ABSTRACT
Introduction:
The objective of our study was to explore the association between sex and clinical outcome in sepsis patients in a large, diverse population.
Materials and Methods:
We analyzed 6,134 adult patients with sepsis from the critical care units of Beth Israel Deaconess Medical Center between 2001 and 2012. Study data were retrospectively extracted from Medical Information Mart for Intensive Care-III, a multiparameter intensive care database.
Results:
There were 2,677 (43.6%) female and 3,457 (56.4%) male patients. Compared with female patients, male patients with sepsis had a higher 1-year mortality rate (55.6% vs. 51.4%, P = 0.001), and so did the 90-day mortality rate (45.1% vs. 42.1%, P = 0.018). 33.8% of male and 31.3% of female patients with sepsis died during hospitalization (P = 0.041). The median length of hospitalization and intensive care unit (ICU) stay for male patients was 19.54 and 7.54 days, while that for female patients was 16.49 and 6.75 days (P < 0.001, P = 0.002, respectively). Male patients were more likely to require dialysis therapy (P = 0.109), ventilation support (P = 0.012) and more vasoactive agents (dopamine P = 0.113, norepinephrine P = 0.016, and epinephrine P = 0.093) during the ICU period than female patients. Our Cox proportional hazard regression model confirmed that the risk of death within 1 year of ICU admission in male patients is 1.083 times that in female.
Conclusion:
Female patients with sepsis have better clinical outcomes than male patients in terms of mortality and length of hospitalization and ICU stay.
Keywords: Big data analytics, gender, medical information mart for intensive care, mortality, septic patients
Abbreviations: MIMIC, Medical Information Mart for Intensive Care, BIDMC, Beth Israel Deaconess Medical Center, MIT, Massachusetts Institute of Technology, ICU, intensive care unit, SICU, surgical intensive care unit, CCU, cardiac care unit, CSRU, cardiac surgery recovery unit, MICU, medical intensive care unit, LOS, length of stay, CI, confidence interval, OR, odds ratio, SAPS, Simplified Acute Physiology Score, SOFA, Sequential Organ Failure Assessment, VECs, vascular endothelial cells, PICS, postintensive care syndrome
INTRODUCTION
Sepsis is a common clinical syndrome characterized by life-threatening organ dysfunction caused by a dysregulated host response to infection (1). Despite increased research efforts and continuous exploration of the pathophysiology of sepsis, this illness is still a major cause of death in the ICU and remains a public health issue that affects quality of life for many sufferers around the world every year (2–4). Previous studies have confirmed that patients of different sex show different clinical manifestations and outcomes during infection with bacteria, viruses, and fungi (5). The literature has documented that the way men and women respond to infection is different. Women can produce better humoural and cellular immune responses after infection than men but are also prone to autoimmune diseases (6, 7). Epidemiological studies consistently report a higher prevalence of sepsis in males relative to that in females (8). However, the influence of sex on the clinical outcomes of sepsis is still highly controversial. The results of human studies focusing on the impact of sex on sepsis-associated mortality are inconsistent. A prospective, observational clinical study (9) in Germany concluded that the mortality of female sepsis patients is higher than that of male sepsis patients. Another prospective study by Nasir et al. (10) reported a completely opposite conclusion; their study showed that higher levels of plasma IL-6 lead to higher mortality in male patients with sepsis. However, a recent study declared that there is no effect from sex on the clinical outcome of patients with sepsis (11). Thus far, there is no conclusive consensus on this issue. Clinicians are often confused by the different perspectives and thus cannot correctly predict the prognosis. Understanding this problem is of great significance to comprehensive prevention and treatment of sepsis. To obtain more convincing results, this problem must be studied in a large, diverse group of people. In our study, we aimed to gain insight into sex-related differences in sepsis outcomes in large sample populations.
