Abstract
Objectives
This study aimed to investigate whether oocyte retrieval from PCOS patients affects the morphokinetics or the incidence of abnormalities in early embryonic development, using time-lapse imaging analysis.
Methods
This was a prospective study in total of 39 PCOS patients and 67 women with normal ovarian function, underwent a GnRH antagonist protocol of controlled ovarian stimulation and fertilization by ICSI. 402 zygotes from the PCOS group and 449 zygotes from the control group were observed by time-lapse monitoring for 48 h following sperm injection.
Results
Patients with PCOS showed a significantly higher number of retrieved oocytes, and a significantly higher number of metaphase II oocytes per cycle than that of the non-PCOS group (18.85 ± 9.41 vs. 11.48 ± 5.51 and 14.97 ± 7.43 vs. 9.51 ± 4.7, respectively). However, oocyte maturation rate and morphologically assessed embryo quality did not differ between two groups. After 48 h of the embryo culture using time-lapse video recording, most of the embryos in both groups had reached the 4-cell stage (353/449 vs. 314/402 embryos). There were no significant differences between the control and PCOS group regarding the time-points of the successive events in early embryonic development from the appearance of 2 polar bodies to the 6-cell stage. However, the percentage of t2 stages which fell in “optimal range” (>24 h and ≤28 h) was significantly lower in the PCOS group than in the control group (37.8% vs. 48.1%, P = 0.004). The proportion of embryos manifesting abnormal fertility, multinucleation, direct cleavage and reverse cleavage were not significantly different between the two groups.
Conclusion
Our data indicated that PCOS does not affect the morphokinetics or incidence of abnormalities in early embryonic development.
Keywords: PCOS, Time-lapse monitoring, Morphokinetics, Embryo cleavage, Early development
Introduction
Polycystic ovary syndrome (PCOS) is the most common disorder and cause of infertility in women of reproductive age with proportion about 5–10% [1]. Women with PCOS may suffer from disturbed ovarian function, with resultant abnormal folliculogenesis, steroidogenesis, and lack of development of a dominant follicle [2].
Metabolic disorders in cases of PCOS can lead to the reduction of glucose in the follicular fluid composition. Hyperinsulinemia and low levels of glucose in oocytes and follicular cells may be caused by defective transport of glucose and altered energy pathways [1]. Furthermore, changes in the fatty acid in follicular fluid results in poor cumulus-oocyte complex (COC) morphology and lipid metabolism affects the quality of oocytes and the development of the embryo. [1]. As a result, PCOS has the potential to interfere with the expression gene of follicles, thereby disrupting the synchronization of cytoplasm and the nuclear maturation of oocytes [2].
Recently, time-lapse devices have been applied invitro fertilization (IVF) laboratories and allow continuous monitoring of embryos inside incubator, record the entire process of embryo development. Monitoring and analysis of embryo division activity improves the evaluation of embryo quality [3]. This information also helps us to identify kinetic and morphologic abnormalities in an embryo undergoing IVF, and allows prediction of aneuploidy of the embryo [4,5]. By these reasons, the present study was aimed to evaluate the fertilization process and the early development of embryos from PCOS patients by time-lapse monitoring.
Materials and methods
This prospective study was conducted from April 2016 to December 2017 in patients undergoing infertility treatment by IVF/ICSI (intracytoplasmic sperm injection), using time-lapse monitoring at the Hue Center for Reproductive Endocrinology and Infertility, Hue University Hospital, Vietnam. The study was approved by the Hue University of Medicine and Pharmacy Ethics Committee. Informed and written consent were obtained from all participants.
Patients with PCOS were identified based on the revised 2003 Rotterdam consensus criteria, in which two out of three of the following conditions must be met: (1) the presence of oligo- and/or anovulation, (2) clinical and/or biochemical signs of hyperandrogenism, and (3) polycystic ovaries on ultrasonography. Other causes of hyperandrogenism, such as congenital adrenal hyperplasia, androgen-secreting neoplasms and Cushing’s syndrome were excluded.
Inclusion criteria in the control group consisted of infertile women without PCOS and regular menstrual cycle, collected at the same time of PCOS recruitment, but excluded any cases with ovarian disease (ovarian cyst/tumor or endometriosis), history of ovarian surgery, and ovarian failure. Exclusion criteria were over 45-years-old female, low responders (retrieved oocytes less than 4), IVF cycles with oocytes donor, sperm donor, severe oligozoospermia (sperm concentration less than 1 million/ml) or cycles with sperm retrieved from surgery.
Ovarian stimulation and oocyte retrieval
All of the patients were treated with the controlled ovarian stimulation by a GnRH antagonist protocol and recombinant FSH (follitropin alfa) was administered with starting dose of 150-225IU (Gonal F®, Merck KGaA, Darmstadt, Germany) on day 2 of the cycle after an ultrasound scan to check antral follicale count (AFC) and rule out the presence of ovarian cysts. Starting dose of rFSH was selected on the basis of the patient’s age, AMH and AFC. GnRH-antagonist 025 mg/day (Cetrorelix, Cetrotide®, Merck KGaA, Darmstadt, Germany) was initiated on day 5 of stimulation according to the fixed protocol until the day of triggering. Oocyte retrieval was performed 35–36 hours after hCG 10000IU IM (Pregnyl®, Merck Sharp & Dohme Limited, UK) administration by ultrasound-guided follicle aspiration with a single-lumen needle (Kitazato, Japan).
