1. Introduction
Microphysiological systems (MPS, also known as ‘organ-on-a-chip’, ‘body-on-a-chip’, or ‘human-on-a-chip’) have emerged over the last fifteen years as attractive systems to probe response to pharmaceutical or chemicals. While applicable to animals, such systems are particularly powerful in predicting human response prior to clinical testing of a drug or as augmentation of clinical studies to test underlying mechanisms. The rate of development of such systems has increased exponentially, particularly over the last three years. Organ-on-a-chip systems have evolved in sophistication and ability to model details of organ physiology, allowing better understanding of underlying mechanisms of response to drugs and chemicals. Body-on-a-chip (BOC) systems are multi-organ systems, often designed to emulate human physiological response to drugs and have the potential to capture both efficacy of a drug and potential toxicity in other organs. While the focus has been on human response to pharmaceuticals, such systems can evaluate response to general chemical exposure, which is important in evaluating the safety of chemicals, food ingredients, and cosmetics. Here, we review recent progresses in the development of model systems over the last three years, with particular focus on BOC systems.
The physiological relevance of the model systems is a key factor that determines the success of drug development.1 Although animal models have been the gold standards for preclinical drug testing, animal models often do not predict human response effectively,2 increasing the demand for more advanced model platforms. In addition to the high cost and the ethical issues associated with using animal models, accurate extrapolation of data from animal experiments to humans is also an important limitation. In vitro cell-based models with a single cell type in static culture require lower cost and are more adaptable to high-throughput format but cannot recapitulate many of the complex biological phenomena as well as BOC systems.
Microphysiological systems (MPS) offer several advantages, such as recapitulation of tissue architecture, diffusion kinetics of drugs and signaling factors, and physiological flow conditions.3 Since the initial development in early 2000s, various organ-on-a-chip (OOC) devices have been developed, some of them showing notable successes in recapitulating the physiological functions of in vivo tissues, which was not possible using traditional cell culture models.4
One of the fundamental issues with current cell-based single tissue/organ in vitro models is that they cannot reproduce complex interactions between different organs or tissues in the body. This can be a critical issue, since such interactions in the body play essential roles in maintaining homeostasis, as well as in many cases of pathologies. Traditional cell culture models or current OOC systems targeting a single organ or tissue cannot achieve this level of complexity. Such a limitation of traditional in vitro models becomes more critical when dealing with complex pathologies, such as metabolic diseases, obesity, and immunological disorders. MPS technology, which relies heavily on microfabrication and microfluidics, is ideal for mimicking such interactions in a reductionist way, by connecting and integrating multiple ‘modules’ of OOC systems.5 These multi-organ systems are often termed as BOC, human-on-a-chip, multi-organ microphysiological systems (MOM), or multi-organ-on-a-chip (MOC), and have emerged as potential tools to evaluate both a drug’s efficacy and side effects that may limit the drug’s usefulness. BOC systems are constructed to mimic the physiological ratios of organ sizes and flow,6 while MOC systems may not necessarily attempt to emulate physiological conditions.
Proof-of-concept studies of multi-organ systems were first reported almost 15 years ago,7–9 and were used to understand the mechanism of toxicity of naphthalene in rodents and to explain differences in response of rats and mice to naphthalene. Since then, recent advances in MPS systems, organoids and stem cell technology, and in vitro vascularization techniques have contributed to the development of improved BOC systems. In this review, we highlight recent progresses in MPS systems aimed at reproducing multi-organ physiology of the human body. We discuss recent advances in novel microfluidic platforms that connect multiple organ modules, and on-chip sensing techniques for real-time analysis of BOC systems, and BOC systems aimed at modeling diseases and drug testing. As described in this review, we have seen remarkable developments in BOC systems. Overcoming several existing challenges promises more advanced systems that can be potentially applicable to clinical situations or incorporated into the drug development process. We will review first advances in the microfluidic platforms for BOCs, then techniques, particularly on-chip systems to monitor responses, then selected single organ systems with multiple cell types, then devices with multiple interacting organ modules.