PATIENTS AND METHODS
Database
This article aimed to reveal the impact of sex on the clinical outcome of sepsis using data from Medical Information Mart for Intensive Care III (MIMIC-III) (12, 13), which is a large, publicly available database that includes unidentified health-related data from approximately sixty thousand patients who were admitted to critical care units of the Beth Israel Deaconess Medical Center (BIDMC). MIMIC is made available largely through the work of researchers at the Massachusetts Institute of Technology (MIT) Laboratory for Computational Physiology and collaborating research groups. After completing the National Institutes of Health web-based training course “Protecting Human Research Participants,” we obtained approval to access the database (Certification Number: 2093226).
Study population
All patients diagnosed with “sepsis,” “severe sepsis,” or “septic shock” in the MIMIC-III clinical database were potentially eligible for the present analysis. The only exclusion criterion was an age less than 18 years old.
Data extraction and management
Data on the following aspects were extracted: age, ethnicity, Simplified Acute Physiology Score (SAPS), sequential organ failure assessment (SOFA) score, admission type and location, first ICU service, date of ICU admission and discharge, date of birth and death, mechanical ventilation time, amount of vasoactive drug applied, and dialysis demand. The database had obscured the true ages of patients over 89 years old. The median age of those patients was 91.5 years, and we used this value as a surrogate age for those patients. Both the SOFA score and SAPS were computed using the code directly provided by Johnson et al. (14).
The primary endpoint in our study was 1-year mortality, which was defined as death observed within 1 year after ICU admission. The hospital and ICU length of stay (LOS) were selected as the secondary outcomes, with the former calculated from the date of admission and discharge and the latter extracted directly from the database. Hospitalization and 90-day mortality were also assessed. For patients who were discharged alive, we extracted the date of death from the social security database to determine out-of-hospital mortality.
Data extraction was accomplished using structure query language (SQL) by PostgreSQL tools (v9.6; PostgreSQL Global Development Group). Data were extracted from the following tables: ADMISSIONS, ICUSTAYS, PATIENTS, CHARTEVENTS, and DIAGNOSIS_ICD.
STATISTICAL ANALYSIS
If the measurement data were normally distributed and the variance was homogeneous, the data were described as the mean ± standard deviation and compared between groups using a t test. If the requirements were not satisfied, data were represented by the median and interquartile range, and the Wilcoxon rank-sum test was used for comparisons between groups. Numeration data were expressed as absolute values and percentages; the chi-square test or Fisher exact test was chosen for statistical analyses as appropriate. Survival analysis was performed with the log-rank test to determine whether sex affects 1-year mortality. Kaplan–Meier survival curves were generated.
Univariate analysis was carried out between survivors and non-survivors to screen variables associated with 1-year mortality by Pearson chi-square test. The variables with P < 0.1 in the univariate analysis were included in the Cox proportional hazard regression model as covariates to determine the factors that impact the 1-year survival rate.
All data analyses were performed by SPSS software (v21.0; IBM, Armonk, NY); a two-tailed P < 0.05 was considered statistically significant.
RESULTS
Demographic and clinical characteristics
Results from the univariate analysis of patients’ basic information stratified by sex are summarized in Table 1. There were 2,677 female and 3,457 male patients, accounting for 43.6% and 56.4% of the total population, respectively. Male patients were slightly younger than female patients, and the proportion of White (75.1%, P = 0.002) and Hispanic or Latino (4.1%, P = 0.001) patients was higher while the proportion of Asian patients was lower among male patients than among female patients. The proportion of male patients from the medical intensive care unit (MICU) was lower than that of female patients (P < 0.001), while in the cardiac surgery recovery unit (CSRU) and surgical intensive care unit (SICU), the proportion of men was higher than that of women (P = 0.003, P = 0.004, respectively). There were differences in admission type and location between men and women (P = 0.003 for each). The SOFA score of the first day in the ICU was higher for men (P < 0.001), while there was no significant difference in the SAPS between the two groups of men and women (P = 0.113).
Table 1.
