Supplemental Digital Content is Available in the Text.
Keywords: brain injuries, Economic evaluation, outcome assessment (Healthcare), Rehabilitation, traumatic
Abstract
Objectives:
To evaluate cost-efficiency of rehabilitation following severe traumatic brain injury (TBI) and estimate the life-time savings in costs of care.
Setting/Participants:
TBI patients (n = 3578/6043) admitted to all 75 specialist rehabilitation services in England 2010–2018.
Design:
A multicenter cohort analysis of prospectively collated clinical data from the UK Rehabilitation Outcomes Collaborative national clinical database.
Main Measures:
Primary outcomes: (a) reduction in dependency (UK Functional Assessment Measure), (b) cost-efficiency, measured in time taken to offset rehabilitation costs by savings in costs of ongoing care estimated by the Northwick Park Dependency Scale/Care Needs Assessment (NPDS/NPCNA), and (c) estimated life-time savings.
Results:
The mean age was 49 years (74% males). Including patients who remained in persistent vegetative state on discharge, the mean episode cost of rehabilitation was £42 894 (95% CI: £41 512, £44 235), which was offset within 18.2 months by NPCNA-estimated savings in ongoing care costs. The mean period life expectancy adjusted for TBI severity was 21.6 years, giving mean net life-time savings in care costs of £679 776/patient (95% CI: £635 972, £722 786).
Conclusions:
Specialist rehabilitation proved highly cost-efficient for severely disabled patients with TBI, despite their reduced life-span, potentially generating over £4 billion savings in the cost of ongoing care for this 8-year national cohort.
SEVERE TRAUMATIC BRAIN INJURY (TBI) can cause life-changing disability. There is now strong evidence from both randomized controlled clinical trials1 and other designs2 that coordinated multidisciplinary inpatient rehabilitation reduces long-term disability. There is also evidence for cost benefits, including reduced long-term care costs and increased return to work.3,4
Within the United Kingdom, specialist in-patient rehabilitation is delivered over regional networks in which a small number of units provide specialist rehabilitation for a selected group of patients with highly complex rehabilitation needs following acquired brain injury. However, these patients are costly to treat and it is necessary to demonstrate that the programs not only are effective in improving independence, but also provide value for money through savings in the cost of ongoing care.
The UK Rehabilitation Outcomes Collaborative (UKROC) provides the national clinical database, systematically collating episode data, and providing benchmarking on quality and outcomes for all specialist (levels 1 and 2) inpatient rehabilitation units in England.* Within the data set, cost-efficiency is routinely calculated in terms of the time taken to offset the cost of inpatient rehabilitation by estimated savings in the cost of ongoing care in the community (either at home or in long-term nursing home/residential care). Previous analyses have demonstrated that the costs of inpatient rehabilitation were offset within about 18 months5,6 after discharge from rehabilitation.
Despite their relatively longer lengths of stay (and often ongoing care needs), patients who were highly dependent on admission proved most cost-efficient to treat with an average “pay-back” time of just 14 months.5,7 However, these most severely disabled patients also have a significantly reduced life span, so that the long-term cost-savings should be considered in relation to their life expectancy.
The Life Expectancy Project (www.LifeExpectancy.org) is a long-standing research group in the United States. Over the last 2 decades, its authors have published extensively on life expectancy in patients with TBI, based on analyses of 2 major prospectively collected US cohorts. In 2007, Shavelle and colleagues8 published life-expectancy figures from the California Department of Developmental Services database, producing tables of the “% of normal life expectancy” in 5 functional categories (based on the patient's walking and feeding ability). Two further recent publications by Brooks et al9,10 updated those figures, based on analysis of both the Californian database and the national US Traumatic Brain Injury Model Systems (TBIMS) database (www.msktc.org/tbi).
In the absence of an equivalent national registry of TBI to provide accurate mortality data, one possible approach to estimating life expectancy in the UK TBI rehabilitation cohort is to apply the US published figures on “% normal life-expectancy” to the normal life expectancy of the UK general population, which is somewhat longer than that in the United States.
This article presents an analysis of functional outcome and cost-efficiency of specialist inpatient rehabilitation following severe TBI from the UKROC data set. Individual estimates of life expectancy using the approach outlined earlier are used to calculate net life-time savings in the cost of ongoing care.
METHODS
Design
A large multicenter national cohort analysis of prospectively collated clinical data from the UKROC national clinical database 2010–2018.
Setting and participants
In England, the majority of patients with mild-moderate TBI receive rehabilitation within their local nonspecialist level 3 services. Those with more complex rehabilitation (Category B) needs, beyond the scope of their local services, are referred to level 2 specialist rehabilitation services, providing for district-based populations of up to 1 million. Those with highly complex (Category A) needs are managed in level 1 (tertiary) services providing for regionally based populations of 2 million to 5 million. The criteria for admission to level 1 and 2 services are set out in the NHS England service specification.11
Participants were all adults (aged 16-plus) who were admitted for specialist inpatient rehabilitation in a level 1 or 2 service in England following severe TBI during the 8-year study period. By definition, these patients have complex neurological disability, presenting with a range of physical, cognitive, communicative, emotional, behavioral, and psychosocial needs.
