Abstract
Objectives
To assess the effect of transcervical endometrial resection on clinical symptoms related to histopathological findings of the junctional zone.
Study design
This prospective study took place at a university hospital. Premenopausal women suffering from abnormal uterine bleeding and/or pelvic pain and scheduled for transcervical endometrial resection were enrolled (n = 112). Histopathological findings of the endomyometrial biopsies were categorized as follows: Adenomyosis of the inner myometrium (intrinsic adenomyosis): ≥ 2 mm myometrial invasion without contact to the basal endometrium, serrated junctional zone: > 3 mm myometrial invasion with contact to the basal endometrium and linear junctional zone: No or marginal myometrial invasion (≤ 3 mm) with contact to the basal endometrium. All study participants received a baseline and two follow-up questionnaires (6 and 18 months after surgery) regarding symptom severity, health-related quality of life and pelvic pain. Data regarding reintervention surgery was obtained from the National Database Patoweb. The rate of reintervention surgery and the improvement in symptom severity, health-related quality of life and pelvic pain were correlated to histopathological findings.
Results
Twenty-four patients had intrinsic adenomyosis, 31 had serrated junctional zone and 57 had linear junctional zone. Fifteen patients (13%) underwent reintervention surgery; three (20%) within 6 months, nine (60%) between 6–18 months and three (20%) > 18 months after transcervical endometrial resection. Reintervention surgery was more common in women with intrinsic adenomyosis compared to women without (33% (95% CI: 16–55) vs 8% (95% CI: 3–16)) (p-value: < .05). Nine patients (38%) with intrinsic adenomyosis were asymptomatic based on low symptom severity score, high health-related quality of life and no pelvic pain at 18 months follow-up. Patients with linear junctional zone had a higher improvement in symptom severity and health-related quality of life than patients with intrinsic adenomyosis or serrated junctional zone at 6 months follow-up after surgery (p-value < .05). However, there was no significant difference in pelvic pain reduction.
Conclusion
The effect of transcervical endometrial resection may depend upon the degree of junctional zone changes, and patients with intrinsic adenomyosis are more likely to undergo reintervention surgery than patients with either linear or serrated junctional zone. However, intrinsic adenomyosis may also be successfully treated with endometrial resection.
Keywords: Adenomyosis, Health-related quality of life, Heavy menstrual bleeding, Pelvic pain
Introduction
Abnormal uterine bleeding can severely affect quality of life [1,2] and is a leading reason for outpatient contacts [3]. Adenomyosis is a common cause of abnormal uterine bleeding [4] and is known as a heterogeneous disease of the junctional zone (JZ) with ectopic endometrium infiltrating varying degrees of the myometrium. A hysterectomy may be the last resort to treat adenomyosis, but attempts with hormonal and minimally invasive treatment such as transcervical endometrial resection (TCRE) are often preferred [[5], [6], [7], [8]]. Adenomyosis is often found in patients who have a hysterectomy following treatment failure of TCRE [[9], [10], [11], [12], [13], [14], [15]]. However, of all studies assessing the effect of TCRE none have a preoperative diagnosis of adenomyosis verified by histopathology [[9], [10], [11], [12], [13], [14], [15]]. Thus, it is not clear whether adenomyosis is induced by TCRE or whether it is the cause of treatment failure. The number of patients with adenomyosis and a good response to the procedure is unknown due to the lack of a preoperative diagnosis of adenomyosis. The degree of JZ changes before TCRE in relation to symptom relief is of particular interest [14]. It is likely that symptoms may persist after TCRE depending on the degree of JZ changes. We hypothesized that women with more extensive down growth of the endometrium may have no or less symptom relief after TCRE compared with women with no or minimal down growth of the endometrium. Therefore, the aim of this study was to investigate the association between outcome of TCRE (symptom severity, health-related quality of life, pelvic pain and reintervention rate) and histopathological findings of the JZ.
Materials and methods
Study population
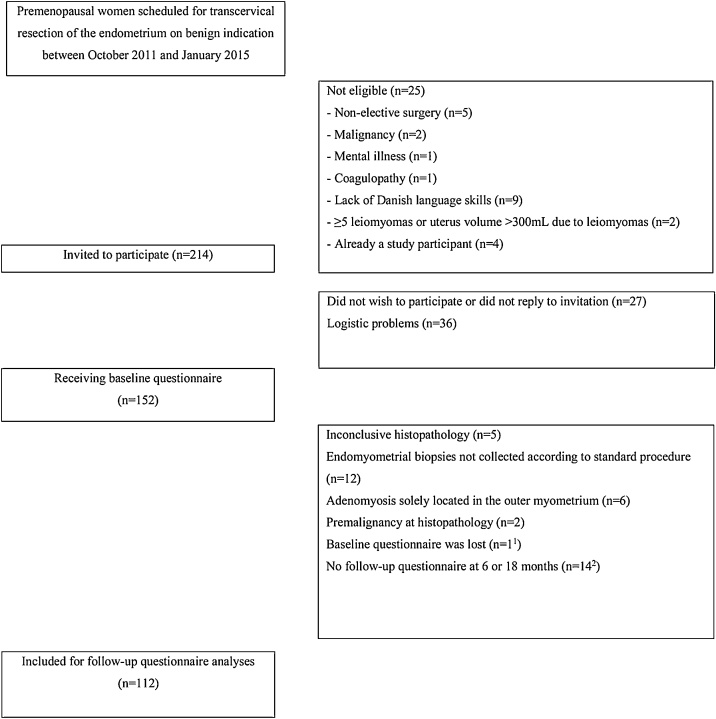
This prospective study was approved by The Central Denmark Region Committee of Health Research Ethics, journal number: 20010175 and The Danish Data Protection Agency, journal number: 2007-58-0010. Written informed consent was obtained from all study participants before study entry. From October 2011 to January 2015, a consecutive sample of premenopausal women undergoing TCRE due to benign abnormal uterine bleeding and/or pelvic pain was recruited at the Department of Gynecology and Obstetrics, Aarhus University Hospital, Denmark. The exclusion criteria were non-elective surgery, malignancy, mental illness, coagulopathy, lack of Danish language skills or presence of five or more leiomyomas / uterine volume > 300 ml due to leiomyomas. Women were only able to participate once in the study project, thus repeat TCRE were excluded (Fig. 1).
