Abstract
Objective
Persistent infection with human papillomavirus (HPV) is recognized as the main risk factor of cervical cancer. Investigation via cytology and colposcopy have lower sensitivity than HPV testing in the diagnosis of high-grade cervical intraepithelial neoplasia (CIN2+). Despite normal cytology and colposcopy findings women with persistent HPV infection have an increased risk of CIN2+. The aim of the study was to evaluate the proportion of histologically confirmed CIN2+ in women with persistent HPV infection and normal Pap smears.
Study design
From April 2013 until March 2016 we prospectively recruited 91 women over 40 years with persistent HPV infection without any abnormalities in cytology. Of these, 40 women attended a gynecological examination including an HPV test, Pap smear, endocervical cytology, colposcopy with biopsies and diagnostic loop electrosurgical excision procedure (LEEP). Biopsy and LEEP samples were subjected to histological examination
Results
CIN2+ was verified by histological examination of the LEEP sample in 6/40 (15%) of the women. All the cytological samples were normal and none of the biopsies confirmed CIN2+. Only 19/40 women still had a persistent HPV infection at the study visit. None of the 21/40 women who had cleared their HPV infection at the study visit had CIN2+ in histology of the LEEP sample.
Conclusions
A persistent HPV infection needs to be monitored despite normal Pap smears, since 6/40 (15%) women older than 40 years, was revealed to have an undiagnosed CIN2+ when LEEP was performed. Counseling women regarding the risk of cervical cancer and the expected effect of an eventual LEEP can help them to make an optimal informed choice.
Keywords: Human papillomavirus, Cervical intraepithelial neoplasia, Colposcopy, Loop electrical excision procedure, Transformation zone
Introduction
Persistent infection with high-risk human papillomavirus (HPV) is a prerequisite for the development of cervical cancer [1]. Persistence of HPV is consistently and strongly associated with the risk of developing high-grade cervical intraepithelial neoplasia (CIN2+) [2] which in turn include an elevated risk to progress to cervical cancer [3]. HPV testing has greater sensitivity in revealing CIN2+ than cytology [4], and is presently recommended as the primary screening method for cervical cancer in Europe [5]. HPV-positive women are recommended cytological triage, the less sensitive method for detection of CIN2 + . This will result in a group of HPV-positive women with normal cytology and the optimal clinical handling of these women is not known [6].
Although some persistent infections clear spontaneously, women with normal cytology who are positive for HPV have a much higher risk of developing CIN3 than HPV-negative women [7]. Katki et al. have found a 7.4% 5-year risk of CIN3+ in women with persistent HPV infection and normal cytology [8]. Kjaer et al did a population based prospective cohort study on HPV positive women <30 years with normal Pap smear at baseline showing risks of developing CIN3 in 12 years in different HPV genotypes as following: HPV16 26,7%, HPV18 19,1% and HPV31/33 over 14% [9]. The Swedescreen study, involving women aged 32–38 years, described that among women with a normal Pap smear attending organized screening, the positive predictive value of HPV persistence as regards detection of biopsy-confirmed CIN2+ was 29% [10]. Long-term follow-up of this study pointed out that all the HPV-positive women with initially normal cytology either become HPV-negative or developed CIN2+ within seven years [11]. Mittal et al additionally describe highest incidence rate of CIN2+ developing from persistent HPV infection in women over 50 years [12].
Cytological screening is less sensitive in women of 50 years of age or more compared with younger women [13]. Colposcopy have moderate sensitivity for detection of CIN2+ in premenopausal women, and the sensitivity decreases further in postmenopausal women [14]. In colposcopic examination the type of the transformation zone (TZ) is crucial for interpretation of the examination. In types 1 and 2 the TZ is fully visible. In TZ type 3 the upper limit is not visible and this is a common finding in the postmenopausal period when the TZ often retracts into the endocervix [15]. Performance of biopsies lacking the TZ is insufficient [16]. It is also more difficult to obtain adequate amounts of tissue from the endocervix and the sensitivity of cytobrush sampling in detecting dysplasia varies between 44-93%, and is even lower with endocervical curettage for histopathological samples [17,18]. In addition, the positive predictive rate of colposcopic examination is better as regards high-grade cervical lesions and less accurate for low-grade cervical lesions [19]. Petry et al have described a higher colposcopy failure rate in the HPV-positive/Pap-normal group than in the HPV-positive/Pap-abnormal group [16]. The most reliable method to obtain representative samples is to excise the whole TZ surgically, for example by using a loop electrosurgical excision procedure (LEEP), for histological analysis. This procedure is already recommended as clinical practice for women with low-grade colposcopic changes or TZ3 in colposcopy associated with high-grade cytological changes [20].
