Abstract
Purpose of review
About one in 650–1000 children is born with an extra X or Y chromosome, referred to as sex chromosome trisomies (SCTs). Studying SCTs may uncover unique insights in neurodevelopmental pathways underlying the risk for neurobehavioral problems and psychopathology. There is also a clinical need for more knowledge about the phenotype of SCT with the recent introduction of noninvasive prenatal screening.
Recent findings
The reviewed studies illustrate an increased vulnerability for psychopathology such as (symptoms of) autism spectrum disorder, attention-deficit/hyperactivity disorder, anxiety, depression and, to a lesser degree, psychotic disorders. Although traditionally the primary focus has been on language and learning problems, recent research suggests that impairments in executive functioning, social cognition and emotion regulation may also be key factors underlying the risk for neurobehavioral problems.
Summary
The research field of SCT is in need of a more longitudinal perspective to identify early markers of ‘at risk’ development, and to assess the effectiveness of early interventions. Neurocognitive markers that signal compromised neurodevelopment may prove to be helpful in this. Variability in the SCT phenotype provides a unique opportunity to identify not only genetic but also environmental factors that shape neurodevelopmental outcome, calling for studies focused on understanding individual differences.
Keywords: behavior, cognition, Klinefelter syndrome, trisomy X, XYY
INTRODUCTION
About one in 650–1000 children is born with an extra X or Y chromosome, resulting in the 47,XXY, 47,XXX or 47,XYY chromosomal pattern. The most common cause is nondisjunction of the X or Y chromosome during early cell division, most often during meiosis. Knowledge about the neurocognitive and behavioral phenotypes remains rather limited, which is somewhat surprising considering the disproportionate amount of genes on the X chromosomes that have been linked to brain functioning [1].
However, in the last two decades, sex chromosome variations have received increased attention, in part driven by an increasing awareness that studying gene–brain–behavior pathways in these genetic conditions may uncover new insights in mechanisms of developmental risk that may lead to psychopathology. It has been argued that this ‘behavioral neurogenetics approach’ [2] may provide a powerful tool that can complement the study of behaviorally defined populations. As sex chromosome trisomies (SCTs) can already be identified as early as prenatally, this offers the opportunity to prospectively study early neurodevelopmental markers of individual differences in outcome in terms of neurobehavioral symptoms and risk for psychopathology. There is also an increasing clinical need for knowledge about SCT. The number of children prenatally diagnosed with SCT is expected to increase with availability of the noninvasive prenatal screening test [3], which calls for more knowledge about the phenotype of SCT to be able to monitor child development, and provide clinical care and early support or intervention if needed.
This need for knowledge may be most pressing for the neurobehavioral domain: so far, the majority of research studies (about 75%) have focused on the somatic/medical phenotype, with only 25% of the studies focusing on the neurobehavioral phenotype [4]. In addition to characterizing the variety and severity of behavioral problems that may be seen in SCT, it is also of great importance to have a better understanding of the mechanisms underlying the behavioral phenotype. Similar behavioral problems may arise from different underlying information processing dysfunctions in the brain. Knowledge about these cognitive dysfunctions that are core to behavioral problems in SCT is crucial for disentangling etiological pathways, and is also essential for identifying the nature of developmental vulnerability, as well as the identification of specific targets for intervention, enabling more tailored mental healthcare.
Here, a compact review is provided of risk for psychopathology and neurocognitive vulnerabilities that may underlie this increased risk in individuals with SCT. Recent developments in this field are highlighted, and directions for future research are provided.
Box 1.
no caption available
AUTISM SPECTRUM DISORDER SYMPTOMS
A systematic review has established a worldwide prevalence rate of autism spectrum disorder (ASD) of 0.6% in the general population [5]. Levels of autism symptoms and risk for ASD have shown to be significantly higher in SCT as compared to the general population. To illustrate, on average, 25% children with XXY (ranging from 12 to 47%) have autism symptoms in the mild/moderate-to-severe range [6–9]. When considering diagnostic classification of ASD, on average 18% of the children meet full ASD criteria, with a range of 10–27% [7,10,11]. When looking at the history of clinical diagnoses based on screening of medical files or parental report, this percentage is somewhat lower at 7% (ranging from 5 to 11%) [6,9,12]. Increased levels of ASD symptoms have also been found in adults with XXY, with 6.5% scoring above clinical cutoff [13]. This fits with a screening of hospital discharges in 860 adults with XXY, showing that ASD was diagnosed 6.2 times more often as compared to men with XY [14].