Fig. 1.
Flowchart.
1 No adenomyosis of the inner myometrium and no reintervention surgery
2 Five with a histopathological diagnosis of adenomyosis of the inner myometrium and no reinterventions surgery. Two with serrated junctional zone had reintervention surgery (“no adenomyosis” verified by hysterectomy)
Patients with at least one follow-up questionnaire (6 or 18 months) were kept for the questionnaire analyses.
Reintervention surgery was recorded for all patients who completed baseline questionnaire (n = 126).
Questionnaire
Right before surgery, all study participants were asked to fill out a comprehensive questionnaire to obtain information on baseline characteristics, symptom severity, health-related quality of life (HR-QoL) and pelvic pain. The questionnaire was in Danish and consisted of the validated fibroid symptom and quality of life questionnaire (UFS-QOL) [[16], [17], [18]] including eight questions related to symptom severity (menstrual bleeding characteristics, pelvic and urinary discomfort, and fatigue) and 29 questions related to HR-QoL (concern, activities, energy/mood, control, self-consciousness, and sexual function). Each question was scored on a scale between 1 and 5. A total score was calculated and transformed into a symptom severity score (SSS) from 0 to 100 (higher score = more symptoms) and an HR-QoL score ranging from 0 to 100 (higher score = better HR-QoL). The UFS-QoL has been used for patients with adenomyosis before [[19], [20], [21], [22]]. Pelvic pain was assessed using the visual analogue scale for pain (VAS pain) [23], which consists of a 100-mm line with the endpoints "no pain" and "worst pain". Study participants were asked to mark the point along the line that expressed their average pain intensity. Identical questionnaires for follow-up were sent by email or post 6 and 18 months after surgery. The changes between baseline and follow-up scores (at 6 and 18 months) were calculated for SSS, HR-QoL, and VAS pain intensity. Women with an SSS < 20, an overall HR-QoL > 80, and no pelvic pain at 18 months follow-up were considered asymptomatic.
In addition to the questionnaires, the rate of reintervention surgery was recorded on January 2018 from the national database Patoweb. This is a database where all surgical pathology tests are registered.
Histopathology
The procedure for obtaining deep endomyometrial biopsies during TCRE was standardized and applied in the same manner to all study participants. The surgical procedure took place in an outpatient setting with patients in local or brief general anesthesia. TCRE was performed with standardized electro current settings to avoid changes by electrocoagulation. The surgeon used a Storz resectoscope (26 F 12 oblique optic) (Karl Storz, Holte, Denmark) with a large monopolar cutting loop. To ensure that the resection could penetrate deep into the myometrium, careful suction curettage was performed immediately before resection, if the endometrium was not in the early proliferative phase. Furthermore, a firm pressure was applied to the resectoscope. Parallel slices representing the entire endometrium were obtained. Each biopsy was removed separately after each pass of the resectoscope, stretched and immediately fastened to a surface with the endometrium pointing downwards to avoid twisting. A needle was placed at the end, representing the beginning of the biopsy and the upper part of the uterus. Each biopsy was numbered according to its origin and then fixed in formalin prior to histopathological analysis.
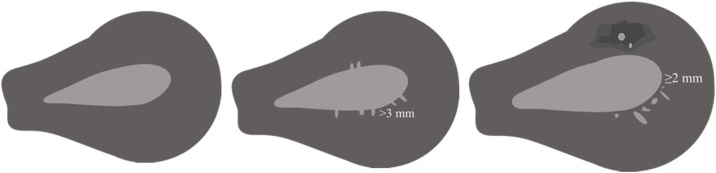
The endometrial-myometrial JZ was defined by histopathology of endomyometrial biopsies from TCRE and defined as: Adenomyosis of the inner myometrium, ≥ 2 mm myometrial invasion without contact to the basal endometrium, serrated JZ, > 3 mm myometrial invasion with contact to the basal endometrium, and linear JZ (no or marginal myometrial invasion ≤ 3 mm with contact to the basal endometrium) (Fig. 2).
Fig. 2.
Left: Linear junctional zone, no or marginal myometrial invasion ≤ 3 mm with contact to the basal endometrium.
Middle: Serrated junctional zone, > 3 mm myometrial invasion with contact to the basal endometrium.
