Abstract
Objective
To evaluate the indication and performance of systematic lymphadenectomy (SL) in Stage I endometrioid endometrial carcinoma (EEC), at intermediate risk (FIGO IAG2/G3, IBG1/G2) on recurrence, disease-free survival (DFS) and survival.
Study design
194 women underwent hysterectomies by laparotomy, with SL (n = 95) or without SL (n = 99) between 1990 and 2014 was evaluated. Diagnosis period, age, BMI, comorbidities, stage, and adjuvant radiotherapy were analyzed. DFS and cancer-specific survival were analyzed by Kaplan-Meier and log-rank test, and recurrences by Cox regression.
Results
SL was performed in 93% (41/44) of women managed before 1998 and decreasing after that (p < 0.001). SL was also more frequent if BMI under 35.0 kg/m2 (p < 0.001) and in women without comorbidities (p = 0.017). Distribution of age, stage and postoperative radiotherapy were not different between groups. There were 14 recurrences (7.4%), concentrated in the SL group (12 cases) and associated with Stage IAG3 (35.7%, p = 0.009). Longitudinal evaluation exhibited 95% of 5-year cancer-specific survival rate for non-SL group vs. 88% for the SL group (p = 0.039), and DFS rate was 97% for the non-SL group vs. 85% for the SL group (p = 0.004). Cox regression analyses exhibited Stage IAG3 (HR 6.48, IC95% 1.88–22.39; p = 0.003) associated with less DFS.
Conclusion
SL in surgical staging of EEC at intermediate risk presented no benefits regarding recurrences, DFS, and cancer-specific survival rate when compared to patients not submitted to complete surgical staging. Stage IAG3 had poor prognosis regardless treatment modality. Our results provide further evidence to support the current trend to avoid SL in the surgical approach to selected women.
Keywords: Endometrial carcinoma, Lymphadenectomy, Recurrence, Disease-free survival, Survival
Introduction
Endometrial cancer is the most frequent malignant gynecologic neoplasm in developed countries, with 70% of cases diagnosed in the initial stages, with good prognosis and a five-year survival rate of 80–85% [[1], [2], [3]]. Standard surgical staging considers the extent of the disease, particularly the presence of myometrial infiltration (MI), histological type, histological differentiation grade (G) and regional (lymph node) or distant metastasis.
Detection of locoregional metastasis is preferably performed by retroperitoneal lymphadenectomy of the pelvic and/or para-aortic region. The presence of compromised lymph nodes is related to worse prognoses and decreased five-year survival rates (44–52%) [4,5], signaling the need for adjuvant therapy [6].
Despite the importance of lymphadenectomy in staging, the procedure is associated with significant morbidity and it could be omitted depending on the extent of the disease and risk factors [2,7,8]. From previous published studies, the American College of Obstetrics and Gynecology (ACOG) advises that lymphadenectomy may be dispensed in early-stages low-risk patients for lymph node metastasis [9,10].
There are three distinct risk groups for lymph node metastasis, defined with some variations depending on the published study: (1) low risk (27% of the cases, with disease in an initial stage and up to 50% MI and G1), with lymph node metastasis found in <5%. In these cases, the staging surgery may be restricted to total hysterectomy and bilateral salpingo-oophorectomy (BSO) [6,[11], [12], [13]]; (2) the opposite group, at high-risk (24% of the cases, with >50% MI and G3), with lymph node metastasis found in 25%–40%. This group has indication for systematic lymphadenectomy (SL) of the pelvic and para-aortic region, for the correct staging of the neoplasm [[13], [14], [15]]; (3) the third group, at intermediate risk (at least 50% of all cases, when considered endometrioid endometrial carcinoma (EEC) with up to 50% MI and G2 or G3 (FIGO Stage IAG2 or IAG3), and tumors with deep MI (>50%) and G1 or G2 (FIGO Stage IBG1 or IBG2) [13,16], with lymph node metastasis rate between 5% and 25%. Uncertainty persists for this third group in respect of the advantages and disadvantages of SL in the accuracy of definitive staging, as regards possible therapeutic outcome.
This study aims to evaluate the indication and performance of SL carried out over time in women with stage I EEC at intermediate risk and its impact on recurrence, disease free survival (DFS) and cancer survival.