Data source and reporting requirements
Following a single-center pilot study in 2006,6 the UKROC database was established in 2009 as part of a 5-year research program funded by the UK National Institute for Health Research (NIHR)12 to gather prospective national data on outcomes, costs, and cost-efficiency.13 It is now commissioned directly by NHS England to provide the national commissioning data set for specialist rehabilitation.
Completed rehabilitation episodes are collected by each provider on local dedicated software with in-built validatory checks, and are uploaded at monthly intervals to a secured NHS server held at Northwick Park Hospital, London. Data are further checked and collated into the central UKROC database by authors K.S. and H.W. Any detected inconsistencies are fed back to the individual provider allowing a 1-month “flex and freeze policy” for correction of errors. There are currently more than 40 000 registered episodes.
The UKROC data set comprises sociodemographic and process data (waiting times, discharge destination, etc.) as well as clinical information on rehabilitation needs, inputs, and outcomes. Full details may be found on the UKROC Web site http://www.csi.kcl.ac.uk/ukroc.html.
The data reporting requirements for level 1 and 2 services have evolved over time and vary somewhat between the different levels of service. Registration with UKROC and reporting of a minimum data set on each inpatient episode of specialist rehabilitation was a requirement for all level 1 and 2 services from 2009.14 Systematic data collection started in April 2010, but reporting of the full data set was initially voluntary. Since April 2013, services commissioned centrally by NHS England are required to report the full UKROC data set for all admitted episodes, but some locally commissioned level 2 services still report only a reduced data set. All units registered with UKROC receive training in the use of the tools to support accurate data collection, and have access to update workshops and telephone support.
Measurements
The UK Functional Assessment measure (UK FIM+FAM) is a global measure of disability.15,16 It includes the 18-item Functional Independence Measure (FIM version 4) and adds further 12 items, mainly addressing psychosocial function giving a total of 30 items (16 motor and 14 cognitive items). Each item is scored on a 7-point ordinal scale from 1 (total dependence) to 7 (complete independence). Further details are published elsewhere.15,16 Collected on admission and discharge the UK FIM+FAM forms the principal measure of outcome (change in physical and cognitive disability) within the UKROC data set.
The Northwick Park Dependency Score and Care Needs Assessment (NPDS/NPCNA). The NPDS is an ordinal scale of dependency on nursing staff time (the number of helpers and time taken to assist with each task). It is shown to be a valid and reliable measure needs for care and nursing in rehabilitation settings.17,18
When entered into the UKROC software, the NPDS translates via a computerized algorithm to the NPCNA that generates a timetable of daily care needs and, from this, estimates both the total care hours per week and the approximate weekly cost of care (£/week) in the community, based on the UK care agency costs. A more detailed description of the algorithm and how it was developed is published elsewhere.19 The NPCNA provides a generic assessment of care needs, regardless of who provides and pays for them. The estimated cost of care is therefore independent of individual circumstances or local policy for the provision continuing care, which varies widely across the United Kingdom.6
Ongoing care costs
A primary aim of rehabilitation is to improve independence for self-care. Recorded on admission and discharge from the program, the NPDS/NPCNA is used to quantify any reduction in care needs and the associated savings in the weekly cost of care. Extrapolated over time, these data may be used to estimate how long it would take for these weekly savings to offset the cost of the original rehabilitation program. Annual savings thereafter, extrapolated over the individual's expected remaining years of life, may be used to estimate anticipated life-time savings in the cost of care. These calculations are now embedded in the UKROC database for prospective patient-level reporting as detailed below.
Computations built in to the UKROC database
Cost of the rehabilitation episode
In the absence of a mandated national tariff for specialist rehabilitation, commissioning prices vary widely. Cost data are collated annually from each service provider. The UKROC data set calculates the cost of each inpatient rehabilitation episode (including all direct treatment costs and central and overhead costs) by applying a complexity-weighted bed-day tariff according to a previously published methodology.20 Tariff prices are updated every 3 to 4 years. For consistency across the different services, in this analysis we applied the indicative tariff prices published by the UK Department of Health in 2013/14 (inclusive of the Marketing Forces Factor to account for unavoidable geographic variation). These represent a reasonable mid-range estimation of service costs for the data collection period.
Cost-efficiency of rehabilitation
Within the UKROC data set, the cost-efficiency is calculated as the time taken (months) to offset the cost of rehabilitation by the resulting savings in the cost of ongoing care in the community as estimated by the NPCNA. This is calculated from the (Cost of the episode of rehabilitation) ÷ (Change in NPCNA-estimated weekly care costs from admission to discharge) × 52/12.