In vitro fertilization and embryo culture
Only Intracytoplastmic Sperm Injection (ICSI) procedure was used to ensure that all oocytes at the MII stage after denudation were fertilized at the same time and Time-lapse timings were then observed exactly. Cumulus-oocyte complexes (COC) were aspirated by conventional single lumen needle 17,107 (Vitrolife, Västra Frölunda, Sweden) 36–38 hours after hCG injection. The oocytes were retrieved and washed in a 35 BD-falcon dish containing 2 ml G-MOPS PLUS (Vitrolife, Västra Frölunda, Sweden), covered with 2 ml OVOIL (Vitrolife, Västra Frölunda, Sweden) and incubated at 370C. After washing, the COC was incubated in 1 ml of G-IVF PLUS (Vitrolife, Västra Frölunda, Sweden), equilibrated to 37 °C and 5.5% CO2. The COC were incubated for 2 h before denudation.
The COC were then immersed in 80 IU of HYASE (Vitrolife, Västra Frölunda, Sweden) for 1 min. These cumulus cells were removed from the oocyte by sucking and slicing in a 135 μm diameter pipette. After denudation the oocytes, they were incubated in 100 μl drops of G-IVF PLUS covered with 3 ml OVOIL for 1 h before ICSI.
Sperm preparation
Sperm was obtained from ejaculate after a 3–5 day abstinence. After the liquid semen was maintained at 370C for 1 h, the sperm was collected by gradient concentration centrifuging, using the Sil-Select Plus™ density gradient system (45%–90% SIP050, Fertipro, Beernem – Belgium). The semen was centrifuged for 10 min at 300–450 xg. After collection, the sperm was washed twice with SpermRinse (10101, Vitrolife, Västra Frölunda, Sweden) and centrifuged for an additional 7 min at 300 xg. After washing but prior to performing ICSI, sperm density and mobility were assessed.
ICSI procedure and time-lapse imaging
All oocytes at the MII stage after denudation were used for the ICSI procedure with prepared spermatozoa. Only ICSI procedure was used to ensure that the fertilization and early development of embryos were observed fully. After fertilization, up to 16 injected oocytes were cultured in 100 μl of G-TL™ (10145, Vitrolife, Västra Frölunda, Sweden) contained in a Primo Vision culture dish (16606, Vitrolife, Viby, Denmark), with 3 ml of OVOIL and equilibrated in 37 °C and 5.5% CO2 overnight. The culture dish was placed in incubator Galaxy 170R (Eppendorf AG, Germany) with an atmosphere of 5.0% O2, 5.5% CO2 and 89.5% N2 at 370C.
Fertilization and cleavage were analyzed using a Primo Vision™ EVO + starter kit (16621, Vitrolife, Denmark). Images of the embryos were recorded every 10 min in 9 planes. For this study, images of the embryos’ development were recorded from injection to 48 h after insemination. Manual annotation of the images was performed at a desktop computer running Primo Vision Software v.5.2 (Vitrolife, Viby, Denmark). Only embryos fertilized with visible pronuclei were evaluated. Morphokinetic events were noted the appearance time of 2 polar bodies (t2PB) or pronuclei (tPNa), time of the fading of both pronuclei (tPNF), the time of cleavage to 2, 3, 4, 5, and 6-cells (t2, t3, t4, t5, t6), and the time interval between stages (tPNa-2PB, tPNF-PNa, t2-PNF, t3-2, t4-3), Abnormal embryo were considered that fertility was abnormal (the number of pronuleus was different from 2 in zygote stage) or multinucleation (MN - the blastomeres showed more than 1 nucleus), direct cleavage (DC - when a single blastomere divided directly from 1 to 3 cells in earlier than 5 h) or reverse cleavage (RC). The morphokinetic data were organized according to the parameters developed by Siristatidis and his colleagues: t2: 24–28 h; t3-2: 8–12 h; t3: 30–38 h; t4-3: ≤45 min; t4: 35–41 h [6].
Embryo selection and transfer and cryopreserved
Embryos were selected for transfer on day 2 according to the morphologic criteria which had 4–6 cell stage embryos, stage-specific cell size (≥ 45 μm),and with ≤ 25% fragmentation, and did not show abnormal cleavage by the time-lapse records [[7], [8], [9]]. Two embryos were selected for embryo transfer regularly, but patients with poor prognosis (over 35 year old, unsuccessful embryo transfer in previous cycles) or medium quality of embryo (grade B) may be received three embryos transferred. Other good quality embryos which were not assigned for transfer were cryopreserved.
Outcome measurement
Maturation rate (defined as number of Metaphase II oocytes/oocytes retrieval), fertilization rate (defined as number of zygotes with 2 pronulei /the number oocytes imseminated by ICSI) and good quality embryo rate (number of good quality embryo/number of zygotes) were calculated. Chemical pregnancy was defined by serum β-hCG concentration more than 50 IU/ml after 14 days from embryo transfer. Clinical pregnancy was approved by the presence of fetal heart activity by transvaginal ultrasonography after 4 weeks from embryo transfer. In case of patients without fresh embryos transfer, the results of the first frozen embryo transfer was monitored and recorded.
Statistical analysis
Variables were analyzed using the Statistical Package for the Social Sciences version 20.0 (SPSS, Chicago, USA). Continuous variables were presented as the mean ± SD, and were compared between treatment groups by means of a two-tailed, unpaired t-test. All variables were compared between groups by the chi-squared test. The results are reported as percentages, with relative risk at 95% confidence intervals. All analyses were considered significant at p < 0.05.