The management of women with persistent HPV infection and no cytological or colposcopic evidence of CIN represents an unsolved clinical problem. We therefore performed LEEP on a group of women over 40 years with HPV persistency but normal cytology, to determine the prevalence of histologically confirmed CIN2+.
Materials and methods
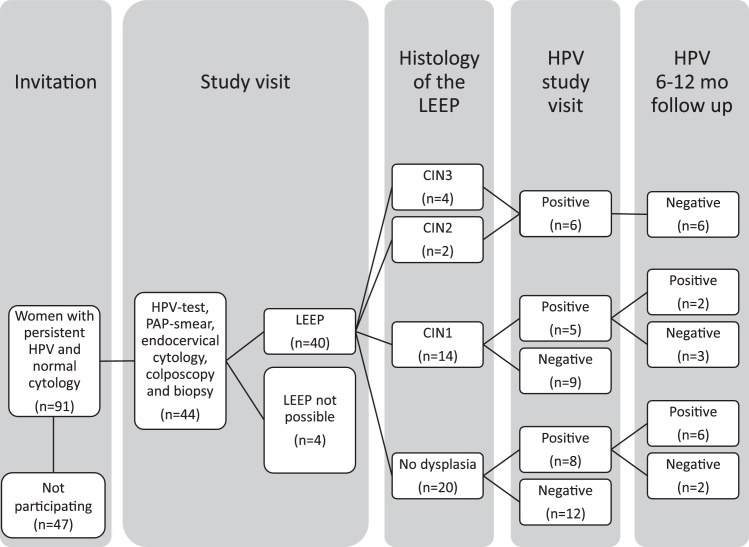
This prospective study was performed from April 2013 until March 2016 and carried out at the gynecological out-patient clinic, Uppsala University Hospital, Sweden. We recruited 91 women with persistent HPV infection but without abnormalities in cytology by sending them an invitation letter (Fig. 1). We excluded women who had plans for future pregnancies, who could not understand the information in Swedish, and where LEEP was regarded as being technically difficult to perform, since we wished to carry it out using local anesthesia. Forty-four women were interested in taking part in the study and these women were contacted by telephone for more information and to schedule a study visit. In four women LEEP could not be performed for anatomical reasons. All postmenopausal women were treated with local estradiol for at least two weeks before the visit to optimize the vaginal mucosa and minimize the possible risk of postoperative cervical stenosis. A signed informed consent document was obtained at the visit. The examination was performed by an experienced colposcopist (RA) and included a) a cytobrush sample for HPV analysis, b) a conventional Pap smear, c) a separate endocervical cytobrush sample on a glass slide, and d) colposcopic evaluation with application of 5% acetic acid and iodine solution, identification of squamocolumnar junction and transformation zone and punch biopsy sampling. The tissues with the most pathological appearance were biopsied and in the absence of abnormality a random biopsy sample was taken. Finally, diagnostic LEEP was performed in local anesthesia for histological analysis. All women that were HPV-positive at the study visit underwent follow-up 6–12 months after LEEP, with a Pap smear and an HPV test. Women that did not participate in the study were followed up with annual Pap smears and HPV tests.
Fig. 1.
Diagram of all the invited women including key results.
All cytology and histology was performed at the Clinic of Pathology and Cytology, Uppsala University Hospital, Uppsala. The highest histological grade found in each patient was used for interpretation of the results. In HPV-testing cervical cytobrush samples were applied to an indicating FTA elute micro-cardTM (GE Healthcare, United Kingdom, art. no WB129308). The FTA cards were processed using a dedicated automated laboratory system (easyPunch STARlet, Hamilton Robotics, USA) which collects each card, takes a photograph of the sample collection area, identifies the parts of the sampling deposition area with the highest amount of cellular material using a machine learning software, and then takes 4 punches with a 3-mm diameter knife from the area containing most material and deposits the punches in a single well in a 96-well microtiter plate. DNA extraction from punches was performed as described earlier [21]. Testing for HPV was performed using a multiplex real-time PCR assay (hpVIR), which detects the following high-risk HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59 (18 and 45 are detected together, and 33, 52 and 58 as one group) and also measures a human single copy gene (HMBS), which serves as a control for that the samples contain sufficient amounts of cellular material for the test to be informative. The limit of detection (LOD) for the nuclear single copy gene HMBS and HPV was both set to 10 copies per PCR. The FTA cards were analyzed at the HPV laboratory, Uppsala University.
