Abstract
Background
Wild type pigs express several carbohydrate moieties on their cell surfaces that differ from those expressed by humans. This difference in profile leads to pig tissue cell recognition of human blood cells causing sequestration, in addition to antibody mediated xenograft injury. One such carbohydrate is N-glycolylneuraminic acid (Neu5Gc), a sialic acid molecule synthesized in pigs but not in humans. Here we evaluate livers with and without Neu5Gc in an ex vivo liver xeno perfusion model.
Methods
Livers from pigs with an α1,3-galactosyl transferase gene knockout (GalTKO), and transgenic for human membrane cofactor (hCD46) with (n=5) or without (n=7) an additional Neu5Gc gene knock out (Neu5GcKO) were perfused ex vivo with heparinized whole human blood. A drug regimen consisting of a histamine inhibitor, thromboxane synthase inhibitor, and a murine anti-human GPIb-blocking antibody fragment was given to half of the experiments in each group.
Results
Liver function tests (AST and ALT) were not significantly different between livers with and without the Neu5GcKO. GalTKO.hCD46.Neu5GcKO livers had less erythrocyte sequestration as evidenced by a higher mean hematocrit over time compared to GalTKO.hCD46 livers (p=0.0003). The addition of Neu5GcKO did not ameliorate profound thrombocytopenia seen within the first 15 minutes of perfusion. TXB2 was significantly less with the added drug regimen (p=0.006) or the presence of Neu5GcKO (p=0.017).
Conclusions
The lack of Neu5Gc expression attenuated erythrocyte loss but did not prevent profound early onset thrombocytopenia or platelet activation, although TXB2 levels were decreased in the presence of Neu5GcKO.
Keywords: N-glycolylneuraminic acid, ex vivo perfusion, liver xenotransplantation, sialic acids
Introduction
The alpha1,3-galactosyltransferase gene knockout (GalTKO) attenuates hyperacute rejection mechanisms driven by anti-Gal antibodies1. However this genetic modification alone has not enabled successful long-term survival in preclinical xenotransplantation models. In addition to terminal alpha1,3-galactose residues, pigs express several carbohydrate moieties on their cell surfaces that are recognized by human preformed antibodies leading to graft injury and conversely different carbohydrates on human cells are recognized by resident pig tissue cells leading to cellular sequestration2,3. One such carbohydrate is N-glycolylneuraminic acid (Neu5Gc), a sialic acid molecule synthesized in pigs but not in humans4. This difference in carbohydrate expression5 appears to contribute to anemia and thrombocytopenia seen in porcine liver xenotransplantation models6–8.
The cytidine monophosphate-N-acetylneuramic acid hydroxylase (CMAH) enzyme catalyzes conversion of N-acetylneuraminic acid (Neu5Ac) to Neu5Gc in most mammals, including pigs and baboons. However, due to an evolutionary mutation resulting in loss of function of CMAH, human cells express only Neu5Ac9. Wild type pig liver cells including endothelial cells and Kupffer cells recognize human Neu5Ac as foreign, triggering cell adhesion and scavenging mechanisms that contribute to thrombocytopenia and anemia. By removing the Neu5Gc antigen from pigs and leaving only Neu5Ac porcine cells should no longer identify human cells as foreign, eliminating a graft versus host type effect. Additionally, humans have pre-formed antibodies to Neu5Gc resulting in graft injury10. Reduced binding of human antibodies to GalTKO.Neu5Gc knock out (Neu5GcKO) pig tissues and red blood cells has been shown11,12. Especially promising is the observation that solid organs from GalTKO.hCD46.Neu5GcKO pigs should have reduced human antibody binding13, and this has proven true on porcine bioprosthetic heart valves prepared similarly to those used clinically for valve replacements14.
In addition, phagocytosis of human platelets has been shown by porcine hepatocytes and liver sinusoidal endothelial cells in both in vitro and ex vivo models6,15. While several other recognition pathways have been implicated in platelet sequestration, recent work has shown a decrease in platelet consumption ex vivo when Neu5GcKO livers were perfused with washed human platelets16. By disabling the CMAH gene and thus removing the Neu5Gc antigen from pigs, the Neu5Ac on human cells should no longer be identified as foreign by porcine cells. Consequently we postulated that CMAH KO in the donor pig would decrease macrophage and endothelial cell recognition of human erythrocytes and platelets and lead to reduced cell loss.
As non-human primates (NHPs) express Neu5Gc and not Neu5AC, the effect of the CMAH KO modification in pigs cannot be studied in NHP models. Here we report the initial use of a modified pig-to-human ex vivo xenoperfusion model to study the effects of genetically engineered Neu5GcKO porcine livers on multiple blood cell lineages in whole human blood.
Materials and Methods
Animals
Ten pigs genetically engineered with α1,3-galactosyl transferase gene knock out and human membrane cofactor (GalTKO.hCD46), and 5 pigs with an additional N-glycolylneuraminic acid knock out (GalTKO.hCD46.Neu5GcKO) were obtained from Revivicor Inc (Blacksburg, VA). All pigs were of blood type O. The Neu5GcKO phenotype associated with each genotype was confirmed by flow cytometry of peripheral blood mononuclear cells (PBMC) and cultured porcine aortic endothelial cells (pAECs) from individual pigs (see below). All animal protocols were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
Cell culture
Porcine aortic endothelial cells were isolated from GalTKO.hCD46.Neu5GcKO experiments (n=4) and GalTKO.hCD46 historical controls (n=3) via scraping and plated on 0.5% gelatin coated culture flasks (Sigma-Aldrich, St. Louis, MO, USA). Culture media consisted of DMEM (Gibco-Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Atlanta Biological, Lawrenceville, GA, USA), gentamicin, amphotericin B, and endothelial cell growth supplement (Becton Dickinson, San Jose, CA, USA). Human aortic endothelial cells (HAECs) were purchased from Lonza (Allendale, NJ, USA) and cultured in presence of Medium 200 + LSGS Kit supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA).
Flow Cytometry Verification of Neu5GcKO
The above cultured pAECs and PBMCs were assessed for Neu5Gc expression using a Neu5Gc Antibody Kit (BioLegend, San Diego, CA, USA) according to kit instructions. Samples were then read on a BD FACSVERSE (Becton Dickinson, Franklin Lakes, NJ, USA). Acquired readings were analyzed using FlowJo software (National Institutes of Health). Results are expressed as the group mean and SD MFI after subtraction of the isotype control.
Experimental Groups
Reference experiments using human blood in a circuit without a liver (anhepatic controls, n=2), were performed. Three allogenic control experiments using GalTKO.hCD46 pig livers perfused with blood from GalTKO.hCD46 porcine blood donors were performed. Twelve experiments using GalTKO.hCD46 porcine livers (n=7) and GalTKO.hCD46.Neu5GcKO porcine livers (n=5) were perfused with human blood (Table 1).