Results
Total of 402 zygotes from 39 women diagnosed with PCOS, and 449 zygotes from 67 women categorized as non-PCOS were included in the data analysis. Age, BMI, FSH level at day 2, and estradiol at day 2 were not significantly different between the two groups. However, PCOS women had significantly higher AMH levels and basal LH levels than non-PCOS women (7.7 IU/L versus 5.49 IU/L and 9.67 ng/ml versus 3.25 ng/ml, respectively). The ovarian stimulation responses indicated that the first FSH and total dosage of recombinant gonadotropins was significantly lower in the PCOS group than the non-PCOS group (229.49 ± 33.38; 1820.69 ± 332.06 vs. 245.15 ± 31.15; 2005.60 ± 379.69, respectively). The patients with PCOS showed significantly higher levels of estradiol on the day of hCG administration, and the number of oocytes and the number of metaphase II oocytes retrieved per cycle was significantly higher than in the control group (3247.1 ± 1992.0; 18.85 ± 9.41; 14.97 ± 7.43 vs. 1971.26 ± 1007.39; 11.48 ± 5.51; 9.51 ± 4.7, respectively). However, the oocyte maturation and morphological markers of embryo quality did not differ between the two groups. The clinical pregnancy outcomes (including biochemical pregnancy, clinical pregnancy, implantation rate and multiple pregnancy) between the study groups were not significantly different when evaluated proportionally to the number of embryo transfers per group.
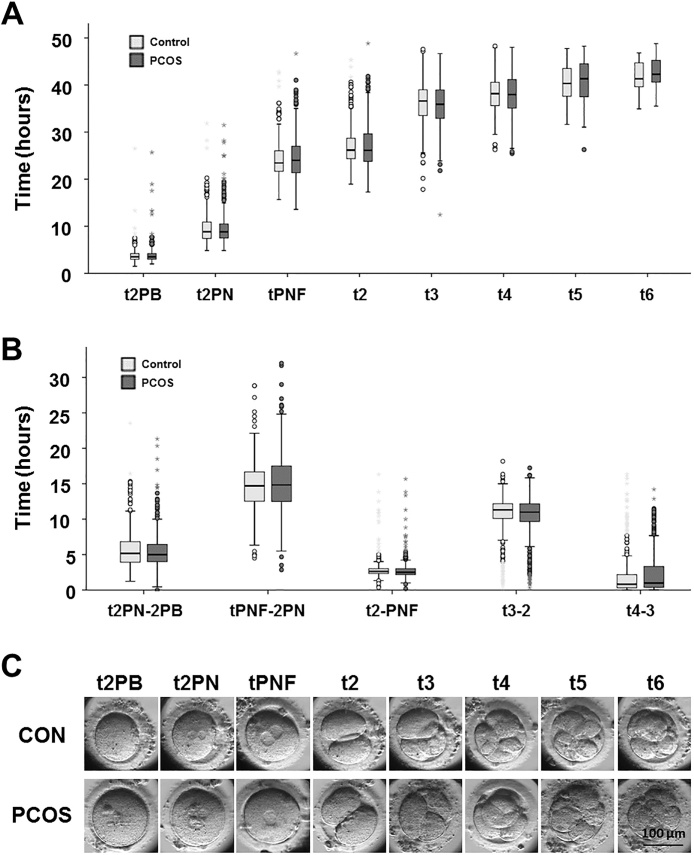
Early embryonic development was recorded by using time-lapse imaging. Morphokinetic events recorded (shown in Table 2 and Fig. 1) were the time of the appearance of 2 polar bodies or 2 pronuclei (t2PB or tPNa), the time of the fading of both pronuclei (tPNF), the time of division to 2, 3, 4, 5, and 6-cell stages (t2, t3, t4, t5, t6), and the time elapsed between stages (tPNa-2PB, tPNF-PNa, t2-PNF, t3-2, t4-3) (Supplementary Video S1). At 48 h, most of embryos had reached the 4-cell stage (353 out of 449 embryos in the control group and 314 out of 402 embryos in PCOS group). The time of events in early embryonic development between the appearance of 2 polar bodies and the 6-cell stage was not significantly different between the control and PCOS groups. The results show that the time-points for embryos from the PCOS patients were equivalent to those for the non-PCOS patients. There was no significant difference in the duration of tPNa-2PB, tPNF-PNa, t2-PNF, t3-2 or t4-3 between embryos from the control-group and the PCOS women (Table 2).
Table 2.
Embryo morphokinetic parameters of PCOS and non-PCOS patients.
| Kinetic marker | Control |
PCOS |
P value | ||
|---|---|---|---|---|---|
| n | Time | n | Time | ||
| t2PB | 449 | 3.77 ± 1.59 | 402 | 3.87 ± 1.99 | 0.42 |
| t2 P N | 449 | 9.52 ± 3.18 | 401 | 9.56 ± 3.49 | 0.86 |
| tPNF | 449 | 24.18 ± 3.89 | 400 | 24.58 ± 4.55 | 0.16 |
| t2 | 420 | 27.02 ± 4.14 | 353 | 27.10 ± 4.67 | 0.80 |
| t3 | 396 | 36.12 ± 4.85 | 353 | 35.77 ± 4:96 | 0.34 |
| t4 | 353 | 38.19 ± 3.81 | 314 | 38.10 ± 4.23 | 0.77 |
| t5 | 63 | 40.12 ± 3.90 | 73 | 40.86 ± 4.45 | 0.31 |
| t6 | 16 | 41.76 ± 3.40 | 33 | 42.47 ± 3.80 | 0.53 |
| t2 P N-2PB | 449 | 5.74 ± 2.77 | 401 | 5.57 ± 2.68 | 0.30 |
| tPNF-2 P N | 449 | 14.65 ± 3.56 | 400 | 15.08 ± 4.19 | 0.11 |
| t2-PNF | 420 | 2.98 ± 1.61 | 353 | 2.95 ± 1.63 | 0.80 |
| t3-2 (CC2) | 367 | 10.29 ± 3.39 | 308 | 10.06 ± 3.54 | 0.39 |
| t4-3 (S2) | 343 | 2.21 ± 3.28 | 306 | 2.58 ± 3.3 | 0.15 |
PCOS: Polycystic Ovary Syndrome; t2PB = time of appearance of 2 polar bodies; t2 P N = time of appearance of 2 pronuclei; tPNF = time by which both pronuclei had faded; t2 = time of division to 2-cell stage; t3 = time of division to 3-cell stage; t4 = time of division to 4-cell stage; t5 = time of division to 5-cell stage; t6 = time of division to 6-cell stage; t2 P N-2PB = duration of the period from appearance of 2 polar bodies to appearance of 2 pronuclei; tPNF-2 P N = duration of the period from appearance of 2 pronuclei to pronuclear fading; t2-PNF = duration of the period as 1-cell; t3-2 (CC2) = time of the second cell cycle; t4-t3 (S2) = time of synchrony of the second cell cycle.
