Abstract
Objective
C-type lectin domain family 2, member D (CLEC2D) is implicated in the immune response. Pre-eclampsia and HIV infection have opposing immune responses. In view of the high prevalence of HIV infection and pre-eclampsia in South Africa, this study assessed the placental immuno-expression of CLEC2D in HIV associated pre-eclampsia.
Method
Placental tissue was obtained from 60 pregnancies which were categorized according to pregnancy type (pre-eclamptic or normotensive) and HIV status (positive or negative). Immunohistochemistry and morphometric image analysis were used to evaluate placental CLEC2D immuno-expression.
Results
CLEC2D expression was significantly decreased in the conducting villi of pre-eclamptic vs normotensive placentae (p = 0.0418) but was increased in the exchange villi, albeit non-significant (p = 0.4948). HIV positive status intensified placental CLEC2D immuno-expression in conducting (p = 0.0312) and exchange (p = 0.0025) villi. CLEC2D expression was significantly different in exchange vs conducting villi (p < 0.0001) and across study groups (p = 0.0003). Normotensive; HIV negative placentae (control) had a non-significant difference in CLEC2D expression across villi types, however significant difference was noted within the remaining groups: normotensive; HIV positive (p < 0.05), pre-eclamptic; HIV positive (p < 0.01 and pre-eclamptic; HIV negative (p < 0.001).
Conclusion
The contrasting expression of CLEC2D in HIV infection and pre-eclampsia is demonstrative of the immunosuppressive and pro-inflammatory roles of the respective pathologies. However, this implication may be confounded by highly active anti-retroviral treatment (HAART).
Abbreviations: CLEC2D, C-type lectin domain family 2, member D; HIV, Human Immunodeficiency Virus; PE, pre-eclampsia; LLT1, lectin like transcript; OCIL, osteoclast inhibiting lectin; NK, natural killer; ITAMs, immunoreceptor tyrosine-based activation motifs; HAART, highly active anti-retroviral treatment
Keywords: CLEC2D, Pre-eclampsia, HIV, LLT1, OCIL, CLEC, Preeclampsia, Hypertension, Pregnancy
Introduction
The top 3 causes of maternal mortality in South Africa are non-pregnancy related infections (HIV and TB), hypertension in pregnancy and obstetric hemorrhage [1]. Moreover, one-fifth of women in their reproductive ages are HIV infected [2].
Pre-eclampsia (PE) is a pregnancy-specific hypertensive disorder, defined as a BP ≥ 140 mmHg systolic and/or ≥90 mmHg diastolic [3]. It is a multifactorial condition, accompanied by one or more of the following: proteinuria, thrombocytopenia, hemolysis, acute kidney injury, neurological features, liver dysfunction, or fetal growth restriction [3]. Pre-eclampsia may be classified as early onset (EOPE) or late onset (LOPE) PE based on gestational age, the former associated with intrauterine growth restriction [4,5]. Pre-eclampsia is a two-stage disorder, the first 1st stage involves inadequate placentation and the non-physiological conversion of spiral arteries [6]. This creates a hypoxic micro-environment, leading to elevated oxidative stress and anti-angiogenic factors [7]. The 2nd stage involves the clinical manifestation of this systemic disease [6].
During normal pregnancy, due to the presence of the placenta there is a shift from Th1 to Th2 immune response, however, in PE this shift does not occur [8,9]. In contrast, during HIV infection, there is a shift from Th1 to Th2 immune response [10]. Hence, it is likely that co-morbidity of these two conditions may stabilize the immune response [11].
C-type lectins (CLECs) contain at least one characteristic C-type lectin-like domain and function physiologically in development, angiogenesis and inflammation because of their ability to identify exogenous and endogenous ligands [12]. CLEC2D transcripts include lectin like transcript (LLT1) and osteoclast inhibiting lectin (OCIL) [13,14].
In PE, there is an increased activation of circulating natural killer (NK) cells, this may be liable for the induction of lysis of trophoblast cells, which lack HLA-G [15]. Nevertheless, NK cells produce angiogenic factors which are imbalanced in PE [16]. HIV infection induces CLEC2D expression on lymphocytes, resulting in the inhibition of NK cell mediated cytotoxic response and the initiation of IFN-γ release by CD8 + T lymphocytes [17,18]. Moreover, CLEC2D is associated with the protection of B lymphocytes following viral infection [19,20]. In addition, HIV-CLEC2 interaction causes platelet activation which mediates the platelet capture of HIV virions [20,21]. The binding of HIV to platelets leads to the internal replication and multiplication of HIV virions [20,22].