2. Recent advances and trends in microfluidic platforms for BOCs
Microfluidic technologies enable integration of multiple organ models in a single system to simulate the dynamic organ-organ interactions in a living body. Various BOC systems generally fall into four major categories based on their configurations of fluidic interconnections: i) static microscale platforms; ii) single-pass microfluidic platforms; iii) pump-driven and iv) pumpless recirculating microfluidic platforms, as have been thoroughly reviewed previously.3 During the last two years, there has been substantial growth in the development of BOC systems. Several previously reviewed multiorgan microfluidic platforms have moved beyond the demonstration stage into the development stage. They have been adapted to develop a broader range of BOC models. Some examples include a two-compartment microtunnel platform used to create a functional neuromuscular junction (NMJ) model for phenotypic screening for motoneuron diseases (Figure 1A).10 A two-chamber, transwell-based recirculating BOC platform driven by pneumatic actuation from the Marx group11 was applied to a pancreatic islet-liver model to simulate insulin and glucose regulation (Figure 1B)12 and to a tumor-skin model to evaluate the safety and efficacy of antibody therapies.13 Multiple organ units or chips have also been connected in series using tubing and/or an integration chip to form BOC systems.14,15 Such configurations have recently been employed to construct a neurovascular unit to evaluate neurotherapeutics (Figure 1C),16 a lung-heart-liver BOC to simulate drug response (Figure 1D),17 as well as a multiorgan model that included the ovary, fallopian tube, uterus, cervix and liver to emulate female reproductive tract and the endocrine loops (Figure 1E).18 The gravity-driven pumpless recirculating platforms developed by the Shuler group19 have been employed for multiple BOC systems of two to four organs, including gut-liver models,20–22 a cardiac-liver chip (Figure 1F),23 a tumor liver metastasis model,24 and a cardiac-liver-neuron-skeletal muscle chip,25 as well as a whole-body BOC model with 13 explicitly considered organs (Figure 1G).26 New platforms are also rapidly emerging. Here we review the most recent (within the last two years) advances and new trends in microfluidic platform development for BOCs, mainly focusing on the areas of fluidic control, platform materials, platform throughput, and compatibility of circulating cells.
Figure 1.
Applying well-established BOC platforms to a broad range of BOC models. A) A NMJ model based on the microtunnel microscale platform. Reprinted from Biomaterials, Vol. 166, Santhanam, N.; Kumanchik, L.; Guo, X.; Sommerhage, F.; Cai, Y.; Jackson, M.; Martin, C.; Saad, G.; McAleer, C. W.; Wang, Y.; Lavado, A.; Long, C. J.; Hickman, J. J. Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics, pp. 64–78 (ref 10). Copyright 2018, with permission from Elsevier. B) A pancreatic islet-liver model driven by pneumatic an on-chip micropump.12 Adapted under the terms of the CC-BY-4.0 license. Copyright 2017, the authors. Individual organ modules have also been connected in series to construct BOC systems. C) A neurovascular unit model.16 Reprinted by permission from Macmillan Publishers Ltd: Nature Biotechnology, Maoz, B. M.; Herland, A.; FitzGerald, E. A.; Grevesse, T.; Vidoudez, C.; Pacheco, A. R.; Sheehy, S. P.; Park, T. E.; Dauth, S.; Mannix, R.; Budnik, N.; Shores, K.; Cho, A.; Nawroth, J. C.; Segre, D.; Budnik, B.; Ingber, D. E.; Parker, K. K. Nat Biotechnol 2018, 36, 865–874 (ref 16). Copyright 2018. D) A multi-tissue system on an integrate plate with fluid circulation driven by electromagnetic actuation to emulate female reproductive tract and the endocrine loops.18 Adapted under the terms of the CC-BY-4.0 license. Copyright 2017, the authors. Pumpless platforms were adopted to construct a cardiac-liver chip (E)23 and a whole-body model (F).26 Panel E was reprinted from Biomaterials 182: 176–190, vol 182, Carlota Oleaga, Anne Riu, Sandra Rothemund, Andrea Lavado, Christopher W. McAleer, Christopher J. Long, Keisha Persaud, Narasimhan Sriram Narasimhan, My Tran, Jeffry Roles, Carlos A. Carmona-Moran, Trevor Sasserath, Daniel H. Elbrecht, Lee Kumanchik, L. Richard Bridges, Candace Martin, Mark T. Schnepper, Gail Ekman, Max Jackson, Ying I. Wang, Reine Note, Jessica Langer, Silvia Teissier, James J. Hickman, Investigation of the effect of hepatic metabolism on off-target cardiotoxicity in a multi-organ human-on-a-chip system (ref 23). Copyright 2018, with permission from Elsevier.