Fig. 1.
Differences in embryo morphokinetics assessed by time-lapse imaging within control and PCOS population. (A) Box plots depicting morphokinetic times. (B) Box plots depicting morphokinetic durations. Light gray: control; dark gray: PCOS. (C) Timeline of early stage of human embryo development. t2PB = time of appearance of 2 polar bodies; t2 P N = time of appearance of 2 pronuclei; tPNF = time by which both pronuclei had faded; t2 = time of division to 2-cell stage; t3 = time of division to 3-cell stage; t4 = time of division to 4-cell stage; t5 = time of division to 5-cell stage; t6 = time of division to 6-cell stage; t2 P N-2PB = duration of the period from appearance of 2 polar bodies to appearance of 2 pronuclei; tPNF-2 P N = duration of the period from the appearance of 2 pronuclei to pronuclear fading; t2-PNF = duration of the period as 1-cell; t3-2 (CC2) = time of the second cell cycle; t4-t3 (S2) = time of synchrony of the second cell cycle.
The time-points for early embryo development were subdivided according to the parameters developed by Siristatidis and colleagues [6]. The proportion of embryos in almost all the subperiods showed no significant differences between the study groups, except for in the optimization range of the 2-cell stage (>24 h and ≤28 h), and the t2 ≤ 24 h stage. The proportion of embryos in the optimization range of stage t2 in the PCOS group was significantly lower than that in the control group (37.8% vs. 48.1%, p = 0.004). By contrast, the proportion of 2-cell embryos at less than 24 h was significantly higher in the PCOS group than in the control group (27.6% vs. 21.2%, p = 0.04) (Table 3).
Table 3.
Comparison of morphokinetic parameters of PCOS and non-PCOS patients.
| Duration | Control n = 67 | PCOS n = 39 | RR | 95%CI | P value |
|---|---|---|---|---|---|
| t2PB≤ 4 h | 69.9% (314) | 70.1% (282) | 1.003 | 0.919-1.110 | 0.95 |
| t2 P N≤ 9 h | 51.9% (233) | 55.0% (221) | 1.062 | 0.937-1.204 | 0.35 |
| tPNF≤ 24 h | 56.1% (252) | 51.2% (206) | 0.918 | 0.810-1.040 | 0.18 |
| t2 ≤ 24 | 21.2% (89) | 27.6% (98) | 1.303 | 1.015-1.672 | 0.04 |
| 24<t2 ≤ 28 | 48.1% (202) | 37.8% (134) | 0.785 | 0.665-0.927 | 0.004 |
| t2 > 28 | 30.7% (129) | 34.6% (123) | 1.128 | 0.921-1.381 | 0.24 |
| t3 ≤ 30 | 11.1% (44) | 11.3% (40) | 1.020 | 0.681-1.527 | 0.92 |
| 30<t3 ≤ 38 | 55.8% (221) | 57.2% (202) | 1.025 | 0.904-1.163 | 0.40 |
| t3 > 38 | 33.1% (131) | 31.6% (111) | 0.951 | 0.772-1.171 | 0.63 |
| t4 ≤ 35 | 20.8% (74) | 23.5% (74) | 1.124 | 0.846-1.493 | 0.42 |
| 35<t4 ≤ 41 | 57.2% (202) | 50.5% (159) | 0.885 | 0.768-1.019 | 0.09 |
| t4 > 41 | 22.0% (78) | 26.0% (82) | 1.181 | 0.902-1.548 | 0.23 |
| t3-2 ≤ 8 | 17.1% (63) | 17.9% (55) | 1.040 | 0.749-1.445 | 0.81 |
| 8<t3-2 ≤ 12 | 55.9% (205) | 55.8% (172) | 1.000 | 0.874-1.144 | 0.99 |
| t3-2 > 12 | 27.0% (99) | 26.3% (81) | 0.975 | 0.758-1.254 | 0.84 |
| t4-3 ≤ 45 | 45.9% (157) | 43.5% (133) | 0.947 | 0.797-1.124 | 0.53 |
PCOS: Polycystic Ovary Syndrome; t2PB = time of appearance of 2 polar bodies; t2 P N = time of appearance of 2 pronuclei; tPNF = time by which both pronuclei had faded; t2 = time of division to 2-cell stage; t3 = time of division to 3-cell stage; t4 = time of division to 4-cell stage; t5 = time of division to 5-cell stage; t6 = time of division to 6-cell stage; t2 P N-2PB = duration of the period from appearance of 2 polar bodies to appearance of 2 pronuclei; tPNF-2 P N = duration of the period from appearance of 2 pronuclei to pronuclear fading; t2-PNF = duration of the period as 1-cell; t3-2 (CC2) = time of the second cell cycle; t4-t3 (S2) = time of synchrony of the second cell cycle.