Materials and methods
After obtaining approval from the Research Ethics Committee at the State University of Campinas (UNICAMP) in Campinas (SP), Brazil, we identified from institutional surgical database 1068 women with EC treated with hysterectomy in the period 1990 to 2014 and with no history of other neoplasms, previous chemotherapy or radiotherapy. From this cohort, 291 were under 75 years old, had preoperative Stage I, and were submitted for surgery by laparotomy as first treatment, and exhibited histopathological evaluation with endometrioid-type histology, with MI, and no lymph-vascular space invasion detected. Final Stage was reclassified according to FIGO-2014 staging system [17]. There were 152 women treated with hysterectomy and BSO, and the final Stage were IAG1 for 50, Stage II (cervical invasion) for three, and 99 were at intermediate risk (IAG2/G3, IBG1/G2 - ‘non-SL’ group) [16,17]. Other 139 women were treated with hysterectomy and BSO plus pelvic and para-aortic lymphadenectomy, and 22 had final Stage IAG1, 15 Stage II and three Stage III (lymph node metastasis), with remaining 95 at intermediate risk (‘SL’ group). In all cases, the hysterectomy was performed according to Paver 1 (Querleu A) [18,19], and the pelvic lymphadenectomy consisted of the removal of adipose tissue around the iliac vessels (common, internal and external) bilaterally and in obturator fossa until visualization of the obturator nerve, while the para-aortic lymphadenectomy comprised the peri aortal and pericaval region below the renal vessels. Surgical procedures were performed or supervised by surgeons specialized in gynecological cancer treatment. Until 1998, the recommendations guided complete surgical staging (with SL) in all cases that clinical conditions permitted it. After 1998, the selection of patients to undergo SL started taking into consideration the risk of lymph node disease and more careful evaluation of clinical and/or technical restrictions, as due to obesity. All lymph nodes removed were processed, counted, and sectioned in their half, with both sections were microscopically analyzed. All pathological evaluations were performed by pathologists specialized in gynecological malignancies.
Adjuvant therapy was determined according to woman performance and tumor pattern and stage and guided by Brazilian National Cancer Institute (INCA). The options were no further treatment, or treatment with brachytherapy or brachytherapy plus teletherapy. Adjuvant pelvic radiation therapy usually started within 4 to 6 weeks after surgery using a conventional four-field technique, with dose ranged from 40 Gy in 23 daily fractions to 50.4 Gy in 28 daily fractions. Brachytherapy in vaginal cuff was performed weekly in four fractions and total dose of 28 Gy.
At follow-up visits, all patients received a complete physical and gynecological examination every four months for the first year and every six months for the next five years. Chest radiography, pelvic and abdominal ultrasound exam, CT scan and biopsy were performed when clinical abnormalities were found. Recurrence was defined as histological presence of tumor cells or enlarged lymph nodes or detection of pelvic tumor or distant metastasis.
Information were obtained from medical files of hospital records regarding diagnosis period (1990–1998, 1999–2008 and 2009–2014), age-group (<50, 50–59, 60–69 and >70 years), body mass index-BMI (<35.0 kg/m2 and ≥35.0 kg/m2), presence of comorbidities (diabetes mellitus and/or systemic arterial hypertension and/or cardiovascular disease), surgical and pathological information, final staging, use of adjuvant radiotherapy, relapse and death in the follow-up and considered until 2016.
Statistical analysis
Data were entered into a computerized database and statistical analysis was performed using the StatsDirect statistical software 3.0 (England, www.statsdirect.com), and p values < 0.05 were considered statistically significant.
Categorical variables between groups were analyzed by Chi-Square or Fisher's exact tests. Kaplan-Meier survival curves were assembled and analyzed using the log-rank test, considering DFS - the interval between the end of the treatment and the relapse and cause-specific survival - the interval between the date of surgery and death, and only deaths due to illness or related to treatment were considered. Follow-up was censored at 120 months. Factors possibly associated with recurrence and DFS (p < 0.20) were selected for Cox regression analyses (univariate and multivariate).