Right: Adenomyosis of the inner myometrium (intrinsic adenomyosis), ≥ 2 mm myometrial invasion without contact to the basal endometrium.
For an accurate evaluation of the JZ, this method required thick endomyometrial biopsies with a depth of ≥ 5 mm into the myometrium. The histopathological evaluation took place within 1 to 3 weeks after surgery. The pathologist documented the findings in a standardized form designed for this study. Histopathology was inconclusive if the biopsies were either twisted or did not contain myometrium of sufficient depth (< 5 mm). Inconclusive histopathology samples were excluded from the study.
All statistical analyses were made using STATA 13.1. The sample size calculation was based on the assumption of at least 30% reintervention among patients with adenomyosis of the inner myometrium and 5% reintervention among patients with normal JZ (linear JZ), with standard errors of 5%. The estimated required sample size was then at least n = 72. The three groups according to histopathology of the JZ (adenomyosis of the inner myometrium, serrated JZ or linear JZ) were compared using the Kruskal-Wallis one-way analysis of variance for data that were not normally distributed and one-way ANOVA for data that were normally distributed. In addition, we used the Wilcoxon signed-rank test (not normally distributed data) and student's t-test (normally distributed data) to compare patients with either adenomyosis of the inner myometrium or serrated JZ to patients with linear JZ. P-values of < .05 were considered statistically significant. Patients who had reintervention surgery within the follow-up period (6 or 18 months) were excluded from the respective analyses. Missing items from the questionnaires were handled as follows: if < 50% of the scale items were missing, the scale would be retained, with the mean scale score of the items present used to impute a score for the missing items (subscale analysis). If ≥ 50% of the items were missing, no scale score was calculated, and the subscale score was considered missing [16].
Results
In total, 112 patients filled out the baseline questionnaire. At 6 and 18 months, follow-up questionnaires were completed by 96.4% and 94,6% of the patients, respectively. Among patients who did not respond to the questionnaire, only two underwent reintervention surgery. Baseline characteristics are shown in Table 1. The presence of intrauterine device (IUD) did not affect the histopathological results. Based on histopathology twenty-four patients (21%) had adenomyosis of the inner myometrium, 31 (28%) had serrated JZ and 57 patients (51%) had linear JZ. There was no patients with a histopathological diagnosis of adenomyomas.
Table 1.
Baseline characteristics of patients scheduled for transcervical resection of the endometrium.
| All (n = 112) N | 95% CI 75% PI% | Adenomyosis of the inner myometrium (n = 24) N | 95% CI 75% PI % | Serrated junctional zone (n = 31) N | 95% CI 75% PI % | Linear junctional zone (n = 57) N | 95% CI 75% PI % | |
|---|---|---|---|---|---|---|---|---|
| Mean age | 45.2 | 44-46 | 45.5 | 44-47 | 45.1 | 43-47 | 45.1 | 43-47 |
| Median BMI1 | 26.1 | 23-29 | 27.1 | 24-28 | 26.5 | 23-29 | 24.7 | 22-29 |
| Mean number of pregnancies | 3 | 3-3 | 4 | 3-4 | 3 | 3-4 | 3 | 2-3 |
| Mean number of labors | 2 | 2-2 | 2 | 2-2 | 2 | 2-3 | 2 | 2-2 |
| Previous hysteroscopic surgery or myomectomy2 | 18 | 16% | 4 | 17% | 6 | 19% | 8 | 14% |
| Previous evacuation | 23 | 21% | 7 | 29% | 6 | 19% | 10 | 18% |
| Previous caesarean section | 16 | 14% | 3 | 13% | 10 | 52% | 3 | 5% |
| IUD or hormonal therapy before TCRE3 | 43 | 38% | 4 | 17% | 16 | 52% | 23 | 40% |
| IUD or hormonal therapy after TCRE4 | 34 | 30% | 6 | 25% | 10 | 32% | 18 | 32% |
| Ultrasound diagnosis of leiomyomas (all types) | 27 | 24% | 4 | 17% | 9 | 29% | 14 | 25% |
| Ultrasound diagnosis of leiomyomas (intramural >3 cm in diameter or type 0-3) |
16 | 24% | 2 | 8% | 5 | 16% | 9 | 16% |
| Ultrasound diagnosis of polyps | 10 | 9% | 3 | 13% | 0 | 0% | 7 | 12% |
| Mean thickness of endomyometrial biopsies | 5.3 | 5-6 | 5.4 | 5-6 | 5.4 | 5-6 | 5.2 | 5-5 |
CI: Confidence interval. PI: Percentile interval. BMI: Body Mass Index. IUD: Intrauterine device, TCRE: Transcervical resection of the endometrium.
There were no statistically significant differences (p-value <.05) between the groups (adenomyosis of the inner myometrium, serrated JZ and linear JZ) in baseline characteristics, except for mean number of pregnancies.
1One missing.
216 had resection of polyps, one had fibroid resection and one had myomectomy.
3Three months or more prior to operation.
4Five missing.