To further investigate the effects of PCOS on oocyte quality and its relationship with embryo abnormalities, the number of embryos showing abnormal fertilization or cleavage (such as multinucleation, direct cleavage and reverse cleavage) was recorded and analyzed (Supplementary Video S1-9). Abnormal fertility trended higher in the control group (3.1%) compared to the PCOS group (1.2%), but this difference was not statistically significantly (p=0.08). Similarly, the proportion of embryos with multinucleation, direct cleavage and reverse cleavage was not significantly different between the PCOS and non-PCOS groups. The data are shown in Table 4.
Table 4.
Comparison of abnormal cleavage of human embryos between PCOS and non-PCOS patients.
| Abnormalities | Control n = 67 | PCOS n = 39 | RR | 95% CI | P value |
|---|---|---|---|---|---|
| Abnormal fertilization | 3.1% (14/449) | 1.2% (5/402) | 0.399 | 0.145-1.099 | 0.08 |
| Multinucleated | 10.2% (46/449) | 7.5% (30/402) | 0.728 | 0.469-1.131 | 0.15 |
| Direct cleavage | 9.4% (42/449) | 11.4% (46/402) | 1.223 | 0.823-1.818 | 0.32 |
| Reverse cleavage | 0.9% (4/449) | 1.7% (7/402) | 1.955 | 0.576-6.063 | 0.28 |
PCOS: Polycystic Ovary Syndrome; RR: Relative risk; CI: confidence interval.
Discussion
The recent study demonstrated the correlation between cumulus cell gene expression and the time-lapse parameters of early developing embryos in PCOS women (Tabibnejad N, Sheikhha MH, et al., 2019). They concluded that cumulus cell genes involved in cytokinesis are influenced by the ovarian microenvironment in metabolic disorders such as PCOS. Furthermore, the over-responsiveness and AMH level may have affected the development of the embryo with negatively correlated with t5, t8, and the third cell cycle in women with PCOS [10].
The present data has showed that women with or without PCOS were comparable in ages, BMI, and basal hormonal levels. Obviously, due to disturbances in folliculogenesis in the PCOS patients, the response to ovarian stimulation was significantly different between the two groups. In the PCOS group, the patients received the similar total FSH doses but more oocytes were retrieved from them than that from the control group (18.85 ± 9.41 vs 11.48 ± 5.51 respectively, a difference significant at p <0.05). The number of mature oocytes in the MII phase was also higher in PCOS patients (14.97 ± 7.43 vs 9.51 ± 4.7, p <0.05), but the percentage of mature oocytes was similar. These results were similar to recent research [10]. However, fertilization rate (79.74 ± 16.8% and 82.24 ± 12.63%) and the quality of the embryos (good quality embryo rate) collected from these two groups were not significantly different.
As a rule, in our center, embryos were selected for transfer on day 2 according to the morphologic criteria [8] which had 4–6 cell stage embryos, stage-specific cell size (≥ 45 μm) with ≤ 25% fragmentation, and did not show abnormal cleavage by the time-lapse records [7,9]. Two embryos were selected for embryo transfer regularly, but patients with advanced age (≥ 35 year old) or poor prognosis may be had three embryos transferred. Table 1 shows that number of transferred embryos were comparable (2.49 ± 0.64 and 2.46 ± 0.76) in two groups with and without PCOS respectively.
Table 1.
Patient characteristics and cycle specifics in the two study groups.
| Parameter | Control n = 67 | PCOS n = 39 | P value |
|---|---|---|---|
| Female age | 32.75 ± 5.06 | 31.13 ± 5.19 | 0.12 |
| BMI | 20.51 ± 2.34 | 20.72 ± 2.69 | 0.68 |
| Basal FSH (IU/l) | 7.43 ± 6.79 | 6.05 ± 1.47 | 0.21 |
| Basal LH (IU/l) | 5.49 ± 2.27 | 7.77 ± 5.44 | 0.02 |
| Basal estradiol (pg/ml) | 40.6 ± 23.86 | 34.92 ± 22.7 | 0.23 |
| AMH (ng/ml) | 3.25 ± 2.25 | 9.67 ± 5.85 | 0.001 |
| Starting dose of FSH | 245.15 ± 31.15 | 229.49 ± 33.38 | 0.02 |
| Total dose of FSH | 2005.60 ± 379.69 | 1820.69 ± 332.06 | 0.01 |
| Estradiol on day of hCG (pg/ml) | 1971.26 ± 1007.39 | 3247.1 ± 1992.0 | 0.001 |
| Nr. of stimulation days | 8.31 ± 1.02 | 8.10 ± 0.82 | 0.27 |
| Nr. of retrieved oocytes | 11.48 ± 5.51 | 18.85 ± 9.41 | 0.001 |
| Nr. of mature oocytes | 9.51 ± 4.7 | 14.97 ± 7.43 | 0.001 |
| Oocyte maturation rate | 83.11 ± 14.74 | 81.24 ± 12.92 | 0.51 |
| Fertilization rate | 79.74 ± 16.8 | 82.24 ± 12.63 | 0.42 |
| Good quality embryo rate (Grade A + B) | 77.86 ± 19.28 | 74.17 ± 18.06 | 0.33 |
| Nr. of Blastomere at day 2 | 3.89 ± 0.89 (449) | 4.01 ± 1.13 (402) | 0.09 |
| Low fragmentation at day 2(< 10%) | 78.6% (353) | 76.4% (307) | 0.35 |
| Nr. of transferred embryos | 2.49 ± 0.64 | 2.46 ± 0.76 | 0.82 |
| Biochemical pregnancy rate | 38.8% (26) | 56.4% (22) | 0.08 |
| Clinical pregnancy rate | 32.8% (22) | 46.2% (18) | 0.17 |
| Implantation rate | 17.41 ± 28.01 | 23.07 ± 26.93 | 0.31 |
| Multiple pregnancy rate | 7.5% (5) | 2.6% (1) | 0.29 |
PCOS: Polycystic Ovary Syndrome; BMI: Body Mass Index; FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; AMH: Anti-Mullerian Hormone; Nr.: number.