Whilst the role of CLECs in immunity is well established, there is a paucity of data regarding the role of CLEC2D in homeostasis and angiogenesis [23]. Furthermore, the role of this protein in the exaggerated immune response of PE remains obscure. Moreover, to our knowledge, there is a lack of data on placental CLEC2D expression in HIV associated PE. Therefore, this study aims to morphometrically analyze CLEC2D immuno-expression in placental conducting and exchange villi, based on pregnancy type (normotensive vs PE), HIV status (HIV- vs HIV+) and across all study groups.
Materials and methods
This prospective study was performed during the period January–December 2018.
Ethical considerations
This study received institutional ethics approval (BCA 338/17), hospital managers consent, and informed consent was obtained from all participants.
Study population
The study population (n = 60) consisted of pre-eclamptic (n = 30; PE) and normotensive (n = 30; N) women attending the obstetric unit of a large regional hospital in Durban, KwaZulu-Natal. Study groups were stratified into HIV negative (n = 15; PE- and N-) and HIV positive (n = 15; PE + and N+).
Inclusion criteria
Pre-eclampsia was defined as a sustained systolic blood pressure ≥140 mmHg and a diastolic blood pressure ≥90 mmHg at least 4 h apart, and proteinuria after 20 weeks’ gestation in a previously normotensive patient [24]. HIV status was determined by a rapid test.
Exclusion criteria
Participants with chronic hypertension, diabetes mellitus, gestational diabetes, heart failure, epilepsy chronic renal disease, unknown HIV status, intrauterine death, abruptio placentae, systemic lupus erythematous, antiphospholipid antibody syndrome, chorioamnionitis, thyroid disease, sickle cell anemia, and participants treated with aspirin, non-steroidal anti-inflammatory drugs and asthma medication were excluded.
Methods
This study utilizes wax embedded placental samples that were retrospectively collected. The original samples were fixed in 10% buffered formaldehyde and embedded into paraffin wax blocks as per standard laboratory procedure [25]. Sections of placental tissue were cut (3 μm) using a rotary microtome and mounted onto coated slides (X-tra Adhesive, Leica Microsystems, Germany). Samples were de-paraffinized and rehydrated.
Immunostaining
Immunostaining was performed using the EnVision FLEX mini kit, high pH, (DAKO, Denmark) as per the manufacturer’s instructions. Antigen retrieval was achieved by preheating (5 min) and incubating in a target retrieval solution (Envision FLEX DAKO, Denmark) for 20 min at 95 °C. Slides were rinsed and washed in wash buffer (5 min). Post encircling sections with a de-limiting pen (pap pen; DAKO, Denmark), endogenous peroxidase was blocked (5 min). Thereafter, sections were incubated in the primary CLEC2D antibody for 2 h (Abcam, ab197341;1:200; diluent-DAKO REAL diluent) at room temperature. Post washing, the sections were incubated with horseradish peroxidase (20 min.). Detection of immunoreactivity was performed with diamino-benzidine (DAKO, Denmark). All sections were counterstained with Mayer’s hematoxylin, dehydrated and mounted. Negative controls were performed by replacing the primary antibody with non-immune sera of the same IgG isotype as the primary antibody. The primary antibody was replaced with PBS in buffer controls. Thyroid cancer served as the positive control.
Morphometric analysis
The Axioscope A1 microscope (Carl Zeiss, Germany) was used to view the prepared placental tissue sample and a random selection of 4 fields of view per slide was analyzed at an objective magnification of ×20. AxioVision Image Analysis Software Version 4.8.3 (Carl Zeiss, Germany) was used to acquire, capture, process and analyze the images. By using the auto-measure mode of the software, CLEC2D immuno-expression was evaluated as a percentage of immunostaining per frame area. Frame size was expressed as field of frame in μm2. The entire frame was selected for exchange villi. The amount of CLEC2D immunostaining (green) within the placental exchange villi (red) was established by a two-phase threshold, thus areas of immunostaining were segmented and defined as a field area percentage for each villi type.
Data analysis techniques
An excel database was created to compare CLEC2D expression across all groups, between pregnancy types and by HIV status.
Statistical analysis
Sample size was determined using Cohens’ formula. Results were tested for normality prior to analysis. Two-way ANOVA was performed to examine the effect of HIV status (HIV- vs HIV+) and pregnancy type (normotensive vs pre-eclamptic) and across all groups. Bonferroni post hoc test was used to further assess the data between subcategories.