Univariate analysis of demographic and hospital characteristics by gender category
| Female (2,677) | Male (3,457) | P value | |
| Age, n (%) | 69 (57,81) | 67 (56,79) | <0.001 |
| <45 | 259 (9.7) | 321 (9.3) | 0.605 |
| 45–64 | 820 (30.6) | 1,187 (34.4) | 0.002 |
| 65–89 | 1,349 (50.4) | 1,766 (51.1) | 0.591 |
| >90 | 249 (9.3) | 181 (5.2) | <0.001 |
| Ethnicity, n (%) | <0.001 | ||
| White | 1,915 (71.5) | 2,597 (75.1) | 0.002 |
| Black | 369 (13.8) | 273 (7.9) | <0.001 |
| Hispanic or Latino | 70 (2.6) | 143 (4.1) | <0.001 |
| Asian | 85 (3.2) | 119 (3.4) | 0.563 |
| Other | 238 (8.9) | 325 (9.4) | 0.492 |
| First ICU service, n (%) | <0.001 | ||
| CCU | 238 (8.9) | 318 (9.2) | 0.677 |
| MICU | 1,854 (69.3) | 2,230 (64.5) | <0.001 |
| CSRU | 78 (2.9) | 151 (4.4) | 0.003 |
| SICU | 507 (18.9) | 758 (21.9) | 0.004 |
| Marital status, n (%) | <0.001 | ||
| Married | 930 (34.7) | 1,883 (54.5) | <0.001 |
| Single/separated/divorced/widow | 1,634 (61.0) | 1,408 (40.7) | <0.001 |
| Unknown | 113 (4,2) | 166 (4.8) | 0.279 |
| Insurance, n (%) | <0.001 | ||
| Government | 45 (1.7) | 72 (2.1) | 0.254 |
| Medicaid/Medicare | 2,039 (76.2) | 2,410 (69.7) | <0.001 |
| Private | 587 (21.9) | 950 (27.5) | <0.001 |
| Self-pay | 6 (0.2) | 25 (0.7) | 0.006 |
| Admission type, n (%) | 0.003 | ||
| Elective | 75 (2.8) | 155 (4.5) | 0.001 |
| Emergency | 2,562 (95.7) | 3,247 (93.8) | 0.002 |
| Urgent | 40 (1.5) | 55 (1.6) | 0.761 |
| Admission location, n (%) | 0.003 | ||
| Referral | 684 (25.6) | 805 (24.7) | 0.432 |
| Emergency | 1,375 (51.4) | 1,578 (48.3) | 0.022 |
| Transfer | 618 (23.1) | 881 (27) | 0.001 |
| SOFA* | 6.97 ± 3.52 | 7.29 ± 3.75 | <0.001 |
| SAPS* | 21.39 ± 5.73 | 21.06 ± 5.60 | 0.113 |
*Score calculated within 24 h after entering ICU.
SAPS indicates the simplified acute physiology score; SOFA, the sequential organ failure score; ICU, intensive care unit; SICU, surgical intensive care unit; CCU, cardiac care unit; CSRU, cardiac surgery recovery unit; MICU, medical intensive care unit.
Univariate analysis of clinical outcomes
Calculations of the mortality rates and the hospital and ICU LOS were stratified by sex and are summarized in Table 2. Among male patients, 55.6% died within 1 year after admission to the ICU, which was significantly higher than the percentage of female patients (51.4%, P = 0.001). Additionally, 33.8% of male and 31.3% of female patients died during hospitalization (P = 0.041). Furthermore, there was a significant difference in the 90-day mortality rate between men and women (45.1% vs. 42.1%, respectively, P = 0.018). Considering that age represents an important issue in the female group, we compared 1-year mortality between males and females in different age groups (Fig. 1). We established four life periods according to the child-bearing, menopause, senescence, and longevity periods in females. The mortality rate of males and females increased with age. In patients younger than 90 years old, males had significantly higher 1-year mortality than females. The median length of hospitalization and ICU stay for male patients with sepsis was 19.54 and 7.54 days, respectively, while that for female patients was 16.49 and 6.75 days (P < 0.001, P = 0.002 respectively). The Kaplan–Meier curve for 1-year survival by sex is shown in Figure 2.
Table 2.
