Abstract
Osteoporosis screening rates by DXA are low (9.5% women, 1.7% men) in the US Medicare population aged 65 years and older. Addressing this care gap, we estimated the benefits of a validated osteoporosis diagnostic test suitable for patients age 65 years and older with an abdominal computed tomography (CT) scan taken for any indication but without a recent DXA. Our analysis assessed a hypothetical cohort of 1000 such patients in a given year, and followed them for 5 years. Separately for each sex, we used Markov modeling to compare two mutually exclusive scenarios: (i) utilizing the CT scans, perform one-time “biomechanical computed tomography” (BCT) analysis to identify high-risk patients on the basis of both femoral strength and hip BMD T-scores; (ii) ignore the CT scan, and rely instead on usual care, consisting of future annual DXA screening at typical Medicare rates. For patients with findings indicative of osteoporosis, 50% underwent 2 years of treatment with alendronate. We found that BCT provided greater clinical benefit at lower cost for both sexes than usual care. In our base case, compared to usual care, BCT prevented hip fractures over a 5-year window (3.1 per 1000 women; 1.9 per 1000 men) and increased quality-adjusted life years (2.95 per 1000 women; 1.48 per 1000 men). Efficacy and savings increased further for higher-risk patient pools, greater treatment adherence, and longer treatment duration. When the sensitivity and specificity of BCT were set to those for DXA, the prevented hip fractures versus usual care remained high (2.7 per 1000 women; 1.5 per 1000 men), indicating the importance of high screening rates on clinical efficacy. Therefore, for patients with a previously taken abdominal CT and without a recent DXA, osteoporosis screening using biomechanical computed tomography may be a cost-effective alternative to current usual care. © 2019 American Society for Bone and Mineral Research.
Keywords: OSTEOPOROSIS, SCREENING, COST EFFECTIVENESS, HEALTH ECONOMICS, DXA
Introduction
As the main gateway to eventual treatment of osteoporosis and prevention of fractures, effective diagnostic screening is a critical element of any osteoporosis management program. Indeed, payors and health systems are increasingly being rated and rewarded by their performance on various clinical metrics, one of which is osteoporosis preventative screening. In the United States, screening postmenopausal women for osteoporosis is recommended via bone mineral density (BMD) testing by dual-energy X-ray absorptiometry (DXA). Such screening, coupled with pharmacologic treatment, reduces hip and other fractures(1) and is cost effective.(2–5) However, managing osteoporosis in this way remains limited in two ways. First, and especially for men,(4,6,7) the screening rate for DXA is low(6,8–10)—only 9.5% of women and 1.7% of men in the US Medicare population aged 65 years and older.(11) Second, the sensitivity of traditional DXA for predicting fracture is modest.(12–17) Thus, any adjunct approaches to DXA that have higher screening rates and sensitivity, even if only for a subset of patients, could improve osteoporosis management.
One recently validated test for diagnostic screening of osteoporosis is “biomechanical computed tomography” (BCT) as applied to abdominal CT scans previously obtained for any medical indication.(17) The general BCT approach, ie, finite element analysis of clinical-resolution CT scans,(18,19) provides noninvasive estimates of the breaking strength of the hip and spine. Combining that measurement with a CT-based measurement of a DXA-equivalent hip BMD T-score,(17,20,21) BCT provides a more comprehensive diagnostic assessment of osteoporosis than using either bone strength or BMD alone. For example, because femoral strength predicts hip fracture independently of hip BMD, BCT has higher sensitivity for hip fracture than traditional DXA, and patients who test positive with BCT are at higher risk of hip fracture than are patients who test positive with traditional DXA.(17) When applied to previously obtained CT scans—an ancillary approach—no extra procedure or office visit is required by the patient to undergo an osteoporosis screen, and no change is required in the clinical imaging protocol.(17,20–23) Thus, in any managed care system, it is highly feasible for ancillary BCT to be offered in a standing-order fashion to all screen-eligible patients undergoing abdominal or hip-containing CT when an osteoporosis screen is medically indicated. With many millions of abdominal CT scans taken annually,(24) such standing-order BCT-based screening in managed care systems could be clinically impactful if exploited for patients already undergoing CT—while continuing to rely on usual-care screening for all other patients.
We sought to assess the clinical and economic benefits of such an ancillary BCT-based, standing-order screening program in women and men age 65 years and older. Two recent cost-effectiveness analyses for women showed that BCT can be cost effective (versus usual care) when used for screening generally, by using both DXA and a dedicated hip CT scan ordered specifically for the BCT analysis.(25,26) In those analyses, the overall cost of the BCT test would be the sum of the costs for acquiring the CT scan and then analyzing the scan with BCT analysis. Those cost-effective analyses did not address an ancillary approach for patients already undergoing CT and did not address men. Here, we focused on one-time ancillary BCT without the need for DXA or for acquiring a CT scan, and offered only to patients who are undergoing abdominal CT as part of their medical care and who have not had a recent DXA (for other patients, usual care DXA screening would be used and was not assessed here); we also assessed performance for both women and men. Mindful of the economic barriers for introducing any new medical practice, we aimed at identifying operating parameters that would make the program cost neutral for the payor compared to current usual care.
Methods
This analysis, conducted from the perspective of the healthcare sector, was aimed at an audience from the managed care setting in which the payor provides the screening and reading as well as the care for the target population. In line with this perspective, given that short-term performance typically drives implementation of new medical practices in managed care systems, the time horizon was a short time period of 5 years.(27,28) We considered a hypothetical cohort of 1000 patients (either women or men) age 65 years or older who received an abdominal CT (in year 1) for any medical reason, who did not have a recent DXA (for example, in the previous 2 years), who were not on osteoporosis treatment, or had no disqualifying conditions for an osteoporosis diagnostic screen according to accepted clinical guidelines.(29) Although clinical guidelines are well established for women age 65 years and older,(29) guidelines are less well established for men and therefore we explored performance for men over the same age range as for women. For each sex separately, we compared the following two mutually exclusive screening strategies for the 1000 patients:
Participate in the BCT program and undergo BCT screening (in year 1);
Rely instead on usual care, ie, annual DXA screening at typical testing rates for women and men over age 65 years.
We also assessed no screening (and no treatment), which enabled us to calculate the absolute number of prevented hip fractures.
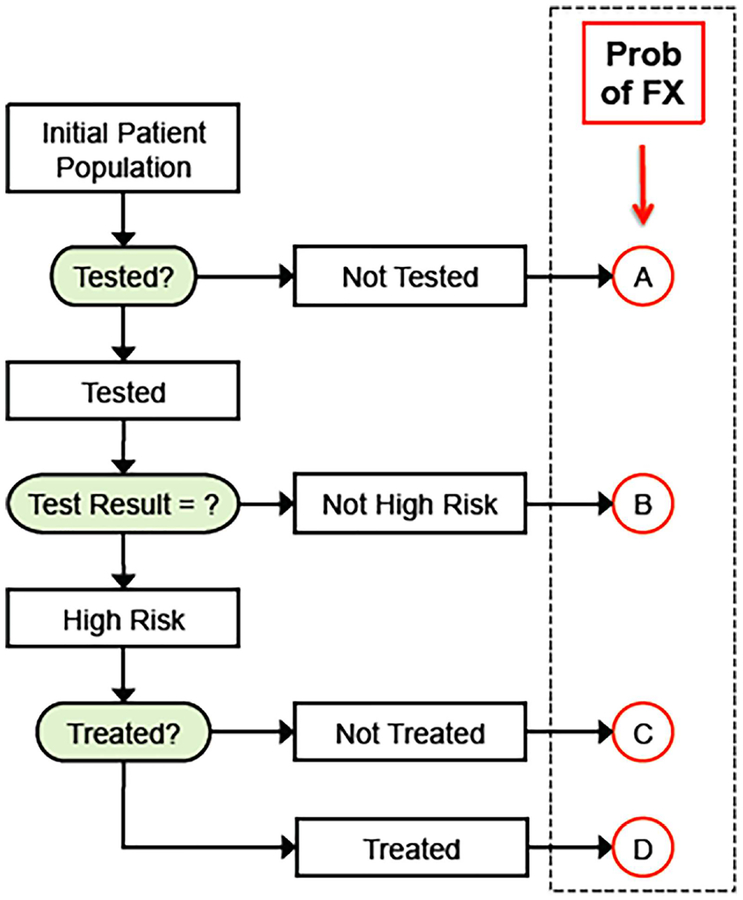
Under the BCT strategy (Fig. 1), the program is set up so that all 1000 patients undergo the BCT test in year 1, which would be feasible in a managed care setting because no new patient procedure is needed and the screen is medically indicated. Typically, 90% of those CT scans yield diagnostic-quality BCT results.(17) Patients who tested positive were classified at high risk and were eligible for 2 years of alendronate treatment,(19) whereas patients who tested negative were not offered any treatment. We assumed that 50% of patients prescribed treatment filled their prescriptions(1,10) and were 100% adherent to it over a 2-year duration.(30)
Fig. 1.