A probability level of p < 0.05 was considered statistically significant. All statistical analyses were conducted using GraphPad Prism™ version 5.01 (San Diego, CA, USA).
Results
Clinical characteristics
Maternal age (p = 0.0046), parity (p = 0.0055) and placental weight (p = 0.0259) were significantly different across groups. As expected, the diastolic (p = <0.0001) and systolic (p = <0.0001) blood pressure differed significantly (Table 1). There was one twin pregnancy in the HIV positive PE group.
Table 1.
Patient demographics in normotensive HIV negative; normotensive HIV positive; pre-eclamptic HIV negative and pre-eclamptic HIV positive pregnant women.
| Normotensive pregnant women |
Pre-eclamptic women |
||||
|---|---|---|---|---|---|
| n | HIV negative (N−) 15 |
HIV positive (N+) 15 |
HIV negative (PE−) 15 |
HIV positive (PE+) 15 |
p value |
| Maternal age (years) | 18 (18–20) | 23 (20–27) | 20 (18–24) | 23 (20–26) | p = 0.0046** |
| Gestational age (weeks) | 38 (37–39) | 40 (39–40) | 38 (35–40) | 38 (37–39) | p = 0.0569 |
| Parity | 1(1) | 1(1) | 1(1) | 1 (1–2) | p = 0.0055** |
| Systolic blood pressure (mmHg) | 120 (116–125) | 117 (112–121) | 152 (146–162) | 144 (136–169) | p = <0.0001*** |
| Diastolic blood pressure (mmHg) | 70 (63–74) | 68 (63–72) | 101 (92–108) | 95 (92–100) | p = <0.0001*** |
| Maternal weight (kg) | 80 (62–85.9) | 80 (68.5–89.1) | 71 (64–82.95) | 71 (64.1–89.7) | p = 0.6304 |
| Maternal BMI (kg/m2) | 32.07 (28.08–35.09) |
29.38 (26.1–33.18) |
29.26 (27.31–33.18) |
29.01 (26.84–36.39) |
p = 0.8118 |
| Placental weight (g) | 450 (400–500) | 550 (490–600) | 510 (450–560) | 500 (480–510) | p = 0.0259* |
| Baby weight (kg) | 3.1 (2.77–3.4) | 3.5 (3–3.65) | 3.2 (2.65–3.5) | 3 (2.8–3.6) | p = 0.3427 |
Summary statistics are presented as median and interquartile range, n = 60.
p < 0.05.
p < 0.01.
p < 0.001.
CLEC2D placental immunolocalization
Conducting (stem) and exchange (intermediate and terminal) were immunostained with CLEC2D across all study groups. CLEC2D immuno-reactivity was observed in syncytiotrophoblast and cytotrophoblast cell populations across villi types [conducting (Fig. 1a–d) and exchange (Fig. 2a–d)]. The mesenchymal core of the villi was non-reactive. The positive control displayed CLEC2D immunostaining while buffer and method controls produced no staining.
Fig. 1.
CLEC2D immunoreactivity in conducting villi. (a) HIV negative, normotensive healthy controls (N−); (b) HIV positive, normotensive (N+); (c) HIV negative, pre-eclamptic (PE−) and (d) HIV positive, pre-eclamptic (PE+).
Fig. 2.
CLEC2D immunoreactivity in exchange villi. (a) HIV negative, normotensive healthy controls (N−); (b) HIV positive, normotensive (N+); (c) HIV negative, pre-eclamptic (PE−) and (d) HIV positive, pre-eclamptic (PE+).
Morphometric image analysis of placental CLEC2D
Conducting villi
The frame area of conducting villi within each group was: PE+: 267036 μm2 ± 17574 μm2; PE-: 266350 μm2 ± 18185 μm2; N+: 239141 μm2 ± 25923 μm2; N-: 253306 μm2 ± 14722 μm2.