Life expectancy and estimated life-time savings
The UKROC database computes the life expectancy for each patient adjusted for TBI according to their age, gender, and functional level on discharge from rehabilitation. “Normal remaining years of life” for people of that age in the United Kingdom are derived from the Life Tables published by the Office of National Statistics (ONS).21 Figures for percentage of normal life expectancy (% Normal LE) are taken from the US Life Expectancy Project publications,8,10 which provide data on % Normal LE by decade of age in 5 “Functional categories” (4: “Walks well alone,” 3: “Some walking ability,” 2: “Does not walk, self feeds,” 1: “Does not walk, fed by others,” and 0: “Permanent vegetative state” [PVS]) based on FIM Eating and Walking scores as shown in the Supplemental Digital Content Appendix 1, available at: http://links.lww.com/JHTR/A292. “Remaining years of life adjusted for TBI” are then calculated individually as “Normal remaining years of life” × “% Normal LE.”
The ONS publishes both period and cohort expectations of life. ‘Period life expectancy’ is the average number of years a person would live, if they experienced the age-specific mortality rates for that time period throughout their life. “Cohort (or ‘projected’) life expectancy” allows for projected changes in mortality taking account of the gradual trend toward longer life in the general population. Cohort life expectancies are generally considered a better measure of how long a person of that age would be expected to live. However, Brooks et al10 have highlighted that these may give overly optimistic values as (in contrast to the general population) the life expectancy of patients with moderate-severe brain injury has not increased over the last 20 years. We adopted the more conservative “period” LE figures to calculate the remaining life years for our primary analysis, but equivalent figures based on the “projected” life expectancy are also presented (see the Supplemental Digital Content Table 1, available at: http://links.lww.com/JHTR/A293).
Estimated life-time savings are computed individually case by case within the UKROC data set using the calculations shown in the Supplemental Digital Content Appendix 1, available at: http://links.lww.com/JHTR/A292.
Data extraction, bias, and sample size
De-identified data were extracted for all recorded inpatient episodes for adults with severe TBI aged 16+ years who were admitted to a level 1 or 2 specialist rehabilitation service and discharged between April 2010 and July 2018. Episodes were included in the TBI specialist rehabilitation data set if they had a length of stay 8 to 400 days (i.e. plausible admissions for rehabilitation, excluding cases admitted for very brief inpatient assessment or for long-term care).
Life expectancy analysis was conducted on the subset for whom the relevant measures had been recorded, which were:
Valid NPDS ratings completed on both admission and discharge, from which to compute reduction in care costs.
Valid FIM scores for Eating and Walking at discharge, from which to compute the functional category for life expectancy estimation.
To minimize bias, all episodes were included that met the above criteria. No patients were excluded on the basis of age, race, ethnicity, gender, or sex.
In this non-interventional observational study, size was not predetermined, but dictated by the accruals to the national data set that met the inclusion criteria over the 8-year period. Missing data were expected because data reporting was initially voluntary and, even now, not all services are required to collect the full data set. No data were imputed, but numbers included in the analyses are given in each table.
Data handling and retrospective analysis
Data were collated in MS Excel and transferred to SPSS v24 (IBM corp, Armonk, New York) for analysis. Given the large size of the data set, the data were analysed using parametric statistics. To minimize the effect of any skewed data, 95% confidence intervals (95% CI) were calculated and multiple comparisons made based on 1000 bootstrapped samples.
Analysis was conducted for the whole sample and within the 5 functional categories described above (based on their FIM Eating and Walking scores at discharge). Paired t tests were used to compare significant differences between admission and discharge. ANOVA tests with post hoc analysis using Bonferroni correction for multiple tests were used to test for between-group differences in life-time cost-savings across the 5 functional categories.
Discounted future costs
Discounting is a technique used to compare costs and benefits that occur in different time periods. It is a separate concept from inflation and is based on the principle that, generally, people prefer to receive goods and services now rather than later.22 In theory, when long-term care costs are valued or paid for in advance, there is an option effectively to set aside capital and use some of the interest to pay for future care, so that the upfront valuation (or ‘Net Present Value’ [NPV]) is lower. There is debate about whether and how to discount future costs in cost-benefit analysis for long-term care (see the Supplemental Digital Content Appendix 1, available at: http://links.lww.com/JHTR/A292). Here, we report a sensitivity analysis using discount rates (r) of both 1.5% and 3.5% to calculate the NPV of total life-time savings, in accordance with current recommendations from the National Institute for Health and Care Excellence.23 These discounted rates were chosen for comparability with other studies that have used this approach.3 NPV was computed using the formula: P = F[(1/r) - (1/[r(1 + r)n])], where F = Average net annual life savings, r = discount rate, and n = the TBI-adjusted remaining years of life (ie, the total number of years over which the costs – or savings – are expected to be incurred). (Note: For simplicity, the costs were conservatively assumed to occur at year-end, rather than at the midpoint.)
RESULTS
Figure 1 illustrates the data extraction process. Of a total of 6277 registered episodes for adults admitted to a level 1 or 2 unit following complex severe TBI, 6043 had a length of stay between 8 and 400 days, representing the data set of patients admitted for specialist rehabilitation. Of these, 3578 (59%) had valid NPDS and FIM scores and were included in the life expectancy analysis (“LE sample”). All 75 registered specialist rehabilitation units in England (19 level 1 tertiary, and 56 level 2 services) provided data.