General strategy for the BCT-based screening program. The patient population (n = 1000) comprises patients who come to the medical center for a hip CT (for any indication) and who have not had a recent DXA and otherwise qualify for an osteoporosis diagnostic screen. The assigned probability of hip fracture for any individual had four values (A–D), which depended on the background risk (no testing or no test result), the test results (positive or negative) and, for those who tested positive, whether or not treatment was initiated. Variables in the analysis were the level of fracture risk of the underlying population (the background risk), the proportion of patients testing positive who went on treatment, the treatment duration, and the sensitivity and specificity of the test. FX = fracture.
Under the usual care strategy, the 1000 patients were assumed to be screened by DXA each year at typical diagnostic screening rates observed for the Medicare population aged 65 years or older—9.5% annually for women and 1.7% annually for men.(11) We note that these screening rates reflect the net effects of such factors as coverage policies, reimbursement rates, clinical guidelines, and patient preferences for current usual care in the 65+ year age demographic. Once any patient was tested with DXA, they were not retested over the 5-year period, but otherwise all patients were eligible to be tested annually. All other parameters and assumptions for usual care were the same as for the BCT program. For both strategies, patients who started treatment did so once tested.
For simplicity of modeling, and because many of the parameters that would be required for more complex modeling are not known (eg, sensitivity of predicting non-hip fractures from hip BCT measurements), we focused on explicitly modeling just hip fractures. Thus, our estimates of clinical efficacy are conservative because they do not include clinical benefits of preventing other types of fractures.
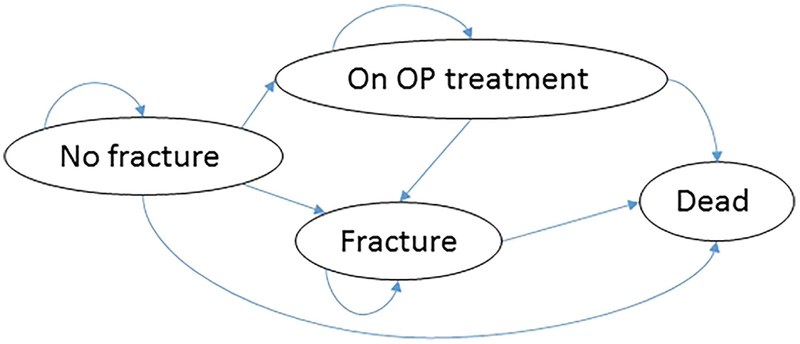
In the model, clinical outcomes and costs were obtained over time using a Markov model with a one-year cycle. The basic model had four states (Fig. 2): (i) no (hip) fracture, (ii) on osteoporosis treatment, (iii) hip fracture, and (iv) death (absorbing state). The cohort would start from the no fracture state and each year have a probability of hip fracture or death. Patients who were screened and tested positive and received treatment would move to the on osteoporosis treatment state, in which the probability of moving to the hip fracture state was reduced due to treatment efficacy. The model estimated the number of hip fractures, quality-adjusted life-years (QALYs), and direct costs in 2016 US dollars at an annual discount rate of 3%. We assumed QALY values for the no fracture state of 0.84 for women and 0.87 for men. Disutility values for the first and subsequent years after hip fracture were obtained using multipliers of 0.797 and 0.899, respectively (Table 1(1,5,7,10,11,17,25,26,30–46)). As noted above, these parameter values are conservative because we did not consider QALY gains associated with preventing any other major osteoporotic fractures (e.g. spine, humeral, pelvic, and wrist). All analyses were performed separately on women (n = 1000) and men (n = 1000) using base-case parameters that in some cases were sex specific and some of which were varied parametrically (Table 1). All probabilities were modeled on the basis of direct data rather than indirectly through modeling changes in BMD, bone strength, or age and thus none of the assumed parameters depended on patient demographics other than as reflected in the assumed risk rates. Analyses were performed using TreeAge Pro 2018 (Williamstown, MA, USA).
Fig. 2.
Simplified depiction of the Markov model used to estimate costs and outcomes over time. The model included four states (No fracture, Fracture, On OP treatment, and Death as an absorbing state) and had a cycle of 1 year. OP = osteoporosis.
Table 1.
Inputs to the Model and Ranges of Any Varied Parameters: Sex-Specific Parameters Noted (Women/Men)
| Input | Value | Range | Reference |
|---|---|---|---|
| BCT testing | |||
| Test rate (all year 1) | 0.90 | Adams and colleagues(17) | |
| Sensitivity/specificity | 0.66/0.66 (W) | x1.2 | Adams and colleagues(17) |
| 0.56/0.76 (M) | x1.2 | ||
| DXA testing | |||
| Annual screening rate (years 1–5) | 0.095 (W) | Zhang and colleagues(11) | |
| 0.017 (M) | |||
| Sensitivity/specificity | 0.59/0.67 (W) | Adams and colleagues(17) | |
| 0.48/0.78 (M) | |||
| Treatment | |||
| Patient adherence | 0.5 | 0.3–0.7 | Siris and colleagues(1,10) |
| Duration (years) | 2 | 1–3 | Durden and colleagues(30) |
| Efficacy (relative risk; alendronate) | 0.47 | Black and colleagues(33) | |
| Risks | |||
| Hip fracture incidence | 10.51 (W) | X2, X3, X4 | Brauer and colleagues(34) |
| (per 1000 persons per year) | 4.57 (M) | X2, X3, X4 | |
| Probability of death after hip fracture | 0.20 | 0.10 | Brauer and colleagues(34); |
| Tajeu and colleagues(35); | |||
| Ray and colleagues(36); | |||
| Abrahamsen and colleagues(37) | |||
| Probability of death due to other causes (by age) | Murphy and colleagues(38) | ||
| Costs | |||
| BCT testing (per test performed) | $100 | $150–250 | Agten and colleagues(25); |
| Viceconti and colleagues(26) | |||
| DXA testing (per test performed) | $100 | Centers for Medicare & Medicaid Services(39) | |
| Hip fracture care | |||
| First year after fracture | $50,000 | Hiligsman and colleagues(40); | |
| Second year after fracture | $12,000 | Nayak and Greenspan(7); | |
| Parthan and colleagues(41); | |||
| Schousboe(5); | |||
| Majumdar and colleagues(42); | |||
| Kilgore and colleagues(43) | |||
| Drug treatment (generic, per year) | $100 | Social Security Administration(44) | |
| Annual discount rate | 3% | Gold(45) | |
| Utility | |||
| Utility weights | 0.84 (W) | Fryback and colleagues(46) | |
| 0.87 (M) | |||
| Disutility multiplier for hip fracture | |||
| First year after fracture | 0.797 | Hiligsman and colleagues(40); | |
| Nayak and Greenspan(7); | |||
| Parthan and colleagues(41) | |||
| Following years | 0.899 |
BCT = biomechanical computed tomography.