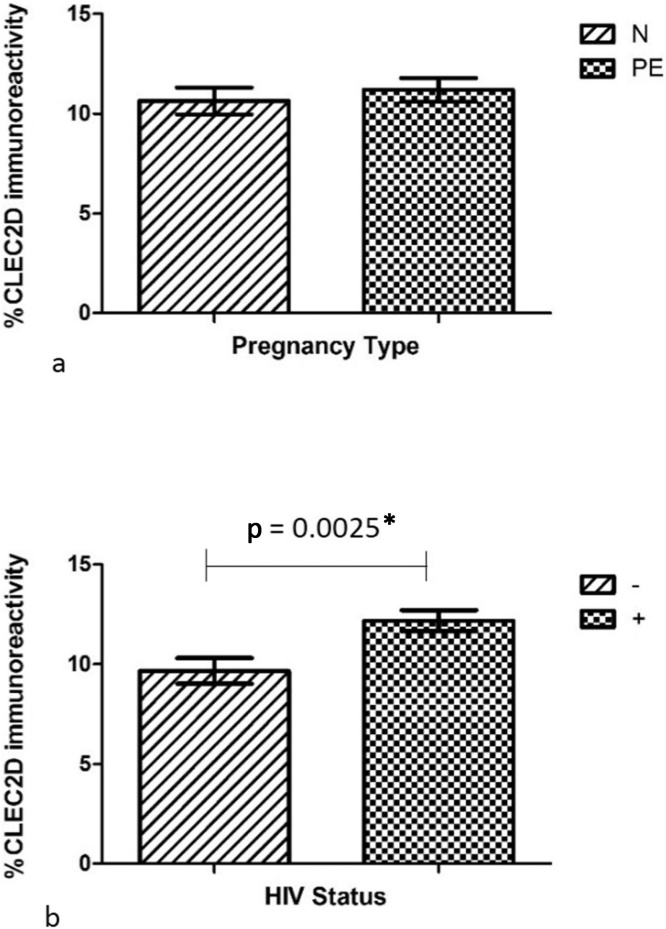
The field area percentage of CLEC2D immunoreactivity within each group was PE+: 7.399 ± 0.6334%; PE-: 6.001 ± 0.5664%; N+: 9.713 ± 1.364%; N-: 7.289 ± 0.6374%. CLEC2D immuno-expression within conducting villi differed based on HIV status (irrespective of pregnancy type) [F(1.56) = 4.89, p = 0.0312] (Fig. 3b). Similarly, CLEC2D immuno-expression within conducting villi was significantly different between normotensive and PE, irrespective of HIV status [F(1.56) = 4.34, p = 0.0418] (Fig. 3a). However, the Bonferroni multiple comparison test showed no interaction across all groups [PE + vs PE-; PE + vs N+; PE- vs N-; N + vs N-; F(1.56) = 0.35, p = 0.5552] (Fig. 5).
Fig. 3.
CLEC2D immunoreactivity in conducting villi based on (a) pregnancy type and (b) HIV status.
Fig. 5.
CLEC2D immunoreactivity based on villi type across all groups.
Exchange villi
The frame area of was kept consistent throughout (598907.2739 μm2), however the area of exchange villi within each group was PE+: 229770 μm2 ± 13392 μm2, PE-: 287870 μm2 ± 13692 μm2, N+: 253084 μm2 ± 12650 μm2, N-: 256649 μm2 ± 13198 μm2.
The field area percentage of CLEC2D immunoreactivity within each group was PE+: 11.40 ± 0.7062%, PE-: 10.99 ± 0.9482%, N+: 12.96 ± 0.7358%, N-: 8.336 ± 0.7628%. CLEC2D immuno-expression within exchange villi was significantly different based on HIV status (irrespective of pregnancy type) [F(1.56) = 10.05, p = 0.0025] (Fig. 4b) and interaction across all groups [F(1.56) = 7.03, p = 0.0104]. However, there was no significant difference in the CLEC2D immuno-expression within exchange villi between PE and normotensive women, irrespective of HIV status [F(1.56) = 0.47, p = 0.4948] (Fig. 4a).
Fig. 4.
CLEC2D immunoreactivity in exchange villi based on (a) pregnancy type and (b) HIV status.
CLEC2D immuno-expression between villi types
A two-way ANOVA was used to assess the interactions based on villi type. CLED2D immuno-expression was significant between exchange and conducting villi irrespective of study group [F(1.112) = 32, p < 0.0001]; the study groups had a very significant effect on CLEC immuno-expression [F(3.112) = 6.78, p = 0.0003]. The Bonferroni post hoc test was used to determine significance between each group (based on percentage CLEC2D immuno-expression of conducting vs exchange villi), [N-: p > 0.05, N+: p < 0.05, PE-: p < 0.001, PE+: p < 0.01] (Fig. 5).
