Abstract
Polycystic ovary syndrome (PCOS) is a heterogeneous condition which is related to an endocrine reproductive disorder of females. It affects females of 18–44 age. The persistent hormonal disbalance leads to the complexities such as numerous cysts, an irregular menstrual cycle that ultimately leads to infertility among females. Many candidate genes have been identified to be one of the causes of PCOS. Different studies have been carried out to find the genetic correlation of PCOS. It is essential to carry out such studies that identify the clear cause of PCOS and its genetic association and hormonal disbalance. This review has highlighted different genes and their correlation with PCOS that leads to hormonal disbalance. Yet not in-depth but an attempt to study the genetic predisposition of PCOS.
Keywords: Polycystic ovary syndrome, Infertility, Hormonal disbalance, Endocrine reproductive disorder
Introduction
Polycystic ovary syndrome (PCOS) increase serious complications among females. One in every 5–6 female is facing serious complications regarding infertility and irregularity in their menstrual cycles. Stress, obesity, fluctuation in hormonal level are the major cause worldwide [1]. This condition is also named as Schlerocystic Ovaries, Multicystic ovaries, Stein Leventhal Syndrome which was named by an American gynecologist Irving F Stein, SR and Michael L. Leventhal [2]. This endocrine disorder affects females under 18–44 age [3]. Globally it affects 5–15% of females [4]. The normal functioning of hormones plays an important role in the ovary functioning and regulation of the menstrual cycle that maintains fertility. If there is a constant disturbance of hormonal level in females then it will disturb ovary functioning which leads to the formation of a cyst inside the sac of an ovary. Whereas androgen which is a male hormone elevated beyond its normal range in females affected with PCOS [5].
Clinical features/ sign & symptoms
The complexity of this condition does not refer to its name, there are many other conditions that are associated with this problem. PCOS patients have numerous cyst 8 mm in size in the sac of their ovary. More than 12 cysts are present in the ovary. About 70% of females are infertile because of this condition [6,7]. As discussed above in PCOS condition, the level of male hormones i.e. androgen elevated that causes hirsutism and acne. There is an insulin resistance which leads to obesity and Type 2 Diabetes. This problem leads to an irregularity in the menstrual cycle that results in infertility. 20% of females often experienced sleep apnea. Depression and anxiety are common [8]. PCOS long term condition is been represented in Pie Chart Fig. 1
Fig. 1.
PCOS long term condition.
Diagnosis
This condition can be diagnosed on the basis of Rotterdam criteria i.e. irregular menstrual cycle, elevated androgen level, the presence of cysts [9]. Diagnostic criteria of PCOS is depicted in Table 1.
Table 1.
Diagnostic criteria for PCOS [11].
| NIH 1990 | Rotterdam 2003 | AE-PCOS society 2006 |
|---|---|---|
| Long-lasting anovulation | Oligo or anovulation | Biochemical and clinical evidence of hyperandrogenism |
| Hyperandrogenism | Hyperandrogenism | Dysfunction ovaries |
| Polycystic ovaries | Polycystic ovary morphology |
Etiology of PCOS