Also, for individuals with XXX, significantly increased rates of ASD diagnoses or clinically significant ASD symptoms have been observed, with an average rate of 15% of the children (ranging from 10.8 to 20%) [7,15].
In individuals with XYY, a clinical diagnostic classification of ASD has been found in on average 30% of the children (ranging from 19 to 43%) [9,10,12,16,17]. Studies using dimensional measures have shown that 35% of the boys have ASD symptoms in the mild–moderate range, and another 50% in the severe range [9].
ATTENTION DEFICIT HYPERACTIVITY DISORDER SYMPTOMS
A meta-analysis of worldwide attention deficit hyperactivity disorder (ADHD) prevalence in children found an overall estimate of 7.2% [18]. As compared to the general population, significantly higher levels of ADHD symptoms as well as increased risk for ADHD have been reported for SCT. Using dimensional measures, clinical levels of ADHD symptoms have been found in on average 35% of the children with XXY (with a range of 27–42%) [9,19,20], with inattentive symptoms more predominant than hyperactive/impulsive symptoms [20]. When considering full diagnostic classification of ADHD (either direct assessment or clinical history), on average 43% of the children and adolescents (with a range of 34–63%) meet diagnostic and statistical manual of mental disorders (DSM) criteria for ADHD [6,9,11,21]. In adults with XXY, ADHD was diagnosed 5.6 times more often according to a screening of hospital discharges in 860 adults with XXY as compared to 86 000 men with XY [14].
A review on XXX has reported an estimated 25–35% of the individuals with XXX having ADHD [19,22]. Somewhat higher rates have also been reported with on average 49% (ranging from 46.7 to 52%) having attention symptoms in the clinical range [20,23], which is in line with a recent study reporting 48.9% of the girls with XXX having a diagnosis of ADHD [15].
For XYY, dimensional measures have shown that on average 69% of the XYY boys (ranging from 62 to 76%) had clinical levels of ADHD symptoms [9,20]. An average of 36% of boys with XYY (ranging from 11 to 52%) met full diagnostic criteria for ADHD, either based on clinical evaluation or history of psychiatric diagnoses [9,16,17].
PSYCHOTIC SYMPTOMS
On the basis of meta-analysis, the median global prevalence of psychotic disorders in the general population has been estimated at 0.46% [24]. A screening of hospital discharges in 860 adults with XXY revealed that psychotic disorders (schizophrenia or bipolar disorder) were diagnosed 7.4 times more often as compared to men with the typical XY pattern [14]. Clinical assessment studies have shown that 12% of the children with XXY [11] and 6.5% of the adults with XXY [25] meet DSM criteria for a psychotic disorder. In those with XXX, bipolar or psychotic disorders have been observed in 13.3% [15]. In individuals with XYY, psychiatric screening revealed a rate of 8% for bipolar disorder [17].
When considering dimensional measures, significantly higher rates of thought problems in SCT have been found in comparison to the general population: 37.8% of the children with XXY or XXY [19] and 30% of the individuals with XXX [23].
ANXIETY/DEPRESSION SYMPTOMS
Meta-analysis has shown global prevalence rates of 7.3% for anxiety disorders [26] and 12.9% for depression [27] in the general population. Data suggest that rates may be higher in SCT, although direct comparisons with control groups are lacking, except for two more recent studies using dimensional measures in SCT groups versus control groups, which showed mixed results [19,23]. In individuals with XXY, the average reported rate of depressive disorders is 26.9% (ranging from 12 to 68%) [6,11,25,28,29]. Clinically significant anxiety symptoms have been observed in on average 20.5% of the children with XXY (ranging from 14 to 27%) [6,19]. The reported rates of depression in XXX range from 17.8 [15] to 54% [28]. Anxiety disorders have been found in 19.5% [15] and clinical levels of anxiety-depression symptoms in 27–30% of the individuals with XXX [19,23]. As for XYY, a reported 13% had a depressive disorder and 26% had an anxiety disorder [17].
NEUROCOGNITIVE VULNERABILITY
Although IQ is typically at the borderline or lower end of the normal range [30], and intellectual disability is not common, the majority of children with SCT have specific cognitive impairments to some degree. Although the majority of studies have traditionally primarily focused on describing academic achievement and global intellectual levels, in recent years more and more studies have relied on comprehensive performance-based assessment of multiple and specific cognitive domains, as outlined below.