Probability of hip fracture
The model assumed four sex-specific values of the probability of hip fracture (Fig. 1). The probability of hip fracture for those without testing or treatment was based on incidence rates from the 1995 Medicare population (age 65 years or older), chosen because this population was presumably free of bisphosphonate treatment.(34) We refer to this sex-specific rate as the background population risk. Possible secular reductions in hip fracture rates in the Medicare population since 1995 are not well understood but are unlikely to exceed about 15%,(34,47) an effect we ignored since that is a small effect compared to the fourfold change in this parameter that we explored in our sensitivity studies. To calculate probability of hip fracture for patients who tested positive or negative from the test (either BCT or DXA), the sex-specific background fracture rate was applied to the 1000 patients in order to calculate a prevalence rate for each year. Then, using that prevalence rate and the test-specific and sex-specific values of sensitivity and specificity (see section on sensitivity and specificity of the tests), we calculated the positive predictive value (PPV) and negative predictive value (NPV), from which we quantified the probability of hip fracture after testing either positive (PPV) or negative (1–NPV). Finally, for those patients testing positive and placed on treatment, the probability of fracture was reduced to reflect the efficacy of the treatment (see section on treatment-related parameters). In sensitivity analyses, we increased the assumed background population risk as much as fourfold to assess performance if the program were limited to higher-risk patient pools; all probabilities of fracture were recalculated for each value of the background population risk.
Treatment-related parameters
As in other cost-effectiveness analyses,(48) we assumed 50% of patients who tested positive actually went on treatment.(28,30,31,49) In sensitivity analyses, we used lower (30%)(1,10) and higher (70%)(50) rates of treatment initiation to assess how that parameter can impact the overall clinical utility and costs. For treatment efficacy, we used a relative risk value of 0.47 for hip fracture for both sexes, based on the pivotal “FIT” trial for postmenopausal women with either a vertebral fracture or hip BMD T-score ≤−2.5 who were treated with alendronate.(33) In that trial, treatment efficacy was observed within 12 months. Because the FIT trial remains by far the largest clinical trial performed to assess clinical efficacy of alendronate versus placebo,(51) we did not vary this point estimate of efficacy in our analysis. However, because gains in BMD after 5 years of treatment with alendronate can be substantially maintained over multiple subsequent years,(52) we modeled this “offset of effect” in a linear fashion,(53,54) reducing treatment efficacy to 75%, 50%, and 25% in the respective 3 years after treatment ended. For treatment duration, we assumed conservatively a total of 2 years of treatment for each patient who went on treatment,(30,32) and also explored a duration of 3 years, which is possible in some managed settings.(55) For simplicity and because changing adherence rates would be equivalent to changing treatment duration, we assumed 100% adherence to treatment as in other cost-effectiveness studies,(2) and used a conservative estimate of total treatment duration for our base case.
Sensitivity and specificity of the tests
Sex-specific values of sensitivity and specificity for both BCT and DXA (Table 1) were taken from the “FOCUS” hip-fracture observational BCT study that was conducted in a large managed-care multicenter clinical setting using routine abdominal CT and DXA scans.(17) For BCT, patients tested positive if they had either BMD-defined osteoporosis at the hip (lower of the femoral neck or total hip BMD T-scores ≤ −2.5, for both sexes)—also measured from the CT scan—or fragile bone strength (femoral strength ≤3000 N for women or ≤3500 N for men).(56) For DXA, patients tested positive if they had BMD-defined osteoporosis at either the hip or spine (lowest T-score), reflecting typical clinical practice. We did not evaluate FRAX, other clinical factors, or the presence of vertebral or other clinical fractures. In sensitivity analyses to gain insight into cost-effectiveness implications of potential future improvements to the BCT test,(26) we also increased the sensitivity or specificity or both for BCT by 20%. We also used the sensitivity and specificity values from DXA testing for the BCT test in the BCT program so as to better understand the relative contributions of the testing rate and test sensitivity/specificity on the overall clinical efficacy of the BCT program.
Cost of testing
We assumed that the BCT test is provided to the healthcare provider by a specialized laboratory that charges a per-patient fee for reading each BCT test performed. In this way, the only cost associated with BCT testing in our analysis was this fee. The costs of the BCT analysis(25) and DXA testing were each nominally set at $100. For DXA, we did not vary the cost because it approximated the hospital-based fee set by CMS.(39) For BCT, pricing has not yet been established in the United States and thus we varied pricing up to $250, the latter being about 25% lower than a price reported in the United Kingdom.(26)
Other costs
For any patients who were treated, we assumed a nominal first course of treatment of generic alendronate (70 mg weekly), with an annual wholesale acquisition cost of $100.(44) For the cost of care for all types of fractures that would be prevented by the osteoporosis drug treatment, we modeled an effective total care cost of $50,000 per hip fracture during the year each hip fracture occurs plus $12,000 in the following year for additional care at home or in a nursing home.(5,7,34,40–43,57,58) We did not account for the costs of any additional clinical program staff, which we assumed could be justified (and paid for) by any cost savings and would be similar for BCT and usual care. We did not include cost for rare, but possible, adverse effects associated with alendronate use, which is considered a small risk.(59)
Outcomes
We report costs, number of hip fractures, and QALYs for each of BCT and usual care strategies relative to no screening, and BCT relative to usual care. In the analysis of the three strategies, we first eliminated dominated strategies, ie, more costly and less effective than another strategy. Among the remaining strategies, if one was more costly and more effective than the other, we calculated the incremental cost effectiveness ratio (ICER) as net cost divided by QALYs gained. Results are also presented for the one-way sensitivity analyses, varying the parameters as discussed above (Table 1).
To help assess BCT pricing levels for payor budgetary purposes, we also calculated a breakeven cost per BCT test performed at which the BCT program would be cost neutral to the payor compared to usual care. This cost was calculated as the total cost savings for BCT relative to usual care, divided by the total number of BCT tests yielding diagnostic-quality results (n = 900), plus the $100 nominal BCT test fee. Because this breakeven cost is based on the cost savings of BCT relative to usual care, any program costs not included in the analysis but common to both the BCT and usual care strategies (eg, program-specific staffing or hospital overhead), would not influence this outcome.
Results
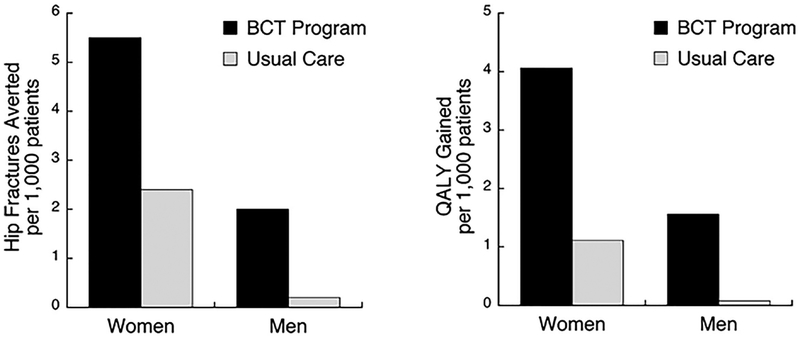
In our base-case analysis, the BCT program was the dominant strategy for both sexes, that is, it provided greater clinical benefit at lower cost compared to both usual care and no screening. With the BCT and 90% of women tested, overall 21% tested positive and were eligible for treatment. With DXA, 37.4% were tested, and 12.5% overall tested positive. For 1000 women, the BCT program could prevent 3.1 hip fractures and result in 2.95 QALYs gained compared to usual care (Table 2). The absolute number of prevented hip fractures compared to no screening for BCT and DXA were 5.5 and 2.4, respectively (Fig. 3). Among men, by testing 90% with the BCT, 21.7% tested positive and were eligible for treatment. With DXA, 7.9% were tested over the 5-year period and 1.7% tested positive. For 1000 men, BCT prevented 1.9 hip fractures compared to usual care, which in turn resulted in 1.48 QALYs gained (Table 2). The absolute number of prevented hip fractures compared to no screening for BCT and DXA were 2.0 and 0.2 respectively (Fig. 3). Thus, the absolute clinical benefit for BCT screening in men over age 65 years was similar to that for DXA screening in women over age 65 years. For women, BCT was cost saving compared to usual care ($113,000) and no screening ($200,000), and for men the strategies had similar costs, but BCT saved $7000 relative to both usual care and no screening (Table 2).