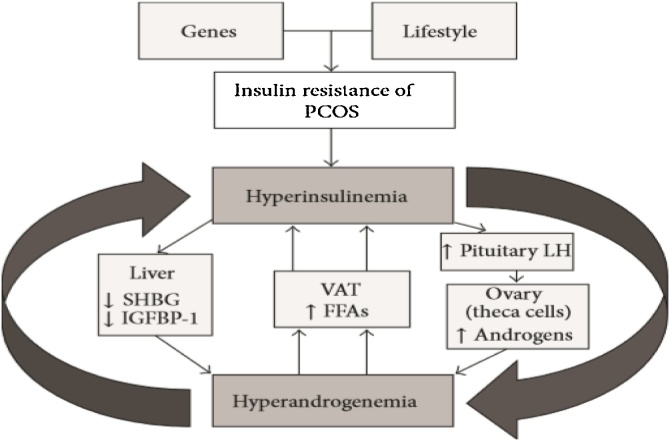
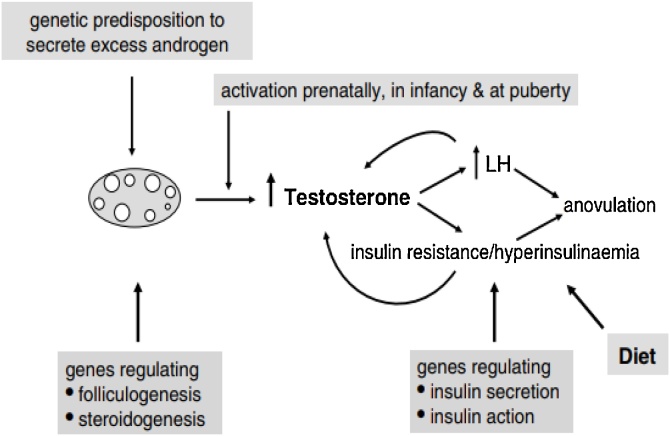
The genetic and environmental factor is responsible for the etiology of this condition. Unhealthy lifestyle, diet or any infectious mediators increase the risk of PCOS [10]. Due to insulin resistance and its elevated level, the ovaries function disturbs that rises androgen level which leads to anovulation [11]. The level of gonadotrophin-releasing hormone, follicular stimulating hormone (FSH), luteinizing-hormone (LH) and prolactin is also disturbed in case of PCOS [12]. Apart from the environmental factors, there are genetic factors that are responsible for the etiology of PCOS. Its cause involves candidate genes, SNP’s. According to databases PCOS etiology involves 241 gene variations [13]. Polymorphism or any nucleotide change cause a defect in the transcriptional activity of a gene that leads to PCOS [14]. Mostly genes that encode for the androgen receptor, Luteinizing Hormone receptors, Follicular Stimulating Hormone receptors, Leptin receptors are responsible [15]. Gene defect perturbs the biochemical pathway and leads to dysfunction of an ovary. Polymorphism such as StAR polymorphs, FSHR polymorphism, FTO polymorphism, VDR polymorphism, IR and IRS polymorphism, GnRHR polymorphism are found to be involved in PCOS cause [16]. PCOS progression and severity increases with the increase in insulin level as well as an androgen. Hyperinsulinemia affects ovarian theca cells and raise androgen level. This condition reduces the hepatic biosynthesis of SHBG and IGFBP-1. Elevated androgen level, on the other hand, stimulates visceral adipose tissue (VAT) that generates free fatty acids (FFA’s) which contributes in insulin resistance Fig. 2 [17]. Genetic predisposition with PCOS, a pathway describes hyperandrogenism Fig. 3.
Fig. 2.
how insulin resistance effects the ovarian theca cells and perturbs its functioning [18].
Fig. 3.
A defect in the pituitary axis elates testosterone and LH. It also leads to insulin resistance. Together insulin resistance and high level of androgen subsidize in the pathway of anovulation [19].
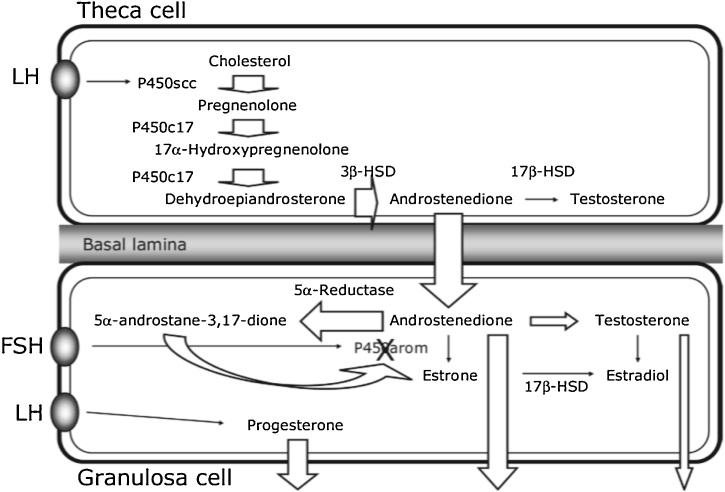
Fig. 4 depicts a pathway that describes how steroidogenesis enzyme affect the theca cells of an ovary. 5ᾳ-reductase activity increased that elevates 5ᾳ-androstane -3, 17 Dione concentrations and inhibit the activity of aromatase in the granulosa cells. In the case of PCOS, LH and progesterone are expressed in the granulosa cells which results in high androgen level and reduced estrogen level [18].
Fig. 4.
effect of steroidogenesis enzyme and theca cells of an ovary.
Hormonal association with PCOS
A study conducted among the Pakistani population in which correlation between hormonal level and PCOS was observed. This cross-sectional study includes affected and healthy individuals. Clinical examination was recorded, and blood samples were drawn for hormonal analysis using immunoradiometric assay and radioimmunoassay. They concluded that BMI, FSH, LH, prolactin level was quite elevated in PCOS as compared to healthy individuals. For the diagnosis of PCOS, the basic parameters that must be considered are FSH, LH and androgen level. Raised LH level leads to an increase in androgen level that gives rise to the progression of PCOS [19].