Reintervention surgery reported in the follow-up questionnaires is shown in Table 2. In total, twelve patients (80%) underwent reintervention surgery within 18 months after TCRE and an additional three patients (20%) underwent reintervention surgery after the 18-month follow-up period and before January 2018. Reintervention surgery was performed in 33% (95% CI: 16–55) patients with adenomyosis of the inner myometrium compared to 13% (95% CI: 4–30) and 5% (95% CI: 1–15) with serrated or linear JZ respectively (p-value < .05). Indications for reintervention surgery were either heavy menstrual bleeding and/or pelvic pain. All patients who had a reintervention surgery had a hysterectomy, but three (20%) had undergone repeated TCRE before hysterectomy. Adenomyosis of the inner myometrium was verified by histopathological examination of hysterectomy specimens in all but one woman. This woman had repeat TCRE prior to hysterectomy, thus it is likely that adenomyosis was removed before hysterectomy.
Table 2.
Surgical reintervention during follow-up according to histopathology and clinical symptoms.
| Baseline symptoms | Symptoms at 6 months follow-up | Use of analgesics after TCREα | Use of IUD after TCRE | Surgical reintervention | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Histopathology | SS | HR-QoL | Pelvic pain (VAS intensity %) | SS | HR-QoL | Pelvic pain (VAS intensity %) | ||||
| #1 | Adenomyosis of the inner myometrium | 37.5 | 56.0 | During (39) and between menstruation (30) | Hysterectomy | |||||
| #2 | Adenomyosis of the inner myometrium | 59.4 | 57.8 | No | Repeat TCRE* | |||||
| #3 | Serrated junctional zone | 53.1 | 40.5 | During (30) and between menstruation (15) | Hysterectomy | |||||
| #41 | Linear junctional zone | 56.3 | 50.0 | During (30) and between menstruation (88) | 21.9 | 93.7 | Between menstruation (87) | Yes | Yes | Hysterectomy |
| #5 | Adenomyosis of the inner myometrium | 62.5 | 41.4 | During (87) and between menstruation (62) | 56.3 | 46.6 | During (90) and between menstruation (70) | Yes | Yes | Repeat TCRE* |
| #6 | Adenomyosis of the inner myometrium | 53.1 | 75.9 | No | 18.8 | 89.7 | During menstruation (22) | Yes | No | Repeat TCRE* |
| #7 | Adenomyosis of the inner myometrium | 78.1 | 42.2 | No | 43.8 | 81.0 | No | No | No | Hysterectomy |
| #8 | Adenomyosis of the inner myometrium | 62.5 | 42.2 | During (71) and between menstruation (65) | 15.6 | 90.5 | Between menstruation (14) | Yes | No | Hysterectomy |
| #92 | Linear junctional zone | 56.3 | 75.0 | During (77) and between menstruation (71) | 53.1 | 82.8 | During (56) and between menstruation (58) | Yes | Yes | Hysterectomy |
| #10 | Serrated junctional zone | 12.5 | 80.2 | No | 9.4 | 69.8 | Between menstruation (23) | No | Yes | Hysterectomy |
| #11 | Serrated junctional zone | 50.0 | 44.8 | During menstruation (78) | Hysterectomy | |||||
| #12 | Serrated junctional zone | 18.8 | 81.9 | During (85) and between menstruation (80) | Hysterectomy | |||||
| #13 | Adenomyosis of the inner myometrium | 71.9 | 63.8 | During (65) and between menstruation (23) | 6.3 | 90.5 | No | No | No | Hysterectomy |
| #14 | Adenomyosis of the inner myometrium | 62.5 | 49.1 | During menstruation (72) | 53.1 | 61.2 | During menstruation (54) | Yes | No | Hysterectomy |
| #15 | Linear junctional zone | 34.4 | 30.2 | No | 65.6 | 7.8 | During (63) and between menstruation (60) | Yes | No | Hysterectomy |
TCRE: Transcervical resection of the endometrium. SSS: Symptom severity. HR-QoL: Health-related quality of life. VAS: Visual analogue scale. IUD: Intrauterine device.
#1-3 had surgical reintervention < 6 months after TCRE, #4-12 had surgical reintervention 6–18 months after TCRE and #13-15 had surgical reintervention > 18 months after TCRE (before January 2018). Follow-up questionnaire missing in #11 and #12.
* All three patients had subsequently hysterectomy due to persisting symptoms.
Presence or absence of adenomyosis of the inner myometrium was verified in all hysterectomy specimens except for patient #2. This patient had one foci of adenomyosis of the inner myometrium, and it is likely that this foci may have been removed by repeat TCRE prior to hysterectomy.
α Reported use at 6 months follow-up, including paracetamol, aspirin, non-steroidal anti-inflammatory drugs.