LANGUAGE
One of the best described and most recognized domains of cognitive vulnerability is language dysfunction. To illustrate, about 70–80% of children with 47,XXY have language difficulties that are typically present already from a young age [31]. This finding has been quite robust, being shown in numerous and unbiased samples [30,31]. Compromised language development is not only characteristic for boys with XXY, but also for boys with XYY and girls with XXX, although the degree of impairment may vary somewhat depending on the karyotype [32]. Difficulties include both receptive and expressive language skills (with the latter typically somewhat more affected), as well as difficulties in communication and language pragmatics [12,33]. Although the majority of studies have focused on school-aged children, more recent neuropsychological studies (of children with XXY and XXX) have started to investigate language development in children as young as 18–24 months, describing smaller vocabulary size and difficulties in lexical skills and emergent syntactic abilities [34▪,35].
With regard to the association between language dysfunction and psychopathology, two studies have shown that more compromised language functioning is directly associated with higher levels of autism symptoms in children, adolescents and adults with XXY [8,36].
EXECUTIVE FUNCTIONING
Executive functions are considered essential for flexible adaptive functioning in complex situations that have a high load of information, for inhibition of irrelevant actions and thoughts, for dealing with changing environmental demands and for organization of actions and thoughts in a goal-directed way. Although some of the early studies on executive function emphasized primarily working memory impairments in SCT [37], there is now increasing evidence for significant difficulties (as compared to the general population) in a range of executive function functions including inhibition, mental flexibility, strategic planning, fluency, working memory and sustained attention regulation, with medium-to-large effect sizes [19,21,38,39]. Although the majority of studies have focused on XXY and XXX, executive function problems have also been found in boys with XYY, with somewhat more compromised scores as compared to XXY [32]. In a study including both boys with XXY and girls with XXX, rates of 16–37% of the children with clinically impaired executive dysfunction were found [19]. A very recent international investigation of boys with XXY showed rates of 23–57% of the children having executive function dysfunctions within the clinically impaired range [40▪]. Vulnerability in executive function also seems to translate to daily life difficulties in executive function as observed by parents [19,41]. Executive function problems may show variability across age [41,42], are observed in both boys with XXY and girls with XXX [19,41] and may be more pronounced in the context of early life stressors [43].
In terms of the role of executive function difficulties in behavioral symptoms and risk for psychopathology in SCT, there is evidence showing that compromised executive functioning is significantly associated with increased externalizing behavior problems [19], increased social difficulties and ASD symptoms [39,44], increased psychotic symptoms such as disorganized thought [45] and increased symptoms of ADHD [21].
SOCIAL COGNITION AND EMOTION
Social cognition refers to the ability to perceive, understand and express social signals, which is crucial for successful social adaptation. Emotions, if well regulated, also have an important role in social functioning by facilitating behaviors that are adaptive to the social environment [33]. In the last decade, several studies in individuals with XXY or XXX have revealed significant impairments in these areas as compared to the general population. These include difficulties in reading social signals from social gaze direction [46] or tone of voice [47] and difficulties in recognizing emotional expressions from faces [48,49▪,50], which may be more pronounced in the context of early life stressors [43]. A reported 16–43% of the children with XXY [40▪] and 33% of the adults with XXY [49▪] have facial affect recognition problems in the clinically impaired range. In addition to understanding facial expressions, research suggests that significantly more children with XXY or XXX have difficulties with theory of mind [48], referring to the attribution of mental states, intentions and emotions to others. A very recent study suggests that children and adults with XXY may have disproportionate difficulties when ‘social load’ of information increases, revealing difficulties in the higher order labeling and interpretation of social cues [49▪].
The processing of facial emotions has been studied in more detail using eyetracking. One of these studies showed that adults with XXY looked less to the eye region of faces and did not show the typical tendency to first fixate on the eyes when presented with a face [51]. Shorter looking times were associated with poorer social functioning. Another eyetracking study in adults with XXY showed decreased attention to the eyes of characters displaying emotions, but increased affective arousal in response to these emotions [52]. This hyperarousal fits with studies showing increase social anxiety in men with XXY [13] and children with XXY or XXX [7], as well as increased subjective emotional arousal in response to emotion-inducing events, and increased influence of emotions during strategic, goal-directed tasks in adults with XXY [50]. Such increased affective arousal levels may negatively affect social interactions, especially as difficulties in recognizing and labeling of own emotions have been found in adults with XXY [50], which likely hampers the implementation of emotion regulation strategies. Indeed, more problems in the regulation of emotions have been reported for children and adolescents with XXY or XXX [41].