Figure 1.
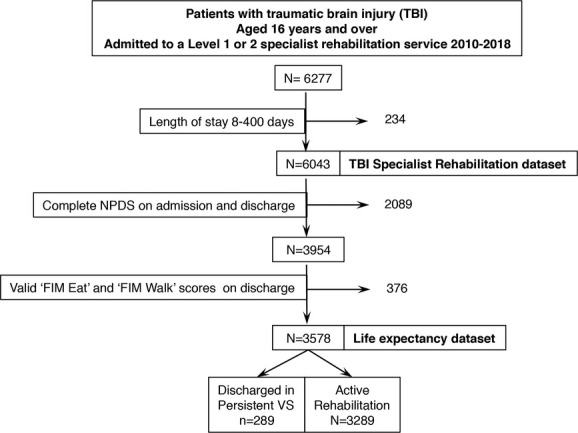
The data extraction process. Of 6043 adults (16 years and older) with severe TBI admitted for specialist rehabilitation in a level 1 or 2 service, 3578 had the requisite NPDS and FIM data to calculate life-time savings. Of these, 289 (8%) were admitted for assessment of disordered conscious only and were discharged still in persistent VS, while the remaining 3289 were the active rehabilitation group. FIM indicates Functional Independence Measure; NPDS, Northwick Park Dependency Score; VS, vegetative state.
Demographics are given in Table 1. Because the LE sample comprised less than 60% of the total TBI Specialist Rehabilitation data set (n = 6043), demographics were compared between the 2 groups. The LE sample comprised approximately 3:1 males:females, with a mean age of 49.3 years at admission and mean length of stay 89 days. The mean time between onset and admission was approximately 3 months. If anything, this analysed LE sample was more dependent at admission, and stayed longer in rehabilitation, and thus cost more to treat than the average patient with severe TBI undergoing specialist rehabilitation in the United Kingdom.
TABLE 1. Demographics of the study population undergoing specialist rehabilitation following severe TBI.
| Parameter | Full TBI cohort (N = 6043) |
Life expectancy sample (N = 3578) |
|---|---|---|
| Age, y | 48.4 (48.0, 48.9) | 49.3 (48.7, 50.0) |
| Mean (95% CI) range | 16-100 | 16-100 |
| Male-to-female ratio, % | 75/25% | 74/26% |
| Time since onset, d | ||
| Mean (95% CI) | 89 (73, 109) | 95 (73, 122) |
| Length of stay, d | ||
| Mean (95% CI) | 80 (78, 82) | 89 (86, 91) |
| Discharge destination, % | ||
| Home/temporary accommodation | 3612 (60%) | 1998 (56%) |
| Nursing/residential home | 791 (13%) | 611 (17%) |
| Further residential rehabilitation | 650 (11%) | 474 (13%) |
| Acute hospital ward | 434 (7%) | 277 (8%) |
| Other | 237 (4%) | 143 (4%) |
| Unknown/missing | 319 (5%) | 75 (2%) |
| FIM score on admission | (n = 5396) | (n = 3512) |
| Mean (95% CI) | Missing 647 (11%) | Missing 66 (2%) |
| FIM-motor | 48.0 (47.3, 48.8) | 42.6 (41.7, 43.6) |
| FIM-cognitive | 18.5 (18.1, 18.6) | 16.9 (16.6, 17.2) |
| FIM-total | 66.3 (65.4, 67.3) | 59.6 (58.4, 60.7) |
Abbreviation: FIM, Functional Independence Measure.
Table 2 shows the overall change in dependency and care costs between admission and discharge for this sample. As expected, significant improvements in independence were seen in both the motor and cognitive subscales of the FIM+FAM, with corresponding reduction in dependency, care hours, and care costs as estimated by the NPCNA. Significant changes were similarly seen within each of the functional category groups except, as expected, for those who remained in PVS at discharge (see the Supplemental Digital Content Table 2, available at: http://links.lww.com/JHTR/A2934). There was no significant temporal bias in main outcomes between early (pre-2013) and later years of the study period.
TABLE 2. Overall change in dependency and care hours between admission and discharge.
| Mean (95% CIs) | Significance, P | |||
|---|---|---|---|---|
| Admission | Discharge | Change | ||
| FIM+FAM Motor | 51.4 (50.3, 52.5) | 75.0 (73.8, 76.0) | 23.5 (22.8, 24.3) | <.001 |
| FIM+FAM Cognitive | 45.9 (45.1, 46.7) | 62.7 (61.8, 63.5) | 16.8 (16.2, 17.3) | <.001 |
| FIM+FAM Total | 97.3 (95.6, 99.0) | 137.6 (135.7, 139.5) | 40.4 (39.2, 41.6) | <.001 |
| Total NPDS score | 33.6 (33.0, 34.3) | 21.7 (21.1, 22.4) | 11.9 (11.4, 12.4) | <.001 |
| NPCNA-estimated care hours per week | 44.4 (43.8, 45.2) | 31.7 (31.0, 32.4) | 12.8 (12.2, 13.3) | <.001 |
| Care costs per week | £1,730 (£1699, £1762) | £1,190 (£1,157, £1,225) | £540 (£512, £568) | <.001 |
Abbreviations: NPCNA, Northwick Park Care Needs Assessment; NPDS, Northwick Park Dependency Score; UK FIM+FAM, UK Functional Assessment Measure.