Univariate analysis of requirement of organ support therapy by gender category
| Female | Male | P value | |
| Ventilation (n, %) | 1,345 (50.2) | 1,848 (53.5) | 0.012 |
| Ventilation duration (h)* | 106.00 (33.88, 236.68) | 109.25 (32.66, 245.01) | 0.606 |
| Dialysis, n (%) | 819 (30.6) | 1,124 (32.5) | 0.109 |
| Vasoactive agent | |||
| Dopamine, n (%) | 279 (10.4) | 341 (9.9) | 0.472 |
| Dopamine (mg/kg)† | 3.07 (0.89, 10.45) | 4.27 (1.15, 12.42) | 0.113 |
| Norepinephrine, n (%) | 1,329 (49.6) | 1,638 (47.4) | 0.079 |
| Norepinephrine (mg/kg)† | 0.15 (0.04, 0.48) | 0.18 (0.05, 0.54) | 0.016 |
| Epinephrine, n (%) | 61 (2.3) | 98 (2.8) | 0.174 |
| Epinephrine (mg/kg)† | 0.03 (0.01, 0.10) | 0.06 (0.01, 0.11) | 0.093 |
*From the beginning of mechanical ventilation to the oxygen therapy start.
†The total amount of vasoactive drugs required per kilogram of weight.
Fig. 1.
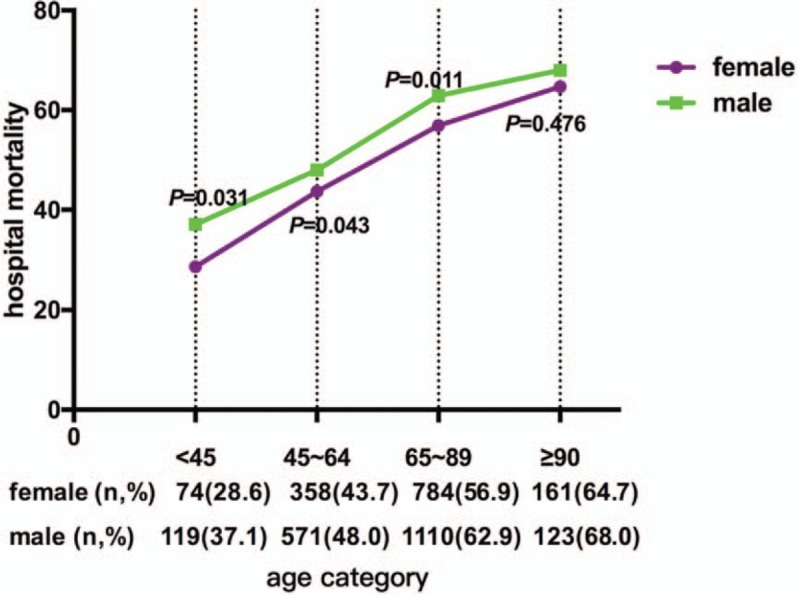
Comparison of 1-year mortality between male and female sepsis patients in different age groups.
Fig. 2.
Kaplan–Meier curve for 1-year survival stratified by gender.
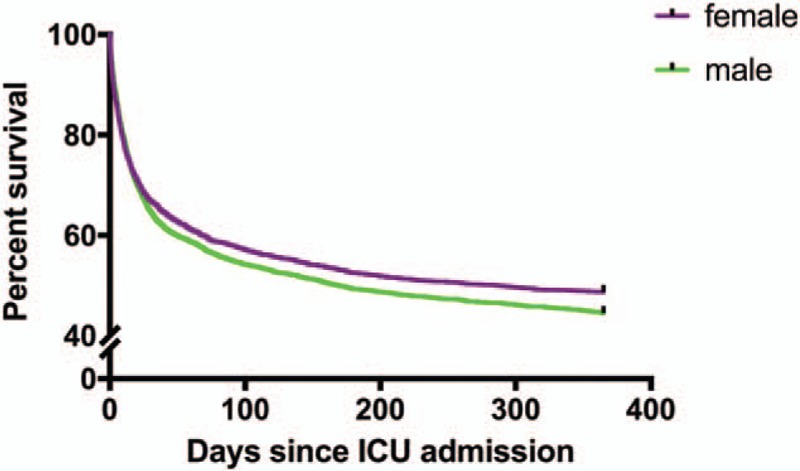
Kaplan–Meier curve for survival stratified by gender over a 1-year period. The survival rate was significantly higher in the female group than in the male group (P = 0.007 by log-rank test).