Discussion
This novel study demonstrates a significant decrease of CLEC2D expression in the conducting villi of pre-eclamptic vs. normotensive placentas, irrespective of HIV status. Whilst there is no existing literature on placental CLEC2D expression in PE, Apps et al., (2011) reported a high expression of CLEC2D in villous and extra-villous trophoblasts in normotensive pregnancy [26]. The CLEC2D down-regulation observed in our study may be attributed to the immune exacerbation that occurs in PE [[27], [28], [29]].
In normal pregnancy, inflammatory modulators such as CLEC2D maintain a balance between maternal-fetal immune responses, by regulating NK cell activity [30]. Whilst NK cells are important for placentation, an exaggerated presence of NK cells may lead to complications during pregnancy [29]. In PE, an exacerbated inflammatory response is a result of elevated NK cells [31]. Since CLEC2D binds to the NK cell receptor- NKR-P1, it is no longer available to mediate its function [30,32].
Suzuki-Inoue, (2014) discussed CLEC2-podoplanin interactions in promoting platelet activation and the impediment of migration and growth of lymphatic endothelial cells [33]. Furthermore, platelet activation by CLEC2-podoplanin interactions assist in remodeling of the spiral arteries during normal placentation [33,34]. Additionally, CLECs facilitate immune suppression, metastasis and tumor invasion by assisting the interactions of cancer cells with platelets, endothelial cells and leukocytes [35]. CLEC2 and cancerous-podoplanin expressing cells may mediate platelet aggregation, thus playing a protective role against leukocytes and shear stress whilst promoting angiogenesis, metastasis and tumor development [36]. In normal pregnancy, the villous stroma of the placenta contains a strong expression of podoplanin [37]. Platelets may be exposed to higher levels of placental podoplanin during angiogenesis, and suppression of CLEC2D may decrease platelet activation [38]. Hence, the diminished presence of CLEC2D in our findings, may also contribute to thrombocytopenia and the anti-angiogenic properties associated with PE.
We further report an up-regulatory, albeit non-significant trend of placental CLEC2D expression in exchange villi of PE vs normotensive pregnancies, regardless of HIV. Lin et al. indicated that villous tree geometry affects the oxygen exchange capabilities, which influences angiogenesis [39]. An increased villi density was observed in PE as a compensatory response to the hypoxic micro-environment [40]. Additionally, Sankar et al., (2013) found that PE has an area reductive effect of conducting villi, but no effect on exchange villi was observed [41]. Notably, a reduced area in conducting villi would result in a decreased CLEC2D expression.
Although the exaggerated micro-environment of PE suppresses the anti-inflammatory effector function of CLEC2D, HIV progression is enhanced by CLEC2D. This is the first demonstration of a significant up-regulation of CLEC2D in both conducting and exchange villi based on HIV status. Viral glycoproteins are recognized by cells expressing CLECs and promotes an anti-viral response. Alternatively, DC-SIGN (CLEC2 family member) recognizes HIV leading to infection of dendritic cells and subsequent trans-infection in CD4 + T cells [42]. C type lectin polymorphisms correlate with viral disease susceptibility and progression [43]. Activated endothelial cell CLECs induce a pro-inflammatory response that contributes to pathology in conditions of viral infection such influenza and Ebola virus [44,45].
CLEC2D is responsible for detecting alterations in the expression of MHC class 1 molecules and thus mediates NK cell activity accordingly [46]. HIV compensates for this by its mimicry of platelet integrins (αIIbβ3) and by the expression of CLEC2 on viral proteins [22]. This result is representative of the immunosuppressive role of HIV and the inhibitory mechanisms of CLEC2D, as well as the protective effect CLEC2D serves for HIV infected lymphocytes.
Our study further demonstrates a significant increase in CLEC2D immune-expression in exchange vs conducting villi, irrespective of study group. Additionally, a significant difference was noted between groups, irrespective of villi type. Furthermore, we demonstrated a significant difference in CLEC2D immuno-expression in villi types, between groups. The control group (normotensive; HIV negative group) yielded a non-significant result suggesting that CLEC2D remains unchanged under non-pathological conditions. Moreover, the highest significance existed between the PE- group (p < 0.001), suggesting that this was an exaggerated inflammatory response, followed by the PE+ group (p < 0.01), and the N+ (p < 0.05).
The combined effect of the exaggerated immune response in PE and the immunosuppressive influence of HIV, the maternal-fetal interface, requires a finessing of the immunological balance between exhibiting the maternal defence to pathogens whilst encouraging tolerance to the allogenic fetus [47]. Thus, the combined actions of CLEC2D play protective and antagonistic roles in HIV associated PE. Additionally, Highly Active Anti-retroviral Therapy (HAART) is the standard of care for HIV infected individuals. Phoswa et al. suggests that although HAART suppresses HIV by restoring NK cell abilities, this activation may lead to dysregulated trophoblast invasion during pregnancy and potentially lead to PE [48].