Table 2.
Base Case Results for Annual Savings and QALY Gained Due to Averted Hip Fractures, and Effects of Changing the Sex-Specific Level of Fracture Risk for the Patient Pool
| Women (n = 1000) | Men (n = 1000) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Population risk of hip fracture | Test | Savings ($1000) | Hip fracture averted | QALY gained | Breakeven BCT cost ($) | Savings ($1000) | Hip fracture averted | QALY gained | Breakeven BCT cost ($) |
| Base case | BCT | 200 | 5.5 | 4.06 | 7 | 2.0 | 1.56 | ||
| UC | 88 | 2.4 | 1.11 | 1 | 0.2 | 0.08 | |||
| BCT-UC | 113 | 3.1 | 2.95 | 225 | 7 | 1.9 | 1.48 | 107 | |
| 2× higher | BCT | 522 | 11.0 | 8.11 | 129 | 4.1 | 3.13 | ||
| UC | 213 | 4.6 | 2.16 | 10 | 0.3 | 0.16 | |||
| BCT-UC | 310 | 6.4 | 5.95 | 444 | 118 | 3.8 | 2.97 | 232 | |
| 3× higher | BCT | 843 | 16.4 | 12.1 | 251 | 6.2 | 4.71 | ||
| UC | 328 | 6.6 | 3.15 | 19 | 0.5 | 0.23 | |||
| BCT-UC | 515 | 9.8 | 8.99 | 672 | 233 | 5.7 | 4.48 | 359 | |
| 4× higher | BCT | 1159 | 21.8 | 16.1 | 376 | 8.3 | 6.30 | ||
| UC | 435 | 8.5 | 4.1 | 28 | 0.7 | 0.31 | |||
| BCT-UC | 724 | 13.4 | 12.0 | 904 | 348 | 7.7 | 5.99 | 487 | |
Results are cumulative per 1000 patients, are annually recurring, and are shown for the proposed BCT program relative to no screening (BCT), usual care relative to no screening (Usual care), and BCT relative to usual care (BCT-UC). The breakeven BCT cost is the average fee per BCT test that would result in the BCT program being cost neutral compared to usual care. In a one-way sensitivity analysis, data are also shown for 2×, 3×, and 4× higher levels of fracture risk. Base case hip fracture rates (annual incidence) are 10.51 per 1000 women and 4.57 per 1000 men. See main text for all assumptions.
BCT = biomechanical computed tomography; UC = usual care.
Fig. 3.
Base-case results for clinical efficacy. Number of hip fractures prevented and QALYs gained compared to no screening (and no treatment), for the proposed BCT program and for DXA-based usual care. These benefits are for 1000 patients in the program in a single year, evaluated over a 5-year window.
Clinical efficacy and cost savings for BCT relative to usual care were improved appreciably when the program was restricted to patients at higher risk of hip fracture (Table 2). For example, restricting the program to a twofold higher risk pool increased the number of prevented hip fractures twofold for BCT compared to usual care, from 3.1 to 6.4 per 1000 women and from 1.9 to 3.8 per 1000 men; cost savings increased proportionally with the numbers of prevented fractures. The absolute clinical benefits for BCT screening for men in the twofold higher risk pool (4.1 prevented hip fractures; 3.13 QALY gained) exceeded the benefits for DXA screening in women for the base case (2.4 prevented hip fractures; 1.11 QALY gained). Depending on the risk level of the pool, the breakeven cost per BCT test for the program to be cost neutral compared to usual care ranged from $225 to 904 for the women, and from $107 to 487 for the men. For example, for a patient pool of women having the base-case risk of hip fracture, the breakeven cost per BCT test was $225; for a pool of men having a twofold higher risk of fracture than the base case, the breakeven cost was $232.
In our one-way sensitivity analyses, when the BCT test fee was increased from the $100 nominal fee up to $250, BCT remained either the dominant strategy or was highly cost effective for women, and was cost effective for the men depending on the fee. For 1000 women, increasing the BCT test fee from $100 to $150 and $250 per test, compared to usual care, cost savings decreased to $68,000 for a BCT fee of $150, and for a $250 fee costs were higher by $22,000, with an ICER of $7622 per QALY gained. For 1000 men, when the BCT test fee was increased to $150, BCT resulted in costs of $38,000 and an ICER of $25,970 per QALY gained compared with usual care (usual care versus no screening resulted in savings of $7000). For a BCT fee of $250 in men, BCT resulted in higher costs of $128,000 compared to usual care and an ICER of $86,981 per QALY gained. When the probability of death due to hip fracture was decreased twofold from 0.20 to 0.10, QALY gained from BCT compared to usual care remained high, reducing from 2.95 to 2.17 for women, and from 1.48 to 1.11 for men.
Increasing the treatment initiation rate or the duration of the treatment increased the benefits of BCT relative to usual care, the effect being larger for women than men (Table 3). For the women, BCT remained the dominant strategy when the proportion of patients treated was reduced from 50% to 30% (Table 3). When the proportion of women treated was increased from 50% to 70%, cost savings for BCT relative to usual care increased from $113,000 to $211,000 and hip fractures prevented from 3.1 to 4.9 (per 1000 patients). For the men, decreasing the proportion of treated patients from 50% to 30% resulted in costs of about $30,000 and 1.1 fractures prevented compared to usual care. For that situation, the ICER of BCT compared to usual care was $34,000 per QALY gained. When 70% of men were treated, BCT was again the dominant strategy, preventing 2.6 more hip fractures (2.8 versus 0.2 per 1000 men) and saving $43,000 compared to usual care. When changing treatment duration by ± 1 year, BCT remained dominant except for 1 year of treatment for men (Table 3). For that situation, BCT incurred costs of about $13,000 compared to usual care, for which BCT had an ICER of $11,000 per QALY gained.
Table 3.