We studied the requirements of the two groups for mechanical ventilation, dialysis, and vasoactive drugs during the ICU period. The results of the comparison are shown in Table 3. Among male patients, 53.5% required mechanical ventilation. In comparison, the ratio of female patients who required mechanical ventilation was lower (50.2%, P = 0.012). There was no difference in the number of patients who required dialysis or vasoactive drugs between the two groups. The total amount of dopamine, norepinephrine, and epinephrine required per kilogram of weight for men during their ICU stay was more than that for women (P = 0.113, P = 0.016, and P = 0.093, respectively) (Fig. 3).
Table 3.
Univariate analysis of clinical outcomes by gender category
| Female | Male | P | |
| Mortality, n (%) | |||
| Hospital mortality | 839 (31.3) | 1,169 (33.8) | 0.041 |
| 30-day mortality | 888 (33.2) | 1,219 (35.3) | 0.087 |
| 90-day mortality | 1,127 (42.1) | 1,571 (45.1) | 0.018 |
| One-year mortality | 1,327 (51.4) | 1,923 (55.6) | 0.001 |
| Length of stay (day) | |||
| Hospital LOS | 10.44 (5.73,20.84) | 12.06 (6.13,24.01) | <0.001 |
| ICU LOS | 3.37 (1.80,8.05) | 3.80 (1.88,8.95) | 0.002 |
ICU indicates intensive care unit; LOS, length of stay.
Fig. 3.
Result of the Cox proportional hazard regression analysis.

Each horizontal line represents the 95% CI range, and the small black spots in the middle of the crosses represent the OR value.
Cox proportional hazards analyses of 1-year mortality
After comparing the variables between 1-year survivors and non-survivors in the univariate analysis (see Table 4), we chose to input sex, race, first ICU service, marital status, insurance, admission location, SAPS and SOFA scores but not admission type into the Cox proportional hazards regression model (see Fig. 2). Our multivariate regression model revealed relations between sex and 1-year mortality; the risk of death within 1 year in men was 0.083-times higher than that in women (P = 0.031, OR = 1.083, 95% CI 1.007–1.167). In addition, advanced age and higher admission SAPS and SOFA score were correlated with increased mortality risk within 1 year (P < 0.001).
Table 4.
Univariate analysis of demographic and hospital characteristics by category of 1-year outcome
| Survival | Non-survival | P | |
| Gender | 67 (55, 79) | 70 (58, 81) | 0.001 |
| Female | 1,300 (48.6) | 1,377 (51.4) | |
| Male | 1,534 (44.4) | 1,923 (55.6) | |
| Age | <0.001 | ||
| <45 | 387 (66.7) | 193 (33.3) | <0.001 |
| 45–64 | 1,080 (53.8) | 929 (46.2) | 0.003 |
| 65–89 | 1,221 (39.2) | 1,894 (60.8) | 0.127 |
| >90 | 146 (34.0) | 284 (66.0) | <0.001 |
| Ethnicity | 0.026 | ||
| White | 2,066 (45.8) | 2,466 (54.2) | 0.354 |
| Black | 321 (50.0) | 321 (50.0) | 0.016 |
| Hispanic or Latino | 110 (51.6) | 103 (48.4) | 0.148 |
| Asian | 100 (49.0) | 104 (51.0) | 0.380 |
| Other | 237 (42.1) | 326 (57.9) | <0.001 |
| First ICU Service | 0.01 | ||
| CCU | 227 (40.8) | 329 (59.2) | 0.088 |
| MICU | 1,888 (46.2) | 2,196 (53.8) | 0.095 |
| CSRU | 99 (43.2) | 130 (56.8) | 0.113 |
| SICU | 620 (49.0) | 645 (51.0) | 0.994 |
| Marital status, n (%) | <0.001 | ||
| Married | 1,275 (45.0) | 1,538 (46.6) | 0.205 |
| Single/separated/divorced/widow | 1,459 (51.5) | 1,583 (48.0) | 0.006 |
| Unknown | 100 (3.5) | 179 (5.4) | <0.001 |
| Insurance, n (%) | <0.001 | ||
| Government | 75 (2.6) | 42 (1.3) | <0.001 |
| Medicaid/Medicare | 1,906 (67.3) | 2,543 (77.1) | <0.001 |
| Private | 837 (29.5) | 700 (21.2) | <0.001 |
| Self-pay | 16 (0.6) | 15 (0.5) | 0.545 |
| Admission type | 0.87 | ||
| Elective | 104 (45.2) | 126 (54.8) | 0.125 |
| Emergency | 2,684 (46.2) | 3,125 (53.8) | 0.076 |
| Urgent | 46 (48.4) | 49 (51.6) | 0.390 |
| Admission location | <0.001 | ||
| Referral | 784 (51.0) | 753 (49.0) | 0.008 |
| Emergency | 1,376 (45.1) | 1,673 (54.9) | 0.004 |
| Transfer | 674 (43.5) | 874 (56.5) | <0.001 |
| SPAS | 19 (15, 23) | 22 (18, 26) | <0.001 |
| SOFA | 5 (3, 8) | 7 (3, 8) | <0.001 |
*The patient is still alive after a year of ICU entry.
†The patient died within 1 year after ICU entry.