The endpoint of the study was histologically identified CIN2+ in LEEP samples from women with persistent HPV infection but without evident CIN in either cytology or colposcopy.
The study was approved by the Regional Ethics Committee in Uppsala (Dnr 2012/460).
Results
In the 40 women who underwent complete examination including LEEP, the mean duration of known HPV persistence was 20 months (median 12, range 4–93). The mean age of the women was 58 years (median 59, range 41–77) and 33/40 women were postmenopausal. Before the study, 25/40 women had taken 1 Pap smear, 13/40 had taken 2 Pap smears, 1/40 had taken 3 Pap smears and 1/40 had taken 7 Pap smears. They were all normal. All the cytological analysis of Pap smears and endocervical samples obtained at the study visit were normal. Only 3/40 Pap smears were reported lacking columnar cells which can be interpreted that in 37/40 cases the junction was reached. None of the biopsy samples confirmed CIN2+, but six showed CIN1. Five cases lack a biopsy sample because it was difficult to obtain due to technical reasons. Notably, for 28/40 (70%) of the women had a TZ type 3. The mean depth of excision was 12.3 mm (median 12 mm, range 8–18 mm) and 34/40 histological samples included the whole TZ. No complications were reported. Histological evaluation of the LEEP samples showed that 20 women had no CIN, 14 women had CIN1 and only 3 of these samples did not include the whole TZ. Further two women had CIN2 and four women had CIN3. None of the LEEP samples displayed invasive disease and five out of six CIN2+ excisions had free endocervical margins. Four out of six women with CIN2+ had TZ type 3 but two women with CIN2+ had a fully visible normal TZ. HPV analysis revealed that 21/40 women had cleared their HPV infection, while 19/40 women still had a persistent HPV infection at the study visit. The most common HPV types that persisted were HPV16 (n = 5) and the HPV33/52/58 group (n = 5). All the women with CIN2+ were HPV-positive at the study visit with different HPV types. The known duration of HPV persistence among the women with CIN2+ was between 7 and 20 months (mean 13 months) and the known duration of HPV persistence among the women showing no CIN was between 7 and 93 months (mean 26 months). None of the 21 women who were HPV-negative at the study visit showed CIN2+ in histology. All results from women that underwent LEEP are presented in Table 1.
Table 1.
Individual data on all the women who underwent LEEP presented in order of CIN findings with highest grade first.
| Baseline data at invitation |
Test result at study visit |
Follow up 6 months after study visit |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Former excision for CIN | Smoking | Persistent HPV type | Persistency (months) | HPV | PAP-smear | Endocervical cytology | TZ | Histology in biopsy | Histology in excision | Margin status cervical/vaginal | Excision height (mm) | HPV | PAP-smear |
| 59 | 0 | 0 | 16 | 20 | 16 | normal | normal | 3 | normal | CIN3 | neg/neg | 13 | neg | normal |
| 56 | 0 | 0 | 31 | 7 | 31 | normal | normal | 3 | normal | CIN3 | neg/neg | 15 | neg | normal |
| 41 | 0 | 1 | 39 | 10 | 39 | normal | normal | 3 | 0 | CIN3 | neg/neg | 15 | neg | normal |
| 59 | 0 | 0 | 33/52/58 | 10 | 33/52/58 | normal | normal | 1 or 2 | CIN1 | CIN3 | pos/pos | 13 | neg | normal |
| 60 | 0 | ? | 56 | 19 | 56 | normal | normal | 3 | 0 | CIN2 | neg/neg | 14 | neg | normal |
| 54 | 0 | 1 | 59 | 11 | 59 | normal | normal | 1 or 2 | 0 | CIN2 | neg/neg | 12 | neg | normal |