One-Way Sensitivity Analysis on Changing Patient Adherence to Treatment and the Duration of Treatment, for BCT and Usual Care Relative to No Screening and for BCT-UC
| Women (n = 1000) | Men (n = 1000) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment-related parameters | Parameter value | Test | Savings ($1000) | Hip fracture averted | QALY gained | Breakeven BCT cost ($) | Savings ($1000) | Hip fracture averted | QALY gained | Breakeven BCT cost ($) |
| Adherence to treatment | 0.3 | BCT | 92 | 3.4 | 2.49 | −30 | 1.2 | 0.95 | ||
| UC | 78 | 2.2 | 0.91 | 0 | 0.2 | 0.07 | ||||
| BCT-UC | 14 | 1.3 | 1.59 | 116 | −30 | 1.1 | 0.88 | 67 | ||
| 0.5a | BCT | 200 | 5.5 | 4.06 | 7 | 2.0 | 1.56 | |||
| UC | 88 | 2.4 | 1.11 | 1 | 0.2 | 0.08 | ||||
| BCT-UC | 113 | 3.1 | 2.95 | 225 | 7 | 1.9 | 1.48 | 107 | ||
| 0.7 | BCT | 308 | 7.5 | 5.62 | 44 | 2.8 | 2.16 | |||
| UC | 97 | 2.6 | 1.31 | 1 | 0.2 | 0.09 | ||||
| BCT-UC | 211 | 4.9 | 4.31 | 334 | 43 | 2.6 | 2.07 | 148 | ||
| Years of treatment | 1 | BCT | 134 | 4.1 | 3.32 | −13 | 1.5 | 1.27 | ||
| UC | 76 | 2.1 | 0.98 | 0 | 0.2 | 0.07 | ||||
| BCT-UC | 59 | 2.0 | 2.33 | 165 | −13 | 1.3 | 1.20 | 86 | ||
| 2a | BCT | 200 | 5.5 | 4.06 | 7 | 2.0 | 1.56 | |||
| UC | 88 | 2.4 | 1.11 | 1 | 0.2 | 0.08 | ||||
| BCT-UC | 113 | 3.1 | 2.95 | 225 | 7 | 1.9 | 1.48 | 107 | ||
| 3 | BCT | 245 | 6.5 | 4.48 | 20 | 2.4 | 1.72 | |||
| UC | 94 | 2.6 | 1.17 | 1 | 0.2 | 0.08 | ||||
| BCT-UC | 151 | 3.9 | 3.31 | 268 | 19 | 2.2 | 1.64 | 121 | ||
Adherence to treatment refers to the proportion of the patients who test positive that go on treatment. Negative values of savings indicate the strategy is not cost saving (see text for associated values of incremental cost-effectiveness ratios). See Table 2 for additional legends.
BCT = biomechanical computed tomography; UC = usual care; BCT-UC = BCT relative to UC.
Base case.
Increasing sensitivity and specificity for the BCT test had modest effects but revealed the important role of the proportion of patients tested for each screening strategy. When sensitivity and specificity for BCT were both increased by 20%, the number of prevented hip fractures per 1000 women increased from 3.1 to 5.0 compared to usual care (Table 4). When the sensitivity and specificity values for DXA testing were used for the BCT test, the number of prevented fractures for BCT compared to usual care were 2.7 for women and 1.5 for men, indicating that a large contributor to the clinical effectiveness of the BCT program was its high testing rate.
Table 4.
Sensitivity Analyses to Assess the Effects of Changing the Values of Sensitivity or Specificity, or Both, for the BCT Test, Assuming Base-Case Values for All Other Parameters
| Women (n = 1000) | Men (n = 1000) | |||||
|---|---|---|---|---|---|---|
| Increase sensitivity and/or specificity for the BCT test | Savings ($1000) | Hip fracture averted | QALY gained | Savings ($1000) | Hip fracture averted | QALY gained |
| Base case values | 113 | 3.1 | 2.95 | 7 | 1.9 | 1.48 |
| Sensitivity and specificity +20% | 235 | 5.0 | 4.10 | 65 | 2.6 | 1.90 |
| Sensitivity only +20% | 189 | 4.4 | 3.80 | 33 | 2.3 | 1.80 |
| Specificity only +20% | 145 | 3.5 | 3.10 | 33 | 2.1 | 1.57 |
| Use DXA sensitivity and specificity | 90 | 2.7 | 2.80 | −9 | 1.5 | 1.25 |
Results are for the BCT program relative to usual care. Base case sensitivity/specificity for BCT was 0.66/0.66 for women and 0.56/0.76 for men; and for DXA was 0.59/0.67 for women and 0.48/0.78 for men. See Table 2 for additional legends.
BCT = biomechanical computed tomography.
Discussion
As a nationally recommended service by the US Preventive Services Task Force,(59) preventative screening for osteoporosis for all women age 65 years and older in Medicare is covered by the Affordable Care Act.(60) Our findings indicate that, for both women and men age 65 years and older who are already undergoing abdominal CT and are without a recent DXA, the proposed BCT program for osteoporosis screening appears to be a cost-effective alternative to usual care—and for many scenarios could be cost saving. For women, the predicted benefits for BCT over DXA-based usual care were large at all risk categories and for all modeling scenarios. For men, although our BCT program does not reflect Medicare coverage or typical clinical practice guidelines for managing osteoporosis, our base-case analysis indicated that BCT screening in men age 65 years and older should have similar clinical efficacy as usual-care DXA screening in women age 65 years and older; and BCT screening in higher risk men would far exceed the clinical efficacy of usual-care DXA screening in women age 65 years and older and would be far more cost effective, even cost saving. DXA-based osteoporosis care is inherently limited because each year only 9.5% of eligible Medicare women and 1.7% of men get screened.(11) The proposed BCT program can help address that care gap by providing a highly effective diagnostic screen(17) utilizing the millions of abdominal CT scans taken annually in the United States for patients age 65 years or older.(24) Ancillary BCT requires no extra patient procedure, is associated with no extra radiation exposure, can be applied to almost all routine clinical abdominal and hip-containing CT scans,(17,20–23) and outperforms traditional DXA for identifying patients at high risk of hip fracture.(17) Thus, our new results indicate that if BCT-based screening programs can be targeted to these CT patients—while continuing to use DXA-based screening for all other patients—BCT programs should pay for themselves while improving patient care.
Although our results for clinical efficacy are theoretical, their feasibility is supported by evidence from real world clinical experience. In the recent SCOOP study of community-based women aged 70 to 85 years in the United Kingdom, screening by FRAX/DXA of all participants in an intervention arm was compared against usual care; this interventional screening led to a net reduction of 54 hip fractures over 5 years versus usual care.(50) In that study, the treatment rate in the identified high-risk patients was 78%, which was approximately 50% higher than for usual care. Scaling our results to those parameters and to the sample sizes used in SCOOP, assuming a 50% elevated risk level for a 70-year-old to 85-year-old cohort compared to our base-case cohort, using DXA values for sensitivity and specificity for the BCT test to simulate screening of all participants by DXA as in SCOOP, our model would predict prevention of 54 fractures per 6200 women compared to usual care. That prediction is consistent with the observed results from SCOOP, supporting our methodology for predicting clinical efficacy.
The improved clinical efficacy of the BCT program versus usual care arose from its high testing rate—as did the SCOOP intervention(50)—and also its improved test sensitivity. A high testing rate is feasible with ancillary BCT because it does not require the patient to undergo any extra procedure or office visit, and it can be used on almost all abdominal CT scans without any change to the imaging protocol. This approach is particularly feasible in a managed system for which a standing-order protocol could be implemented. The improved sensitivity of BCT compared to traditional DXA is a unique feature of BCT,(17) providing confidence that, as an adjunct to DXA, BCT is at least as good as the clinical standard. In addition, women who test positive with BCT are at significantly higher risk of hip fracture due to low bone strength than are women who test positive with traditional DXA.(17) Because alendronate is thought to be more effective in patients having lower bone strength,(61) BCT-positive patients may be more responsive to alendronate than are DXA-positive patients, a possible effect that was not explored in our analysis. Related, from the perspective of responding to an osteoporosis drug treatment, testing positive with BCT but with low bone mass (osteopenia) by BMD criteria, identifies patients at high risk due solely to weakened bone; this may be preferable to testing positive by having low bone mass but with other risk factors that cannot be modified by an osteoporosis drug treatment, such as sex, advanced age, or a parental history of hip fracture.