Table 1:
Experimental groups by liver genotype and drug regimen used
| Group | Experiment | Duration (min) | Liver Genotype | Perfusion Blood Treatment |
|---|---|---|---|---|
| Anhepatic controls | C1 | 480* | Anhepatic circuit | Famotidine, BIA |
| C2 | 900* | Anhepatic circuit | Famotidine, BIA, αGPIb | |
|
Allogenic controls (porcine blood) |
A1 | 480* | GalTKO.hCD46 | Famotidine, BIA, αGPIb |
| A2 | 480* | GalTKO.hCD46 | Famotidine, BIA, αGPIb | |
| A3 | 660* | GalTKO.hCD46 | Famotidine, BIA, αGPIb | |
| Group 1A | 816−11 | 115 | GalTKO.hCD46 | Famotidine, BIA, αGPIb |
| 850−12 | 597 | GalTKO.hCD46 | Famotidine, BIA, αGPIb | |
| 850−06 | 202 | GalTKO.hCD46 | Famotidine, BIA, αGPIb | |
| Group 1B | 850−04 | 208 | GalTKO.hCD46 | |
| 851−05 | 600 | GalTKO.hCD46 | ||
| 857−03 | 195 | GalTKO.hCD46 | ||
| 857−04 | 600 | GalTKO.hCD46 | ||
| Group 2A | 727−01 | 125 | GalTKO.hCD46. Neu5GcKO |
Famotidine, BIA, αGPIb |
| 726−04 | 480 | GalTKO.hCD46. Neu5GcKO |
Famotidine, BIA, αGPIb | |
| Group 2B | 720−01 | 160 | GalTKO.hCD46. Neu5GcKO |
|
| 724−02 | 240 | GalTKO.hCD46. Neu5GcKO |
||
| 724−01 | 480 | GalTKO.hCD46. Neu5GcKO |
Experiments were electively terminated
Liver Procurement
Pigs between 20 and 30 kg were sedated with intramuscular ketamine (10–15mg/kg; Midwest Veterinary Supply, Lakeville, MN, USA) and xylazine (1–2.2mg/kg; Midwest Veterinary Supply, Lakeville, MN, USA) and kept under inhalation anesthesia (isoflurane 1–4%) throughout the procedure. Liver harvest was completed under aseptic conditions via a midline laparotomy. The aorta and vena cava were isolated. Heparin (1000IU/kg; Midwest Veterinary Supply, Lakeville, MN, USA) was given intravenously, followed by standard clinical cannulation of the infrarenal aorta. The liver was flushed with cold (4°C) UW solution (Organ Recovery Systems, Chicago, IL, USA) and ice was placed into the abdominal cavity while the supraceliac aorta was cross clamped and the suprahepatic vena cava was vented. The liver was removed with care to preserve the celiac artery with an aortic Carrel patch, common bile duct, portal vein at its origin, and suprahepatic vena cava above the diaphragm. On the back table the liver was flushed via the portal vein with additional UW solution at 4°C. The infrahepatic vena cava was tied, and cannulas were placed into the suprahepatic vena cava, celiac artery, portal vein, and common bile duct. The liver was stored in perfusion solution on ice until ex vivo perfusion. Two experiments in each of the GalTKO.hCD46 and GalTKO.hCD46.Neu5GcKO xenogeneic groups included donor animal pretreatment with desmopressin (DDAVP) (Diamondback, Scottsdale, AZ, USA), to deplete endothelial cell von Willebrand factor. An intravenous dose (3μg/kg) was given the day prior to surgery and the day of surgery immediately prior to organ procurement.
Blood Collection
Human blood was obtained from type A or O healthy volunteers into citrated blood bags (Terumo Medical corporation, Somerset, NJ, USA) according to University of Maryland School of Medicine Institutional Review Board-approved protocols and used within four hours of collection. For allogenic controls fresh whole porcine blood was collected on the day of perfusion into citrated bags. Additional whole blood was collected prior to the day of perfusion and centrifuged to yield platelet poor plasma which was frozen and stored. This fresh frozen porcine plasma was used in allogenic experiments.
Blood Treatment and Drug Regimen
All human blood was diluted with blood type compatible human plasma (Indiana Blood Center, Indianapolis, IN, USA) in a 1:1 ratio, and all porcine blood for allogenic experiments was also diluted in a 1:1 ratio. The blood was heparinized (7U/L) (Midwest Veterinary Supply), recalcified with calcium chloride (8.8mmol) (Midwest Veterinary Supply) to reverse citrate, and treated with a prostaglandin I2 analogue as a vasodilator (Remodulin 2mg/hr, United Therapeutics, Silver Spring, MD, USA). Based on extensive prior work in a pig-to-human lung xeno perfusion model, we presumptively adopted a “complete drug regimen” targeting proinflammatory (thromboxane, histamine) and adhesive (GPIb) pathways in some experiments. Experiments using the complete drug regimen (Groups 1A and 2A) additionally received a histamine blocker (Famotidine 12mg/unit of blood, Midwest Veterinary Supply), thromboxane synthase inhibitor (BIA 120mg/unit of blood) (Sigma-Aldrich), and a murine anti-human GPIb-blocking antibody (6B4) fragment (αGPIb) (Fab, 10mg/unit of blood, provided by H. Deckmyn, Laboratory for Thrombosis Research, University of Leuven, Belgium)17 (see Table 1). One anhepatic reference experiment did not receive αGPIb due to transient drug unavailability. During circuit perfusion continuous heparin infusion was titrated to maintain a target activated clotting time (ACT) > 400 seconds. Continuous insulin (Midwest Veterinary Supply) infusion was titrated based on glucometer readings. Sodium bicarbonate (Midwest Veterinary Supply) boluses and/or continuous infusion and CO2 gas flow rate to the oxygenator were titrated based on serial arterial and venous reservoir blood gas measurements (Medtronic, Edgewater, MD, USA).
Liver Perfusion
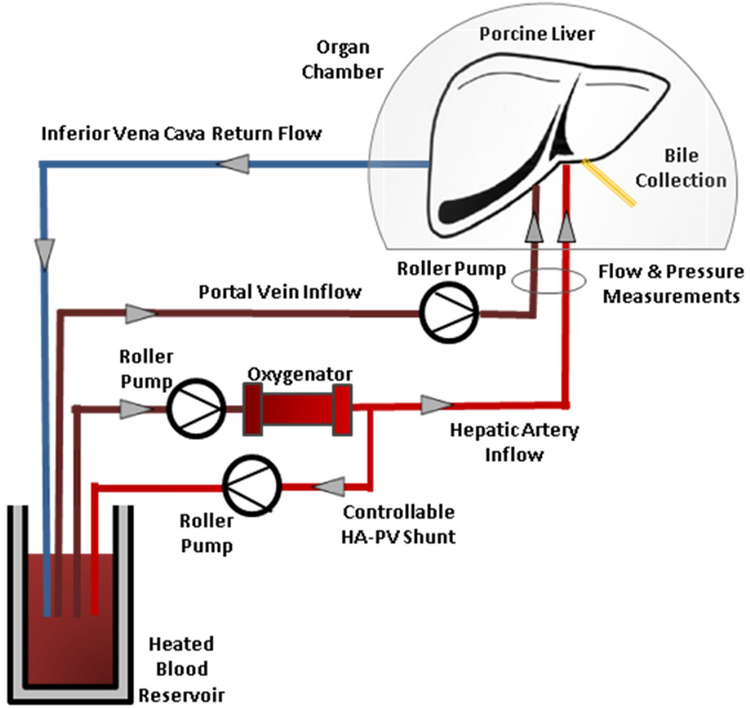
After storage at 4°C livers were flushed with 500mL of room temperature Hetastarch (Midwest Veterinary Supply) to remove preservation solution and then perfused with human blood. The ex vivo perfusion circuit used is illustrated in Figure 1. Briefly, blood was placed in a heated 37°C water-jacketed reservoir. Blood from the reservoir was pumped through an oxygenator (Dideco neonatal oxygenator, Sorin Group, Arvada, CO, USA) then pumped both through the hepatic artery and a shunt back to the reservoir. Blood from the reservoir, partially oxygenated by the circuit shunt, was pumped through the portal vein and returned to the reservoir via the suprahepatic vena cava. Portal vein flow was adjusted to maintain a portal venous pressure of 5–10mmHg and hepatic artery flow was adjusted to maintain a mean arterial pressure of 60–80mmHg, corresponding to acceptable physiologic human ranges for central venous pressure and mean arterial pressure respectively, and reflecting parameters used in prior xenoperfusion studies. Pressure in the IVC was not measured as the reservoir was an open system. Portal vein and hepatic artery pressure, flow, and calculated resistance were continuously recorded using The Digimed System Integrator (Micro-Med, Louisville, KY, USA) and LabChart 7 Pro software (AD Instruments, Colorado Springs, CO, USA). Resistance was calculated by dividing the measured pressure by the flow rate. Xenogeneic perfusions were terminated when elevated pressure and resistance led to lack of flow through either the portal vein or hepatic artery (flow rate of zero), or if there was uncorrectable acidosis, hyperkalemia, or other metabolic derangements. “Survival” was defined as the time from liver reperfusion to termination of perfusion using the criteria listed above. Anhepatic and allogenic perfusions were electively terminated.