Fertilization process
We investigated the fertilization process and early embryonic development to look for further differences between the two groups. To explore the hypothesis that the morphokinetics of fertilization can predict embryo quality, various time intervals between discrete fertilization events were assessed in relation to the number of cells and degree of fragmentation of the resulting embryos, blastocyst formation rate, and results of IVF treatment. Commonly, clinical embryologists have looked for the appearance of two pronuclei (2 P N) at 16–18 h post-insemination. During microscopic observation outside the incubator, it causes exposing the embryo to uncontrolled atmospheric conditions. The evaluation of fertilization at a single time point, such as by observation of patterns of nucleolar precursor bodies (NPBs) or the presence of halos in cytoplasm, may predict IVF treatment outcome, but the effect is not always as expected [11,12]. Time-lapse embryo imaging enables non-invasive observation and setting of evaluation markers such as polar body extrusion, pronuclear formation, and cleavage times and patterns, as well as enabling identification of embryo quality and prediction of the pregnancy/implantation rate [13,14]. In-depth study of the fertilization process by time-lapse imaging shows a difference between normal and abnormal embryos in the mean duration of pronuclei fading (PNF) [15,16]. The time from appearance to fading of pronuclei has been found to be related to genetic abnormalities and the quality of day 5 embryos, but the difference is not statistically significant [17,7,18].
Fertilization is affected by many factors such as the age of the woman, the cause of infertility, and the stimulation process. A study by Akhter & Shahab showed a significant difference in the times of pronuclear appearance across different age groups [19]. Time-lapse analysis documented a significant delay in two-pronuclear fading in embryos from ICSI patients with PCOS compared to embryos from other women [20]. However, our study did not find any differences in the time-points or progress of fertilization of PCOS patients and other patients. This result is consistent with the study of Sundvall 2015 [21].
Classification based on the position of embryo time-points
Several groups have tried to find significant kinetic markers or to build algorithms predicting ploidy through the TLM and PGT-A techniques. The morphokinetic algorithm is based on regression analysis, a set of statistical processes for estimating the relationship between the ploidy stage and embryo cleavage [22]. Chavez et al. were the first to observe chromosomally normal embryos by analysis of morphokinetic parameters, fragmentation patterns and blastomere symmetry behavior at the four-cell stage of human embryo development. Differences in the timing of embryo cleavage between chromosomally normal and abnormal embryos were recorded and analyzed. In this model, embryos were classified into three categories according to high, medium or low risk of being aneuploid. Apart from predicting aneuploidy, some time-lapse studies in human IVF have shown that faster-developing embryos have higher implantation rates [23].
Siristatidis and colleagues used time-lapse image monitoring of the cleavage process to predict implantation success. Evaluation of embryos was made based on the position of their time-points within the “normal” range. These embryos which showed the most optimal morphokinetic parameters were higher implantation ability than others [6].
Smoking, BMI, certain types of subfertility (such as PCOS) and the type of ovarian stimulation used have been found to affect embryo development and morphokinetics, as well as reproductive outcomes following IVF/ICSI [6]. In case of PCOS patients, the mean time of all events were significantly delayed in terms of developmental timings when compared with the controls [9,24]. However in the present study, we did not find differences in the cleavage of embryos of patients with and without PCOS, except at the t2 time-point. In the PCOS group, we found that the proportion of embryo reached 2 cell before 24 h was higher than in the non-PCOS group (27.6% vs 21.2% respectively, significant at p = 0.04). However, there were fewer embryos showing a t2 in optimal range in the PCOS group than in the non-PCOS group (37.7% vs 48.1% respectively, significant at p = 0.004). These results were similar to the research of Sundvall and colleagues [21].
Abnormal embryo cleavage
The appearance and disappearance of the third pronuclei outside the range of 16–18 h following ICSI and presented 1 pronuleus was only detected by time-lapse monitoring. Using ICSI for fertilization, the rate of abnormal zygotes was about 6%. These embryos were still able to become blastocysts, but the implantation rate was very low (only 1.2%) and commonly resulted in early miscarriage. Several studies on the genetic quality of these abnormal zygotes show that most of them carry abnormalities in the number of chromosomes. In the PCOS group, abnormal zygotes were found in 5 out of 402 embryos (Supplementary Video S6, S7), lower than the rate in the non-PCOS group: 14 out of 449 (Supplementary Video S2, S3).