Genetic predisposition & PCOS
PCOS has a strong genetic association. Genes like CAPN10, Cytochrome family p450, Insulin gene, AR, FTO, FSHR have been discussed.
Androgen Receptor Gene (AR)
This gene is present on chromosome Xq12 and has 11 exons, it codes for more than 90 kb long protein that has total of three functional domain [20]. Androgen receptor AR is also linked with PCOS. X Inactivation disrupts androgen signaling pathway and elevated. AR is an X linked gene and a single copy of X chromosome perturbs the whole pathway. It is possible to conduct Genome-Wide Association for PCOS to identify the novel mutations and other genetic variants that is associated with the cause of PCOS [21].
Follicular stimulating hormone receptor (FSHR)
Cytogenic location of FSHR is at chromosome 2p16.3 and it has total of 14 exons. This gene encodes a protein named as G coupled receptors and plays an important role in gonad development [22]. The disturb hormonal levels effects endocrine reproductive system. Apart from other hormones imbalance level of FSH also responsible for PCOS severity. FSH is encoded by Follicular Stimulating Hormone receptor. Follicular and ovary functioning disturbs due to any abnormality present in FSHR. On the basis of statistical analysis and RFLP using restriction enzymes Eam11051, a great difference was observed among healthy and affected individual in a study conducted in the North of Iraq [23].
Fat Mass Obesity (FTO)
FTO gene is also known as alpha-ketoglutarate dependent dioxygenase, its cytogenic location is 16q12.2 and has 14 exons. Different studies have shown that FTO is associated with obesity, BMI and type 2 Diabetes [24]. Polymorphism in the FTO gene among PCOS patients was also identified via a study conducted in Pakistan. PCOS patients have rs9939609 SNP in its intronic variant. The genetic and statistical analysis has revealed that affected individuals have a significant difference of BMI as compared to healthy individuals. SNP’s in the FTO gene was also associated with PCOS among Pakistani population [25].
Capn10
CAPN10 also known as Caplain10 that is calcium-dependent cysteine proteases. It is present on chromosome 2q37.3 and has 12 exons. Its protein is a heterodimer, this gene is associated with type 2 diabetes. Its location is in non-insulin dependent Diabetes Mellitus type 1 region [26]. Chromosomal locus that has CAPN10 encodes cysteine proteases calpain 10. Calpain 10 is found to play a role in insulin action and secretion. Any abnormality or polymorphism in CAPN10 leads to PCOS because insulin resistance and type 2 diabetes are associated with PCOS, therefore it is also a candidate gene that is known to be responsible for PCOS [27].
Aromatase and PCOS
Steroidogenesis enzymes include aromatase which belongs to a complex Cytochrome P450 family that normally plays a vital role in steroid conversion. It assists in the conversion of androgen into estrogen. Aromatase deficiency leads to a defect in the pathway that ceases its conversion [28]. This deficiency will affect ovary functioning and elevates androgen level due to no alteration of C19 into C18. Aromatase genes that are reported in PCOS databases are CYP11A1, CYP11B2, CYP17A1, CYP19A1, CYP1A1, CYP21A2, CYP3A7 [13]. Any abnormality in Cytochrome P450 increased risk in PCOS progression.
CYPA1A
Gene named as CYPA1A is also known to be a causative gene in the etiology of PCOS. It is abbreviated as Cytochrome P450, family 1, subfamily A, member 1 and is located on chromosome 15q24.1. It is comprised of 7 exons. It encodes Cytochrome P450 proteins that are present in the endoplasmic reticulum and its expression is mainly induced by polycyclic aromatic hydrocarbons (PAHs) [29]. A study conducted on PCOS patients and healthy individuals which concluded that PCOS patients have a high rate of Isoleucine/valine as compared to normal individuals whereas it was further addressed through statistical analysis that isoleucine is replaced by valine in PCOS and they have the genotype for Valine. Hence they concluded that there was7.8-fold higher frequency of CYP1A1 isoleucine/valine genotype whereas the rate of CYP1A1 of valine genotype was 7.4-fold [30]. Polymorphism in phase 1 and phase 2 enzyme may lead to an increased toxification and detoxification. Any alteration in those enzyme leads to abnormal functioning of ovaries and cyst formation. The genetic polymorphism T6235C in phase 1 enzyme that’s encoded by CYP1A1 is strongly associated with the susceptibility of PCOS. the presence of this mutant genotype will cause a disturbance in the enzymatic pathway and become a risk factor for PCOS predisposition and progression [31].