CONCLUSION
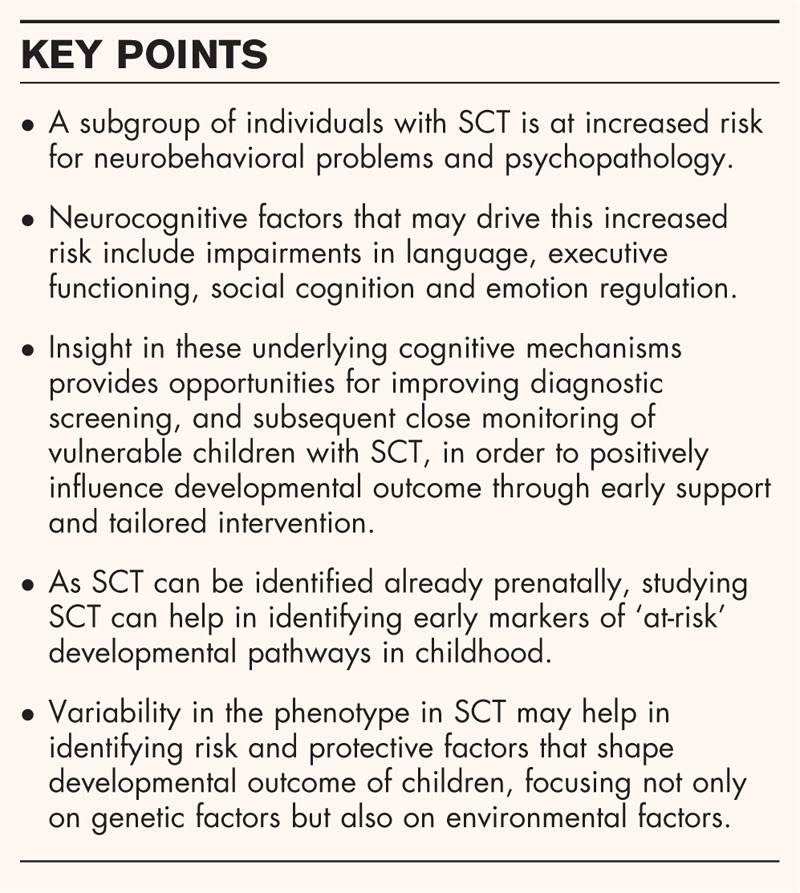
The reviewed studies illustrate an increased risk for social, emotional and behavioral problems in individuals with SCT. Although traditionally the primary focus has been on language and learning problems, more recent research suggests that impairments in executive functioning, social cognition and emotion regulation may also be key factors underlying the risk for behavioral problems and psychopathology. Insight in these underlying cognitive mechanisms provides opportunities for improving diagnostic assessment, and subsequent close monitoring of vulnerable children with SCT, in order to positively influence developmental outcome through early support and tailored intervention. Early implementation of preventive interventions, delivered during key windows of opportunity when neurodevelopment is most sensitive for environmental influences, has the potential to optimize quality of life and reduce risk for mental health problems later in life [53].
Ascertainment and recruitment biases are always important factors in SCT research, and contribute to the variance in the described vulnerabilities and lack of representation for the full population. In interpreting study findings, it is important to distinguish cohorts recruited for research purposes versus those that represent clinical samples. Also, cohorts identified through postnatal identification likely overestimate severity of the SCT phenotype (because of preselection of more severe cases), whereas cohorts identified based on prenatal screening may underestimate severity of the phenotype (because of higher socioeconomic status and implementation of early support and intervention). Increased awareness of these biases and increasing attempts to investigate the impact of these biases in studies with mixed prenatal/postnatal samples are helpful in determining the full range of outcomes in SCT.
The research field of SCT is in need of a more longitudinal perspective to study the described neurocognitive and behavioral aspects of the phenotype of SCT already from an early age onward. SCT can already be diagnosed prenatally, providing the responsibility and at the same time unique opportunity to identify early markers of ‘at risk’ development. Neurocognitive assessments that signal compromised neurodevelopment may prove to be helpful in this.