Another phenomenon of abnormal embryo development is the presence of multinucleation. Although multinucleation can be observed by morphological assessment of alpha consensus, the appearance of the nucleus may be short-lived, and sometimes occurs beyond the evaluation period. Through continuous imaging of the embryo within the incubator, the presence of multinucleated embryos was completely screened by the time-lapse system. In our study we observed 30 out of 402 embryos that exhibited multinucleation in the PCOS group (Supplementary Video S8). The rate of multinucleated embryos in the control group was higher (42 out of 449), but this difference was not significant (p = 0.15) (Supplementary Video S4). The presence of multinucleation is often associated with chromosomal abnormalities and therefore affects the outcome of embryo transfer. Several studies have shown that the presence of multinucleation in early stage (2-cell) embryos may still be followed by normal embryonic implantation. This indicates that self-repair occurs during the development of the embryo. The self-repair mechanism allows the embryos to restore the chromosomes to their normal state. However, when multinucleated embryos are present in later-stage (4-cell) embryos, the self-repair mechanism is no longer effective. In this circumstance, multinucleated embryos had low implantation ability when transferred.
Direct cleavage (1 cell into 3 cells) is an abnormal occurrence only detected with the time-lapse system. This abnormality usually occurs with a frequency of about 10%. In the PCOS group, direct cleavage was observed in 46 out of 402 embryos (Supplementary Video S6, S9), a rate similar to the control group (42 out of 449 embryos) (Supplementary Video S2, S4). These abnormal embryos carry severe genetic abnormalities due to the uneven distribution of the blastomeres. These embryos had extremely low implantation ability, so that these abnormal embryos are often not recommended for embryo transfer to patients. These abnormal embryos might develop into the blastocyst stage (at a rate of about 21.6%), but when examined for genetic quality, 75% of these embryos had abnormalities in the number of chromosomes.
The time-lapse system can help detect reverse cleavage, which occurs with a frequency of approximately 7%. Reverse cleavage is the fusion of two blastomeres into one, followed by cleavage into two blastomeres. This abnormal cleavage may be observed in the first 3 days of embryonic development and these embryos are often also multinucleated. These embryos have extremely low implantation ability, so the embryos are usually removed. Monitoring the morphokinetics of these embryos from PCOS patients, we observed 7 out of 402 manifesting reverse cleavage (Supplementary Video S9). The rate reverse cleavage in control group was lower 4 out of 449 embryos (Supplementary Video S5) but this different was not significantly in our study (p=0.28).
In conclusion, our data indicate that there are significant differences in the level of AMH, LH, FSH, estradiol and ovarian stimulation response in PCOS women. However, the morphokinetics and rate of abnormal cleavage of embryos derived from PCOS and non PCOS women was not significantly different from the rate found in non-PCOS women.
Ethics approval
This study was approved by the Ethics Committee of Hue University of Medicine and Pharmacy. All information and data were encrypted and confidential.
Consent for publication: All authors have been involved in the research work and consent for publication
Availability of data and material
All data are available and would be shown as requested
Competing interests
The authors have no competing financial or other interests to declare in relation to this manuscript.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Authors’ contributions
L.M.T, N.T.T.T, N.T.T, N.V.T participated in the study design, data collection, data analysis, manuscript drafting and critical discussion. N.V.Q.H, N.T.T.A and C.N.T participated in the study design, interpretation of data and critical discussion. All authors have been involved in drafting the work or revising it critically for important intellectual content of the final manuscript.
Acknowledgements
We sincerely thank Mr. Elihu Welber and Dr. Jan Herr of San Anselmo, CA, USA, from Health Volunteers Overseas (HVOUSA), for their assistance in English editing.
Footnotes
The work was carried out at: Hue University Of Medicine And Pharmacy, Hue University Center For Reproductive Endocrinology And Infertility 06 Ngo Quyen Street, Hue City, Vietnam.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eurox.2019.100045.
Contributor Information
Minh Tam Le, Email: leminhtam@huemed-univ.edu.vn.
Trung Van Nguyen, Email: nvtrung.huecrei@huemed-univ.edu.vn.
Tung Thanh Nguyen, Email: nttung@huemed-univ.edu.vn.
Thai Thanh Thi Nguyen, Email: nttthanh.huecrei@huemed-univ.edu.vn.
Tam An Thi Nguyen, Email: nttan.huecrei@huemed-univ.edu.vn.
Quoc Huy Vu Nguyen, Email: nvqhuy@huemed-univ.edu.vn.