Figure 1: Ex vivo perfusion circuit.
Blood is stored in a heated jacketed reservoir at physiologic temperature. Blood is pumped through an oxygenator and the outflow split to provide oxygenated blood to the liver via the hepatic artery and back to the reservoir via a shunt. The partially oxygenated blood in the reservoir is pumped into the portal vein. Flow and pressure measurements of the arterial and venous systems are obtained and recorded. Outflow is collected from the suprahepatic vena cava and returned by gravity to the reservoir.
Sampling
Blood samples were obtained from the reservoir prior to initiation of perfusion (pre), and from the venous return at 5, 15, 30, 60, 120, 240, 360, and 480 minutes, and at termination of perfusion. Blood was circulated through the system for 5 minutes before placing the liver in line and this sample was identified as time zero.
Hematologic Analysis
Peripheral blood leukocyte, neutrophil, hemoglobin, hematocrit, and platelet levels were measured by hemocytometer (Hemavet, Drew Scientific, Miami Lakes, FL, USA) and confirmed via commercial laboratory (Antech, Rockville, MD, USA). Measurement of plasma electrolyte and liver function test values was also performed by commercial laboratory (Antech).
βTG and TXB2 Assays
For βTG, blood perfusate was collected in CTAD vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA) after breaking the vacuum and left on ice for 20 min until centrifugation at 1430g for 20 minutes. For TXB2, blood perfusate was collected in EDTA vacutainer tubes (Becton Dickinson) supplemented with meclofenamate (10μg/mL) (Sigma-Aldrich) and immediately centrifuged at 514g for 15 minutes. Plasma was collected and stored at −80C until assayed. Assays for beta-thromboglobulin (βTG; Asserachrom β-TG, Diagnostica Stago, Asnieres, France) and thromboxane B2 (TXB2; TXB2 ELISA Kit, Cayman Chemicals, Ann Arbor, MI, USA) were performed according to manufacturer’s instructions. The normal reference ranges for human βTG and TXB2 are <50 IU/mL and 1–2 pg/mL respectively.
P-selectin measurement
Expression of CD62P was measured on fixed platelets by flow cytometry as described previously18.
Histology
Liver tissue samples were obtained prior to perfusion (pre) and at 10, 30, 60, 120, 240, 360, 480 minutes, and at termination of perfusion. Both core needle and peripheral wedge biopsies were obtained. Samples were formalin fixed and paraffin embedded. Tissue blocks were cut to make slides for light microscopy and stained with hematoxylin and eosin.
Statistical Analysis
All data are presented as mean and standard error of the mean unless otherwise noted. For time points up to four hours where groups sizes were greater or equal to n=3, two tailed t-tests were used to compare groups, with a p value <0.05 considered significant (GraphPad Prism software, GraphPad, Inc., La Jolla, CA, USA). For later time points and groups with smaller sizes where group size was not consistent and values were not available for all time points, mixed effects modeling was used to analyze change over time and examine the effects of the drug regimen (SAS 9.4, R Studio). One experiment in the GalTKO.hCD46 group, 857–04 (Group 1B), had evidence of liver injury manifested by atypically high AST and ALT values for unclear reasons and was censored from all analysis.
Results
Neu5Gc Expression
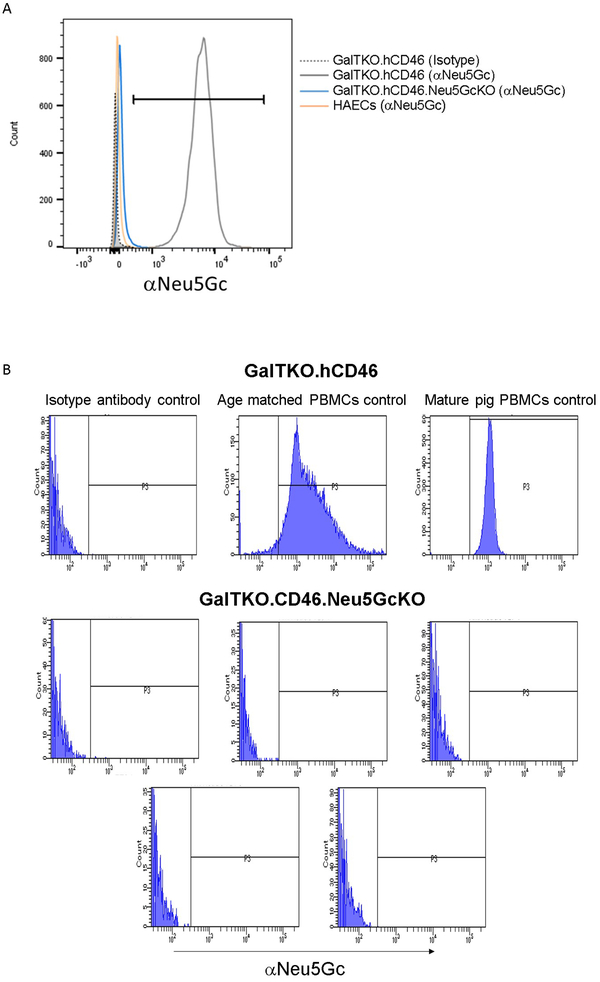
Human aortic endothelial cells stained minimally for Neu5Gc (Figure 2A), likely as a result of culture media containing fetal bovine serum. Cultured porcine aortic endothelial cells from GalTKO.hCD46 pigs stained strongly for Neu5Gc (ΔMFI vs. isotype control: 17981 ±11389) compared to absent or trace staining (−6.4±93) in GalTKO.hCD46.Neu5GcKO cells. Complete absence of Neu5Gc expression was confirmed on PBMCs isolated from GalTKO.hCD46.Neu5GCKO pigs (Figure 2B).
Figure 2: Neu5Gc expression.
Expression of Neu5Gc was assessed on human and pig cells by flow cytometry. A. Human aortic endothelial cells (orange) stained minimally for Neu5Gc (likely from culture media containing fetal bovine serum. Cultured porcine aortic endothelial cells from GalTKO.hCD46 pigs (gray) stained strongly for Neu5GCc compared minimal staining in GalTKO.hCD46.Neu5GcKO endothelial cells (blue). B. Pattern of Neu5Gc expression on peripheral blood mononuclear cells (PBMCs) from GalTKO.hCD46 pigs as a function of pig age; absence of Neu5GcKO expression in PBMCs from GalTKO.hCD46.Neu5GcKO pigs.
Graft Survival and Functional Parameters
Successful perfusion was achieved in all experiments as evidenced by physiologic vascular pressures and flows, bile production, and potassium normalization over the first hour (Figure S1A). The production of ascites was noted by all livers, and grossly experiments did not appear to vary in amount produced, but this amount was not quantified. Anhepatic and allogeneic perfusions demonstrated portal vein and hepatic artery pressures consistently within normal physiologic ranges. In the xenogeneic perfusions pressures of both the hepatic artery and portal vein were initially maintained within physiologic ranges until approximately thirty minutes prior to termination when both arterial and portal resistance increased and potassium increased (Figure S1B). There was no significant difference observed in the xenogeneic experiments by liver phenotype or treatment group in terminal physiologic behavior. The mean survival was similar in the GalTKO.hCD46 (319 minutes, [standard error of the mean (SEM) 89, range 115–600]) and GalTKO.hCD46.Neu5GcKO group (297 minutes, SEM 77, range 125–480), and not obviously influenced by additional drug treatment.