Studying SCT may also help in generating specific genetic hypotheses. Although few studies so far have attempted to link specific genes on the X or Y chromosomes to the neurobehavioral phenotype, recent studies have yielded very promising results [54]. Nonetheless, it is important to acknowledge that it is not all about the genes, and that is only in interaction with the environment that the phenotype is shaped, contributing to variability in outcome in SCT. This calls for a more integrated perspective considering developmental vulnerabilities in children with this genetic syndrome in the context of environmental factors, which may also help in identifying protective factors that could be targeted to reduce developmental risk as much as possible. Focusing on subgroups and individual differences rather than relying on group averages may also help uncover key mechanisms that contribute to risk for specific and different types of psychopathology.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by a personal grant (to S.v.R., grant number 016.165.397) from the Netherlands Organization for Scientific Research (NWO).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Zechner U, Wilda M, Kehrer-Sawatzki H, et al. A high density of X-linked genes for general cognitive ability: a run-away process shaping human evolution? Trends Genet 2001; 17:697–701. [DOI] [PubMed] [Google Scholar]
- 2.Reiss AL, Eliez S, Schmitt JE, et al. Brain imaging in neurogenetic conditions: realizing the potential of behavioral neurogenetics research. Ment Retard Dev Disabil Res Rev 2000; 6:186–197. [DOI] [PubMed] [Google Scholar]
- 3.Samango-Sprouse C, Keen C, Sadeghin T, et al. The benefits and limitations of cell-free DNA screening for 47, XXY (Klinefelter syndrome). Prenat Diagn 2017; 37:497–501. [DOI] [PubMed] [Google Scholar]
- 4.Pieters JJ, Kooper AJ, van Kessel AG, et al. Incidental prenatal diagnosis of sex chromosome aneuploidies: health, behavior, and fertility. ISRN Obstet Gynecol 2011; 2011:807106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsabbagh M, Divan G, Koh Y-J, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res 2012; 5:160–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tartaglia N, Cordeiro L, Howell S, et al. The spectrum of the behavioral phenotype in boys and adolescents 47,XXY (Klinefelter syndrome). Pediatr Endocrinol Rev 2010; 8 Suppl 1:151–159. [PMC free article] [PubMed] [Google Scholar]
- 7.van Rijn S, Stockmann L, Borghgraef M, et al. The social behavioral phenotype in boys and girls with an extra X chromosome (Klinefelter syndrome and Trisomy X): a comparison with autism spectrum disorder. J Autism Dev Disord 2014; 44:310–320. [DOI] [PubMed] [Google Scholar]
- 8.Cordeiro L, Tartaglia N, Roeltgen D, et al. Social deficits in male children and adolescents with sex chromosome aneuploidy: a comparison of XXY, XYY, and XXYY syndromes. Res Dev Disabil 2012; 33:1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross JL, Roeltgen DP, Kushner H, et al. Behavioral and social phenotypes in boys with 47, XYY syndrome or 47, XXY Klinefelter syndrome. Pediatrics 2012; 129:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tartaglia NR, Wilson R, Miller JS, et al. Autism spectrum disorder in males with sex chromosome aneuploidy: XXY/Klinefelter syndrome, XYY, and XXYY. J Dev Behav Pediatr 2017; 38:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruining H, Swaab H, Kas M, et al. Psychiatric characteristics in a self-selected sample of boys with Klinefelter syndrome. Pediatrics 2009; 123:e865–e870. [DOI] [PubMed] [Google Scholar]
- 12.Bishop DV, Jacobs PA, Lachlan K, et al. Autism, language and communication in children with sex chromosome trisomies. Arch Dis Child 2011; 10:954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rijn S, Swaab H, Aleman A, et al. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. J Autism Dev Disord 2008; 38:1634–1641. [DOI] [PubMed] [Google Scholar]
- 14.Cederlof M, Gotby AO, Larsson H, et al. Klinefelter syndrome and risk of psychosis, autism and ADHD. J Psychiatr Res 2014; 48:128–130. [DOI] [PubMed] [Google Scholar]
- 15.Wigby K, D’Epagnier C, Howell S, et al. Expanding the phenotype of Triple X syndrome: a comparison of prenatal versus postnatal diagnosis. Am J Med Genet A 2016; 170:2870–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geerts M, Steyaert J, Fryns JP. The XYY syndrome: a follow-up study on 38 boys. Genet Couns 2003; 14:267–279. [PubMed] [Google Scholar]
- 17.Bardsley MZ, Kowal K, Levy C, et al. 47,XYY Syndrome: clinical phenotype and timing of ascertainment. J Pediatr 2013; 163:1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas R, Sanders S, Doust J, et al. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics 2015; 135:e994–e1001. [DOI] [PubMed] [Google Scholar]