Ngoc Thanh Cao, Email: thanhykhue@yahoo.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Niu Z., Lin N., Gu R., Sun Y., Feng Y. Associations between insulin resistance, free fatty acids, and oocyte quality in polycystic ovary syndrome during in vitro fertilization. J Clin Endocrinol Metab. 2014;99(11):2269–2276. doi: 10.1210/jc.2013-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao S., Li Y., Li T., Chen M., Xu Y., Wen Y. Evidence for decreased expression of ADAMTS-1 associated with impaired oocyte quality. PCOS. 2014;99(June):1015–1021. doi: 10.1210/jc.2013-4177. [DOI] [PubMed] [Google Scholar]
- 3.Milewski R., Ajduk A. Time-lapse imaging of cleavage divisions in embryo quality assessment. Reproduction. 2017;154(2):R37–R53. doi: 10.1530/REP-17-0004. [DOI] [PubMed] [Google Scholar]
- 4.Zaninovic N., Irani M., Meseguer M. Assessment of embryo morphology and developmental dynamics by time-lapse microscopy: is there a relation to implantation and ploidy? Fertil Steril. 2017;108(5):722–729. doi: 10.1016/j.fertnstert.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Mumusoglu S., Yarali I., Bozdag G., Ozdemir P., Polat M., Sokmensuer L.K. Time-lapse morphokinetic assessment has low to moderate ability to predict euploidy when patient– and ovarian stimulation–related factors are taken into account with the use of clustered data analysis. Fertil Steril. 2017;107(2):413–421. doi: 10.1016/j.fertnstert.2016.11.005. e4. [DOI] [PubMed] [Google Scholar]
- 6.Siristatidis C., Komitopoulou M.A., Makris A., Sialakouma A., Botzaki M., Mastorakos G. Morphokinetic parameters of early embryo development via time lapse monitoring and their effect on embryo selection and ICSI outcomes: a prospective cohort study. J Assist Reprod Genet. 2015;32(4):563–570. doi: 10.1007/s10815-015-0436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond E.R., Stewart B., Peek J.C., Shelling A.N., Cree L.M. Assessing embryo quality by combining non-invasive markers: early time-lapse parameters reflect gene expression in associated cumulus cells. Hum Reprod. 2015;30(8):1850–1860. doi: 10.1093/humrep/dev121. [DOI] [PubMed] [Google Scholar]
- 8.Balaban B., Brison D., Calderón G., Catt J., Conaghan J., Cowan L. Istanbul consensus workshop on embryo assessment. Proceedings of an Expert Meeting. 2011;22(6):632–646. doi: 10.1016/j.rbmo.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Wissing M.L., Olesen A.I.G., Hoest T., Mikkelsen A.L. Impact of PCOS on early embryo cleavage kinetics. Reprod Biomed Online. 2014;28(4):508–514. doi: 10.1016/j.rbmo.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Tabibnejad N., Soleimani M., Aflatoonian A. Serum Anti-Mullerian hormone and embryo morphokinetics detecting by time-lapse imaging: a comparison between the polycystic ovarian syndrome and tubal factor infertility. Int J Reprod Biomed. 2018;16(8):483–490. [PMC free article] [PubMed] [Google Scholar]
- 11.Coticchio G., Renzini M.M., Novara P.V., Lain M., Ponti E., De Turchi D. Focused time-lapse analysis reveals novel aspects of human fertilization and suggests new parameters of embryo viability. Hum Reprod. 2018;33(1):23–31. doi: 10.1093/humrep/dex344. [DOI] [PubMed] [Google Scholar]
- 12.Faramarzi A., Khalili M.A., Omidi M., Agha-Rahimi A., Taheri F. Pronuclear pattern does not predict morphokinetics behavior in human embryos. Gynecol Endocrinol. 2017:1–4. doi: 10.1080/09513590.2017.1388365. 0(0) [DOI] [PubMed] [Google Scholar]
- 13.Carrasco B., Arroyo G., Gil Y., Gómez M., aJ, Rodríguez I., Barri P.N. Selecting embryos with the highest implantation potential using data mining and decision tree based on classical embryo morphology and morphokinetics. J Assist Reprod Genet. 2017;34(8):983–990. doi: 10.1007/s10815-017-0955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandawala A.A., Harvey S.C., Roy T.K., Fowler K.E. Time-lapse embryo imaging and morphokinetic profiling: towards a general characterisation of embryogenesis. Anim Reprod Sci. 2016;174:2–10. doi: 10.1016/j.anireprosci.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Patel D., Shah P., Kotdawala A., Herrero J., Rubio I., Banker M. Morphokinetic behavior of euploid and aneuploid embryos analyzed by time-lapse in embryoscope. J Hum Reprod Sci. 2016;9(2):112. doi: 10.4103/0974-1208.183511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freour T., Basile N., Barriere P., Meseguer M. Systematic review on clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring. Hum Reprod Update. 2015;21(1):153–154. doi: 10.1093/humupd/dmu054. [DOI] [PubMed] [Google Scholar]
- 17.Basile N., Nogales M.D.C., Bronet F., Florensa M., Riqueiros M., Rodrigo L. Increasing the probability of selecting chromosomally normal embryos by time-lapse morphokinetics analysis. Fertil Steril. 2014;101(3):699–704. doi: 10.1016/j.fertnstert.2013.12.005. e1. [DOI] [PubMed] [Google Scholar]
- 18.Chawla M., Fakih M., Shunnar A., Bayram A., Hellani A., Perumal V. Morphokinetic analysis of cleavage stage embryos and its relationship to aneuploidy in a retrospective time-lapse imaging study. J Assist Reprod Genet. 2014;32(1):69–75. doi: 10.1007/s10815-014-0372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akhter N., Shahab M. Morphokinetic analysis of human embryo development and its relationship to the female age : a retrospective time-lapse imaging. Cell Mol Biol. 2017;63(8):84–92. doi: 10.14715/cmb/2017.63.8.18. [DOI] [PubMed] [Google Scholar]
- 20.Palomba S., Daolio J., Battista G., Sala L. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab. 2017;28(3):186–198. doi: 10.1016/j.tem.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Sundvall L., Kirkegaard K., Ingerslev H.J., Knudsen U.B. 2015. Unaltered timing of embryo development in women with polycystic ovarian syndrome (PCOS): a time-lapse study; pp. 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pennetta F., Lagalla C., Borini A. Embryo morphokinetic characteristics and euploidy. Curr Opin Obstet Gynecol. 2018;1 doi: 10.1097/GCO.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 23.Petersen B.M., Boel M., Montag M., Gardner D.K. Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on Day 3. Hum Reprod. 2016;31(10):2231–2244. doi: 10.1093/humrep/dew188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabibnejad N., Sheikhha M.H., Ghasemi N., Fesahat F., Soleimani M., Aflatoonian A. Association between early embryo morphokinetics plus cumulus cell gene expression and assisted reproduction outcomes in polycystic ovary syndrome women. Reprod Biomed Online. 2019;38(2):139–151. doi: 10.1016/j.rbmo.2018.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available and would be shown as requested