). The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review, 111(4), 931–959. 10.1037/0033-295X.111.4.931 [DOI] [PubMed] [Google Scholar]
- Zlojutro M, Manz N, Rangaswamy M, Xuei X, Flury-Wetherill L, Koller D, … Almasy L (2011). Genome-wide association study of theta band event-related oscillations identifies serotonin receptor gene HTR7 influencing risk of alcohol dependence. American Journal o f Medical Genetics, Part B: Neuropsychiatric Genetics, 156(1), 44–58. 10.1002/ajmg.b.31136 [DOI] [PMC free article] [PubMed] [Google Scholar]