CYP11A1
CYP11A1 named as Cytochrome P450, family 11, subfamily A, member 1. It encodes superfamily of cytochrome p450. It is present in the mitochondrial inner membrane. The main function is in the catalysis of cholesterol to pregnenolone. It also plays a vital role in the steroid synthesis pathway. This gene is located on chromosome 15q24.1 and composed of 10 exons [32]. Polymorphism in the promotor pentanucleotide (TTTTA)n is known to be another genetic predispose to PCOS. It is reported that CYP11A1 polymorphism is found to be a risk molecular marker for PCOS. the risk increased when there is an interaction between genetics and environmental factors. A study conducted on South Indian population concluded about 15 allele variations ranging from 2 to 16 repeats and the most common was 8 repeat alleles. This study also addressed the presence of >8 repeat allele in PCOS affected females which indicates 3 fold risk for PCOS predisposition than control [33]. A case-control study in China depicted that polymorphism in CYP11A1 is known to be responsible for the cause of PCOS. SNP rs4077582 in CYP11A1 is significantly associated with PCOS and it also elevates androgen level through the regulation of Luteinizing hormone in various genotypes [34].
CYP11b2
It is abbreviated as Cytochrome P450, family 11, subfamily B, member 2. This gene is located on chromosome 8q24.3 and composed of 9 exons. Its function is to deliver commands for the synthesis of aldosterone synthetases which is present in the adrenal glands [35]. It is another gene that is reported and responsible for PCOS progression. A case-control study concludes that polymorphism in the promoter region of aldosterone synthetase is responsible for the etiology of PCOS. The frequency of polymorphism in PCOS patients was quite high as compared to normal individuals. Whereby the level of aldosterone and testosterone were also significantly raised in PCOS affected individual which increased PCOS risk [36].
CYP17A1
Cytochrome P450, Family 17, subfamily A, member 1 is another steroidogenesis enzyme that is monooxygenases. Its cytogenic location is on chromosome 10q24.32 and has 8 exon count [37]. CYP17 is reported as a causative gene in the pathogenesis of PCOS. A study conducted on the Chilean population concluded that polymorphism C > T in the CYP17 is responsible for PCOS progression. The comparison of body weight and insulin resistance with polymorphism was also performed through hormonal and clinical shreds of evidence. It was further addressed that due to the polymorphism in CYP17 and the defect found in the gene after performing RFLP PCR, it leads to an increase in the body weight, insulin resistance and excessive lipid. Hence, it is associated with PCOS along with metabolic pathways [38]. In another study T/C polymorphism in the CYP17A1 gene was also observed among the Chinese population. The clinical and genetic parameters depicted TC, TT, CC genotype which was 43.71%, 49.69% and, 6.6%. Affected females that have CC genotype had elevated testosterone as compared to the individuals who have TT, TC genotype. It was further concluded that T/C polymorphism may not directly be associated with the PCOS. The association may rely on the polymorphism when there is increased level of testosterone and insulin resistance [39].
CYP1A1
The full name of CYP1A1 is Cytochrome P450, Family 1, Subfamily A, member 1. Its cytogenic location is on chromosome 15q24.1 and has 7 exons [40]. The assessment of variant alleles on CYP1A1 and its association with PCOS has revealed that in affected individuals the rate of CYP1A1 isoleucine/Valine was quite high as compared to healthy individuals. The biochemical and genetic analysis further concluded that patients with PCOS have 7.8 fold higher frequency of isoleucine/ valine and 7.4 fold frequency of another genotype of Valine i.e. Val/Val and Ile/Val [30].
CYP21A2
Cytochrome P450, Family 21, subfamily A member 2, is another gene that is reported as a risk factor gene for PCOS progression and development. Its chromosomal location is 6p21.33 and comprised of 10 exons [41]. In the pathogenesis of PCOS, a heterozygous mutation in CYP21A2 may play its role. About 14 molecular anomalies have been reported in CYP21A2 for PCOS progression. The frequency of mutation in control and affected individual were 5.9% and 7.6%. However, still it’s not a satisfactory answer in case of CYP21A2 [42].
CYP3A7
CYP3A7 also named as Cytochrome P450, Family 3, Subfamily A, Member 7. It mainly expresses in the liver. Its chromosomal location is 7q22.1 and has 13 exons [43] It is reported that an abnormal level of androgen in females is due to inheritance. CYP3A7 assist in the metabolism of DHEAS. The variant allele in the promoter region of CYP3A7 reduces the activity of the metabolic pathway. The total frequency of the variant in a study conducted was found to be 2.7%. In affected individual, it was 2.2% and 3.6% in control. Therefore, it was confirmed that variant allele in CYP3A7 is associated with lower DHEAS in females suffering from PCOS. Henceforth, polymorphism plays a role in the androgen metabolic pathway and it can reduce the severity of elevated androgen and PCOS phenotype [44].