Our results for cost effectiveness extend previous studies that used BCT in different ways. Agten and colleagues(25) recently reported on a cost-effective analysis for the United States using DXA combined with BCT for all patients, which was performed on dedicated hip CT scans at an assumed fee of $100 for the BCT analysis. Assuming all patients got screened, they found that the most cost-effective strategy was combined DXA and BCT screening starting at age 55 years with BCT repeated every 5 years thereafter (ICER = $2000/QALY compared to DXA usual care). Such a low value of ICER—almost cost saving—is consistent with our findings because their program required extra payment for a dedicated CT scan whereas ours did not. Others have calculated that BCT could be cost-effective in the United Kingdom—at a cost of £14,656/QALY—if offered at a fee of $100 to all patients who test positive for osteopenia by DXA, the BCT analysis also requiring payment for a dedicated CT exam.(26) Again, our results are consistent with that low value of ICER, considering overall care costs are lower in the United Kingdom than the United States and our proposed (ancillary) BCT program does not require extra payment for a dedicated CT. We emphasize that these prior analyses assessed general use of BCT with DXA, which would increase radiation exposure and cost by requiring a dedicated CT exam for the BCT analysis. By contrast, our proposed program is typically only offered to patients already having abdominal CT for other medical indications and therefore would not involve any extra radiation exposure nor require a DXA exam; usual-care DXA screening could be used for all other patients.
Our results suggest various plausible implementations of the proposed ancillary BCT program that would be cost saving to the payor while potentially covering costs for providing the BCT service, especially if the BCT test can be streamlined as volume and efficiencies increase over time. Based on census population figures and age-related hip fracture incidence rates for white women and men in the pre-alendronate era,(62) a twofold higher risk pool, compared to the sex-specific base case level (age 65+ years), would be one aged approximately 75+ years for both sexes, and a threefold higher risk pool would be one aged approximately 80+ years for men and 82+ years for women. We found that for screening women age 65 years or older in a general setting, the clinical benefits were over twice those for usual care and the breakeven cost for BCT compared to usual care was $225 per BCT test. A similar level of clinical effectiveness compared to usual care and a similar breakeven cost ($232) was achieved when the BCT test was limited to men in a twofold higher risk group (eg, age 75 years or older). Thus, in both these cases, the BCT program would be cost neutral and multiple-fold more clinically effective than usual care if the BCT test were priced at a fee of $225 and offered to all women age 65 years or older and all men aged 75 years or older. This fee is less than the initial price levels set in the United Kingdom (~$325),(26) reflecting that, although a software-based test, the BCT analysis is not fully automated and requires highly specialized software and computational infrastructure, unique engineering expertise, attention to detail by the analyst, and substantial quality controls; plus the test requires transfer of CT data from archive and clinical assessment of the results. In the fourfold higher risk setting of a nursing home facility,(63) the high breakeven costs ($904 for women, $487 for men) suggest appreciable savings are possible in such a setting. Deployment of a case manager(27,28,64) can increase the proportion of positive-testing patients who start and stay on treatment.(64–67) Importantly, our results indicate that the additional cost savings for BCT over usual care that would result from increasing treatment initiation and extending treatment duration would pay for such a case manager, further improving clinical efficacy at no extra net cost.
The main limitation of this study is its theoretical nature, although as noted above, the results for clinical efficacy are consistent with the available real-world clinical data.(50) One large driver of overall cost effectiveness is the cost of treating hip and other fractures that would otherwise occur without the screening and treatment program. Our effective total care cost of $50,000 during the first year after hip fracture is supported by numerous studies,(5,7,34,40–42,57,58,68,69) and may even be conservative. We based this cost largely on the “incremental” cost of hip fracture—those care costs beyond those that would have occurred without the fracture.(43,68) In particular, Kilgore and colleagues(43) reported incremental costs for just hip fracture, within 6 months of the fracture, to be $31,310 for the Medicare population, in 2007 dollars, equivalent to about $42,000 in 2016 dollars. Others have reported that the incremental cost of hip fracture represents only one-third of the incremental costs for all types of osteoporotic fracture,(68) at least some of which would also be prevented by treatment. Thus, although we only explicitly modeled hip fracture, we lumped care costs for all types of prevented fracture into this effective cost of a hip fracture for the 12 months after fracture. Our assumed total effective care cost is therefore likely conservative. Likewise, our estimates of QALY gained are also likely conservative because they were based on the effects of only hip fracture and only included a 5-year time horizon without accounting for long-term care costs following hip fracture; and our sensitivity studies showed that reducing excess mortality risk twofold still resulted in appreciable benefits for BCT relative to usual care. That said, until implemented in practice in different settings, true efficacy and cost effectiveness cannot be determined with certainty.
Other limitations are noted. We caution about generalizing our cost results to specific instances due to potentially large variations across specific healthcare systems. For example, low care costs in the United Kingdom likely explain why the SCOOP intervention was highly cost-effective but not quite cost saving.(70) Likewise, because the population hip fracture risk rate varies by race,(62,71) region,(71) and even socioeconomic status,(72) so do our results, and we did not address individual risk factors such as low body weight(5) that might place an individual patient at a higher risk than the pool average; nor did we address younger patients who would generally be at lower risk. For women, insight into lower risk can be gleaned from the results for the men, who were at over twofold lower risk than the women for the base case. Although most of our modeling assumptions would affect BCT and usual care in a similar manner, some could introduce small biases that could favor either BCT or usual care. For example, regarding the assumed background population risk, we do not know if BCT patients aged 65 years or older would generally be at different risk of hip fracture than the general population from which the background population risk was estimated. Although BCT patients would not include higher-risk patients already on osteoporosis treatment or referred for (and with a recent) DXA, all BCT patients would have a medical need for a CT scan and, therefore, many may also have poor bone health. We note also that any secular decreases in population fracture rates since the pre-bisphosphonate era were also ignored. Such changes are thought to be on the order of 15%,(34,47) and ignoring this may have produced a small bias favoring BCT. For usual care for men, if one instead assumed men are not screened until age 70 years, the baseline population risk for men would increase, which would favor BCT. Somewhat offsetting this effect, the DXA screening rate is likely higher in men age 70+ years than our assumed rate, which would favor usual care. Despite these small bidirectional biases, given the large positive effects of BCT over usual care for almost all the modeling scenarios, it is unlikely that the uncertainty regarding these various factors would change the overall conclusions. In considering BCT as an adjunct approach to DXA for osteoporosis screening, we note that patient selection criteria may be unique for a BCT program. For example, not all recipients of abdominal CT scans may be medically suitable for osteoporosis treatment because of the substantial comorbidities that these patients may experience. There may also be a higher competing risk of mortality for these patients, compared to the average osteoporosis population, although that is unlikely to appreciably alter our 5-year results.
If extrapolating our results beyond our simulated managed care setting, in which a standing order implementation would be feasible, some additional issues are noted. First, although the effort and expense required to implement a standing-order ancillary BCT program may be greater in certain health systems, our results can still provide estimates of benefits over usual care in a more open setting. One such setting could be a fracture liaison service that expanded to also include patients who were referred to radiology for a CT scan and meet the eligibility requirements for an osteoporosis screen with or without experiencing a fracture. In that scenario, the ancillary BCT test would be ordered by a bone healthcare professional from the expanded fracture liaison service that coordinates with one or more radiology facilities, and results from the BCT test would be interpreted by that individual, not by a radiologist. Although performed ancillary to a CT scan acquired for another medical condition, results from the BCT test are not incidental findings attached to the original exam notes. Instead the BCT test is a quantitative diagnostic-quality test ordered specifically for the patient by the bone healthcare professional. In such a scenario, the results from the ancillary BCT test would be acted upon in terms of follow-up patient care just as would results from a DXA test.
As with DXA screening, we do not advocate BCT screening without ensuring that the results go to the healthcare professional who ordered up the test and who is charged with interpreting the results diagnostically and following up with patient care. Second, in considering consequences at the national level, one would need to consider overlap between DXA and CT populations, a longer timeframe for analysis, and account for QALY gained for all major osteoporotic fractures—all beyond the scope of this study but appropriate for future research. For application beyond the United States, although per capita usage of CT in many countries is comparable to US usage, ancillary BCT would clearly have less impact where CT is not as widely used. Likewise, different countries have different clinical guidelines and payment and coverage policies for osteoporosis screening, which would be an additional issue to consider in assessing potential impact or feasibility of ancillary BCT.