1 Concomitant uterine polyps.
2 Concomitant leiomyomas.
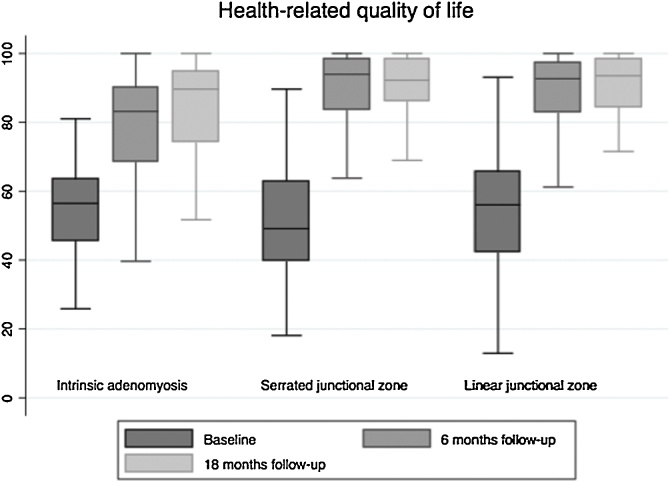
SSS and HR-QoL results at baseline, at 6 months follow-up (median (75% PI), 7.0 months (6–9)), 18 months follow-up (median (75% PI) and 18.4 months (18–20)) after TCRE are shown in Table 3. In addition, Fig. 3 illustrates the HR-QoL at baseline, 6 and 18 months follow-up. There was no statistically significant difference in the baseline SSS and HR-QoL between patients with adenomyosis of the inner myometrium, serrated JZ or linear JZ. When patients with concomitant pelvic pathology (intramural leiomyomas > 3 cm in diameter or type 0–3 leiomyomas, endometriosis and uterine polyps) were excluded from analyses, there was still no statistically significant difference between the groups (S1).
Table 3.
Symptom severity and health-related quality of life at baseline and follow-up after transcervical resection of the endometrium.
| Baseline (n = 112) | 6 months follow-up (n = 105)1 | 6 months improvement | 18 months follow-up (n = 96)2 | 18 months improvement | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | 75% PI | N | Median | 75% PI | Mean | 95% CI | Difference | N | Median | 75% PI | Mean | 95% CI | Difference | |
| SSS | |||||||||||||||
| Adenomyosis of the inner myometrium | 24 | 59.4 | 48-66 | 22 | 18.8 | 9-44 | 34.4 | 25-44 | p-value >.05* | 15 | 18.8 | 6-19 | 39.8 | 30-50 | p-value > .05 |
| Serrated junctional zone | 31 | 50.0 | 34-63 | 29 | 9.4 | 6-25 | 33.4 | 25-41 | 28 | 10.9 | 3-19 | 35.0 | 28-42 | ||
| Linear junctional zone | 57 | 56.3 | 44-72 | 54 | 7.8 | 0-25 | 41.6 | 35-48 | 53 | 9.4 | 3-22 | 42.3 | 36-48 | ||
| HR-QoL total | |||||||||||||||
| Adenomyosis of the inner myometrium | 24 | 56.5 | 46-63 | 22 | 85.8 | 69-91 | 22.9 | 17-29 | p-value ≤ .05 | 15 | 90.5 | 76-95 | 28.6 | 21-37 | p-value > .05 |
| Serrated junctional zone | 31 | 56.0 | 42-66 | 29 | 93.1 | 83-97 | 31.0 | 22-40 | 28 | 93.1 | 80-97 | 30.7 | 22-39 | ||
| Linear junctional zone | 57 | 49.1 | 40-63 | 54 | 94.0 | 84-98 | 36.3 | 31-41 | 53 | 92.2 | 86-98 | 35.4 | 29-42 | ||
SSS: Symptom severity score. HR-QoL: Health-Related Quality of Life. PI: Percentile interval, CI: Confidence interval.
No subscale score was considered missing.
1 6 months follow-up questionnaire missing in four patients (these patients did not have surgical reintervention), three patients excluded due to reintervention surgery within the follow-up period.
2 18 months follow-up questionnaire missing in six patients (one of the patients had reintervention), ten patients exclude due to reintervention surgery within the follow-up period.
No statistically significant difference in baseline SSS and HR-QoL between the groups (p-value > 0.05).
*Statistically significant difference in 6 months follow-up SSS and HR-QoL between patients with either serrated junctional zone or adenomyosis of the inner myometrium and linear junctional zone (p-value ≤ .05).
Fig. 3.
Health-related quality of life on a scale from 0 to 100 at baseline, 6 and 18 months follow-up.
There was a statically significant difference in mean improvement of SSS and HR-QoL following TCRE between patients with either adenomyosis of the inner myometrium or serrated JZ and linear JZ at 6 months follow-up, with the most pronounced improvement among patients with linear JZ. At 18 months, when patients undergoing reintervention surgery were removed from analyses, there was no longer a statistically significant difference between the groups.
Presence and intensity of pelvic pain at baseline, and 6 and 18 months after TCRE is shown in Table 4. There were no statistically significant differences in the distribution of patients with pain or the pain intensity between the groups at baseline. Excluding patients with concomitant pelvic pathology did not change the results significantly (S2). At 18 months follow-up, when patients with reintervention surgery were excluded, the number of patients with pelvic pain was considerably reduced and there was no significant difference in pain reduction according to histopathological findings.
Table 4.