CYP19A1
It was also addressed that hyperandrogenism is also due to the SNP rs2414096 found in CYP19 gene in the Chinese population. The genotype (AG, AA, GG) for rs2414096 were expressively different in PCOS patients as compared to control individuals which suggest that SNP in CYP19 is also associated with the vulnerability of PCOS [45]. Another aromatase encoded gene named as CYP19A1 is also responsible for PCOS development. CYP19A1 is abbreviated as Cytochrome P450, Family 19, Subfamily A, Polypeptide 1. It is monooxygenase that is involved in the biosynthesis of cholesterol, steroids and lipids. It plays a very important role in estrogen biosynthesis pathway and present in the endoplasmic reticulum. Any abnormality in CYP19A1 gene cause disturbance in the estrogen pathway and in aromatase activity, the chromosomal location is 15q21.2, it has a total 18 exons and 17 introns [46]. This gene has two SNP ID’s rs700519(C/T) in its exon region and rs710059(C/T) in its intronic region [13]. CYP19A1 spans more than 123 kb in which 93 kb is regulatory region and 30 kb covers the coding region [47]. Beside PCOS progression there is an increased risk of other conditions like endometrial cancer, breast cancer and prostate cancer due to polymorphism in the CYP19A1 gene is also observed [48]. SNP’s identification among the Korean population is also observed that is found to play a vital role in the disruption of the estrogen pathway. Nineteen variations have been recognized which are present in 10 introns, 4 exons, one SNP in 3′ UTR and 6 SNP in 5′ untranslated region [49]. Among South Indian population SNP at exon region, rs700519 and two intronic region rs2414096 and rs60271534 were also reported. These variants are responsible for the cause of PCOS. Statistical analysis has revealed a strong association of variation in exon region Arg264Cys. Whereas In-silico analysis has shown a destabilized structure of aromatase near substrate recognition site 3 which reduced enzymatic activity [5]. Cytogenic location and genetic anomalies of PCOS is depicted in Table 2.
Table 2.
Cytogenic location and anomalies found in genes associated with PCOS.
| S no | Gene | Cytogenic location | Anomalies | Author | Reference |
|---|---|---|---|---|---|
| 1 | AR | Xq12 | X inactivation | Urbanek | [23] |
| 2 | FSHR | 2p16.3 | Gene variation | Aesha Sh | [25]. |
| 3 | FTO | 16q12.2 | SNP rs9939609 | Rizwan S | (Rizwan S, 2018) |
| 4 | CAPN10 | 2q37.3 | Polymorphism | Margrit Urbanek | [29] |
| 5 | CYPA1A | 15q24.1 | T6235C | K Arvind Babu | [33] |
| 6 | CYP11A1 | 15q24.1 | SNP rs4077582 | Cheng-wei zhang | [36]. |
| 7 | CYP17A1 | 10q24.32 | T > C | Li Li | [41]. |
| 8 | CYP1A1 | 15q24.1 | Ile/Val | Ibrahim Esinler | [32]. |
| 9 | CYP21A2 | 6p21.33 | Heterozygous mutation | Settas N | [44]. |
| 10 | CYP3A7 | 7q22.1 | Variant allele | Mark O goodarzi | [46] |
| 11 | CYP19A1 | 15q21.2 | Arg264Cys | K Ranjith reddy | [6]. |
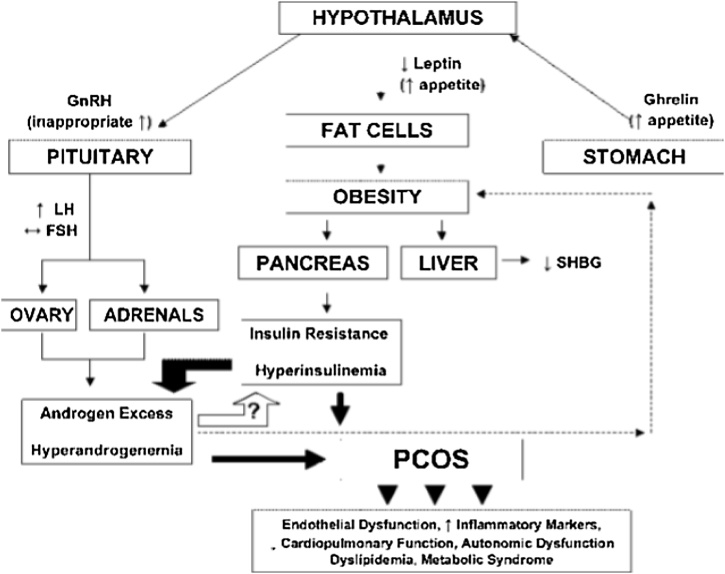
Besides those factors that are involved in the etiology of PCOS, due to unknown single cause there is no such treatment that overcomes this condition. Whereas the symptoms and severity of this condition can be reduced to some extent. Adopting a healthy lifestyle is the first and foremost method of controlling PCOS severity. The severity of symptoms can be reduced if affected females lose weight. Losing weight up to 5–10% control the symptoms [50]. PCOS woman should follow a balanced and healthy diet that includes low fat to moderate proteins. Fiber-rich containing food, fruits, vegetables, cereals should be taken along with regular exercise. High-caloric food should be avoided [51]. Medicines like Oral Contraceptive Pills OCP, Metformin, Cyclin Progestin are recommended to PCOS patients to reduce PCOS progression. It also regulates the menstrual cycle and hyperandrogenism [52]. For hirsutism and acne, laser and cosmetic treatment are recommended. For infertile woman invitro fertilization IVF and gonadotrophins are recommended [3]. Prevention of insulin resistance using certain drugs: a flow chart is represented in Fig. 5.