Across the 5 functional categories, 1235 (34.5%) were able to walk well alone at discharge from rehabilitation, and 648 (18.1%) had some walking ability. A total of 1405 were unable to walk; 444 (12.4%) were fed by others and 961 (26.9%) could feed themselves. The remaining 289 (8.1%) were admitted for assessment of consciousness and remained in PVS at discharge. Table 3 shows the mean reduction in ongoing costs and estimated life-time savings. As expected, PVS patients had almost no change in care costs. In order to provide conservative figures, our primary cost analysis presents data for the full Life Expectancy data set (n = 3578), including PVS patients. However, because most rehabilitation programs would not include patients admitted for PDOC assessment only, we have also provided a parallel cost analysis for the “Active Rehabilitation” sample (n = 3289) only (see Table 3 and the Supplemental Digital Content Table 1, available at: http://links.lww.com/JHTR/A293). Including patients discharged in PVS, the mean episode cost was £42 894. The time to offset the cost of rehabilitation through savings in the weekly cost of ongoing care was approximately 18 months, and the net average annual saving in care costs was £31 513. Based on period life expectancy, this sample had a mean of 21.6 remaining years of life, giving a mean estimated net life-time savings of £679 776 per patient after deduction of rehabilitation costs. If the PVS patients were excluded, the cost savings were slightly higher (net average annual cost saving £32 672; mean remaining years of life 22.7; net total lifetime savings £740 929). Either way these savings translated to more than £2.4 billion for the analysed LE sample and over £4.1 billion extrapolated to the full TBI specialist rehabilitation cohort (see Table 3 and the Supplemental Digital Content Table 1, available at: http://links.lww.com/JHTR/A293).
TABLE 3. Mean individually calculated reduction in ongoing costs and life-time savings based on period life expectancy.
| Life Expectancy Group | PDOC assessment 0 PVS (n = 289) | Active Rehabilitation Group: Functional category | Total Active Rehabilitation Group (excluding PDOC assessment) (N = 3289) | Whole Life expectancy Sample (N = 3578) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 Cannot walk, Fed by others (N = 444) | 2 Cannot walk, Self feeds (N = 961) | 3 Some walking ability (N = 648) | 4 Walks well alone (N = 1235) | ||||||
| Sampling | n = 249 | n = 404 | n = 900 | n = 603 | n = 1103 | n = 3010 | n = 3259 | ||
| Missing (%) | 40 (14%) | 40 (9%) | 61 (6%) | 45 (7%) | 132 (11%) | 279 (8%) | 319 (9%) | ||
| Mean | Mean | Mean | Mean | Mean | Mean | 95% CIa | Mean | 95% CIa | |
| Cost of rehabilitation episode | £70 491 | £70 703 | £52 455 | £31 266 | £25 226 | £40 612 | £39 261, £41 957 | £42 894 | £41 512, £44 235 |
| Annual savings in care costsb | £674 | £4872 | £32 478 | £39 865 | £33.443 | £30 603 | £28 934, £32 185 | £28 317 | £26 889, £29 802 |
| Time to offset the cost of rehabilitation, moc | − | 172.6 | 19.4 | 9.4 | 9.1 | 15.9 | 15.6, 16.3 | 18.2 | 17.8, 18.5 |
| Life-time savings | |||||||||
| Remaining years adjusted for TBI | 8.2 | 12.6 | 19.0 | 22.6 | 29.4 | 22.7 | 22.2, 23.2 | 21.6 | 21.1, 22.0 |
| Estimated total life-time savings | £11 024 | £63 523 | £683 126 | £956 668 | £1 029 093 | £781 541 | £734 998, £832 604 | £722 670 | £679 189, £765 372 |
| Net total life time savings after deduction of rehabilitation costs | −£59 467 | −£6 549 | £630 671 | £925 402 | £1 003 827 | £740 929 | £694 621, £791 256 | £679 776 | £635 972, £722 786 |
| Extrapolated for the sample | £2 436 916 242 | £2 432 238 192 | |||||||
| Extrapolated for the study population | £4 115 861 881 | £4 107 885 801 | |||||||
| Discounted lifetime savings | |||||||||
| Net average annual savingsc | −£7225 | −£521 | £33 120 | £40 912 | £34 169 | £32 672 | £31 303, £34 148 | £31 513 | £30 160, £32 783 |
| Net total life-time savings discounted at 1.5% | −£55 556 | −£5 928 | £545 075 | £779 856 | £807 045 | £624 141 | £587 141, £664 231 | £577 103 | £541 762, £611 560 |
| Net total life-time savings discounted at 3% | −£50 932 | −£5226 | £454 780 | £632 076 | £620 91 | £505 634 | £477 512, £536 08 | £471 685 | £444 534, £479 940 |
Abbreviations: PDOC, prolonged disorder of consciousness; PVS, persistent vegetative state; TBI, traumatic brain injury.
aBootstrapped with 1000 bootstrapping samples.
bAs estimated by the NPCNA.
cCalculated on aggregated data.