Transaminase Elaboration
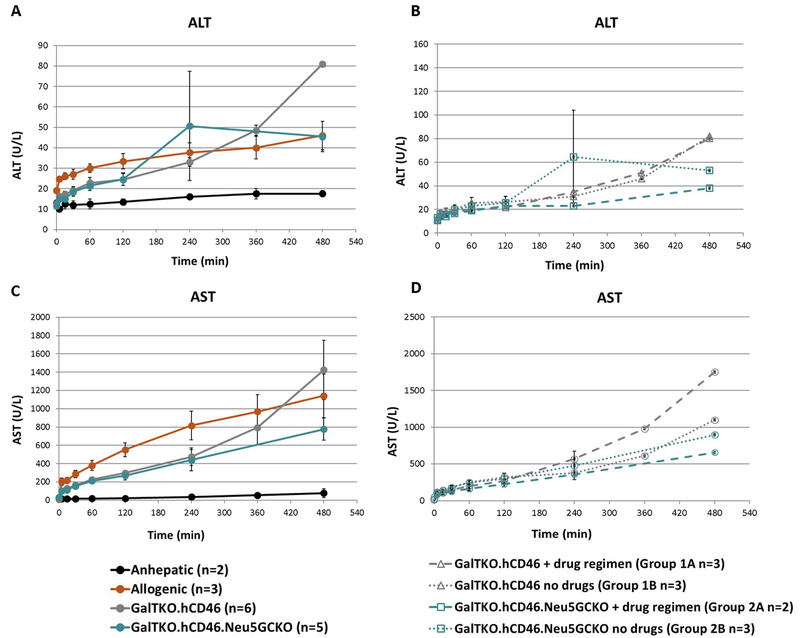
Alanine transaminase (ALT) levels trended up but remained within the normal range in all groups (Figure 3A). There was no significant difference in ALT values in association with xenogeneic liver phenotype or drug regimen (Figure 3B). Aspartate transaminase (AST) levels increased progressively over the perfusion interval in all experiments (Figure 3C) and were not significantly attenuated in association with drug treatment or Neu5Gc phenotype (Figure 3D).
Figure 3: Transaminase elaboration.
A. Measured ALT values comparing GalTKO.hCD46 and GalTKO.hCD46.Neu5GcKO livers to anhepatic controls. ALT levels in all experiments were within the normal range and there was no significant difference in ALT values between the two xenogeneic groups. B. Subgroup analysis of ALT values within xenogeneic experiments based on addition of drug regimen. Within the GalTKO.hCD46 group there was no difference based on drug regimen (Groups 1A and 1B) and within the GalTKO.hCD46.Neu5GcKO group where was no difference based on drug regimen (Groups 2A and 2B). C. AST levels rose well above normal range in all xenogeneic experiments. D. Subgroup analysis of AST values within xenogeneic experiments based on addition of drug regimen. Within the GalTKO.hCD46 group there was no difference based on drug regimen (Groups 1A and 1B) and within the GalTKO.hCD46.Neu5GcKO group where was also no difference based on drug regimen (Groups 2A and 2B). Of note due to different perfusion lengths standard error could not be calculated after 4 hours.
Hematocrit
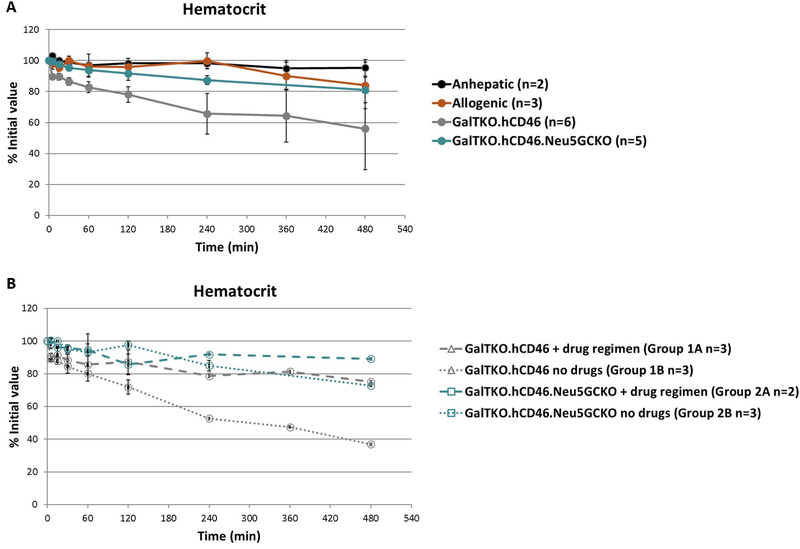
The anhepatic circuit showed a minimal decrease in hematocrit over 8 hours of perfusion. The average hematocrit in the allogenic group after 8 hours was 84% of the initial value. The hematocrit of the GalTKO.hCD46.Neu5GCKO group showed a similar small decrease to 81% of the initial value at 8 hours. In contrast, the GalTKO.hCD46 experiments exhibited a progressing decrease in hematocrit over the course of the perfusion with an 8 hour average of 56% of the initial (Figure 4A). Using a mixed-effects model to estimate the difference in the mean level of hematocrit over time the GalTKO.hCD46.Neu5GcKO group tended to higher average mean hematocrits than the GalTKO.hCD46 group (P =.0003); and the rate of change in hematocrit over time is larger in the GalTKO.hCD46 group (−0.08) than in the GalTKO.hCD46.Neu5GcKO group (−0.04) (difference 0.04, SEM 0.01, and 95% CI −0.069,−0.015; P=.0003). The drug regimen was not associated with a significant difference in hematocrit for either xeno liver phenotype (Figure 4B).
Figure 4: Hematocrit levels.
A. The anhepatic circuit showed a minimal decrease in hematocrit. All xenoperfusions showed a decrease in hematocrit over the course of the perfusion. The decrease in hematocrit of the GalTKO.hCD46 group was more than that of the GalTKO.hCD46.Neu5GcKO group (p=0.0003). B. Subgroup analysis based on drug regimen showed no difference in the GalTKO.hCD46.Neu5GcKO group (Groups 2A and 2B). The GalTKO.hCD46 group with the drug regimen (Group 1A) showed improvement over the group without (Group 1B). Of note due to different perfusion lengths standard error could not be calculated after 4 hours.
Lactate Dehydrogenase
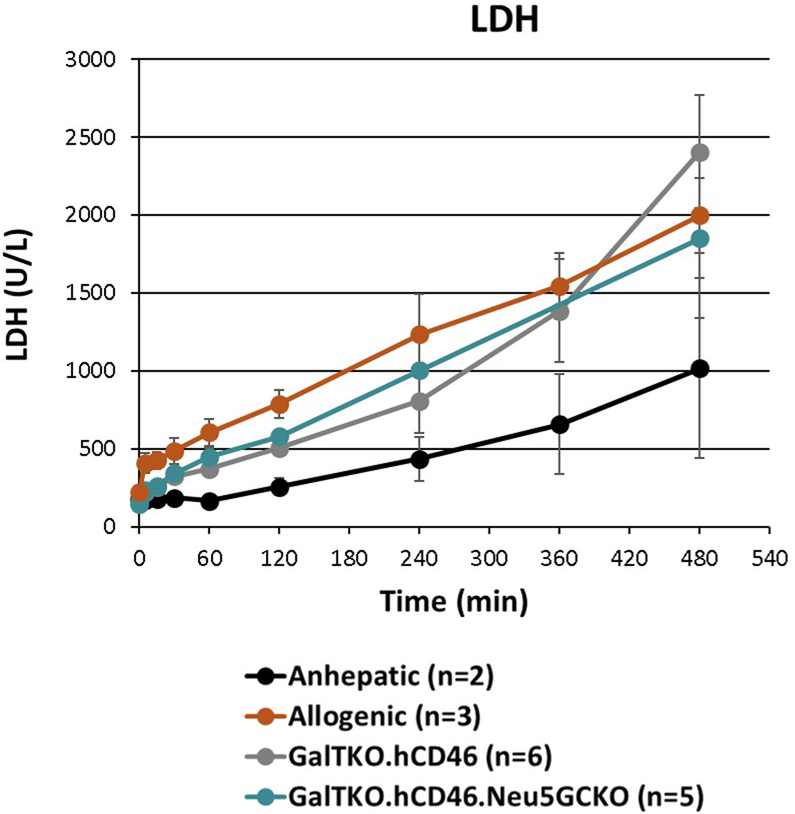
Lactate Dehydrogenase levels increased above normal in the anhepatic controls, but increased twofold to threefold higher in the allogenic and xenogeneic perfusions (Figure 5) with no difference observed between genotypes.