Fig. 5.
Flowchart that illustrate how insulin resistance leads to elevated level of androgen. It also explains decrease level of androgen that can be possible by using drugs that prevents insulin resistance (55).
Conclusion
Apart from environmental factors, many candidate genes are involved in the etiology of the PCOS, Alteration in the metabolic pathway due to a defect in the gene leads to the progression of PCOS and ovary dysfunction. The severity can only be reduced when follows proper precautionary measures i.e. weight loss, healthy diet and recommended medications.
Contributor Information
Nida Ajmal, Email: nidaajmal99@gmail.com.
Sanam Zeib Khan, Email: sanamzeibkhan@gmail.com.
Rozeena Shaikh, Email: drrozeenashaikh@gmail.com.
References
- 1.Torie Comeaux Plowden M.D. Eunice Kennedy Shriver National Institute of Child Health and Human Development; 2016. M.P.H. Reproductive endocrinology and infertility. [Google Scholar]
- 2.Legro R.S. 2009. Stein-Leventhal syndrome. Encyclopedia britannica. [Google Scholar]
- 3.Teede H.D.A., Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8(1) doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricardo Azziz M.D. M.P.H. Introduction: determinants of polycystic ovary syndrome. Fertil Steril. 2016;106(July (1)) doi: 10.1016/j.fertnstert.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 5.ranjith reddy K. POLYCYSTIC Ovary Syndrome: ROLE OF AROMATASE GENE VARIANTS IN SOUTH INDIAN WOMEN. Int J Pharma Bio Sci. 2015;6(2) [Google Scholar]
- 6.Diamanti-Kandarakis E.D.A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocrinology. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization. 2011. Anonymous. ABOUT PCOS FOUNDATION. n-pc, editor. [Google Scholar]
- 8.Helvaci N., Karabulut E. Polycystic ovary syndrome and the risk of obstructive sleep apnea: a meta-analysis and review of the literature. Endocr Connect. 2017;6(437):437–445. doi: 10.1530/EC-17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.group TREA-sPcw Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2003;10(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 10.Goodarzi Mea. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 11.Diamanti-Kandarakis E.K.H., Legro R.S. The role of genes and environment in the etiology of PCOS. Endocrine. 2006;30:19–26. doi: 10.1385/ENDO:30:1:19. [DOI] [PubMed] [Google Scholar]
- 12.Marx T.L.M.A. Polycystic ovary syndrome: Pathogenesis and treatment over the short and long term. Cleve Clin J Med. 2003;70(1):5. doi: 10.3949/ccjm.70.1.31. 31–3, 6–41. [DOI] [PubMed] [Google Scholar]
- 13.Joseph S.B.R., Bhujbalrao R., Idicula-Thomas S. PCOSKB: a KnowledgeBase on genes, diseases, ontology terms and biochemical pathways associated with PolyCystic Ovary Syndrome. Nucl Acids Res. 2015 doi: 10.1093/nar/gkv1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.2003. T S. Instability of the human genome: mutation and DNA repair. [Google Scholar]
- 15.Xita N., GaAT I. The genetic basis of polycystic ovary syndrome. Eur J Endocrinol. 2002;147:717–725. doi: 10.1530/eje.0.1470717. [DOI] [PubMed] [Google Scholar]
- 16.Fang Shu-ying, Ca Yao. Potential genetic polymorphisms predicting polycystic ovary syndrome. Endocr Connect. 2018;7:187–195. doi: 10.1530/EC-18-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NuzhatShaikh R., Mukherjee Srabani. Genetic markers of polycystic ovary syndrome: emphasis on insulin resistanc. Int J Med Genet [review article] 2014 [Google Scholar]
- 18.denis Am. ovarian enzyme activities in woman with polycystic ovary syndrome FertilOvarian enzyme activities in woman with polycystic ovary syndrome. Fertil Steril. 2006;86(1):9–11. [Google Scholar]