| 59 | 0 | 1 | 16 | 7 | 16 | normal | normal | 1 or 2 | CIN1 | CIN1 | neg/neg | 12 | neg | normal |
| 45 | 0 | 0 | 18/45 | 57 | 18/45 | normal | normal | 3 | normal | CIN1 | neg/neg | 11 | 35 | 0 |
| 45 | 0 | 0 | 18/45 | 11 | 18/45 | normal | normal | 3 | CIN1 | CIN1 | neg/neg | 13 | neg | normal |
| 61 | 0 | 0 | 33/52/58 | 29 | 33/52/58 | normal | normal | 3 | CIN1 | CIN1 | pos/pos | 10 | neg | normal |
| 71 | 1 | 0 | 33/52/58 | 18 | 33/52/58 | normal | normal | 1 or 2 | normal | CIN1 | neg/neg | 9 | 33/52/58 | ASCUS |
| 62 | 0 | 0 | 59 | 33 | neg | normal | normal | 1 or 2 | CIN1 | CIN1 | neg/neg | 12 | 0 | 0 |
| 67 | 1 | 0 | 16 | 8 | neg | normal | normal | 3 | CIN1 | CIN1 | neg/pos | 12 | neg | normal |
| 60 | 0 | 0 | 51 | 12 | neg | normal | normal | 1 or 2 | normal | CIN1 | neg/neg | 18 | 0 | 0 |
| 62 | 0 | 0 | 16 | 4 | neg | normal | 0 | 3 | normal | CIN1 | neg/neg | 12 | 0 | 0 |
| 60 | 0 | 1 | 39 | 12 | neg | normal | normal | 3 | 0 | CIN1 | neg/neg | 11 | neg | normal |
| 58 | 1 | 0 | 56 | 10 | neg | normal | normal | 3 | normal | CIN1 | neg/neg | 14 | 0 | 0 |
| 59 | 0 | ? | 56 | 12 | neg | normal | normal | 3 | normal | CIN1 | neg/neg | 15 | neg | normal |
| 47 | 1 | 0 | 33/52/58 | 12 | neg | normal | normal | 1 or 2 | normal | CIN1 | neg/neg | 12 | 0 | normal |
| 59 | 0 | 0 | 18/45 | 12 | neg | normal | normal | 1 or 2 | normal | CIN1 | neg/neg | 13 | neg | normal |
| 59 | 0 | 0 | 16 | 45 | 16 | normal | normal | 3 | normal | normal | 12 | 16 | normal | |
| 68 | 0 | 0 | 16 | 33 | 16 | normal | normal | 1 or 2 | normal | normal | 15 | 16 | normal | |
| 46 | 1 | 0 | 16 | 7 | 16 | normal | normal | 3 | normal | normal | 15 | 16 | normal | |
| 56 | 0 | 0 | 39 | 12 | 39 | normal | normal | 3 | normal | normal | 8 | neg | normal | |
| 69 | 0 | 0 | 56 | 29 | 56 | normal | normal | 3 | normal | normal | 14 | 56 | ASCUS | |
| 53 | 0 | 1 | 56 | 10 | 56 | normal | not possible | 3 | normal | normal | 11 | neg | normal | |
| 54 | 0 | 0 | 33/52/58 | 25 | 33/52/58 | normal | normal | 3 | 0 | normal | 9 | 33/52/58 | normal | |
| 62 | 0 | 0 | 33/52/58 | 93 | 33/52/58 | normal | not possible | 3 | normal | normal | 9 | 33/52/58 | ASCUS | |
| 61 | 0 | 0 | 16 | 44 | neg | normal | normal | 3 | normal | normal | 14 | 0 | 0 | |
| 62 | 0 | 0 | 16 | 30 | neg | normal | normal | 1 or 2 | normal | normal | 10 | 0 | 0 | |
| 73 | 0 | 0 | 16 | 18 | neg | normal | normal | 3 | normal | normal | 11 | 0 | 0 | |
| 64 | 0 | 0 | 18/45 | 28 | neg | normal | normal | 3 | normal | normal | 11 | 0 | 0 | |
| 58 | 0 | 1 | 16 | 24 | neg | normal | normal | 1 or 2 | normal | normal | 12 | 0 | 0 | |
| 41 | 1 | 0 | 51 | 12 | neg | normal | 0 | 1 or 2 | normal | normal | 9 | 0 | 0 | |
| 62 | 0 | 0 | 33/52/58 | 7 | neg | normal | normal | 3 | normal | normal | 10 | 0 | 0 | |
| 77 | 1 | 0 | 33/52/58 | 16 | neg | normal | not possible | 3 | normal | normal | 10 | 0 | 0 | |
| 56 | 0 | 0 | 31 | 10 | neg | normal | normal | 3 | normal | normal | 15 | neg | normal | |
| 43 | 0 | ? | 16 | 10 | neg | normal | normal | 3 | normal | normal | 15 | 0 | 0 | |
| 53 | 0 | 0 | 56 | 10 | neg | normal | normal | 3 | normal | normal | 14 | 0 | 0 | |
| 59 | 0 | 0 | 35 | 12 | neg | normal | normal | 3 | normal | normal | 13 | 0 | 0 | |
At follow-up 6–12 months after LEEP, all six women with CIN2+ had become HPV-negative and showed normal cytology. Among eight HPV-positive women with no dysplasia in the LEEP sample six women still had a persistent HPV-infection at follow-up (Fig. 1).