Limitations of this study include the heterogeneity of the study population; early and late onset PE. The duration and time of initiation of HAART therapy was absent. It is possible that HAART may have confounded immuno-expression of CLEC2D.
In conclusion, this novel study highlights a down-regulation of CLEC2D immuno-staining in the conducting villi of PE which may be attributable to the CLEC2D- NKR-P1 binding that occurs in the high inflammatory milieu of PE. Moreover, this decline may contribute to thrombocytopenia and the anti-angiogenic state associated with PE. We also demonstrate an enhanced trend of placental CLEC2D expression in exchange villi of PE occurring as a compensatory response of the villous tree geometry in the hypoxic micro-environment. Moreover, we show significant difference in CLEC2D expression based on villi type. Additionally, we have established a significant increase of CLEC2D in botconducting and exchange villi based on HIV status validating the anti-viral response of CLEC2D. It is plausible that antagonistic action of CLEC2D are dependent on the mediation of PE, HIV infection and PE co-morbid with HIV infection, and more importantly may be confounded by HAART. Future recommendations include investigating polymorphisms and SNP analysis of CLEC2D on a larger sample size and considering gestational age of PE.
Conflict of interest
The authors have no conflicts of interest to declare.
Contributor Information
Deneshree Varaden, Email: dvaraden@gmail.com.
Thajasvarie Naicker, Email: naickera@ukzn.ac.za.
References
- 1.2016. Saving mothers report. Saving mothers 2014–2016: seventh triennial report on confidential enquiries into maternal deaths in South Africa: short report. [Google Scholar]
- 2.Statistics South Africa . 2018. Mid-year population estimates.www.statssa.gov.zainfo@statssa.gov.za [Google Scholar]
- 3.Brown M.A., Magee L.A., Kenny L.C., Karumanchi S.A., Mccarthy F.P., Saito S. Hypertensive disorders of pregnancy hypertensive disorders of pregnancy. ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 4.Tranquilli A.L., Brown M.A., Zeeman G.G., Dekker G., Sibai B.M. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Pregnancy Hypertens: An Int J Women’s Cardiovasc Health. 2013;3(1):44–47. doi: 10.1016/j.preghy.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Van Der Merwe J.L., Hall D.R., Wright C., Schubert P., Grové D. Are early and late preeclampsia distinct subclasses of the disease—what does the placenta reveal? Hypertens Pregnancy. 2010;29:457–467. doi: 10.3109/10641950903572282. [DOI] [PubMed] [Google Scholar]
- 6.Roberts J.M., Hubel C.A. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30:32–37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redman C.W.G., Staff A.C. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol. 2015;213(4):S9. doi: 10.1016/j.ajog.2015.08.003. e1-S9.e4. [DOI] [PubMed] [Google Scholar]
- 8.Laresgoiti-servitje E., Go N., Olson D.M. An immunological insight into the origins of pre-eclampsia. Hum Reprod Update. 2010;16(5):510–524. doi: 10.1093/humupd/dmq007. [DOI] [PubMed] [Google Scholar]
- 9.Hu W., Wang H., Wang Z., Huang H., Dong M. Elevated serum levels of interleukin-15 and interleukin-16 in preeclampsia. J Reprod Immunol. 2007;73:166–171. doi: 10.1016/j.jri.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Fiore S., Newell M., Trabattoni D., Thorne C., Gray L., Savasi V. Antiretroviral therapy-associated modulation of Th1 and Th2 immune responses in HIV-infected pregnant women. J Reprod Immunol. 2006;70:143–150. doi: 10.1016/j.jri.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Bendtsen F. Pre-eclampsia, antiretroviral therapy, and immune reconstitution. Lancet. 2002;360:2000–2002. doi: 10.1016/s0140-6736(02)11195-0. [DOI] [PubMed] [Google Scholar]
- 12.Brown G.D., Willment J.A., Whitehead L. C-type lectins in immunity and homeostasis. Nat Rev Immunol. 2018;18(6):374–389. doi: 10.1038/s41577-018-0004-8. [DOI] [PubMed] [Google Scholar]
- 13.Aust J.G., Gays F., Mickiewicz K.M., Buchanan E., Brooks C.G. The expression and function of the NKRP1 receptor family in C57BL/6 mice. J Immunol. 2009;183(1):106–116. doi: 10.4049/jimmunol.0804281. [DOI] [PubMed] [Google Scholar]