Pelvic pain at baseline and follow-up after transcervical resection of the endometrium.
| Baseline (n = 112) | 6 months follow-up (n = 105)1 | 18 months follow-up (n = 96)2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | With pain N (%) | VAS* Median % | 75% PI | N | With pain N (%) | VAS* Median % | 75% PI | N | With pain N (%) | VAS* Median % | 75% PI | |
| Pelvic pain during menstruation | ||||||||||||
| Adenomyosis of the inner myometrium | 24 | 18 (75) | 67 | 51-73 | 22 | 7 (32) | 52 | 22-67 | 15 | 2 (13) | 26 | 17-35 |
| Serrated junctional zone | 31 | 21 (68) | 60 | 38-68 | 29 | 5 (17) | 35 | 32-57 | 28 | 7 (25) | 59 | 50-70 |
| Linear junctional zone | 57 | 45 (79) | 57 | 36-70 | 54 | 15 (28) | 56 | 11-63 | 53 | 11 (21) | 31 | 20-39 |
| Pelvic pain between menstruations | ||||||||||||
| Adenomyosis of the inner myometrium | 24 | 11 (46) | 30 | 17-62 | 22 | 5 (23) | 42 | 19-56 | 15 | 0 | 0 | |
| Serrated junctional zone | 31 | 14 (45) | 40 | 24-50 | 29 | 8 (28) | 25 | 21-39 | 28 | 3 (11) | 35 | 15-53 |
| Linear junctional zone | 57 | 29 (51) | 39 | 25-63 | 54 | 15 (28) | 37 | 5-60 | 53 | 11 (21) | 40 | 12-47 |
VAS: Visual Analogue Scale. PI: Percentile interval, CI: Confidence interval.
*Average pain.
1 6 months follow-up questionnaire missing in four patients (these patients did not have reintervention surgery), three patients excluded due to reintervention surgery within the follow-up period.
2 18 months follow-up questionnaire missing in six patients (one of the patients had reintervention), ten patients exclude due to reintervention surgery within the follow-up period.
There was no statistically significant difference in baseline or follow-up pelvic pain and VAS intensity between groups (p-value > 0.05).
Before surgery more than 50% in each of the three groups used analgesic drugs; this was reduced to less than 20% at 18 months follow-up.
In total, 31 patients had IUD’s inserted after TCRE (six with adenomyosis of the inner myometrium, eight with serrated JZ and 17 with linear JZ). There was no statistically significant difference in SSS, HR-QoL and pelvic pain between the groups who had an IUD or between those with and without IUD at 18 months follow-up. Finally, at 18 months follow-up, nine patients with adenomyosis of the inner myometrium had no pelvic pain, a symptom severity score < 20 and an HR-QoL > 80 and were therefore considered asymptomatic.
Comment
Surgical reintervention after TCRE was required among 12% of the study participants, and more commonly among patients who had adenomyosis of the inner myometrium before initial TCRE than those who did not (p-value < .05). However, TCRE completely resolved symptoms in 1/3 of all the patients with adenomyosis of the inner myometrium. A serrated JZ was rarely associated with treatment failure, although it was associated with less symptom improvement after surgery compared to linear JZ. However, the majority of all patients including those with adenomyosis of the inner myometrium and serrated JZ who did not require reintervention surgery experienced a considerable improvement in SSS and HR-QoL as well as a reduction in pelvic pain.
Previous studies have shown that TCRE is an effective treatment for abnormal uterine bleeding, particularly for heavy menstrual bleeding [6,7], but 20% of patients might require surgical reintervention [24]. This may be due to the presence of adenomyosis [[9], [10], [11], [12], [13], [14], [15]]. However, to the best of our knowledge, no previous studies have evaluated the effect of TCRE according to a histopathologically proven preoperative diagnosis of adenomyosis of the inner myometrium. Moreover, outcome in relation to serrated JZ and linear JZ has not been reported. Our results suggest that TCRE does not cause adenomyosis of the inner myometrium but in fact, it seems to be an acceptable treatment option for some patients with adenomyosis of the inner myometrium and for most patients with serrated JZ or linear JZ. The cutting loop was able to penetrate at least 5 mm into the myometrium and destroy additional 1–2 mm of the myometrium due to electro-currency [14], thus we may assume that TCRE is an effective treatment option in patients with 0–7 mm endometrial penetration.
The presence of concomitant pelvic pathology did not affect our results as demonstrated in the supplementary tables (S1 + S2). This may be explained by the selection of patients without large or multiple leiomyomas. In patients with both adenomyosis and small leiomyomas, adenomyosis may be the main contributor to the clinical symptoms [25]. Furthermore, there was no difference in baseline symptoms between patients with either adenomyosis of the inner myometrium or serrated JZ and linear JZ. Again, this may be explained by the selection of only premenopausal women scheduled for surgery; anovulatory bleeding may also contribute to heavy menstrual bleeding [26] no matter the presence of adenomyosis of the inner myometrium. We were surprised not to find any difference in baseline pelvic pain between the groups. We expected that especially patients with adenomyosis of the inner myometrium and serrated JZ would have more menstrual pain because JZ changes may contribute to dysperistalsis or hyperperistalsis [27].
There are some limitations to this study. The study population was small and we did not perform a systematic preoperative imaging to determine the JZ appearance prior to surgery. A recent study suggests that preoperative two- and three-dimensional transvaginal ultrasound may differentiate between adenomyosis of the inner myometrium, serrated JZ and linear JZ [28]. Unfortunately, there is still no consistent definition of how deep the endometrial penetration must be to be diagnosed as adenomyosis of the inner myometrium. Based on previously published textbooks, we chose a definition of > 2 mm with or without surrounding smooth muscle hyperplasia/hypertrophia [29,30]. To determine the effect of TCRE, we assessed several subjective parameters (questionnaire) but only one objective parameter (surgical reintervention). Additional objective parameters such as the amount of menstrual blood loss may have been assessed as well. Finally, the number of patients with IUDs was very limited which may explain why we did not find a difference in symptom improvement between patients with and without IUDs.