Figure 5: LDH levels.
LDH levels were noted to increase in all experiments. An increasing in LDH over time in the anhepatic experiments was likely due to hemolysis from the system. The xenoperfusions showed increased levels of LDH release pointing to increased red blood cell and hepatocyte injury in these experiments compared to the anhepatic control.
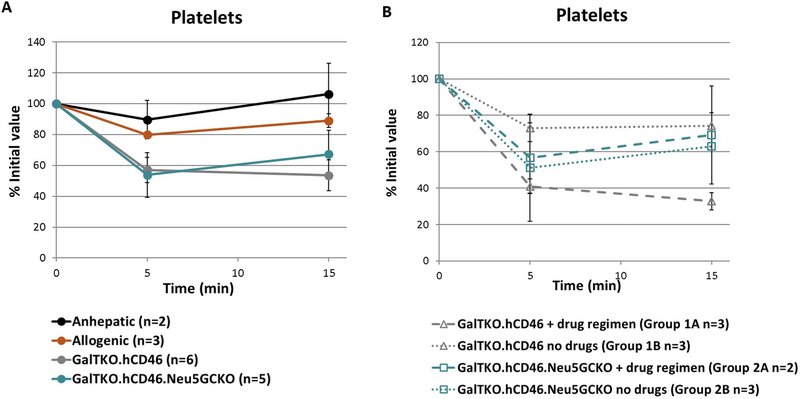
Platelet Count
Marked thrombocytopenia was noted within 5 minutes in all xeno liver perfusion experiments regardless of liver Neu5Gc phenotype, whereas platelet counts remained relatively stable in the anhepatic and allogeneic experiments (Figure 6A). Significantly lower platelet counts were associated with drug treatment in GalTKO.hCD46 liver perfusions (P=.0083, Group 1A vs 1B at 15 min), but not with GalTKO.hCD46.Neu5GcKO livers (Figure 6B).
Figure 6: Platelet counts.
A. Marked thrombocytopenia was noted within 5 minutes in all xenoperfusions while platelet counts remained stable in the anhepatic controls. B. Subgroup analysis showed no difference with or without the drug regimen in the GalTKO.hCD46.Neu5GcKO cohort (Groups 2A and 2B). Platelet counts were lower in the GalTKO.hCD46 group with the drug regimen compared to without (Groups 1A and 1B) (p=0.0083).
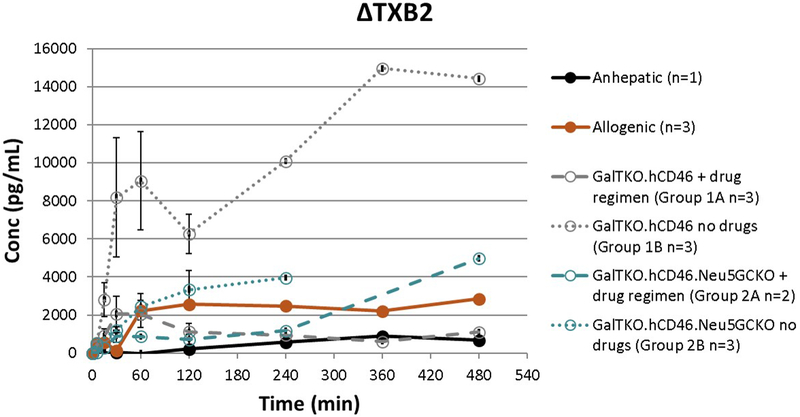
TXB2 Quantification
Prolific TXB2 elaboration, reported as measured TXB2 minus baseline level (ΔTXB2), in all xenoperfusions was significantly attenuated by drug treatment that included a thromboxane synthase inhibitor in the GalTKO.hCD46 group throughout the duration of perfusion (P=.006, Group 1A vs 1B). The addition of Neu5GcKO also showed a significant decrease in ΔTXB2 in the absence of the drug regimen throughout the duration of perfusion (P=.017, Group 1B vs 2B) (Figure 7). The anhepatic and allogeneic experiments showed some TXB2 elaboration as well, though much lower than the untreated xeno groups.
Figure 7: ΔTXB2 elaboration.
TXB2 levels, expressed as the change from time zero (ΔTXB2), increased in all xenoperfusions to much higher than the normal range. The addition of the drug regimen to the GalTKO.hCD46 group showed a significant decrease in ΔTXB2 (p=0.006). The addition of the Neu5GcKO phenotype in the absence of the drug regimen also had a significantly lower level of ΔTXB2 (p=0.017). Of note due to different perfusion lengths standard error could not be calculated after 4 hours.
Platelet Activation
Beta-thromboglobulin elaboration was prolific and higher, though not significantly so, in association with Neu5GcKO without drug treatment relative to GalTKO.hCD46, an effect that tended to be attenuated by drug treatment with or without Neu5Gc expression, although βTG elaboration increased over perfusion time in all groups (Figure S1C). P-selectin expression by circulating platelets increased minimally over time as compared to baseline (Δ <10% in all perfusions). The percentage of platelets expressing P-selectin increased more steadily after two hours of blood circulation in the anhepatic experiments. The GalTKO.hCD46 experiments all had low levels of expression throughout (<2%); the GalTKO.hCD46.Neu5GcKO without drug regiment group had a spike in CD62P expression at 15 minutes, but otherwise was similarly low throughout (Figure S1D).
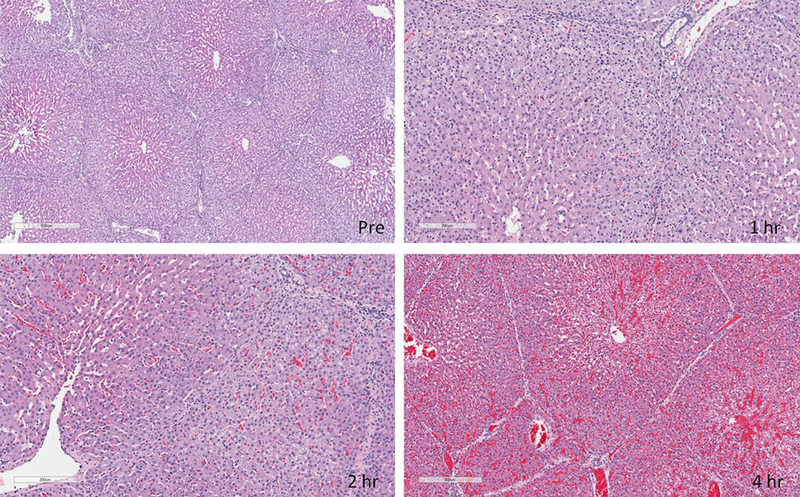
Histology
Pre-perfusion biopsies showed normal liver architecture in all experiments. Progressive periportal vacuolization extending towards the central veins and edema was noted starting at 1 hour and progressing through perfusion. Sinusoidal spaces became less clear starting around 2 hours. At about 4 hours there were areas of red blood cell congestion and hemorrhage (Figure 8). By the end of perfusion there were areas of apoptosis and centrilobular necrosis. These findings were similar in GalTKO.hCD46 and GalTKO.hCD46.Neu5GcKO livers.
Figure 8: H&E staining of liver samples.
Pre-perfusion biopsies showed normal liver architecture in all experiments. Progressive periportal vacuolization extending towards the central veins and edema was noted starting at 1 hour and progressing through perfusion. Sinusoidal spaces became less clear starting around 2 hours. At about 4 hours there were areas of red blood cell congestion and hemorrhage.