In summary, this modeling analysis indicated that a proposed ancillary BCT-based program for osteoporosis diagnostic screening—offered in a standing-order fashion to patients with a previously taken abdominal CT and without a recent DXA—appears to be cost-effective compared to usual care, and for many scenarios could also be cost saving to the payor. Because millions of patients age 65 years and older already undergo abdominal CT each year in the United States, the current results suggest that use of ancillary BCT may have a positive impact on the management of osteoporosis.
Acknowledgments
This work was supported by a grant from the NIH (R44 AR057616 to DLK). The funding agency had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Authors’ roles: Study design: MP, DLK, KGS, and TMK. Data analysis: MP. Data interpretation: MP, DLK, CEL, KGS, and TMK. Drafting manuscript: MP and TMK. Revising manuscript content: MP, DLK, CEL, KGS, and TMK. Approving final version of manuscript: MP, DLK, CEL, KGS, and TMK. MP takes responsibility for the integrity of the data analysis.
Disclosures
MP: Salary support paid to institution from NIH R44 AR057616 awarded to O.N. Diagnostics. CEL: Salary support paid to institution from NIH R44 AR057616 awarded to O.N. Diagnostics. DLK: Employee of O.N. Diagnostics, which offers the BCT test clinically. KGS: Salary support paid to institution from NIH R44 AR057616 awarded to O.N. Diagnostics; Investigator for Amgen, Mereo, Radius; Consultant for Amgen, Lilly, and Radius. TMK: Consultant for Amgen, AgNovos Healthcare, and O.N. Diagnostics; Equity in O.N. Diagnostics. All authors state that they have no conflicts of interest.
References
- 1.Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001–2008. J Bone Miner Res. 2011;26(1):3–11. [DOI] [PubMed] [Google Scholar]
- 2.Schousboe JT, Ensrud KE, Nyman JA, Melton LJ 3rd, Kane RL. Universal bone densitometry screening combined with alendronate therapy for those diagnosed with osteoporosis is highly cost-effective for elderly women. J Am Geriatr Soc. 2005;53(10):1697–704. [DOI] [PubMed] [Google Scholar]
- 3.Schwenkglenks M, Lippuner K. Simulation-based cost-utility analysis of population screening-based alendronate use in Switzerland. Osteoporos Int. 2007;18(11):1481–91. [DOI] [PubMed] [Google Scholar]
- 4.Schousboe JT, Taylor BC, Fink HA, et al. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. JAMA. 2007;298(6):629–37. [DOI] [PubMed] [Google Scholar]
- 5.Schousboe JT, Gourlay M, Fink HA, et al. Cost-effectiveness of bone densitometry among Caucasian women and men without a prior fracture according to age and body weight. Osteoporos Int. 2013;24(1):163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim SY, Lim JH, Nguyen D, et al. Screening for osteoporosis in men aged 70 years and older in a primary care setting in the United States. Am J Mens Health. 2013;7(4):350–4. [DOI] [PubMed] [Google Scholar]
- 7.Nayak S, Greenspan SL. Cost-Effectiveness of Osteoporosis Screening Strategies for Men. J Bone Miner Res. 2016;31(6):1189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewiecki EM, Adler RA, Curtis JR. Hip fracture trends in the United States, 2002 to 2015. Osteoporos Int. 2018;29(3):717–22. [DOI] [PubMed] [Google Scholar]
- 9.King AB, Fiorentino DM. Medicare payment cuts for osteoporosis testing reduced use despite tests’ benefit in reducing fractures. Health Aff (Millwood). 2011;30(12):2362–70. [DOI] [PubMed] [Google Scholar]
- 10.Siris ES, Modi A, Tang J, Gandhi S, Sen S. Substantial under-treatment among women diagnosed with osteoporosis in a US managed-care population: a retrospective analysis. Curr Med Res Opin. 2014;30(1): 123–30. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Delzell E, Zhao H, et al. Central DXA utilization shifts from office-based to hospital-based settings among medicare beneficiaries in the wake of reimbursement changes. J Bone Miner Res. 2012;27(4):858–64. [DOI] [PubMed] [Google Scholar]
- 12.Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34(1):195–202. [DOI] [PubMed] [Google Scholar]
- 13.Orwoll ES, Marshall LM, Nielson CM, et al. Finite element analysis of the proximal femur and hip fracture risk in older men. J Bone Miner Res. 2009;24(3):475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainwright SA, Marshall LM, Ensrud KE, et al. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab. 2005;90(5):2787–93. [DOI] [PubMed] [Google Scholar]
- 15.Oden A, McCloskey EV, Johansson H, Kanis JA. Assessing the impact of osteoporosis on the burden of hip fractures. Calcif Tissue Int. 2013;92(1):42–9. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Gruner M, Tremollieres F, et al. Diagnostic accuracy of FRAX in predicting the 10-year risk of osteoporotic fractures using the USA treatment thresholds: A systematic review and meta-analysis. Bone. 2017;99:20–5. [DOI] [PubMed] [Google Scholar]
- 17.Adams AL, Fischer H, Kopperdahl DL, et al. Osteoporosis and hip fracture risk from routine computed tomography scans: The Fracture, Osteoporosis, and CT Utilization Study (FOCUS). J Bone Miner Res. 2018;33(7):1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keaveny TM. Biomechanical computed tomography-noninvasive bone strength analysis using clinical computed tomography scans. Ann N Y Acad Sci. 2010;1192:57–65. [DOI] [PubMed] [Google Scholar]
- 19.Zysset P, Qin L, Lang T, et al. Clinical use of quantitative computed tomography-based finite element analysis of the hip and spine in the management of osteoporosis in adults: the 2015 ISCD official positions-part II. J Clin Densitom. 2015;18(3):359–92. [DOI] [PubMed] [Google Scholar]
- 20.Weber NK, Fidler JL, Keaveny TM, et al. Validation of a CT-derived method for osteoporosis screening in IBD patients undergoing contrast-enhanced CT enterography. Am J Gastroenterol. 2014; 109(3):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fidler JL, Murthy NS, Khosla S, et al. Comprehensive assessment of osteoporosis and bone fragility with CT colonography. Radiology. 2016;278(1):172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DC, Hoffmann PF, Kopperdahl DL, Keaveny TM. Phantomless calibration of CT scans for measurement of BMD and bone strength-inter-operator reanalysis precision. Bone. 2017;103:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwaiger BJ, Kopperdahl DL, Nardo L, et al. Vertebral and femoral bone mineral density and bone strength in prostate cancer patients assessed in phantomless PET/CT examinations. Bone. 2017;101: 62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agten CA, Ramme AJ, Kang S, Honig S, Chang G. Cost-effectiveness of virtual bone strength testing in osteoporosis screening programs for postmenopausal women in the United States. Radiology. 2017;285(2):506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viceconti M, Qasim M, Bhattacharya P, Li X. Are CT-based finite element model predictions of femoral bone strengthening clinically useful? Curr Osteoporos Rep. 2018;16(3):216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dell RM, Greene D, Anderson D, Williams K. Osteoporosis disease management: what every orthopaedic surgeon should know. J Bone Joint Surg Am. 2009;91 Suppl 6:79–86. [DOI] [PubMed] [Google Scholar]
- 28.Olenginski TP, Maloney-Saxon G, Matzko CK, et al. High-risk osteoporosis clinic (HiROC): improving osteoporosis and postfracture care with an organized, programmatic approach. Osteoporos Int. 2015;26(2):801–10. [DOI] [PubMed] [Google Scholar]