A major strength of the study is the prospective study design of consecutively enrolled study participants undergoing the same standardized procedure for obtaining deep endomyometrial biopsies. This unique method allowed for a detailed histopathological evaluation of the entire JZ circumference. We focused not only on adenomyosis of the inner myometrium but also included serrated JZ as we hypothesized that this finding may also be associated with inferior outcome. Patients had a long-term follow-up and the response rate was high. Furthermore, we were able to gain access to information from Patoweb regarding surgical reintervention, even for the patients who did not complete the questionnaire.
For successful management of women suffering from chronic abnormal uterine bleeding with or without pelvic pain, it is important to consider a preoperative diagnosis of adenomyosis, as this may negatively affect the outcome of TCRE. Nevertheless, in the present study, most women with adenomyosis of the inner myometrium experienced improved clinical symptoms after TCRE. Thus, it seems possible to treat adenomyosis of the inner myometrium with a minimally invasive approach. Future studies should include a large population of women with a preoperative transvaginal ultrasound measure of endometrial penetration before undergoing TCRE. Ultrasound-guided resection [31] and routine IUD insertion after resection may further improve the outcome after endometrial ablation [32,33].
Women with intrinsic adenomyosis are more likely to undergo reintervention surgery after transcervical endometrial resection due to persisting pelvic pain or heavy menstrual bleeding. On the other hand, approximately 1/3 of women with intrinsic adenomyosis may be asymptomatic 18 months after surgery. Overall, women with serrated and linear JZ responded well to TCRE though the highest improvement in symptoms, HR-QoL and pelvic pain was observed among women with linear JZ.
Disclosure
The authors have no conflicts of interest to declare.
Acknowledgments
This study was supported by Aarhus University, Denmark and by funding from A.P. Moeller, Helga and Peter Korning, The Family Hede Nielsen and Manager Jacob Madsen and his wife.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eurox.2019.100029.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Matteson K.A., Raker C.A., Clark M.A., Frick K.D. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the medical expenditures panel survey. J Womens Health (Larchmt). 2013;22:959–965. doi: 10.1089/jwh.2013.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z., Doan Q.V., Blumenthal P., Dubois R.W. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health. 2007;10:183–194. doi: 10.1111/j.1524-4733.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 3.Kadir R.A., Aledort L.M. Obstetrical and gynaecological bleeding: a common presenting symptom. Clin Lab Haematol. 2000;22(Suppl 1) doi: 10.1046/j.1365-2257.2000.00007.x. 12-6; discussion 30-2. [DOI] [PubMed] [Google Scholar]
- 4.Naftalin J., Hoo W., Pateman K., Mavrelos D., Holland T., Jurkovic D. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod. 2012;27:3432–3439. doi: 10.1093/humrep/des332. [DOI] [PubMed] [Google Scholar]
- 5.Dueholm M. Minimally invasive treatment of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2018 doi: 10.1016/j.bpobgyn.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Fergusson R.J., Lethaby A., Shepperd S., Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD000329.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Preutthipan S., Herabutya Y. Hysteroscopic rollerball endometrial ablation as an alternative treatment for adenomyosis with menorrhagia and/or dysmenorrhea. J Obstet Gynaecol Res. 2010;36:1031–1036. doi: 10.1111/j.1447-0756.2010.01251.x. [DOI] [PubMed] [Google Scholar]
- 8.Levgur M. Therapeutic options for adenomyosis: a review. Arch Gynecol Obstet. 2007;276:1–15. doi: 10.1007/s00404-006-0299-8. [DOI] [PubMed] [Google Scholar]
- 9.McCausland A.M., McCausland V.M. Depth of endometrial penetration in adenomyosis helps determine outcome of rollerball ablation. Am J Obstet Gynecol. 1996;174 doi: 10.1016/s0002-9378(96)70211-9. 1786-93; 93-4. [DOI] [PubMed] [Google Scholar]
- 10.Mengerink B.B., van der Wurff A.A., ter Haar J.F., van Rooij I.A., Pijnenborg J.M. Effect of undiagnosed deep adenomyosis after failed NovaSure endometrial ablation. J Minim Invasive Gynecol. 2015;22:239–244. doi: 10.1016/j.jmig.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Simon R.A., Quddus M.R., Lawrence W.D., Sung C.J. Pathology of endometrial ablation failures: a clinicopathologic study of 164 cases. Int J Gynecol Pathol. 2015;34:245–252. doi: 10.1097/PGP.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 12.Busca A., Parra-Herran C. The role of pathologic evaluation of endometrial ablation resections in predicting ablation failure and adenomyosis in hysterectomy. Pathol Res Pract. 2016;212:778–782. doi: 10.1016/j.prp.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Wishall K.M., Price J., Pereira N., Butts S.M., Della Badia C.R. Postablation risk factors for pain and subsequent hysterectomy. Obstet Gynecol. 2014;124:904–910. doi: 10.1097/AOG.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 14.McCausland V., McCausland A. The response of adenomyosis to endometrial ablation/resection. Hum Reprod Update. 1998;4:350–359. doi: 10.1093/humupd/4.4.350. [DOI] [PubMed] [Google Scholar]