- 19.van Rijn S, Swaab H. Executive dysfunction and the relation with behavioral problems in children with 47,XXY and 47,XXX. Genes Brain Behav 2015; 14:200–208. [DOI] [PubMed] [Google Scholar]
- 20.Tartaglia NR, Ayari N, Hutaff-Lee C, et al. Attention-deficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: XXY, XXX, XYY, and XXYY. J Dev Behav Pediatr 2012; 33:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee NR, Wallace GL, Clasen LS, et al. Executive function in young males with Klinefelter (XXY) syndrome with and without comorbid attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc 2011; 17:522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tartaglia N, Howell S, Sutherland A, et al. A review of trisomy X (47, XXX). Orphanet J Rare Dis 2010; 5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freilinger P, Kliegel D, Hanig S, et al. Behavioral and psychological features in girls and women with triple-X syndrome. Am J Med Genet A 2018. [DOI] [PubMed] [Google Scholar]
- 24.Moreno-Kustner B, Martin C, Pastor L. Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS One 2018; 13:e0195687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boks MP, de Vette MH, Sommer IE, et al. Psychiatric morbidity and X-chromosomal origin in a Klinefelter sample. Schizophr Res 2007; 93:399–402. [DOI] [PubMed] [Google Scholar]
- 26.Baxter AJ, Scott KM, Vos T, et al. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol Med 2013; 43:897–910. [DOI] [PubMed] [Google Scholar]
- 27.Lim GY, Tam WW, Lu Y, et al. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci Rep 2018; 8:2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bender BG, Harmon RJ, Linden MG, et al. Psychosocial adaptation of 39 adolescents with sex chromosome abnormalities. Pediatrics 1995; 96:302–308. [PubMed] [Google Scholar]
- 29.Turriff A, Levy HP, Biesecker B. Prevalence and psychosocial correlates of depressive symptoms among adolescents and adults with Klinefelter syndrome. Genet Med 2011; 13:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leggett V, Jacobs P, Nation K, et al. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: a systematic review. Dev Med Child Neurol 2010; 52:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boada R, Janusz J, Hutaff-Lee C, et al. The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Dev Dis Res Rev 2009; 15:284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross JL, Zeger MPD, Kushner H, et al. An extra X or Y chromosome: contrasting the cognitive and motor phenotypes in childhood in boys with 47,XYY syndrome or 47,XXY Klinefelter syndrome. Dev Dis Res Rev 2009; 15:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bender B, Fry E, Pennington B, et al. Speech and language-development in 41 children with sex-chromosome anomalies. Pediatrics 1983; 71:262–267. [PubMed] [Google Scholar]
- 34▪.Zampini L, Burla T, Silibello G, et al. Early communicative skills of children with Klinefelter syndrome. Clin Linguist Phon 2018; 32:577–586. [DOI] [PubMed] [Google Scholar]; This study is of special interest because it is one of the very few studies that focuses on very young children, that is 18 month olds, with sex chromosome trisomy.
- 35.Zampini L, Draghi L, Silibello G, et al. Vocal and gestural productions of 24-month-old children with sex chromosome trisomies. Int J Lang Commun Disord 2018; 53:171–181. [DOI] [PubMed] [Google Scholar]
- 36.van Rijn S, Swaab H. Vulnerability for psychopathology in Klinefelter syndrome: age-specific and cognitive-specific risk profiles. Acta Paediatr 2011; 100:908–916. [DOI] [PubMed] [Google Scholar]
- 37.Fales CL, Knowlton BJ, Holyoak KJ, et al. Working memory and relational reasoning in Klinefelter syndrome. J Int Neuropsychol Soc 2003; 9:839–846. [DOI] [PubMed] [Google Scholar]
- 38.Kompus K, Westerhausen R, Nilsson LG, et al. Deficits in inhibitory executive functions in Klinefelter (47, XXY) syndrome. Psychiatry Res 2011; 189:135–140. [DOI] [PubMed] [Google Scholar]
- 39.Skakkebæk A, Moore PJ, Pedersen AD, et al. The role of genes, intelligence, personality, and social engagement in cognitive performance in Klinefelter syndrome. Brain Behav 2017; 7:e00645. 10.1002/brb3.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪.Samango-Sprouse C, Stapleton E, Chea S, et al. International investigation of neurocognitive and behavioral phenotype in 47,XXY (Klinefelter syndrome): predicting individual differences. Am J Med Genet A 2018; 176:877–885. [DOI] [PubMed] [Google Scholar]; This study is of special interest as it focuses on understanding factors that contribute to individual differences in cognitive and behavioral outcome, an international cohort of children with XXY.