As expected, there were some marked differences among the 5 life expectancy groups, with the 2 higher functioning groups having the greatest life-time savings. A breakdown for the 5 functional categories is shown in Table 3, which also includes the discounted values for net total life-time savings at 1.5% and 3.5%. Full details including 95% confidence intervals are given (see the Supplemental Digital Content Table 1, available at: http://links.lww.com/JHTR/A293). One-way ANOVA showed significant differences for total life savings across the 4 groups (P < .001). Because of overlapping confidence intervals, post hoc tests with Bonferroni correction showed no significant differences between the lower 2 functioning groups (P = 1.0) or between the higher 2 functioning groups (P = 1.0), but significant differences between all other groups (P < .001) (see the Supplemental Digital Content Table 3, available at: http://links.lww.com/JHTR/A295).
DISCUSSION
This large multicenter cohort study of prospectively collated clinical data from the UK national clinical database presents an analysis of the cost-efficiency of specialist inpatient rehabilitation following severe TBI and estimates the life-time savings, taking account of the reduced life expectancy of severely brain-injured patients. Between admission and discharge, significant improvements in independence were seen in both the motor and cognitive subscales of the UK FIM+FAM, with corresponding reduction in dependency, care hours, and care costs as estimated by the NPCNA. These were evident across the whole rehabilitation sample and in all the functional category groups except, as expected, the patients who remained in PVS at discharge. The mean net annual savings in care costs were just over £30 000. The mean estimated net life-time savings were between £679 772 and £740 929 per individual, depending on whether patients discharged in PVS were included.
As expected, there were some marked differences between the 5 life expectancy groups, with the 2 higher functioning groups making the greatest life-time savings, and the patients who remain in PVS making none at all. These latter patients were admitted for assessment of consciousness only with no expectation of delivering rehabilitation toward a goal of improved independence. In most countries they would not be managed in a rehabilitation unit, but in skilled nursing facilities and long-term care settings. For this reason we presented analyses of lifetime cost savings both including and excluding the patients admitted for PDOC assessment only. Either way, total life-time savings amounted to approximately £2.4 billion for the patients in whom costing and life expectancy data were available, or more than £4 billion for the full TBI national rehabilitation cohort from which they were drawn.
Although a number of previous authors have reported on cost benefits of rehabilitation,24–26 few have attempted to estimate life-time savings from rehabilitation following TBI: Those who have mainly reported small case studies27 or single-center studies.3,28–30 Three analyses3,28,29 have reported figures from the same post-acute neurobehavioral program in the United Kingdom. The most recent of these (Oddy et al3) demonstrated reduction in direct care costs amounting to life-time savings of between £0.96 milllion and 1.13 million in a largely ambulant population of patients who would generally be most likely to fit into the highest functional category of “walks well alone.” His figures are very similar to ours for that group. From the United States, Griesbach et al30 compared total life care costs between stroke and TBI patients and identified mean rehabilitation savings of $2 267 967.71 (SD $680 823.31) in the 36 TBI patients, but these included medical, equipment, and housing costs as well as care.
Strengths of our analysis are that the systematic prospective collection of data in the course of routine care is reflective of real-life clinical practice. Importantly, the cohort (n = 6043) from which the LE sample was drawn comprises the entire national population of patients with severe TBI admitted to specialist level 1 and 2 rehabilitation units in England during the period. This enhances the generalizability findings across this patient group and supports the estimation of life-time savings on a population basis.
We also recognize a number of potential weaknesses. Our approach is based on 2 assumptions. The comparator condition for “rehabilitation” is discharge straight to the community without it. The first assumption is that, without rehabilitation, care costs would remain the same as on admission, rather than improving spontaneously—a problem for any observational study. It is, to some extent, justified by the strong evidence from controlled clinical trials that inpatient multidisciplinary rehabilitation improves independence compared with routine care.1,2 But importantly, by the time of admission, these patients were on average 3 months postinjury, and (by meeting the criteria for specialist referral) they were already failing to progress with the help of their local services. The second assumption is that the level of independence achieved during the rehabilitation program will be sustained after discharge. Support for this comes from several quarters. First, this is generally a young patient group and TBI is a single incident injury from which an overall trajectory of stability or improvement may be expected. Second, there is evidence from a follow-up study of patients discharged from specialist rehabilitation in the London area31 that NPDS/NPCNA scores were maintained, if not improved at 1 year after discharge.