- 15.McLucas B. Does endometrial resection cause adenomyosis? J Am Assoc Gynecol Laparosc. 1994;1:S21. doi: 10.1016/s1074-3804(05)80937-5. [DOI] [PubMed] [Google Scholar]
- 16.Spies J.B., Coyne K., Guaou Guaou N., Boyle D., Skyrnarz-Murphy K., Gonzalves S.M. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol. 2002;99:290–300. doi: 10.1016/s0029-7844(01)01702-1. [DOI] [PubMed] [Google Scholar]
- 17.Coyne K.S., Margolis M.K., Bradley L.D., Guido R., Maxwell G.L., Spies J.B. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health. 2012;15:135–142. doi: 10.1016/j.jval.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Harding G., Coyne K.S., Thompson C.L., Spies J.B. The responsiveness of the uterine fibroid symptom and health-related quality of life questionnaire (UFS-QOL) Health Qual Life Outcomes. 2008;6:99. doi: 10.1186/1477-7525-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J.S., Hong G.Y., Lee K.H., Kim T.E. Changes in anti-mullerian hormone levels as a biomarker for ovarian reserve after ultrasound-guided high-intensity focused ultrasound treatment of adenomyosis and uterine fibroid. BJOG. 2017;124(Suppl 3):18–22. doi: 10.1111/1471-0528.14739. [DOI] [PubMed] [Google Scholar]
- 20.Liang E., Brown B., Rachinsky M. A clinical audit on the efficacy and safety of uterine artery embolisation for symptomatic adenomyosis: results in 117 women. Aust N Z J Obstet Gynaecol. 2018 doi: 10.1111/ajo.12767. [DOI] [PubMed] [Google Scholar]
- 21.Nijenhuis R.J., Smeets A.J., Morpurgo M., Boekkooi P.F., Reuwer P.J., Smink M. Uterine artery embolisation for symptomatic adenomyosis with polyzene F-coated hydrogel microspheres: three-year clinical follow-up using UFS-QoL questionnaire. Cardiovasc Intervent Radiol. 2015;38:65–71. doi: 10.1007/s00270-014-0878-1. [DOI] [PubMed] [Google Scholar]
- 22.de Bruijn A.M., Smink M., Hehenkamp W.J.K., Nijenhuis R.J., Smeets A.J., Boekkooi F. Uterine artery embolization for symptomatic adenomyosis: 7-Year clinical follow-up using UFS-Qol questionnaire. Cardiovasc Intervent Radiol. 2017;40:1344–1350. doi: 10.1007/s00270-017-1686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen M.P., Chen C., Brugger A.M. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–414. doi: 10.1016/s1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 24.Longinotti M.K., Jacobson G.F., Hung Y.Y., Learman L.A. Probability of hysterectomy after endometrial ablation. Obstet Gynecol. 2008;112:1214–1220. doi: 10.1097/AOG.0b013e31818c1766. [DOI] [PubMed] [Google Scholar]
- 25.Taran F.A., Weaver A.L., Coddington C.C., Stewart E.A. Characteristics indicating adenomyosis coexisting with leiomyomas: a case-control study. Hum Reprod. 2010;25:1177–1182. doi: 10.1093/humrep/deq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munro M.G., Critchley H.O., Broder M.S., Fraser I.S. Disorders FWGoM. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3–13. doi: 10.1016/j.ijgo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Aguilar H.N., Mitchell B.F. Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update. 2010;16:725–744. doi: 10.1093/humupd/dmq016. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen C.K., Hansen E.S., Dueholm M. Two- and three-dimensional ultrasonographic features related to histopathology of the uterine endometrial-myometrial junctional zone (Accepted for publication) Acta Obstet Gynecol Scand. 2018 doi: 10.1111/aogs.13484. [DOI] [PubMed] [Google Scholar]
- 29.Gompel C.S.S. The corpus uteri. In: Anonymous, editor. Pathology in gynecology and obstetrics. JB Lippincott; Philadelphia: 1985. [Google Scholar]
- 30.Bird C.C., McElin T.W., Manalo-Estrella P. The elusive adenomyosis of the uterus--revisited. Am J Obstet Gynecol. 1972;112:583–593. doi: 10.1016/0002-9378(72)90781-8. [DOI] [PubMed] [Google Scholar]
- 31.Xia W., Zhang D., Zhu Q., Zhang H., Yang S., Ma J. Hysteroscopic excision of symptomatic myometrial adenomyosis: feasibility and effectiveness. BJOG. 2017;124:1615–1620. doi: 10.1111/1471-0528.14673. [DOI] [PubMed] [Google Scholar]
- 32.Maia H., Jr., Maltez A., Coelho G., Athayde C., Coutinho E.M. Insertion of mirena after endometrial resection in patients with adenomyosis. J Am Assoc Gynecol Laparosc. 2003;10:512–516. doi: 10.1016/s1074-3804(05)60158-2. [DOI] [PubMed] [Google Scholar]
- 33.Sheng J., Zhang W.Y., Zhang J.P., Lu D. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception. 2009;79:189–193. doi: 10.1016/j.contraception.2008.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.