- 14.Kveberg L., Dai K.Z., Westgaard I.H., Daws M.R., Fossum S., Naper C. Two major groups of rat NKR-P1 receptors can be distinguished based on chromosomal localization, phylogenetic analysis and Clr ligand binding. Eur J Immunol. 2009;39(2):541–551. doi: 10.1002/eji.200838891. [DOI] [PubMed] [Google Scholar]
- 15.Moffett A. 2015. Co-evolution of NK receptors and HLA ligands in humans is driven by reproduction; pp. 283–297. [DOI] [PubMed] [Google Scholar]
- 16.Fukui A., Yokota M., Funamizu A., Nakamua R., Fukuhara R., Yamada K. 2012. Changes of NK cells in preeclampsia. [DOI] [PubMed] [Google Scholar]
- 17.Ramalingam D., Kieffer-Kwon P., Ziegelbauer J.M. Emerging themes from EBV and KSHV microRNA targets. Viruses. 2012;4(9):1687–1710. doi: 10.3390/v4091687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew S.O., Chaudhary P., Powers S.B., Jamboor K., Mathew P.A. Overexpression of LLT1 (OCIL, CLEC2D) on prostate cancer cells inhibits NK cell-mediated killing through LLT1-NKRP1A (CD161) interaction. Oncotarget. 2016;7(42) doi: 10.18632/oncotarget.11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkham C.L., Carlyle J.R. Complexity and diversity of the NKR-P1: Clr (Klrb1: Clec2) recognition systems. Front Immunol. 2014;5:1–16. doi: 10.3389/fimmu.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaipan C., Soilleux E.J., Simpson P., Hofmann H., Gramberg T., Marzi A. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80(18):8951–8960. doi: 10.1128/JVI.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onyangunga O., Moodley J., Odun-ayo F., Naicker T. 2018. Immunohistochemical localization of podoplanin in the placentas of HIV-positive preeclamptic women; pp. 1–11. [DOI] [PubMed] [Google Scholar]
- 22.Nurden A.T., Nurden P., Sanchez M., Andia I., Eduardo A. Platelets and wound healing. Front Biosci. 2008;2(i):3532–3548. doi: 10.2741/2947. [DOI] [PubMed] [Google Scholar]
- 23.Dambuza I.M., Brown G.D. C-type lectins in immunity: recent developments. Curr Opin Immunol. 2015;32:21–27. doi: 10.1016/j.coi.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.2009. SAICD coding. South African ICD-10 coding standards; p. 3. [Google Scholar]
- 25.Burton G.J., Sebire N.J., Myatt L., Tannetta D., Wang Y., Sadovsky Y. Optimising sample collection for placental research. Placenta. 2014;35(1):9–22. doi: 10.1016/j.placenta.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Apps R., Sharkey A., Gardner L., Male V., Trotter M., Miller N. Genome-wide expression profile of first trimester villous and extravillous human trophoblast cells. Placenta. 2011;32(1):33–43. doi: 10.1016/j.placenta.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann S.C., Schellack C., Textor S., Konold S., Schmitz D., Cerwenka A. Identification of CLEC12B, an inhibitory receptor on myeloid cells. J Biol Chem. 2007;282(31):22370–22375. doi: 10.1074/jbc.M704250200. [DOI] [PubMed] [Google Scholar]
- 28.Thebault P., Lhermite N., Tilly G., Le Texier L., Quillard T., Heslan M. The C-type lectin-like receptor CLEC-1, expressed by myeloid cells and endothelial cells, is up-regulated by immunoregulatory mediators and moderates T cell activation. J Immunol. 2009;183(5):3099–3108. doi: 10.4049/jimmunol.0803767. [DOI] [PubMed] [Google Scholar]
- 29.Bonney E.A. Preeclampsia: a view through the danger model. J Reprod Immunol. 2007;76(1–2):68–74. doi: 10.1016/j.jri.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannibal R.L., Baker J.C. Selective amplification of the genome surrounding key placental genes in trophoblast giant cells. Curr Biol. 2016;26(2):230–236. doi: 10.1016/j.cub.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 31.Loewendorf A.I., Nguyen T.A., Yesayan M.N., Kahn D.A. Preeclampsia is characterized by fetal NK cell activation and a reduction in regulatory t cells. Am J Reprod Immunol. 2015;74(3):258–267. doi: 10.1111/aji.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartel Y., Bauer B., Steinle A. Modulation of NK cell function by genetically coupled C-type lectin-like receptor/ligand pairs encoded in the human natural killer gene complex. Front Immunol. 2013;4:1–10. doi: 10.3389/fimmu.2013.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki-Inoue K. CLEC1B (C-type lectin domain family 1, member B) Atlas Genet Cytogenet Oncol Haematol. 2014;18(7):457–460. [Google Scholar]