Discussion
In our study 6/40 (15%) women with persistent HPV infection and no detectable abnormalities in cytology had CIN2+ verified by histological examination of the LEEP sample. Two thirds of these women had a TZ3. HPV genotyping showed different high-risk types in all six of these women, a finding also noticed in an earlier study from our group [22], which indicates that in order to cover the HPV types associated with persistence, it is necessary to genotype for more than HPV16/18 in this older age group. An important notice is that only 19 women in this cohort still tested HPV-positive at the study visit and all the CIN2+ women were in this group. By contrast, none of the 21 women with cleared HPV infection had CIN2+, a finding that was also noted in follow-up in the Swedescreen study [11]. However, a recent Dutch post-hoc analysis describes higher risk for CIN3+ in five years for women that were HPV positive in the first screening round and then turned HPV negative at follow-up [23]. A continued surveillance of these women might thus be needed. Our results further highlight the importance of carrying out follow-up of women with persistent HPV infection and a normal cytology, since a moderate proportion have CIN2+ and thus are at risk of developing cervical cancer.
The optimal management of women with histological CIN1 is surveillance, since at least 70% of these lesions will resolve spontaneously and only few will progress [24]. While CIN2+ is considered as a true precursor of cervical cancer and is associated with oncogenic types of HPV, CIN1 is merely an insensitive histopathological sign of HPV infection [25] that can be caused even by low-risk HPV types [26]. Even if LEEP performed because of CIN tends to eliminate HPV infection [27], treatment of CIN1 is associated with a higher HPV-positive rate at follow-up when compared with treatment of CIN2+ [28]. In our study also, LEEP performed in connection with CIN2+ tended to clear the HPV infection earlier than in women with CIN1. In addition, it does not seem possible to treat a persistent HPV infection without CIN by means of LEEP, since most of these women still tested HPV-positive at follow-up. In women with CIN1 or normal histology in the excision the future risk of development of CIN or cancer remains unclear, but excision samples including the whole TZ (29/34 women) exclude an existing CIN2+.
The number of study subjects is rather small since only 44/91 (48%) of the invited women chose to participate. We do not find this unexpected since the study visit included an invasive treatment demanding local anesthesia. Also the usually self-healing nature of the HPV-infection and a trust to a normal Pap smear could explain the low participation rate. We do however believe that the obtained data gives a quite fair estimate of the CIN2+ prevalence in this group of women.
The present cohort represents a highly selected group with previously identified persistent HPV infection and no former evidence of CIN. Currently it is not clear how to manage these women and even though a persistent HPV infection is a risk factor of cervical cancer, very few HPV-positive women will develop the disease. About half or more of HPV infections will clear within a year [29] as they also did in this study. It is important to consider the balance between overtreatment of women with transient infections against the risk of not detecting those women who may develop cervical cancer. Unnecessary treatments are not only uncomfortable but have short-term risks such as infection or bleeding and can in the long-term also cause preterm labor [30] or cervical stenosis [31]. Knowledge of having a potentially dangerous HPV infection can also cause anxiety [32]. However, the remaining HPV infections may persist longer and the risk of progression from CIN to cancer is considerable [33] and especially in case of TZ3 the LEEP must kept in mind.