ICU indicates intensive care unit; SICU, surgical intensive care unit; CCU, cardiac care unit; CSRU, cardiac surgery recovery unit; MICU, medical intensive care unit.
DISCUSSION
At present, there are many studies on the relationship between sex and clinical outcomes in patients with sepsis, but the results are still controversial. Hence, it is necessary to further study this problem using a database. All data in the MIMIC database are directly imported by the medical record system or recorded by the medical staff. We then extracted the relevant data according to our research requirements; the only inclusion criterion was an adult patient diagnosed with sepsis, irrespective of his or her primary disease, disease severity, and special treatment. Data storage and assessment were performed by different teams. Since data were already recorded in the database before we formed the research plan, our subjective consciousness did not interfere with patient inclusion and treatment, which ensures that our data are true and that our results are credible. However, selection bias or subjective interference were difficult to avoid in previous retrospective studies and may affect the research results to some extent. In addition, the population number in our research study is four times or more than that in previous studies, and our study duration was also longer. Therefore, some of the confounding factors are more balanced among the two groups of men and women. Animal studies have also indicated that females appear to have a survival advantage in terms of both immunologic and cardiovascular responses (15).
The LOS in the ICU or hospital was longer in male sepsis patients than in female sepsis patients, which has been overlooked by many studies. We agree with Sakr et al.'s (16) interpretation of this finding that there may be a sex-related bias in the treatment choice. Some studies have reached a consensus that male patients require higher intensity ICU support treatment than female patients (17). The ICU admission rates among men were higher than those among women, and men were also more likely than women to receive advanced therapy and undergo invasive procedures. In addition, male patients required more norepinephrine than women to correct hemodynamic abnormalities. That is, hypotension in male patients with sepsis is more difficult to correct than that in female patients. Some researchers have reported that fewer females receive antibiotics than males (18), and females were significantly less likely than males to receive antibiotics within 3 h (19). The difference in the use of antibiotics may be related to the different infection location. Some studies paid attention to this issue and observed that there were clear differences in infection sites based on sex. Two primary sites of infection emerged: the chest (including pneumonia and respiratory tract infection) and the urinary tract. Females were less likely to have respiratory infections than males, but females more commonly developed sepsis from genitourinary sources than males (18, 20). This outcome may be caused by lifestyle and anatomical differences between men and women.
After comprehensive analysis of our data and related literature, we concluded that the difference in 1-year mortality between men and women may be due to postintensive care syndrome (PICS). PICS describes new or worse health problems after critical illness that remain after patients leave the hospital (21). These problems can be related to the body, thoughts, feelings, or mind of patients and may cause physical weakness and affect disease recovery, further influencing the long-term survival rate of patients. Some surveys have shown that the incidence of PICS in males is significantly higher than that in females (22). Sepsis, mechanical ventilation, and invasive operation are high-risk factors for PICS occurrence (23). As mentioned above, male patients are more likely to receive more aggressive treatment in the ICU, including mechanical ventilation, deep venous and artery puncture, and dialysis, than female patients. These treatments also increase the risk of PICS occurrence in male patients.