- 29.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10): 2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durden E, Pinto L, Lopez-Gonzalez L, Juneau P, Barron R. Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos. 2017;12(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meadows ES, Mitchell BD, Bolge SC, Johnston JA, Col NF. Factors associated with treatment of women with osteoporosis or osteopenia from a national survey. BMC Womens Health. 2012;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balasubramanian A, Brookhart MA, Goli V, Critchlow CW. Discontinuation and reinitiation patterns of osteoporosis treatment among commercially insured postmenopausal women. Int J Gen Med. 2013;6:839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000;85(11): 4118–24. [DOI] [PubMed] [Google Scholar]
- 34.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures inthe United States. JAMA. 2009;302(14):1573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tajeu GS, Delzell E, Smith W, et al. Death, debility, and destitution following hip fracture. J Gerontol A Biol Sci Med Sci. 2014;69(3): 346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray NF, Chan JK, Thamer M, Melton LJ 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12(1):24–35. [DOI] [PubMed] [Google Scholar]
- 37.Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int. 2009;20(10):1633–50. [DOI] [PubMed] [Google Scholar]
- 38.Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: final data for 2015. Natl Vital Stat Rep. 2017;66(6):1–75. [PubMed] [Google Scholar]
- 39.Centers for Medicare & Medicaid Services. Hospital Outpatient Prospective Payment System (PPS) Addendum B Update. Baltimore, MD, USA: Centers for Medicare & Medicaid Services; 2019. [cited 2019 Mar 2]. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Addendum-A-and-Addendum-B-Updates.html. [Google Scholar]
- 40.Hiligsmann M, Ben Sedrine W, Bruyere O, Evers SM, Rabenda V, Reginster JY. Cost-effectiveness of vitamin D and calcium supplementation in the treatment of elderly women and men with osteoporosis. Eur J Public Health. 2015;25(1):20–5. [DOI] [PubMed] [Google Scholar]
- 41.Parthan A, Kruse M, Yurgin N, Huang J, Viswanathan HN, Taylor D. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy. 2013;11(5):485–97. [DOI] [PubMed] [Google Scholar]
- 42.Majumdar SR, Lier DA, McAlister FA, et al. Cost-effectiveness of osteoporosis interventions for ‘incidental’ vertebral fractures. Am J Med. 2013;126(2):169.e9–17. [DOI] [PubMed] [Google Scholar]
- 43.Kilgore ML, Morrisey MA, Becker DJ, et al. Health care expenditures associated with skeletal fractures among Medicare beneficiaries, 1999–2005. J Bone Miner Res. 2009;24(12):2050–5. [DOI] [PubMed] [Google Scholar]
- 44.Social Security Administration. Red Book: a summary guide to employment supports for persons with disabilities under the Social Security Disability Insurance and Supplemental Security Income programs (SSA Pub. No. 64–030). Washington, DC: US Government Printing Office; 2017. [Google Scholar]
- 45.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 46.Fryback DG, Dunham NC, Palta M, et al. US norms for six generic health-related quality-of-life indexes from the National Health Measurement study. Med Care. 2007;45(12):1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright NC, Saag KG, Curtis JR, et al. Recent trends in hip fracture rates by race/ethnicity among older US adults. J Bone Miner Res. 2012;27(11):2325–32. [DOI] [PubMed] [Google Scholar]
- 48.Nayak S, Roberts MS, Greenspan SL. Impact of generic alendronate cost on the cost-effectiveness of osteoporosis screening and treatment. PLoS One. 2012;7(3):e32879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majumdar SR, Beaupre LA, Harley CH, et al. Use of a case manager to improve osteoporosis treatment after hip fracture: results of a randomized controlled trial. Arch Intern Med. 2007;167(19):2110–5. [DOI] [PubMed] [Google Scholar]
- 50.McCloskey E, Johansson H, Harvey NC, et al. Management of patients with high baseline hip fracture risk by FRAX reduces hip fractures—a post hoc analysis of the SCOOP study. J Bone Miner Res. 2018; 33(6):1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis S, Martyn-St James M, Sanderson J, et al. A systematic review and economic evaluation of bisphosphonates for the prevention of fragility fractures. Health Technol Assess. 2016;20(78):1–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–38. [DOI] [PubMed] [Google Scholar]
- 53.Jonsson B, Kanis J, Dawson A, Oden A, Johnell O. Effect and offset of effect of treatments for hip fracture on health outcomes. Osteoporos Int. 1999;10(3):193–9. [DOI] [PubMed] [Google Scholar]
- 54.Tosteson AN, Jonsson B, Grima DT, O’Brien BJ, Black DM, Adachi JD. Challenges for model-based economic evaluations of postmenopausal osteoporosis interventions. Osteoporos Int. 2001;12(10):849–57. [DOI] [PubMed] [Google Scholar]
- 55.Dell RM, Adams AL, Greene DF, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27(12):2544–50. [DOI] [PubMed] [Google Scholar]
- 56.Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res. 2014;29(3):570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bass E, French DD, Bradham DD. A national perspective of Medicare expenditures for elderly veterans with hip fractures. J Am Med Dir Assoc. 2008;9(2):114–9. [DOI] [PubMed] [Google Scholar]
- 58.Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51(3):364–70. [DOI] [PubMed] [Google Scholar]
- 59.US Preventive Services Task Force, Curry SJ, Krist AH, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(24):2521–31. [DOI] [PubMed] [Google Scholar]
- 60.HealthCare.gov. Preventive care benefits for women. Baltimore, MD, USA: U.S. Centers for Medicare & Medicaid Services; 2018. September 15 [cited 2019 Mar 2]. Available from: https://www.healthcare.gov/preventive-care-women/. [Google Scholar]
- 61.Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280(24):2077–82. [DOI] [PubMed] [Google Scholar]
- 62.Jacobsen SJ, Goldberg J, Miles TP, Brody JA, Stiers W, Rimm AA. Hip fracture incidence among the old and very old: a population-based study of 745,435 cases. Am J Public Health. 1990;80(7):871–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugarman JR, Connell FA, Hansen A, Helgerson SD, Jessup MC, Lee H. Hip fracture incidence in nursing home residents and community-dwelling older people, Washington State, 1993–1995. J Am Geriatr Soc. 2002;50(10):1638–43. [DOI] [PubMed] [Google Scholar]
- 64.Majumdar SR. A T-2 translational research perspective on interventions to improve post-fracture osteoporosis care. Osteoporos Int. 2011;22 Suppl 3:471–6. [DOI] [PubMed] [Google Scholar]
- 65.Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296(4):427–40. [DOI] [PubMed] [Google Scholar]
- 66.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–18. [DOI] [PubMed] [Google Scholar]
- 67.Ayanian JZ. The elusive quest for quality and cost savings in the Medicare program. JAMA. 2009;301(6):668–70. [DOI] [PubMed] [Google Scholar]
- 68.Gabriel SE, Tosteson AN, Leibson CL, et al. Direct medical costs attributable toosteoporoticfractures. OsteoporosInt.2002;13(4):323–30. [DOI] [PubMed] [Google Scholar]
- 69.Leibson CL, Tosteson AN, Gabriel SE, Ransom JE, Melton LJ. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50(10): 1644–50. [DOI] [PubMed] [Google Scholar]
- 70.Turner DA, Khioe RFS, Shepstone L, et al. The cost-effectiveness of screening in the community to reduce osteoporotic fractures in older women in the UK: economic evaluation of the SCOOP study. J Bone Miner Res. 2018;33(5):845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–7. [DOI] [PubMed] [Google Scholar]
- 72.Bacon WE, Hadden WC. Occurrence of hip fractures and socioeconomic position. J Aging Health. 2000;12(2):193–203. [DOI] [PubMed] [Google Scholar]