There is still no consensus concerning the definition of persistency of an HPV infection. A Columbian prospective study on HPV persistence proposed the definition that persistent infections are those lasting more than the median duration, which was for example 9,5 months for HPV16 in women >30 years [34]. In a large meta-analysis, Koshiol et al. [2] remarked that even testing intervals of ≤ six months produce strong summative relative risks as regards the association between HPV persistence and CIN2+. It is therefore suggested that repeat HPV testing at six months is a valuable way to identify women at increased risk of cervical precancer and cancer. In our study the women with CIN2+ had a detected HPV persistence for seven to 20 months. Follow-up in the Swedescreen study pointed out that all initially cytology-negative women with persistent HPV infection developed colposcopically verified CIN2+ within seven years [11]. The women in that study were between 32 and 38 years of age at entry, in contrast to our study where the majority were postmenopausal. There is a need for further studies on different age groups to form a firm basis for future decisions on how to handle these patients.
Conclusion
A persistent HPV infection needs to be monitored despite normal Pap smears, since 6/40 (15%) women older than 40 years, was revealed to have an undiagnosed CIN2+ when LEEP was performed. Counseling women regarding the risk of cervical cancer and the expected effect of an eventual LEEP can help them to make an optimal informed choice.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgement
Lions Cancer Foundation, Uppsala, Sweden. Grant ID 1050023.
References
- 1.Walboomers J.M., Jacobs M.V., Manos M.M. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Koshiol J., Lindsay L., Pimenta J.M., Poole C., Jenkins D., Smith J.S. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol. 2008;168(2):123–137. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostör A.G. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12(2):186–192. [PubMed] [Google Scholar]
- 4.Mayrand M.H., Duarte-Franco E., Rodrigues I. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 5.second edition. 2015. European guidelines for quality assurance in cervical cancer screening.http://www.gisci.it/documenti/news/EW0115451ENN_002.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katki H.A., Kinney W.K., Fetterman B. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12(7):663–672. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rozendaal L., Walboomers J.M., van der Linden J.C. PCR-based high-risk HPV test in cervical cancer screening gives objective risk assessment of women with cytomorphologically normal cervical smears. Int J Cancer. 1996;68(6):766–769. doi: 10.1002/(SICI)1097-0215(19961211)68:6<766::AID-IJC13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Katki H.A., Schiffman M., Castle P.E. Five-year risks of CIN 3+ and cervical cancer among women who test Pap-negative but are HPV-positive. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S56–63. doi: 10.1097/LGT.0b013e318285437b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjaer S.K., Frederiksen K., Munk C., Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102(19):1478–1488. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elfgren K., Rylander E., Radberg T. Colposcopic and histopathologic evaluation of women participating in population-based screening for human papillomavirus deoxyribonucleic acid persistence. Am J Obstet Gynecol. 2005;193(3 Pt 1):650–657. doi: 10.1016/j.ajog.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 11.Elfgren K., Elfstrom K.M., Naucler P., Arnheim-Dahlstrom L., Dillner J. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol. 2017;216(3):e1–e7. doi: 10.1016/j.ajog.2016.10.042. 264. [DOI] [PubMed] [Google Scholar]
- 12.Mittal S. Risk of high-grade precancerous lesions and invasive cancers in high-risk HPV-positive women with normal cervix or CIN 1 at baseline-A population-based cohort study. Int J Cancer. 2017;140(8):1850–1859. doi: 10.1002/ijc.30609. [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson L., Sparen P., Gustafsson M. Low efficiency of cytologic screening for cancer in situ of the cervix in older women. Int J Cancer. 1995;63(6):804–809. doi: 10.1002/ijc.2910630610. [DOI] [PubMed] [Google Scholar]
- 14.Costa S., Nuzzo M.D., Rubino A. Independent determinants of inaccuracy of colposcopically directed punch biopsy of the cervix. Gynecol Oncol. 2003;90(1):57–63. doi: 10.1016/s0090-8258(03)00202-6. [DOI] [PubMed] [Google Scholar]
- 15.Gilani S.M., Mazzara P.F. Cytohistologic correlation in premenopausal and postmenopausal women. Acta Cytol. 2013;57(6):575–580. doi: 10.1159/000353769. [DOI] [PubMed] [Google Scholar]