At present, the clinical interpretation of sex differences in the outcome of sepsis is still undetermined. The mainstream view regards the difference in the outcome of sepsis based on sex to be caused by different levels of hormones in the body (24, 25). Animal studies have confirmed that oestrogen, especially oestradiol, can inhibit the inflammatory response and regulate the immune system to achieve a balance of inflammatory and anti-inflammatory reactions and promote the repair of damaged tissues, thereby protecting the organs (26, 27). Oestrogen has also been proven to have a direct protective effect on vascular endothelial cells (VECs), which play an important role in microcirculation. It was reported that oestrogen can promote the growth of VECs and protect the normal function of blood vessels (28). Oestrogen can also inhibit endothelial cell apoptosis, induce endothelial cell proliferation and migration, and promote microvessel regeneration (29). In addition to the effect of oestrogen on the inflammatory response, whether the protective effect of oestrogen on endothelial cells underlies the differences in sex-based sepsis outcomes requires more scientists to investigate.
Although our research is based on a large, diverse population, this is actually a retrospective single-center study. Limited by our current capabilities and the extent to which the database can be used, our research discovered a phenomenon but cannot fully explain its pathophysiological mechanism. The inflammatory response has a strong effect on the pathogenesis of sepsis and is closely related to the mortality rate. However, in this study, we did not extract indicators of the inflammatory response, such as the level of inflammatory factors, in patients. Therefore, we cannot directly compare the inflammatory response between male and female patients. In patients with sepsis, the indicators of VEC function are not routinely tested. Thus, we should focus on whether there is a difference in endothelial cell function between male and female patients with sepsis in a future study.
The mechanism of the relationship between sex and clinical outcomes in patients with sepsis also requires further study. A large number of animals and molecular experiments must be carried out to discover the relevant mechanisms, as an understanding of these mechanisms will be of great significance for the prevention and treatment of sepsis.
CONCLUSION
The clinical outcome in women with sepsis is better than that in men with sepsis in terms of mortality and the length of hospitalization and ICU stay.
Footnotes
JX and LT contributed equally to the article and should be considered co-first authors.
This study was supported by two Guangzhou Science and Technology Programs (Grant Nos. 201607010186, 201704020153), the Guangdong Provincial Key Laboratory Construction Projection on Organ and Transplant Immunology (Grant No. 2013A061401007) and the Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (Grant No. 2015B050501002). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no conflicts of interest.
REFERENCES
- 1.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 45 3:486–552, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Marshall JC, Namendys-Silva SA, Francois B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, et al. Assessment of the worldwide burden of critical illness: The intensive care over nations (ICON) audit. Lancet Respir Med 2 5:380–386, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Sakr Y, Moreira CL, Rhodes A, Ferguson ND, Kleinpell R, Pickkers P, Kuiper MA, Lipman J, Vincent JL. The impact of hospital and ICU organizational factors on outcome in critically ill patients: Results from the Extended Prevalence of Infection in Intensive Care study. Crit Care Med 43 3:519–526, 2015. [DOI] [PubMed] [Google Scholar]
- 4.van Vught LA, Klein Klouwenberg PM, Spitoni C, Scicluna BP, Wiewel MA, Horn J, Schultz MJ, Nurnberg P, Bonten MJ, Cremer OL, et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA 315 14:1469–1479, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Geurs TL, Hill EB, Lippold DM, French AR. Sex differences in murine susceptibility to systemic viral infections. J Autoimmun 38 (2–3):J245–J253, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berletch JB, Yang F, Xu J, Carrel L, Disteche CM. Genes that escape from X inactivation. Hum Genet 130 2:237–245, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med 19 5:548–556, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348 