Because of evolution of reporting requirements over the data collection period, the outcomes of interest were collected in less than 60% of the full TBI specialist rehabilitation data set, so selection bias cannot be excluded. As the analysed sample was on average slightly more disabled (on both admission and discharge), the life-time savings may be underestimated for the group as a whole. Finally, this highly selected population of patients with severe complex disability is somewhat atypical compared with populations described in published analyses from other large data sets, which encompass the more general group of patients undergoing rehabilitation following TBI at all levels. However, the findings have potential relevance for other health systems that offer tertiary specialist rehabilitation services for patients with particularly complex needs.
CONCLUSION
Specialist rehabilitation proved highly cost-efficient for patients severely disabled by severe TBI, despite their reduced life expectancy, generating an estimated total of over £4 billion savings in the cost of ongoing care for this 8-year national cohort. This makes rehabilitation one of the most cost-effective interventions in healthcare and supports the case for increased access to specialist in-patient rehabilitation services nationally.
The UKROC program is registered as a multicenter service evaluation and Payment by Results Improvement Project. Collection and reporting of the UKROC data set is a commissioning requirement according to the NHSE service specification for Level 1 and 2 Rehabilitation Services. According to the UK Health Research Authority, the publication of research findings from de-identified data gathered in the course of routine clinical practice does not require research ethics permission. The program was registered with the NIHR Comprehensive Local Research Network: ID number 6352.
The authors gratefully acknowledge the hard work of the clinical teams in the level 1 and 2 rehabilitation units that contribute to the UKROC data set. Special thanks are due to the UKROC programme steering group and co-applicants and to David Strauss and Jordan Brooks of the US Life Expectancy Project for their helpful advice and information regarding the US life-expectancy project data. Lynne Turner-Stokes, Heather Williams, and Keith Sephton have received financial support from the NIHR to attend conferences to disseminate the findings from the UKROC program. This article presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research program (RP-PG-0407-10185). The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Financial support for the preparation of this manuscript was also provided by the Dunhill Medical Trust. Copies of the tools used in this study are available free of charge from the authors. Please visit our website for more details and contact information: http://www.kcl.ac.uk/lsm/research/divisions/cicelysaunders/research/studies/ukroc/tools.aspx. As the UKROC data set is a live clinical data set, for reasons of confidentiality and data protection data sharing is not available at the current time.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.headtraumarehab.com).
The authors declare no conflicts of interest.
REFERENCES
- 1.Turner-Stokes L, Disler PB, Nair A, Wade DT. Multi-disciplinary rehabilitation for acquired brain injury in adults of working age. Cochrane Database Syst Rev. 2005;(3):CD004170. [DOI] [PubMed] [Google Scholar]
- 2.Turner-Stokes L. Evidence for the effectiveness of multi-disciplinary rehabilitation following acquired brain injury: a synthesis of two systematic approaches. J Rehabil Med. 2008;40(9):691–701. [DOI] [PubMed] [Google Scholar]
- 3.Oddy M, da Silva Ramos S. The clinical and cost-benefits of investing in neurobehavioural rehabilitation: a multi-centre study. Brain Inj. 2013;27(13/14):1500–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner-Stokes L. The evidence for the cost-effectiveness of rehabilitation following acquired brain injury. Clin Med. 2004;4(1):10–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner-Stokes L, Williams H, Bill A, Bassett P, Sephton K. Cost-efficiency of specialist inpatient rehabilitation for working-aged adults with complex neurological disabilities: a multicentre cohort analysis of a national clinical data set. BMJ Open. 2016;6(2):e010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner-Stokes L, Paul S, Williams H. Efficiency of specialist rehabilitation in reducing dependency and costs of continuing care for adults with complex acquired brain injuries. J Neurol Neurosurg Psychiatr. 2006;77(5):634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner-Stokes L. Cost-efficiency of longer-stay rehabilitation programmes: can they provide value for money? Brain inj. 2007;21(10):1015–1021. [DOI] [PubMed] [Google Scholar]
- 8.Shavelle RM, Strauss DJ, Day SM, Ojdana KA. Life expectancy. In: Zasler ND, Katz DI, Zafonte RD, ed. Brain Injury Medicine: Principles and Practice. New York: Demos Medical Publishing; 2007:247–261. [Google Scholar]