Discussion
Up until recently work in xenotransplantation has taken advantage of a small pool of genetic modifications focused on modulating the deleterious effects associated with complement, coagulation and inflammation. While these have been helpful in attenuating hyperacute rejection, issues with platelet sequestration, anemia, and coagulation dysregulation persist19, and are particularly prominent for the liver17,20,21. With emerging genome editing techniques it is possible to rapidly generate pigs with a larger number of deleted, “humanized”, or added genes. Meanwhile, evaluation of outcomes associated with GalTKO pig organs enables the study of the effects of “non-Gal” antigens such as Neu5Gc and β4Gal. Assessing the value of grafts and tissues with these novel genetics depends on the ability of models to test them under clinically relevant conditions. In vivo pig to baboon transplant models are inadequate to test the CMAH KO modification as baboons and pigs both express CMAH and thus Neu5Gc22. In addition, an extensive screen of nonhuman primates suggests that they are inappropriate models because porcine macrophages do not bind nonhuman primate cells as they do human cells5, a phenomenon at least partially attributable to Neu5Gc-mediated mechanisms.
Here we show that the CMAH gene KO was associated with a significant decrease in red blood cell sequestration in the liver model, supporting our hypothesis that Neu5GcKO porcine livers would demonstrate reduced human erythrocyte binding. It has been shown that porcine Kupffer cells and human red blood cells interact via recognition of sialic acid by sialoadhesin. Specifically, Rees et al first demonstrated that porcine Kupffer cells bound human red blood cells and postulated it was due to lectin-carbohydrate binding23. They stated “perhaps the receptor on the surface of the macrophage inadvertently recognizes the altered glycosylation pattern on the xenogeneic cell”. This was validated by Burlak et al who showed in a series of in vitro experiments that porcine recognition of human red blood cells was decreased by de-sialation or addition of free Neu5Ac suggesting the importance of this recognition pathway. Additionally, while pigs do express both Neu5Gc and Neu5Ac, it is principally the Neu5Gc form that is found on their red blood cells24. Looking at the role of porcine sialoadhesin on Kupffer cells, Brock et al confirmed that blocking porcine sialoadhesin led to reduced human erythrocyte binding in vitro25 and this was subsequently confirmed in ex vivo models26. The porcine livers in our experiments, and by extension the Kupffer cells present in them, indirectly appeared to demonstrate reduced recognition of human red blood cells as evidenced by reduced erythrocyte loss over time. It is known that human macrophages do not bind Neu5Ac on human red blood cells, and we would postulate that a similar explanation exists in our CMAH KO pigs. Given that this genetic manipulation is not a lethal one and our pigs are not anemic the porcine Kupffer cells may not recognize Neu5Ac as foreign leading to a loss of binding. However, we do not have direct evidence that this occurs in our model. In previous studies red blood cell loss was not completely resolved by blockade of porcine sialoadhesin, demonstrating that additional mechanisms of red blood cell sequestration or lysis exist that are not Neu5Gc mediated. Our finding that red blood cell sequestration is decreased in the absence of Neu5Gc and with (though not significantly) the combination of drugs tested here suggests that one or more alternative, Neu5Gc-independent pathways exist for the sequestration of xenogeneic erythrocytes. Once defined these additional pathways of erythrocyte sequestration will likely need to be addressed.
In this study we did not observe relative preservation of platelets in the Neu5GcKO group. Porcine Kupffer cells and liver sinusoidal endothelial cells have been shown to phagocytose human platelets as well as erythrocytes. Several pathways including ASGR1 and CD18-receptor mediated recognition of human platelets by porcine cells have been identified and may account for Neu5Gc-independent sequestration of platelets in our model27,28. Additionally, alteration of sialic acid profiles on platelet oligosaccharides likely contributes to thrombocytopenia in porcine xenotransplantation; however an exact role for Neu5Gc has not been explained29. Interestingly, and somewhat surprisingly, Butler et al16 showed that GalTKO.Neu5GcKO pig livers sequestered significantly fewer platelets than GalTKO livers. One major difference in our study is the use of whole human blood as opposed to washed platelets. Clearly there are multiple signaling pathways that contribute to platelet sequestration, and the use of washed platelets instead of whole blood would remove important known mediators, in particular Fc-mediated- and coagulation cascade mediated-platelet binding. Specifically, when looking at sialic acid profiles and their effects it is important to note that sialidases are found both on various cell types as well as non-cell bound in plasma30. Isolated platelets would be expected to act differently than whole blood, therefore it is not surprising to us that our results differ from those previously reported. We infer that ultimately solving thrombocytopenia will require a multifaceted approach of both pharmacologic and genetic interventions tested in a stringent model such as ours that simulates clinical application by using whole human blood to perfuse the pig organ.
We found that a steep decline in platelet numbers occurred in all xenogeneic experiments within the first 5 minutes, but that after one hour platelet counts appeared to increase over time in all groups (data not shown). In these experiments and in parallel lung perfusion studies we find that, after the first hour of a xenogeneic perfusion, automated hemocytometer data becomes unreliable in reporting platelet counts due to the presence of erythrocyte and other cell fragments that are similar in size to platelets (manuscript in preparation). Flow cytometry data reveals that while apparent platelet recovery beyond one hour is ephemeral, values within the first hour are concordant between hemocytometer and flow cytometer data31. For this reason, in this report we have focused only on the first fifteen minutes of perfusion, where the change in platelets is the most substantial, and the hemocytometer platelet data reliable. Platelet counts were unexpectedly lower in the GalTKO.hCD46 group with drug regimen versus without. We speculate that drug treatment may result in improved microvascular perfusion, exposing an increased liver endothelial surface area available for platelet interaction. This effect was not seen with GalTKO.hCD46.Neu5GcKO, demonstrating that this genetic modification is partially protective with respect to platelet sequestration, as predicted by Butler’s previous work with washed platelets. Similarly, βTG elaboration occurring earlier during perfusion in association with Neu5GcKO may paradoxically reflect an indirect consequence of reduced Neu5Gc-mediated erythrocyte phagocytosis, leaving porcine endothelial and Kupffer cells available to bind to and activate human platelets.
Thromboxane B2 is elaborated both by activated platelets and by macrophages, which have the highest levels of thromboxane synthase32. In our study, the addition of the Neu5GcKO in the absence of the drug regimen (Group 1B vs Group 2B) shows benefit in decreasing TXB2 production. Previous work in our lab investigated the effect of anti-GPIb antibody on blocking constitutive activation of human platelets by porcine von Willebrand’s factor in liver and lung models, finding some benefit on platelet sequestration and activation17,18. Thromboxane synthase inhibition (which affects both blood platelets, monocytes, and tissue macrophages) and anti-histamines attenuated lung injury33. When added to our liver model the drug regimen reduced TXB2 release within each genotype (Group 1A vs 1B and 2A vs 2B). Therefore, we conclude that while Neu5GcKO does not have a dramatic independent effect on platelet sequestration, it may assist in reducing platelet activation, as do the combination of GPIb, thromboxane, and histamine. We predict that this effect will be augmented if other known pathways that mediate platelet sequestration and activation are also addressed, in particular ASGR1, de-sialation, and selectins and other adhesive interactions (GPIb, CD18).
We did not see increases in P-selectin expression, which reflects platelet activation, on the platelets that remained circulating in the perfusate, as we expected to based on our lung model. We attribute this difference to relatively avid scavenging of activated platelets by pig liver relative to the lung, given the profound thrombocytopenia that we saw in all liver perfusion experiments. The isolated peak in CD62P expression at 15 minutes in the GalTKO.hCD46.Neu5GcKO group without drug treatment reflects a single, high result in one experiment, likely spurious.
Transaminase elaboration is used clinically to quantify liver injury. Censoring the one experiment that had excessively high AST and ALT values for unknown reasons, there was a significant increase in AST in all experiments, with no difference between the two pig liver genotypes or in associated with the drug regimen. The gradual rise in ALT throughout pig liver perfusions did not exceed normal physiologic levels. The disproportionate rise in AST with levels in the thousands confirms prior observations34. We hypothesize that AST elaboration in part reflects RBC hemolysis as well as hepatocellular injury, an inference supported by elevated LDH levels and an increasing red color of plasma isolates at later experimental time points.
To maximize donor organ utilization, several parallel large animal transplants were performed concurrently. By necessity this did result in relatively small group numbers based on drug regimens. To compensate for this we have addressed this by analyzing experimental subgroups based on drug regimen. In cases in which there is not a difference based on drug regimen we have combined groups based on genotype, and in cases where drug regimen does appear to exert a difference we have commented on these as separate groups. The unfortunate corollary to this practice is small sample sizes in some cases.