16:1546–1554, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Nachtigall I, Tafelski S, Rothbart A, Kaufner L, Schmidt M, Tamarkin A, Kartachov M, Zebedies D, Trefzer T, Wernecke KD, et al. Gender-related outcome difference is related to course of sepsis on mixed ICUs: a prospective, observational clinical study. Crit Care 15 3:R151, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasir N, Jamil B, Siddiqui S, Talat N, Khan FA, Hussain R. Mortality in Sepsis and its relationship with Gender. Pak J Med Sci 31 5:1201–1206, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Vught LA, Scicluna BP, Wiewel MA, Hoogendijk AJ, Klein Klouwenberg PMC, Ong DSY, Cremer OL, Horn J, Franitza M, Toliat MR, et al. Association of gender with outcome and host response in critically ill sepsis patients. Crit Care Med 45 11:1854–1862, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, Moody B, Szolovits P, Celi LA, Mark RG. MIMIC-III, a freely accessible critical care database. Sci Data 2016; 3:160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 101 23:E215–E220, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AE, Stone DJ, Celi LA, Pollard TJ. The MIMIC Code Repository: enabling reproducibility in critical care research. J Am Med Inform Assoc 25 1:32–39, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuebler JF, Jarrar D, Toth B, Bland KI, Rue L, 3rd, Wang P, Chaudry IH. Estradiol administration improves splanchnic perfusion following trauma-hemorrhage and sepsis. Arch Surg 137 1:74–79, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Sakr Y, Elia C, Mascia L, Barberis B, Cardellino S, Livigni S, Fiore G, Filippini C, Ranieri VM. The influence of gender on the epidemiology of and outcome from severe sepsis. Crit Care 17 2:R50, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuelsson C, Sjoberg F, Karlstrom G, Nolin T, Walther SM. Gender differences in outcome and use of resources do exist in Swedish intensive care, but to no advantage for women of premenopausal age. Crit Care 19:129, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adrie C, Azoulay E, Francais A, Clec’h C, Darques L, Schwebel C, Nakache D, Jamali S, Goldgran-Toledano D, Garrouste-Orgeas M, et al. Influence of gender on the outcome of severe sepsis: A reappraisal. Chest 132 6:1786–1793, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Madsen TE, Simmons J, Choo EK, Portelli D, McGregor AJ, Napoli AM. The DISPARITY study: Do gender differences exist in Surviving Sepsis Campaign resuscitation bundle completion, completion of individual bundle elements, or sepsis mortality? J Crit Care 29 3:e7–e11, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit Care Med 34 10:2576–2582, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med 40 2:502–509, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Hogue CW, Jr, Sundt T, 3rd, Barzilai B, Schecthman KB, Dávila-Román VG. Cardiac and neurologic complications identify risks for mortality for both men and women undergoing coronary artery bypass graft surgery. Anesthesiology 95 5:1074–1078, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Weinert CR, Sprenkle M. Post-ICU consequences of patient wakefulness and sedative exposure during mechanical ventilation. Intensive Care Med 34 1:82–90, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Sharawy N, Pavlovic D, Wendt M, Cerny V, Lehmann C. Evaluation of the effects of gender and estradiol treatment on the intestinal microcirculation during experimental sepsis. Microvasc Res 82 3:397–403, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Plackett TP, Cory RD, Jessica LP, Richard LG, Elizabeth JK. Effects of estrogen on bacterial clearance and neutrophil response after combined burn injury and wound infection. J Burn Care Res 37 5:328–333, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang TL, Yang YM. Sex differences in response to immunonutrition in sepsis. Nutrition 24 (7–8):761–766, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Jia YL, Liu X, Yan JY, Chong LM, Li L, Ma AC, Zhou L, Sun ZY. The alteration of inflammatory markers and apoptosis on chronic prostatitis induced by estrogen and androgen. Int Urol Nephrol 47 1:39–46, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacol Ther 135 1:54–70, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montt-Guevara MM, Palla G, Spina S, Bernacchi G, Cecchi E, Campelo AE, Shortrede JE, Canu A, Simoncini T. Regulatory effects of estetrol on the endothelial plasminogen pathway and endothelial cell migration. Maturitas 99:1–9, 2017. [DOI] [PubMed] [Google Scholar]