- 16.Petry K.U., Luyten A., Scherbring S. Accuracy of colposcopy management to detect CIN3 and invasive cancer in women with abnormal screening tests: results from a primary HPV screening project from 2006 to 2011 in Wolfsburg, Germany. Gynecol Oncol. 2013;128(2):282–287. doi: 10.1016/j.ygyno.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman M.S., Sterghos S., Jr, Gordy L.W., Gunasekaran S., Cavanagh D. Evaluation of the cervical canal with the endocervical brush. Obstet Gynecol. 1993;82(4 Pt 1):573–577. [PubMed] [Google Scholar]
- 18.Boardman L.A., Meinz H., Steinhoff M.M., Heber W.W., Blume J. A randomized trial of the sleeved cytobrush and the endocervical curette. Obstet Gynecol. 2003;101(3):426–430. doi: 10.1016/s0029-7844(02)02713-8. [DOI] [PubMed] [Google Scholar]
- 19.Hopman E.H., Kenemans P., Helmerhorst T.J. Positive predictive rate of colposcopic examination of the cervix uteri: an overview of literature. Obstet Gynecol Surv. 1998;53(2):97–106. doi: 10.1097/00006254-199802000-00021. [DOI] [PubMed] [Google Scholar]
- 20.McCord M.L., Stovall T.G., Summitt R.L., Jr, Ling F.W. Discrepancy of cervical cytology and colposcopic biopsy: is cervical conization necessary? Obstet Gynecol. 1991;77(5):715–719. [PubMed] [Google Scholar]
- 21.Gustavsson I., Lindell M., Wilander E., Strand A., Gyllensten U. Use of FTA card for dry collection, transportation and storage of cervical cell specimen to detect high-risk HPV. J Clin Virol. 2009;46(2):112–116. doi: 10.1016/j.jcv.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Gyllensten U., Gustavsson I., Lindell M., Wilander E. Primary high-risk HPV screening for cervical cancer in post-menopausal women. Gynecol Oncol. 2012;125(2):343–345. doi: 10.1016/j.ygyno.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 23.Polman N.J., Veldhuijzen N.J., Heideman D.A.M., Snijders P.J.F., Meijer C., Berkhof J. HPV-positive women with normal cytology remain at increased risk of CIN3 after a negative repeat HPV test. Br J Cancer. 2017;117(10):1557–1561. doi: 10.1038/bjc.2017.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petry K.U. Management options for cervical intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol. 2011;25(5):641–651. doi: 10.1016/j.bpobgyn.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Schiffman M., Castle P.E., Jeronimo J., Rodriguez A.C., Wacholder S. Human papillomavirus and cervical cancer. Lancet (London, England) 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 26.Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117(2 Suppl):S5–10. doi: 10.1016/j.ygyno.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Kucera E., Sliutz G., Czerwenka K., Breitenecker G., Leodolter S., Reinthaller A. Is high-risk human papillomavirus infection associated with cervical intraepithelial neoplasia eliminated after conization by large-loop excision of the transformation zone? Eur J Obstet Gynecol Reprod Biol. 2001;100(1):72–76. doi: 10.1016/s0301-2115(01)00457-2. [DOI] [PubMed] [Google Scholar]
- 28.Legood R., Smith M., Lew J.B. Cost effectiveness of human papillomavirus test of cure after treatment for cervical intraepithelial neoplasia in England: economic analysis from NHS Sentinel Sites Study. BMJ. 2012;345:e7086. doi: 10.1136/bmj.e7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez A.C., Schiffman M., Herrero R. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst. 2008;100(7):513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tempfer C., Polterauer S., Grimm C., Bentz E.K., Reinthaller A., Hefler L.A. Endocervical cytobrush for the detection of cervical dysplasia before large loop excision of the transformation zone (LLETZ) Anticancer Res. 2008;28(5B):3131–3134. [PubMed] [Google Scholar]
- 31.Penna C., Fambrini M., Fallani M.G., Pieralli A., Scarselli G., Marchionni M. Laser CO2 conization in postmenopausal age: risk of cervical stenosis and unsatisfactory follow-up. Gynecol Oncol. 2005;96(3):771–775. doi: 10.1016/j.ygyno.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Hendry M., Pasterfield D., Lewis R. Are women ready for the new cervical screening protocol in England? A systematic review and qualitative synthesis of views about human papillomavirus testing. Br J Cancer. 2012;107(2):243–254. doi: 10.1038/bjc.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plummer M., Schiffman M., Castle P.E., Maucort-Boulch D., Wheeler C.M. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195(11):1582–1589. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 34.Munoz N., Hernandez-Suarez G., Mendez F. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer. 2009;100(7):1184–1190. doi: 10.1038/sj.bjc.6604972. [DOI] [PMC free article] [PubMed] [Google Scholar]