Additionally, our perfusion durations were shorter than those previously reported by other groups, raising the concern for technical errors. Recent work has used isolated blood components (washed platelets6,16, or isolated red blood cells26) which as discussed above is different from our whole blood system, and the lack of plasma components likely removes deleterious immunologic and complement mediators. Previous studies of human whole blood perfusion of transgenic human decay-accelerating factor (hDAF) pig livers did achieve 72 hours of perfusion35. In these works the hematocrit was noted to reach a nadir of 5.7% of the initial value at 72 hours. This was not dissimilar to our observed sharp decreases in hematocrit, however over a shorter time period. A potential explanation for this is that due to our lower starting hematocrits, smaller changes in values were magnified when expressed as a percentage remaining. Additionally, metabolic derangements similar to our study, such as acidosis and hyperkalemia, were observed towards the end of these perfusions. Our shorter perfusion durations likely resulted from a combination of excess plasma, lower starting hematocrits, stricter termination criteria, and technical aspects.
Limited clinical application of ex vivo liver perfusion has been reported. Chari et al. published one experience of four patients with irreversible hepatic failure who underwent wild type porcine ex vivo liver perfusions. All four patients demonstrated transient improvement of mental status and biochemical markers, however only one was successfully bridged to allotransplant. In all cases marked thrombocytopenia was observed36. Another small case report using transgenic pigs (CD55/CD59) included two patients who were both bridged to allotransplantation following ex vivo perfusion37. Information regarding platelet and red blood cells counts was not reported. In parallel, large animal pig to non-human-primate in vivo liver transplant studies had been plagued by short survivals attributed to anemia and thrombocytopenia requiring transfusions8,20. However, long term survival (25 days) after GalTKO pig-to-baboon liver xenotransplantation (in the presence of coagulation factor supplementation) has recently been reported38. In this case anemia requiring transfusions was still present, but platelet transfusions were not required and platelet counts recovered spontaneously after eleven days. These results coupled with genetic manipulations leading to decreased platelet consumption during ex vivo porcine liver xenoperfusions, such as asialoglycoprotein receptor 1 knock out39, have reaffirmed the potential of transgenic pig livers for clinical use.
In summary, the addition of Neu5GcKO to GalTKO.hCD46 pig liver xenografts appears to contribute a benefit with respect to erythrocyte biology and TXB2 elaboration, without improving survival, modulating platelet sequestration or activation, or attenuating βTG elaboration. Ultimately we conclude that a combination of additional genetic and pharmacologic strategies will likely be required to achieve successful therapeutic application of liver xenotransplantation.
Supplementary Material
Supplemental Figure 1:
A. Potassium levels were noted to normalize within the first hour in all perfusions. B. Potassium levels rose terminally. C. βTG elaboration was prolific and higher in association with Neu5GcKO without drug treatment relative to GalTKO.hCD46, an effect that was attenuated by drug treatment with or without Neu5Gc expression, although βTG elaboration increased over perfusion time in all groups. Of note due to different perfusion lengths standard error could not be calculated after 4 hours. D. P-selectin expression was <10% in all perfusions. The percentage of platelets expressing P-selectin increased over time in the anhepatic experiment. The GalTKO.hCD46 experiments all had low levels of expression throughout (<2%), and the GalTKO.hCD46.Neu5GcKO with drug regiment group had a spike in expression at 15 minutes (likely due to one spurious value), but otherwise was similarly low throughout.
Acknowledgements:
The authors wish to express their gratitude to the General Clinical Research Center at the University of Maryland for collaboration and support with blood procurement. This work is supported by U19 AI090959 as well as unrestricted gifts from Revivicor and United Therapeutics.
Abbreviations:
- ACT
activated clotting time
- ALT
alanine transaminase
- ASGR1
asialoglycoprotein receptor 1
- AST
aspartate transaminase
- BIA
1-Benzylimidazole
- CMAH
cytidine monophosphate-N-acetylneuramic acid hydroxylase
- DDAVP
desmopressin
- GalTKO
alpha1,3-galactosyltransferase gene-knockout
- GPIb
glycoprotein Ib
- HAECs
human aortic endothelial cells
- hCD46
human membrane cofactor
- hDAF
human decay-accelerating factor
- LDH
lactate dehydrogenase
- Neu5Ac
N-acetylneuraminic acid
- Neu5GcKO
N-glycolylneuraminic acid knock out
- Neu5Gc
N-glycolylneuraminic acid
- NHP
non-human primate
- PAECs
porcine aortic endothelial cells
- SEM
standard error of the mean
- TXB2
thromboxane B2
- αGPIb
murine anti-human GPIb-blocking antibody (6B4) fragment
- βTG
beta-thromboglobulin
Footnotes
Disclosures:
Richard N. Pierson serves on the Scientific Advisory Board of Revivicor. David Ayares is an employee of Revivicor.
References:
- 1.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 2003;299:411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rees MA, Butler AJ, Davies HF, Bolton E, Wight DG, Skepper J, White DJ, Friend PJ. Porcine livers perfused with human blood mount a graft-versus-“host” reaction. Transplantation 2002; 73:1460. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DK. Modifying the sugar icing on the transplantation cake. Glycobiology 2016;26:571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padler-Karavani V, Varki A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation 2011;18(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldman JP, Brock LG, Rees MA. A human-specific mutation limits nonhuman primate efficacy in preclinical xenotransplantation studies. Transplantation 2014;97:385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burlak C, Paris LL, Chihara RK, Sidner RA, Reyes LM, Downey SM, Tector AJ. The fate of human platelets perfused through the pig liver: implications for xenotransplantation. Xenotransplantation 2010;17:350–61. [DOI] [PubMed] [Google Scholar]
- 7.Ekser B, Markmann JF, Tector AJ. Current status of pig liver xenotransplantation. Int J Surg 2015;23:240–6. [DOI] [PubMed] [Google Scholar]
- 8.Kim K, Schuetz C, Elias N, Veillette GR, Wamala I, Varma M, Smith RN, Robson SC, Cosimi AB, Sachs DH, Hertl M. Up to 9-day survival and control of thrombocytopenia following alpha 1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation 2012;19:256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou H-H, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA 1998:95:11751–11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salama A, Evanno G, Harb J, Soulillou JP. Potential deleterious role of anti-Neu5Gc antibodies in xenotransplantation. Xenotransplantation 2015;22:85–94. [DOI] [PubMed] [Google Scholar]
- 11.Burlak C, Paris LL, Lutz AJ, Sidner RA, Estrada J, Li P, Tector M, Tector AJ. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am J Transplant 2014;14:1895–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang ZY, Martens GR, Blankenship RL, Sidner RA, Li P, Estrada JL, Tector M, Tector AJ. Eliminating xenoantigen expression on swine RBC. Transplantation 2017;101:517–23. [DOI] [PubMed] [Google Scholar]