- 34.Lowe K.L., Finney B.A., Deppermann C., Hägerling R., Gazit S.L., Frampton J. Podoplanin and CLEC-2 drive cerebrovascular patterning and integrity during development. Blood. 2015;125(24):3769–3777. doi: 10.1182/blood-2014-09-603803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding D., Yao Y., Zhang S., Su C., Zhang Y. C-type lectins facilitate tumor metastasis (Review) Oncol Lett. 2017;13(1):13–21. doi: 10.3892/ol.2016.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirai T., Inoue O., Tamura S., Tsukiji N., Sasaki T., Endo H. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J Thromb Haemost. 2017;15(3):513–525. doi: 10.1111/jth.13604. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y., Sun J., Gu Y., Zhao S., Groome L.J., Steven J. D2-40/podoplanin expression in the human placenta. Placenta. 2012;32(1):27–32. doi: 10.1016/j.placenta.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardy A.T., Palma-Barqueros V., Watson S.K., Malcor J.D., Eble J.A., Gardiner E.E. Significant hypo-responsiveness to GPVI and CLEC-2 agonists in pre-term and full-term neonatal platelets and following immune thrombocytopenia. Thromb Haemost. 2018;118(6):1009–1020. doi: 10.1055/s-0038-1646924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin M., Mauroy B., James J.L., Tawhai M.H., Clark A.R. A multiscale model of placental oxygen exchange: the effect of villous tree structure on exchange efficiency. J Theor Biol. 2016;408:1–12. doi: 10.1016/j.jtbi.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 40.Ducray J.F., Naicker T., Moodley J. Pilot study of comparative placental morphometry in pre-eclamptic and normotensive pregnancies suggests possible maladaptations of the fetal component of the placenta. Eur J Obstet Gynecol Reprod Biol. 2011;156(1):29–34. doi: 10.1016/j.ejogrb.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 41.Sankar K.D., Bhanu P.S., Ramalingam K., Kiran S., Ramakrishna B.A. Histomorphological and morphometrical changes of placental terminal villi of normotensive and preeclamptic mothers. Anat Cell Biol. 2013;46(4):285–290. doi: 10.5115/acb.2013.46.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pohlmann S., Soilleux E.J., Baribaud F., Leslie G.J., Morris L.S., Trowsdale J. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc Natl Acad Sci U S A. 2001;98(5):2670–2675. doi: 10.1073/pnas.051631398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaeger M., Stappers M.H.T., Joosten L.A.B., Gyssens I.C., Netea M.G. Genetic variation in pattern recognition receptors: functional consequences and susceptibility to infectious disease. Future Microbiol. 2015;10(6):989–1008. doi: 10.2217/fmb.15.37. [DOI] [PubMed] [Google Scholar]
- 44.Teng O., Chen S.-T., Hsu T.-L., Sia S.F., Cole S., Valkenburg S.A. CLEC5A-mediated enhancement of the inflammatory response in myeloid cells contributes to influenza virus pathogenicity in vivo. J Virol. 2017;91(1):e01813–16. doi: 10.1128/JVI.01813-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao D., Han X., Zheng X., Wang H., Yang Z., Liu D. The myeloid LSECtin is a DAP12-coupled receptor that is crucial for inflammatory response induced by ebola virus glycoprotein. PLoS Pathog. 2016;12(3):1–20. doi: 10.1371/journal.ppat.1005487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Germain C., Bihl F., Zahn S., Poupon G., Dumaurier M., Rampanarivo H.H. Characterization of alternatively spliced transcript variants of CLEC2D gene. J Biol Chem. 2010;285(46):36207–36215. doi: 10.1074/jbc.M110.179622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koga K., Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63(6):587–600. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phoswa W.N., Naicker T., Ramsuran V., Moodley J. Pre-eclampsia: the role of highly active antiretroviral therapy and immune markers. Inflamm Res. 2019;63(1):47–57. doi: 10.1007/s00011-018-1190-3. [DOI] [PubMed] [Google Scholar]