Results
The comparison between the groups studied is shown in Table 1. SL was performed in 93% (41/44) of women managed before 1998 and decreasing the proportion after that (p < 0.001). Lymphadenectomy was also more frequent if BMI under 35.0 kg/m2 (p < 0.001) and in women without comorbidities (p = 0.017). There was no difference in age-group distribution (p = 0.128), cancer stage (p = 0.174) and adjuvant radiotherapy (p = 0.156) regarding lymphadenectomy done or not. The mean number of lymph nodes removed in SL group was 12 for the pelvic region and 5 for the para-aortic region (data not shown).
Table 1.
Comparison of women with endometrioid-type endometrial carcinoma at intermediate risk (IAG2G3 and IBG1G2) managed by hysterectomy with or without lymphadenectomy, and according to diagnosis period, the women’s characteristics, cancer stage and adjuvant radiotherapy.
| Characteristics | Lymphadenectomy |
||||
|---|---|---|---|---|---|
| No (n = 99) | Yes (n = 95) | p* | |||
| n | % | n | % | ||
| Period of diagnosis | |||||
| 1990–1998 | 3 | 3.0 | 41 | 43.2 | |
| 1999–2008 | 44 | 44.4 | 35 | 36.8 | |
| 2009–2014 | 52 | 52.6 | 19 | 20.0 | < 0.001 |
| Age-group (in years) | |||||
| <50 | 4 | 4.0 | 8 | 8.4 | |
| 50–59 | 26 | 26.3 | 33 | 34.8 | |
| 60–69 | 46 | 46.5 | 42 | 44.2 | |
| ≥70 | 23 | 23.2 | 12 | 12.6 | 0.128 |
| BMI (kg/m2) | |||||
| <35.0 | 52 | 58.4 | 79 | 83.2 | |
| ≥35.0 | 37 | 41.6 | 16 | 16.8 | < 0.001 |
| Missing information | 10 | 0 | |||
| Comorbidities§ | |||||
| Yes | 73 | 77.7 | 58 | 61.7 | |
| No | 21 | 22.3 | 36 | 38.3 | 0.017 |
| Missing information | 5 | 1 | |||
| Stage (FIGO) | |||||
| IA G2 | 57 | 57.6 | 46 | 48.4 | |
| IA G3 | 5 | 5.0 | 11 | 11.6 | |
| IB G1 | 18 | 18.2 | 13 | 13.7 | |
| IB G2 | 19 | 19.2 | 25 | 26.3 | 0.174 |
| Radiotherapy | |||||
| EBRT (±brachytherapy) | 41 | 41.4 | 49 | 51.6 | |
| Brachytherapy/None | 58 | 58.6 | 46 | 48.4 | 0.156 |
FIGO: International Federation of Gynecology and Obstetrics. EBRT: external beam radiotherapy. BMI: body mass index.
Chi-square test or Fisher's exact test.
Diabetes mellitus and/or systemic arterial hypertension and/or cardiovascular disease.
Four cases (one from the SL group) did not return after performing vaginal cuff brachytherapy and were not followed up. There was one intraoperative death due to uncontrollable bleeding in the non-SL group. Follow-up of the remaining 189 cases, exhibited two deaths (one in each group) caused by bowel complications, four to six months after the end of the radiotherapy and six deaths not related to the disease or treatment (two from the SL group). There were 14 (7.4%) recurrences, nine of which were pelvic (including peritoneal cavity), and five as distant metastases (2-lung, 2-CNS and one neck lymph node, all cases in the SL group; Table 2). All 14 recurred women died, 10 had short post-relapse survival up to 12 months (Table 2).
Table 2.
Cases with recurrence of endometrioid-type endometrial carcinoma at intermediate risk, according to some patient characteristics, management, and recurrence pattern.