- 41.Lee NR, Anand P, Will E, et al. Everyday executive functions in Down syndrome from early childhood to young adulthood: evidence for both unique and shared characteristics compared to youth with sex chromosome trisomy (XXX and XXY). Front Behav Neurosci 2015; 9:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross JL, Roeltgen DP, Stefanatos G, et al. Cognitive and motor development during childhood in boys with Klinefelter syndrome. Am J Med Genet A 2008; 146a:708–719. [DOI] [PubMed] [Google Scholar]
- 43.van Rijn S, Barneveld P, Descheemaeker MJ, et al. The effect of early life stress on the cognitive phenotype of children with an extra X chromosome (47,XXY/47,XXX). Child Neuropsychol 2018; 24:277–286. [DOI] [PubMed] [Google Scholar]
- 44.van Rijn S, Bierman M, Bruining H, et al. Vulnerability for autism traits in boys and men with an extra X chromosome (47,XXY): the mediating role of cognitive flexibility. J Psychiatr Res 2012; 46:1300–1306. [DOI] [PubMed] [Google Scholar]
- 45.Van Rijn S, Aleman A, De Sonneville L, et al. Cognitive mechanisms underlying disorganization of thought in a genetic syndrome (47,XXY). Schizophr Res 2009; 112:91–98. [DOI] [PubMed] [Google Scholar]
- 46.van ’t Wout M, van Rijn S, Jellema T, et al. Deficits in implicit attention to social signals in schizophrenia and high risk groups: behavioural evidence from a new illusion. PLoS One 2009; 4:e5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Rijn S, Aleman A, Swaab H, et al. What it is said versus how it is said: comprehension of affective prosody in men with Klinefelter (47,XXY) syndrome. J Int Neuropsychol Soc 2007; 13:1065–1070. [DOI] [PubMed] [Google Scholar]
- 48.van Rijn S, Stockmann L, van Buggenhout G, et al. Social cognition and underlying cognitive mechanisms in children with an extra X chromosome: a comparison with autism spectrum disorder. Genes Brain Behav 2014; 13:459–467. [DOI] [PubMed] [Google Scholar]
- 49▪.van Rijn S, de Sonneville L, Swaab H. The nature of social cognitive deficits in children and adults with Klinefelter syndrome (47,XXY). Genes Brain Behav 2018; 17:e12465. [DOI] [PubMed] [Google Scholar]; This study is of special interest as it emphasizes social cognitive deficits in 47,XXY
- 50.van Rijn S, Swaab H, Aleman A, et al. X Chromosomal effects on social cognitive processing and emotion regulation: a study with Klinefelter men (47,XXY). Schizophr Res 2006; 84:194–203. [DOI] [PubMed] [Google Scholar]
- 51.van Rijn S. Social attention in 47,XXY (Klinefelter syndrome): visual scanning of facial expressions using eyetracking. J Int Neuropsychol Soc 2015; 21:364–372. [DOI] [PubMed] [Google Scholar]
- 52.van Rijn S, Barendse M, van Goozen S, et al. Social attention, affective arousal and empathy in men with Klinefelter syndrome (47,XXY): evidence from eyetracking and skin conductance. PLos One 2014; 9:e84721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herlihy AS, McLachlan RI. Screening for Klinefelter syndrome. Curr Opin Endocrinol Diabetes Obes 2015; 22:224–229. [DOI] [PubMed] [Google Scholar]
- 54.Ross JL, Tartaglia N, Merry DE, et al. Behavioral phenotypes in males with XYY and possible role of increased NLGN4Y expression in autism features. Genes Brain Behav 2015; 14:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]