- 9.Brooks JC, Shavelle RM, Strauss DJ, Hammond FM, Harrison-Felix CL. Long-term survival after traumatic brain injury part I: external validity of prognostic models. Arch Phys Med Rehabil. 2015;96(6):994–999.e2. [DOI] [PubMed] [Google Scholar]
- 10.Brooks JC, Shavelle RM, Strauss DJ, Hammond FM, Harrison-Felix CL. Long-term survival after traumatic brain injury part II: life expectancy. Arch Phys Med Rehabil. 2015;96(6):1000–1005. [DOI] [PubMed] [Google Scholar]
- 11.NHS Standard Contract for Specialist Rehabilitation for Patients with Highly Complex Needs (all ages): D02. London, England: NHSE; 2013. http://www.england.nhs.uk/wp-content/uploads/2014/04/d02-rehab-pat-high-needs-0414.pdf [Google Scholar]
- 12.Turner-Stokes L. Cost-efficient Service Provision in Neurorehabilitation: Defining Needs, Costs and Outcomes for People With Long Term Neurological Conditions (RP-PG-0407-10185). London: Northwick Park Hospital, National Institute for Health Research Programme Grant for Applied Research; 2008. [Google Scholar]
- 13.Turner-Stokes L, Vanderstay R, Eagar K, Dredge R, Siegert RJ. Cost-Efficient Service Provision in Neurorehabilitation: Defining Needs, Costs and Outcomes for People With Long-term Neurological Conditions: Programme Grant Report (RP-PG-0407-10185). London: National Institute of Health Research; 2015. [Google Scholar]
- 14.National Definition Set for Specialised Services No 7: “Complex Specialised Rehabilitation for Brain Injury and Complex Disability (Adult),” 3rd ed. London: Department of Health; 2009. [Google Scholar]
- 15.Turner-Stokes L, Nyein K, Turner-Stokes T, Gatehouse C. The UK FIM+FAM: development and evaluation. Clin Rehabil. 1999;13(4):277–287. [DOI] [PubMed] [Google Scholar]
- 16.Turner-Stokes L, Siegert RJ. A comprehensive psychometric evaluation of the UK FIM + FAM. Disabil Rehabil. 2013;35(22):1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner-Stokes L, Tonge P, Nyein K, Hunter M, Nielson S, Robinson I. The Northwick Park Dependency Score (NPDS): a measure of nursing dependency in rehabilitation. Clin Rehabil. 1998;12(4):304–318. [DOI] [PubMed] [Google Scholar]
- 18.Siegert RJ, Turner-Stokes L. Psychometric evaluation of the Northwick Park Dependency Scale. J Rehabil Med. 2010;42(10):936–943. [DOI] [PubMed] [Google Scholar]
- 19.Turner-Stokes L, Nyein K, Halliwell D. The Northwick Park Care Needs Assessment (NPCNA): a directly costable outcome measure in rehabilitation. Clin Rehabil. 1999;13(3):253–267. [DOI] [PubMed] [Google Scholar]
- 20.Turner-Stokes L, Sutch S, Dredge R. Healthcare tariffs for specialist inpatient neurorehabilitation services: rationale and development of a UK case mix and costing methodology. Clin Rehabil. 2011;26(3):264–279. [DOI] [PubMed] [Google Scholar]
- 21.2014-16-Based Period and Cohort Life Expectancy Tables. Fareham: UK: Government Office for National Statistics; 2017. [Google Scholar]
- 22.Torgerson DJ, Raftery J. Economic notes. Discounting. Br Med J. 1999;319(7214):914–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute for Health and Care Excellence. Guide to methods of technology appraisal: process and methods 2013. https://http://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. [PubMed]
- 24.Faul M, Wald MM, Rutland-Brown W, Sullivent EE, Sattin RW. Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline: testing the Brain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J Trauma. 2007;63(6):1271–1278. [DOI] [PubMed] [Google Scholar]
- 25.van Heugten CM, Geurtsen GJ, Derksen RE, Martina JD, Geurts AC, Evers SM. Intervention and societal costs of residential community reintegration for patients with acquired brain injury: a cost-analysis of the Brain Integration Programme. J Rehabil Med. 2011;43(7):647–652. [DOI] [PubMed] [Google Scholar]
- 26.Cooney MT, Carroll A. Cost effectiveness of inpatient rehabilitation in patients with brain injury. Clin Med. 2016;16(2):109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashley MJ, Schultz JD, Bryan VL, Krych DK, Hayes DR. Justification of posttraumatic brain injury rehabilitation using net present value techniques: a case study. J Rehabil Outcomes Meas. 1997;1(5):33–41. [Google Scholar]
- 28.Wood RL, McCrea JD, Wood LM, Merriman RN. Clinical and cost effectiveness of postacute neurobehavioural rehabilitation. Brain Inj. 1999;13(2):69–88. [DOI] [PubMed] [Google Scholar]
- 29.Worthington AD, Matthews S, Melia Y, Oddy M. Cost-benefits associated with social outcome from neurobehavioural rehabilitation. Brain Inj. 2006;20(9):947–957. [DOI] [PubMed] [Google Scholar]
- 30.Griesbach GS, Kreber LA, Harrington D, Ashley MJ. Post-acute traumatic brain injury rehabilitation: effects on outcome measures and life care costs. J Neurotrauma. 2015;32(10):704–711. [DOI] [PubMed] [Google Scholar]
- 31.Siegert RJ, Jackson DM, Playford ED, Fleminger S, Turner-Stokes L. A longitudinal, multicentre, cohort study of community rehabilitation service delivery in long-term neurological conditions. BMJ Open. 2014;4(2):e004231. [DOI] [PMC free article] [PubMed] [Google Scholar]