| Case (Age) | First treatment |
Recurrence |
Survival post-relapse§ (months) | ||||
|---|---|---|---|---|---|---|---|
| Year | Lymph-adenectomy | Stage* | Adjuvant radiotherapy | Site | Time after treatment (months) | ||
| 1 (75y) | 1996 | No | IBG2 | EBRT | Pelvic | 50 | 4 |
| 2 (73y) | 2014 | No | IAG3 | EBRT | Upper abdomen | 24 | 1 |
| 3 (61y) | 1990 | Yes | IAG2 | VBT | Upper abdomen | 31 | 18 |
| 4 (57y) | 1991 | Yes | IAG2 | EBRT | Neck limph node | 59 | 9 |
| 5 (57y) | 1992 | Yes | IBG2 | EBRT | Lung | 6 | 23 |
| 6 (64y) | 1993 | Yes | IAG2 | EBRT | Pelvic | 26 | 10 |
| 7 (67y) | 1993 | Yes | IAG3 | VBT | CNS | 5 | 1 |
| 8 (68y) | 1993 | Yes | IAG3 | EBRT | Pelvic | 48 | 2 |
| 9 (61y) | 1995 | Yes | IBG1 | EBRT | Upper abdomen | 20 | 5 |
| 10 (70y) | 1996 | Yes | IAG3 | EBRT | Lung | 6 | 3 |
| 11 (47y) | 2004 | Yes | IBG2 | EBRT | Para-aortic | 64 | 34 |
| 12 (65y) | 2008 | Yes | IAG3 | EBRT | CNS | 20 | 7 |
| 13 (62y) | 2009 | Yes | IAG2 | VBT | Vagina | 11 | 7 |
| 14 (64y) | 2010 | Yes | IAG2 | VBT | Para-aortic | 185 | 24 |
EBRT: external beam radiotherapy; VBT: vaginal brachytherapy; CNS: central nervous system; NA: not available.
FIGO Stage/2014 [16]: A=myometrial invasion <50% deep; B=≥50% deep; G=histological grade.
Survival post-relapse: time after recurrence and until death.
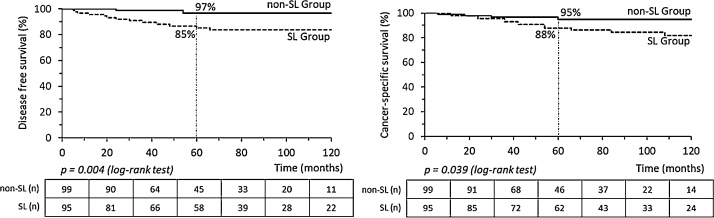
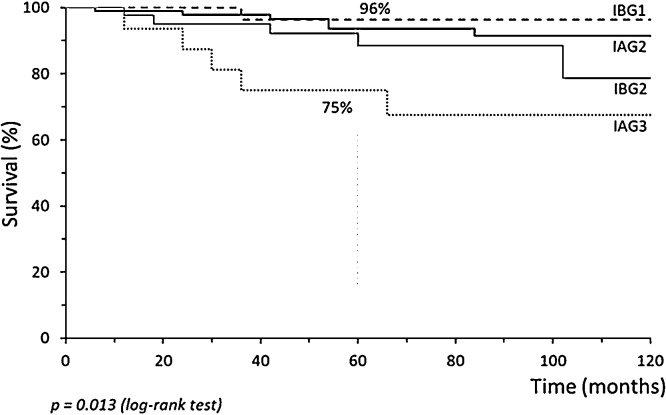
Recurrences were associated with FIGO Stage IAG3 (35.7%, p = 0.009) and concentrated in the SL group (12/14 recurrences; Table 3). Age-group, BMI, comorbidities, and adjuvant radiotherapy were not associated with relapses (Table 3). Survival and DFS time were short when recurrence happened. Cox regression analyses considered the stage, radiotherapy, and lymphadenectomy. Only Stage IAG3 (Hazard Ratio 6.48, IC95% 1.88–22.39, p = 0.003) was associated with less DFS in univariate analysis. In the longitudinal evaluation, DFS of women with intermediate-risk EEC exhibited 97% 5-year survival rate for non-SL group and 85% for SL group (p = 0.004, Fig. 1). Cancer-specific survival curves present significant differences according to whether SL was performed. After five years of follow-up, the survival rate was 95% for the non-SL group vs. 88% for the SL group (p = 0.039, Fig. 1). Comparing cancer stage and tumor grade, stage IAG3 had a worse survival time rate (75% at 5-year vs. 89–97% for other stage, p = 0.013, Fig. 2).
Table 3.