- 19.Muhammad Akram N.R. Endocrine correlates of polycystic ovary syndrome in pakistani women. J Coll Physicians Surg Pak. 2015;25(1):22–26. [PubMed] [Google Scholar]
- 20.2018. AR androgen receptor [Homo sapiens (human)] [database on the Internet]https://www.ncbi.nlm.nih.gov/gene/367 Available from: [Google Scholar]
- 21.Urbanek M. The genetics of the polycystic ovary syndrome. Endocrinol Metab. 2014;3(2) doi: 10.1038/ncpendmet0400. [DOI] [PubMed] [Google Scholar]
- 22.2018. FSHR follicle stimulating hormone receptor [Homo sapiens (human)] [database on the Internet]https://www.ncbi.nlm.nih.gov/gene/2492 Available from: [Google Scholar]
- 23.Baban Aesha Sh.Sh., Korsheed S.H., Hayawi Anas Y.Al. The FSHR polymorphisms association with polycystic ovary syndrome in women of Erbil, Kurdistan in North of Iraq. Ibn Al-Haitham J Pure Appl Sci. 2017 [Google Scholar]
- 24.FTO FTO . 2018. Alpha-ketoglutarate dependent dioxygenase [Homo sapiens (human)] [database on the Internet]https://www.ncbi.nlm.nih.gov/gene/79068 Available from: [Google Scholar]
- 25.Rizwan S.G.S., Rasheed N., Ullah M.I. Association of FTO common RS9939609 polymorphism with obesity and polycystic ovarian syndrome in Pakistani. Women J Med Res Biol Stud. 2018;1:101. [Google Scholar]
- 26.2018. CAPN10 calpain 10 [Homo sapiens (human)] [database on the Internet]https://www.ncbi.nlm.nih.gov/gene/11132 Available from: [Google Scholar]
- 27.urbanek margrit. The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab. 2006;3(2) doi: 10.1038/ncpendmet0400. [DOI] [PubMed] [Google Scholar]
- 28.Harada N.O.H., Shozu M., Yamada K. Genetic studies to characterize the origin of the mutation in placental aromatase deficiency. Am J Hum Genet. 1992;52:666–672. [PMC free article] [PubMed] [Google Scholar]
- 29.2018. CYP1A1 cytochrome P450 family 1 subfamily A member 1 [Homo sapiens (human)] [database on the Internet]https://www.ncbi.nlm.nih.gov/gene/1543 Available from: [Google Scholar]
- 30.Ibrahim Esinler D.A., Otegen Umit, Alikasifoglu Mehmet, Yarali Hakan. Ergul Tuncbilek CYP1A1 gene polymorphism and polycystic ovary syndrome. Reprod BioMed. 2008;16(3):356–360. doi: 10.1016/s1472-6483(10)60596-2. [DOI] [PubMed] [Google Scholar]
- 31.Arvind Babu K., Rao K.L., Kanakavalli M.K., Suryanarayana V.V., Deenadayal Mamata, Singh Lalji. CYP1A1, GSTM1 and GSTT1 genetic polymorphism is associated with susceptibility to polycystic ovaries in South Indian women reproductive. BioMedicine. 2004;9(2) doi: 10.1016/s1472-6483(10)62129-3. [research article] [DOI] [PubMed] [Google Scholar]
- 32.2018. CYP11A1 cytochrome P450 family 11 subfamily A member 1 [Homo sapiens (human)] [database on the Internet]https://www.ncbi.nlm.nih.gov/gene/1583 Available from: [Google Scholar]
- 33.k ranjith reddy M.L.N.D., Supriya K. CYP11A1 microsatellite (tttta)n polymorphism in PCOS women from South India. J Assist Reprod Genet. 2014;31:857–863. doi: 10.1007/s10815-014-0236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.X-lZea Cheng-wei Zhang. Association between polymorphisms of the CYP11A1 gene and polycystic ovary syndrome in Chinese women. Mol Biol Rep. 2012;39(8) doi: 10.1007/s11033-012-1688-7. [DOI] [PubMed] [Google Scholar]
- 35.2018. CYP11B2 cytochrome P450 family 11 subfamily B member 2 [Homo sapiens (human)] [database on the Internet]https://www.ncbi.nlm.nih.gov/gene/1585 Available from: [Google Scholar]
- 36.Zhao S.P.T.X., Shao D.H., Dai H.Y., Dai S.Z. Association study between a polymorphism of aldosterone synthetase gene and the pathogenesis of polycystic ovary syndrome. Zhonghua Fu Chan Ke Za Zhi. 2003;38(2):94–97. [PubMed] [Google Scholar]