- 13.Lee W, Hara H, Ezzelarab MB, Iwase H, Bottino R, Long C, Ramsoondar J, Ayares D, Cooper DKC. Initial in vitro studies on pig tissues and cells from GTKO/CD46/NeuGcKO pigs. Xenotransplantation 2016;23:137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee W, Long C, Ramsoondar J, Ayares D, Cooper DKC, Manji RA, Hara H. Human antibody recognition of xenogeneic antigens (NeuGc and Gal) on porcine heart valves: could genetically modified pig heart valves reduce structural valve deterioration? Xenotransplantation 2016;23:370–80. [DOI] [PubMed] [Google Scholar]
- 15.Peng Q, Yeh H, Wei L, Enjyoj K, Machaidze Z, Csizmad E, Schuetz C, Lee KM, Deng S, Robson SC, Markmann J, Buhler L. Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells. PLoS One 2012;7:e47273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler JR, Paris LL, Blankenship RL, Sidner RA, Martens GR, Ladowski JM, Li P, Estrada JL, Tector M, Tector AJ. Silencing porcine CMAH and GGTA1 genes significantly reduces xenogeneic consumption of human platelets by porcine livers. Transplantation 2016;100:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaMattina JC, Burdorf L, Zhang T, Rybak E, Cheng X, Munivenkatappa R, Salles II, Broos K, Sievert E, McCormick B, Decarlo M, Ayares D, Deckmyn H, Azimzadeh, AM, Pierson RN III, Barth RN. Pig-to-baboon liver xenoperfusion utilizing GalTKO.hCD46 pigs and glycoprotein 1b blockade. Xenotransplantation 2014;21:274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burdorf L, Riner A, Rybak E, et al. Platelet sequestration and activation during GalTKO.hCd46 pig lung perfusion by human blood is primarily mediated by GPIb, GPIIb/IIIa, and von Willebrand Factor. Xenotransplantation 2016;23:222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azimzadeh AM, Kelishadi SS, Ezzelarab MB, Singh AK, Stoddard T, Iwase H, Zhang T, Burdorf L, Sievert E, Avon C, Cheng X, Ayares D, Horvath KA, Cocoran PC, Mohiuddin MM, Barth RN, Cooper DKC, Pierson III RN. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation 2015;22:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekser B, Long C, Echeverri GJ, Hara H, Ezzelarab MB, Lin CC, de Vera ME, Wagner R, Klein E, Wolf RF, Ayares D, Cooper DKC, Gridelli B. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant 2010;10:273–285. [DOI] [PubMed] [Google Scholar]
- 21.Navarro-Alvarez N, Shah JA, Zhu A, Ligocka J, Yeh H, Elias N, Rosales I, Colvin R, Cosimi AB, Markmann JF, Hertl M, Sachs DH, Vagefi PA. The effects of exogenous administration of human coagulation factors following pig-to-baboon liver xenotransplantation. Am J Transplant 2016;16:1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrne GW, McGregor CGA, Michael EB. Recent investigations into pig antigen and anti-pig antibody expression. Int J Surg 2015;23:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rees MA, Butler AJ, Brons IG, Negus MC, Skepper JN, Friend PJ. Evidence of macrophage receptors capable of direct recognition of xenogeneic epitopes without opsonization. Xenotransplantation 2005;12:13–9. [DOI] [PubMed] [Google Scholar]
- 24.Burlak C, Twining LM, Rees MA. Terminal sialic acid residues on human glycophorin A are recognized by porcine Kupffer cells. Transplantation 2005;80:344–52. [DOI] [PubMed] [Google Scholar]
- 25.Brock LG, Delputte PL, Waldman JP, Nauwynck HJ, Rees MA. Porcine sialoadhesin: a newly identified xenogeneic innate immune receptor. Am J Transplant 2012;12:3272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldman JP, Vogel T, Burlak C, Coussios C, Dominguez J, Friend P, Rees MA. Blocking porcine sialoadhesin improves extracorporeal porcine liver xenoperfusion with human blood. Xenotransplantation 2013;20:239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chihara RK, Parris LL, Reyes LM, Sidner RA, Estrada JL, Downey SM, Wang Z, Tector AJ, Burlak C. Primary porcine Kupffer cell phagocytosis of human platelets involves the CD18 receptor. Transplantation 2011;92:739–44. [DOI] [PubMed] [Google Scholar]
- 28.Paris LL, Chihara RK, Reyes LM, Sidner RA, Estrada JL, Downey SM, Milgrom DA, Tector AJ, Burlak C. ASGR1 expressed by porcine enriched liver sinusoidal endothelial cells mediates human platelet phagocytosis in vitro. Xenotransplantation 2011;18:245–51. [DOI] [PubMed] [Google Scholar]
- 29.Paris LL, Chihara RK, Sidner RA, Tector AJ, Burlak C. Differences in human and porcine platelet oligosaccharides may influence phagocytosis by liver sinusoidal endothelial cells in vitro. Xenotransplantation 2012;19:31–9. [DOI] [PubMed] [Google Scholar]
- 30.Yang WH, Aziz PV, Douglas MH, Mahan MJ, Smith JW, Marth JD. An intrinsic mechanism of secreted protein aging and turnover. Proc Natl Acad Sci USA 2015;112:3657–13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sendil S, Braileanu G, Sun W, Hassanein W, Parsell D, Crum H, Burdorf L, Pierson RN III, Azimzadeh AM. An Improved Flow Cytometry Based Platelet Counting in Xeno Perfusion Models. Am J Transplant 2016;16:suppl 3. [Google Scholar]
- 32.Nusing R, Ullrich V. Immunoquantification of thromboxane synthase in human tissues. Eicosanoids 1990;3:175–80. [PubMed] [Google Scholar]
- 33.Burdorf L, Rybak E, Zhang T, Harris D, Dahi S, Ali F, Parsell D, Braileanu G, Cheng X, Sievert E, Phelps C, Ayares D, Azimzadeh AM, Pierson RN III. Combined thromboxane synthase inhibition and H2-recpetor blockade prevents PVR elevation during GalTKO.hCD46.hCD55 pig lung perfusion with human blood. J Health Lung Transplant 2014;33:S257–S258. [Google Scholar]
- 34.Ekser B, Gridelli B, Cooper DK. Porcine alanine transaminase after liver allo-and xenotransplantation. Xenotransplantation 2012;19:52–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rees MA, Butler AJ, Chavez-Cartaya G, Wight DG, Casey ND, Alexander G, Khudner SA, White DJ, Friend PJ. Prolonged function of extracorporeal hDAF transgenic pig livers perfused with human blood. Transplantation 2002;73:1194–202. [DOI] [PubMed] [Google Scholar]
- 36.Chari RS, Collins BH, Magee JC, DiMaio JM, Kirk AD, Harland RC, McCann RL, Platt JL, Meyers WC. Treatment of hepatic failure with ex vivo pig-liver perfusion followed by liver transplantation. N Engl J Med 1994;331:234–7. [DOI] [PubMed] [Google Scholar]
- 37.Levy M, Crippin J, Sutton S, George N, McCormack J, Curiel T, Goldstein RM, Newman JT, Gonwa TA, Banchereau J, Diamod LE, Byrne G, Logan J, Klintmalm GB. Liver allotransplantation after extracorporeal hepatic support with transgenic (CD55/CD59) porcine livers: clinical results and lack of pig-to-human transmission of the porcine endogenous retrovirus. Transplantation 2000;69:272–80. [DOI] [PubMed] [Google Scholar]
- 38.Shah JA, Navarro-Alvarez N, Defazio M, Rosales IA, Elias N, Yeh H, Colvin RB, Cosimi AB, Markmann JF, Hertl M, Sachs DH, Vagefi PA. A bridge to somewhere: 25-day survival after pig-to-baboon liver xenotransplantation. Ann Surg 2016;263:1069–71. [DOI] [PubMed] [Google Scholar]
- 39.Paris LL, Estrada JL, Li P, Blankenship RL, Sidner RA, Reyes LM, Montgomery JB, Burlak C, Butler JR, Downey SM, Wang Z-Y, Tector M, Tector AJ. Reduced human platelet uptake by pig livers deficient in the asialoglycoprotein receptor 1 protein. Xenotransplantation 2015;22:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1:
A. Potassium levels were noted to normalize within the first hour in all perfusions. B. Potassium levels rose terminally. C. βTG elaboration was prolific and higher in association with Neu5GcKO without drug treatment relative to GalTKO.hCD46, an effect that was attenuated by drug treatment with or without Neu5Gc expression, although βTG elaboration increased over perfusion time in all groups. Of note due to different perfusion lengths standard error could not be calculated after 4 hours. D. P-selectin expression was <10% in all perfusions. The percentage of platelets expressing P-selectin increased over time in the anhepatic experiment. The GalTKO.hCD46 experiments all had low levels of expression throughout (<2%), and the GalTKO.hCD46.Neu5GcKO with drug regiment group had a spike in expression at 15 minutes (likely due to one spurious value), but otherwise was similarly low throughout.