Recurrence rate in women with endometrioid-type endometrial carcinoma at intermediate risk (IAG2G3 and IBG1G2) according to diagnosis period, the women's characteristics, cancer stage, adjuvant radiotherapy and surgery staging, with or without lymphadenectomy.
| Characteristics | Recurrence |
||||
|---|---|---|---|---|---|
| No (n = 175) |
Yes (n = 14) |
p* | |||
| n | % | n | % | ||
| Period of diagnosis | |||||
| 1990–1998 | 34 | 19.4 | 9 | 64.3 | |
| 1999–2008 | 76 | 43.4 | 3 | 21.4 | |
| 2009–2014 | 65 | 37.2 | 2 | 14.3 | 0.002 |
| Age-group (in years) | |||||
| <50 | 11 | 6.3 | 1 | 7.1 | |
| 50–59 | 57 | 32.6 | 2 | 14.3 | |
| 60–69 | 77 | 44.0 | 8 | 57.2 | |
| ≥70 | 30 | 17.1 | 3 | 21.4 | 0.465 |
| BMI (kg/m2) | |||||
| <35.0 | 119 | 72.1 | 11 | 78.6 | |
| ≥35.0 | 46 | 27.9 | 3 | 21.4 | 0.454 |
| Missing information | 10 | ||||
| Comorbidities§ | |||||
| Yes | 116 | 68.6 | 10 | 71.4 | |
| No | 53 | 31.4 | 4 | 28.6 | 0.546 |
| Missing information | 6 | ||||
| Stage (FIGO) | |||||
| IA G2 | 95 | 54.3 | 5 | 35.7 | |
| IA G3 | 11 | 6.3 | 5 | 35.7 | |
| IB G1 | 30 | 17.1 | 1 | 7.1 | |
| IB G2 | 39 | 22.3 | 3 | 21.4 | 0.009 |
| Radiotherapy | |||||
| EBRT (±brachytherapy) | 79 | 45.1 | 10 | 71.4 | |
| Brachytherapy/none | 96 | 54.9 | 4 | 28.6 | 0.052 |
| Lymphadenectomy | |||||
| Yes | 82 | 46.9 | 12 | 85.7 | |
| No | 93 | 53.1 | 2 | 14.3 | 0.004 |
FIGO: International Federation of Gynecology and Obstetrics. EBRT: external beam radiotherapy. BMI: body mass index.
Chi-square test or Fisher’s exact test.
Diabetes mellitus and/or systemic arterial hypertension and/or cardiovascular disease.
Fig. 1.
Disease-free survival (DFS) and cancer-specific survival curves of women with endometrioid-type endometrial carcinoma at intermediate-risk according to whether they underwent systematic lymphadenectomy (SL).
Fig. 2.
Cancer-specific survival curve of women with endometrioid-type endometrial carcinoma at intermediate-risk according to cancer stage and tumor grade.
Comment
SL in EEC of intermediate risk (IAG2G3, IBG1G2) did not bring advantages regarding less relapses or increasing survival over time as compared with women who underwent surgery without lymphadenectomy.
By contrast, according to the possible therapeutic outcome of SL, the SL group exhibited DFS, and cancer-specific survival curves always below the curves of the group treated with hysterectomy and BSO without lymphadenectomy, even though some of these latter cases may have been down-staged.
This worse result observed in SL group can be related to more liberation to perform complete surgical staging in the first period (93.2% of the cases up to 1998 vs. 26.8% after 2008), following the guidelines at that time, which advised complete staging surgery for all candidate patients. Maybe, in that first period, we were less selective and managed cases with worse prognosis, such as those with larger tumors. Tumor size is considered associated with prognosis [13,20,21], but we did not have access to consistent information about it, as in preoperative exams as in pathologic reports.
Additionally, another independent risk factor for lymph node involvement or worse prognosis is histologic grade 3 [13,21] and the cases G3 studied (all Stage IA with MI) were twice more frequent at SL group than non-SL group. Regarding all 14 women with recurrence, five were Stage IAG3 and four performed SL (three with distant metastasis). Although the SL group may have worse cases selected, such as in stage IAG3, even with lymphadenectomy performed, they presented lower survival rate, with no evidence of benefit of complete staging surgery.
Currently, several studies have diverged from the idea of performing SL to obtain more accurate staging. From our 139 women submitted to SL, only three (2.2%, two G3) had lymph node positive and final Stage III. Vargas et al. (2014) adopting some specific risk strata, analyzed almost 20 thousand cases of SEER data from 1988 to 2010 and observed 1.4% of lymph node positive, if low-risk EC, and 6.4% if high-risk [21].