- 37.2018. CYP17A1 cytochrome P450 family 17 subfamily A member 1 [Homo sapiens (human)] [database on the Internet]https://www.ncbi.nlm.nih.gov/gene/1586 Available from: [Google Scholar]
- 38.Barbara Echiburu F.P.-B. Polymorphism T → C (−34 base pairs) of gene CYP17 promoter in women with polycystic ovary syndrome is associated with increased body weight and insulin resistance: a preliminary study. Metab Clin Exp. 2008;57(12):1765–1771. doi: 10.1016/j.metabol.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Li Li Z.-P.Gea. Association of CYP17A1 gene -34T/C polymorphism with polycystic ovary syndrome in Han Chinese population. Gynecol Endocrinol. 2014;31(1):1–4. doi: 10.3109/09513590.2014.947948. [DOI] [PubMed] [Google Scholar]
- 40.2018. CYP1A1 Gene [database on the Internet]. Human gene databases.https://www.genecards.org/cgi-bin/carddisp.pl?gene=CYP1A1 Available from: [Google Scholar]
- 41.2018. NCBI. CYP21A2 cytochrome P450 family 21 subfamily A member 2 [Homo sapiens (human)] [Google Scholar]
- 42.D-VM Settas N., Dastamani A., Katsikis I., Chrousos G., Panidis D., Dacou-Voutetakis C. CYP21A2 mutations in women with polycystic ovary syndrome (PCOS) Horm Metab Res. 2013;45(5):383–386. doi: 10.1055/s-0033-1333727. [DOI] [PubMed] [Google Scholar]
- 43.2018. CYP3A7 cytochrome P450 family 3 subfamily A member 7 [Homo sapiens (human)] [database on the Internet]https://www.ncbi.nlm.nih.gov/gene/1551 Available from: [Google Scholar]
- 44.Mark O., Goodarzi N.X., Ricardo Azziz. Association of CYP3A7*1C and serum dehydroepiandrosterone sulfate levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;93(7):2909–2912. doi: 10.1210/jc.2008-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.jsea jia-lijin. Association between CYP19 gene SNP rs2414096 polymorphism and polycystic ovary syndrome in chinese women. BMC Med Genet. 2009;10(139) doi: 10.1186/1471-2350-10-139. 2009 5 august. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.2018. CYP19A1 cytochrome P450 family 19 subfamily A member 1 [Homo sapiens (human)] [database on the Internet]https://www.ncbi.nlm.nih.gov/gene/1588 Available from. [Google Scholar]
- 47.Serdar E., Bulun S.S., Takayama Kazuto, Suzuki Takashi, Sasano Hironobu, Shozu Makio. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem. 2003;86(3-5):219–224. doi: 10.1016/s0960-0760(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 48.Tsuchiya Norihiko. Impact of IGF-I and CYP19 gene polymorphisms on the survival of patients with metastatic prostate cancer. J Clin Oncol. 2006;24(13):1982–1989. doi: 10.1200/JCO.2005.02.9439. [DOI] [PubMed] [Google Scholar]
- 49.Su-Jun Lee W.-Y.K., Choi Ji-Yeob. Sang Seop Lee and Jae-Gook Shin1. Identification of CYP19A1 single-nucleotide polymorphisms and their haplotype distributions in a Korean population. J Hum Genet. 2010;55:189–193. doi: 10.1038/jhg.2010.6. [DOI] [PubMed] [Google Scholar]
- 50.Galletly C., Aea C. A group program for obese infertile woman; weight loss and improved psychological health. J Psychosom Obstet Gynaecol. 1996;17:125–128. doi: 10.3109/01674829609025672. [DOI] [PubMed] [Google Scholar]
- 51.poehlman E.T. Effects of resistance training and endurance training on insulin sensitivity in nonobese young woman a control randomized trial. J Clin Endocrinol Metab. 2000;85:2463–2468. doi: 10.1210/jcem.85.7.6692. [DOI] [PubMed] [Google Scholar]
- 52.Fauser C.J.M. B. Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome (PCOS) Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]