Two meta-analyses have shown that the therapeutic value of SL remains controversial as regards survival rate [3,22] and another recent study found no differences in the reduction of recurrences or mortality related to the performance of SL in stage I endometrial cancer [23]. Two randomized clinical trials with stage I cancer found that performing SL did not improve DFS or cancer-specific survival and the patients could have been spared the procedure and its possible complications [5,6].
While studies tend to point to a lack of benefits of SL in cases of type 1 cancer restricted to the uterine body [[22], [23], [24], [25], [26], [27], [28]], there is no consensus about how to proceed when the EC had an intermediate risk, as the women evaluated here. Longitudinal evaluation of our data exhibited high 5-year survival rate for both groups, although significantly worse for women performed complete surgical staging, including DFS.
The absence of positive impact over SL on cancer-specific survival observed in this study in women with EEC at intermediate risk agrees with some other published studies [3,6,[23], [24], [25]]. Besides the lymph node status, these studies showed that survival is mainly related to stage and risk factors such as myometrial invasion, tumor grade, and histological type. Our results reinforcing what already been put forward in the literature aimed to suppress SL in surgical staging in EEC at intermediate risk [4,8,9,13,23].
Another result worth highlighting was the maintenance of the use of adjuvant radiotherapy even in women who underwent SL, which is currently less indicated in cases of negative lymphadenectomy and without associated risk factors, as guided by more recent studies [13,[26], [27], [28], [29], [30], [31]]. In this study, even in the SL group and considering only women without lymph node involvement, 52% received pelvic radiotherapy, although these were performed before 2010. Recurrences occurred even in women submitted to external beam radiotherapy (EBRT), and this approach did not provide any benefits concerning DFS and cancer-specific survival rates, since the outcomes were no different between the groups. Furthermore, the association between SL and EBRT can increase complications in the follow-up.
Stage I EC presents a good prognosis and the treatment based on surgery and/or radiotherapy is usually associated with non-negligible morbidity and mortality. To these negative events add other prevalent in the pattern of the women with EC, i.e., resulting of older age, obesity and frequent comorbidities. Morbidity and mortality may be partially controlled by reducing the extent of staging surgery and individualize the treatment. Therefore, it is necessary to consider whether SL adds information about disease extension, especially if performed by laparotomy, as evaluated in this study, and how much is used to avoid radiotherapy in cases of negative lymph node involvement. As expected, our cases had less indicated SL in older women, with higher BMI and comorbidities.
Limitations of this study were its retrospective pattern and not considered some relevant risk factors, as lymph-vascular space involvement and tumor size. Strengths of this study were its relatively uniform management, surgery by laparotomy, number of lymph nodes removed, pathological evaluation by pathologists specialized in gynecological malignancies, long-term follow up with high adhesion, and applying established algorithms in a real world.
Relapses were predominated in the first two years of follow up and generally found after symptoms had been reported, similar of the pattern described by Salani et al. in 2011 [32]. Three cases with distant metastasis had G3 primary tumor and happened in the SL group. It is striking to note the poor prognosis of relapses in most of the cases, even treated, which resulted in death after a few months.
Although additional studies in the future can define parameters to select women with Stage I EEC at intermediate risk candidate to perform complete surgical staging, image exams, biomolecular markers, and minimally invasive surgery seems to be a reasonable alternative to control operative complications and costs. Tumors G3 need to be managed more carefully and indication of adjuvant therapeutic, even systemic, can be evaluated.
In conclusion, systematic lymphadenectomy in the surgical staging of EEC patients at intermediate risk presented no benefits regarding recurrence, disease-free survival, cancer-specific survival or mortality when compared to patients who did not undergo complete surgical staging. Our results provide further evidence to support the current trend to avoid systematic lymphadenectomy in the surgical approach to selected women, particularly those with intermediate risk neoplasms.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgement
None [The authors provided the financial support for this study].
References
- 1.IARC; Lyon: 2018. Cancer today. International agency for research on Cancer [Internet]http://gco.iarc.fr/today/online-analysis-table [cited 2018 Oct 21]. Available from. [Google Scholar]
- 2.National Cancer Institute Web site; 2019. Endometrial Cancer Treatment - General Information about Endometrial Cancer.https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf [cited 2018 Oct 27]. Available from. [Google Scholar]
- 3.Kim H.S., Suh D.H., Kim M.K., Chung H.H., Park N.H., Song Y.S. Systematic lymphadenectomy for survival in patients with endometrial cancer: a meta-analysis. Jpn J Clin Oncol. 2012;42:405–412. doi: 10.1093/jjco/hys019. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S., Mariani A., Bakkum-Gamez J.N., Weaver A.L., McGree M.E., Keeney G.L., et al Risk factors that mitigate the role of paraaortic lymphadenectomy in uterine endometrioid cancer. Gynecol Oncol. 2013;130:441–445. doi: 10.1016/j.ygyno.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S., Podratz K.C., Bakkum-Gamez J.N., Dowdy S.C., Weaver A.L., McGree M.E., et la Prospective assessment of the prevalence of pelvic paraaortic and high paraaortic lymph node metastasis in endometrial cancer. Gynecol Oncol. 2014;132:38–43. doi: 10.1016/j.ygyno.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ASTEC study group, Kitchener H., Swart A.M., Qian Q., Amos C., Parmar M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomized study. Lancet. 2009;373(9658):125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward K.K., Shah N.R., Saenz C.C., McHale M.T., Alvarez E.A., Plaxe S.C. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol. 2012;126:176–179. doi: 10.1016/j.ygyno.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Bell J.G., Patterson D.M., Klima J., Harvison M., Rath K., Reid G. Outcomes of patients with low-risk endometrial cancer surgically staged without lymphadenectomy based on intraoperative evaluation. Gynecol Oncol. 2014;134:505–509. doi: 10.1016/j.ygyno.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Frost J.A., Webster K.E., Bryant A., Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. 2015;9 doi: 10.1002/14651858.CD007585.pub3. CD007585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowery W.J., Gehrig P.A., Ko E., Secord A.A., Chino J., Havrilesky L.J. Surgical staging for endometrial cancer in the elderly - is there a role for lymphadenectomy? Gynecol Oncol. 2012;126:12–15. doi: 10.1016/j.ygyno.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Pelikan H.M., Trum J.W., Bakers F.C., Beets-Tan R.G., Smits L.J., Kruitwagen R.F. Diagnostic accuracy of preoperative tests for lymph node status in endometrial cancer: a systematic review. Cancer Imaging. 2013;13:314–322. doi: 10.1102/1470-7330.2013.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teixeira A.M., Ribeiro R., Schmeler K.M., Herzog T.J., Nicolau S.M., Marques R.M. A preoperative and intraoperative scoring system to predict nodal metastasis in endometrial cancer. Int J Gynecol Obstet. 2017;137:78–85. doi: 10.1002/ijgo.12103. [DOI] [PubMed] [Google Scholar]
- 13.Mariani A., Dowdy S.C., Cliby W.A., Gostout B.S., JonesT. Wilson M.B.O. Prospective assessment of lymphatic dissemination in endometrial cancer a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombo N., Creutzberg C., Amant F., Bosse T., González-Martín A., Ledermann J. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Radiother Oncol. 2015;117:559–581. doi: 10.1016/j.radonc.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Ouldamer L., Bendifallah S., Body G., Canlorbe G., Touboul C., Graesslin O. Call for surgical nodal staging in women with ESMO/ESGO/ESTRO high-intermediate risk endometrial cancer a multicentre cohort analysis from the FRANCOGYN study group. Ann Surg Oncol. 2017 doi: 10.1245/s10434-016-5731-0. [DOI] [PubMed] [Google Scholar]
- 16.Dursun P., Ayhan A. Gynecologic oncologist perspective about ESMO-ESGO-ESTRO consensus conference on endometrial cancer. Int J Gynecol Cancer. 2017;27:826–831. doi: 10.1097/IGC.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 17.FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynecol Obstet. 2014;125:97–98. doi: 10.1016/j.ijgo.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Piver M.S., Rutledge F., Smith J.P. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol. 1974;44:265–272. [PubMed] [Google Scholar]
- 19.Querleu D., Morrow C.P. Classification of radical hysterectomy. Lancet Oncol. 2008;9:297–303. doi: 10.1016/S1470-2045(08)70074-3. [DOI] [PubMed] [Google Scholar]
- 20.Sozzi G., Uccella S., Berretta R. Tumor size, an additional risk factor of local recurrence in low-risk endometrial cancer: a large multicentric retrospective study. Int J Gynecol Cancer. 2018;28:684–691. doi: 10.1097/IGC.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 21.Vargas R., Rauh-Hain J.A., Clemmer J. Tumor size, depth of invasion, and histologic grade as prognostic factors of lymph node involvement in endometrial cancer: a SEER analysis. Gynecol Oncol. 2014;133:216–220. doi: 10.1016/j.ygyno.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Cripe J., Handorf E., Brown J., Jain A., Rubin S., Mantia-Smaldone G. National cancer database report of lymphadenectomy trends in endometrial cancer. Int J Gynecol Cancer. 2017;27:1408–1415. doi: 10.1097/IGC.0000000000001005. [DOI] [PubMed] [Google Scholar]
- 23.May K., Bryant A., Dickinson H.O., Kehoe S., Morrison J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst Rev. 2010;1 doi: 10.1002/14651858.CD007585.pub2. CD007585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frost J.A., Webster K.E., Morrison J. Lymphadenectomy for treatment of early-stage endometrial Cancer. JAMA Oncol. 2017;3:117–118. doi: 10.1001/jamaoncol.2016.4873. [DOI] [PubMed] [Google Scholar]
- 25.Benedetti Panici P., Basile S., Maneschi F., Alberto Lissoni A., Signorelli M., Scambia G. Systematic pelvic lymphadenectomy vs. No lymphadenectomy in early-stage endometrial carcinoma randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 26.C.L. Creutzberg, W.L. van Putten, P.C. Koper, M.L. Lybeert, J.J. Jobsen, C.C. Wárlám-Rodenhuis, et al.,Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomized trial. PORTEC Study Group. Post-Operatived Radiation Therapy in Endometrial Carcinoma Lancet 2000; 355: (9213) 1404–1411. [DOI] [PubMed]
- 27.Karalok A., Turan T., Basaran D., Turkmen O., Comert Kimyon G., Tulunay G. Lymph node metastasis in patients with endometrioid endometrial cancer overtreatment is the main issue. Int J Gynecol Cancer. 2017;27:748–753. doi: 10.1097/IGC.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 28.Boyraz G., Salman M.C., Gultekin M., Basaran D., Cagan M., Ozgul N. Incidence of lymph node metastasis in surgically staged FIGO IA G1/G2 endometrial cancer With a tumor size of more than 2 cm. Int J Gynecol Cancer. 2017;27:486–492. doi: 10.1097/IGC.0000000000000919. [DOI] [PubMed] [Google Scholar]
- 29.Creutzberg C.L., van Stiphout R.G., Nout R.A., Lutgens L.C., Jürgenliemk-Schulz I.M., Jobsen J.J. Nomograms for prediction of outcome with or without adjuvant radiation therapy for patients with endometrial cancer: a pooled analysis of PORTEC-1 and PORTEC-2 trials. Int J Radiat Oncol Biol Phys. 2015;91:530–539. doi: 10.1016/j.ijrobp.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Wortman B.G., Creutzberg C.L., Putter H., Jürgenliemk-Schulz I.M., Jobsen J.J., Lutgens L.C.H.W. Ten-year results of the PORTEC -2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer Oct. 2018 doi: 10.1038/s41416-018-0310-8. Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kupets R., Le T., Bentley J., Farrell S., Fortier M.P., Giede C. The role of adjuvant therapy in endometrial cancer. J Obstet Gynaecol Can. 2013;35:375–379. doi: 10.1016/S1701-2163(15)30968-3. [DOI] [PubMed] [Google Scholar]
- 32.Salani R., Nagel C.I., Drennen E., Bristow R.E. Recurrence patterns and surveillance for patients with early stage endometrial cancer. Gynecol Oncol. 2011;123:205–207. doi: 10.1016/j.ygyno.2011.07.014. [DOI] [PubMed] [Google Scholar]