2.1. Innovations in microfluidic control for BOCs
2.1.1. Configurable BOC platforms
Configurable BOC platforms are valuable development tools with which researchers can usually customize the number of organ modules and/or the fluid connections among them. Culture conditions (e.g. culture medium ingredients) for multiorgan systems often differ from single organ modules, especially those organ models that need in situ differentiation and maturation. A general approach is to establish tissue cultures in individual organ chips or chambers before assembling them into an integrated BOC device12,13,26,27 or connecting them into a BOC system.17,28 Configurable BOC platforms allow swift switching between individual organ culture mode and multiorgan culture mode. Such on-chip reconfiguration has previously been demonstrated in a hanging drop array-based BOC system (Figure 2A).29 Neighboring hanging drops are interconnected through open channels. By filling and establishing perfusion through each column of a 6 × 4 array of hanging drops, different types of cells can be loaded in different columns for the individual organ culture. The microfluidic network can then be reconfigured into the multiorgan culture mode by switching to perfusion through each row of hanging drops. A simple microfluidic reconfiguration has also been achieved in a single-pass integrated liver-kidney two-organ system by using different inlets/outlets combinations for loading different tissue cells (Figure 2B-i) and for interconnected multiorgan culture (Figure 2B-ii).29
Figure 2.
Configurable BOC platforms. A) A hanging drop array-based BOC platform.29 Neighboring hanging drops are connected with open channels. Hanging drops in each column (1, 2, 3, or 4) are connected in series and loaded with rLi cells (columns 1–3) or with HCT-116 cells (column 4). The fluid network is then reconfigured to support multiorgan culture by filling channels connecting neighboring drops in a row. Reprinted by permission from Macmillan Publishers Ltd: Nature Communications, Frey, O.; Misun, P. M.; Fluri, D. A.; Hengstler, J. G.; Hierlemann, A. Nat Commun 2014, 5, 4250 (ref 29). Copyright 2014. B) A single-pass integrated liver-kidney system40. i) Live cells were loaded to the liver chamber using ports 3 and 2; Kidney cells were loaded to the kidney chamber using ports 4 and 5. ii) Liver-kidney co-culture was established by switching to sing-pass perfusion using ports 2 and 5. All ports not in use were blocked. Reproduced from Theobald, J.; Ghanem, A.; Wallisch, P.; Banaeiyan, A. A.; Andrade-Navarro, M. A.; Taškova, K.; Haltmeier, M.; Kurtz, A.; Becker, H.; Reuter, S.; Mrowka, R.; Cheng, X.; Wölfl, S. ACS Biomaterials Science & Engineering 2017, 4, 78–89 (ref 40). Copyright 2017 American Chemical Society. C) A 7-MPS platform driven by pneumatically actuated micropumps.30 These on-chip individually addressable micropumps allow precise fluid control and differential flow distribution towards different organ modules. Reprinted from Edington, C. D.; Chen, W. L. K.; Geishecker, E.; Kassis, T.; Soenksen, L. R.; Bhushan, B. M.; Freake, D.; Kirschner, J.; Maass, C.; Tsamandouras, N.; Valdez, J.; Cook, C. D.; Parent, T.; Snyder, S.; Yu, J.; Suter, E.; Shockley, M.; Velazquez, J.; Velazquez, J. J.; Stockdale, L., et al. Sci Rep 2018, 8, 4530 (ref 30) under the terms of the Creative Commons Attribution 4.0 International License. Copyright 2018, the authors.
More recent development of configurable BOC platforms often involves an integration plate with individually addressable on-chip micropumps driven by electromagnetic14 or pneumatic actuation.30 The portable and reconfigurable BOC platforms developed by the Borenstein group allow integration of up to 10 transwell-based microtissue modules.14,18 While so far only demonstrated for serial single-pass or recirculating perfusion (Figure 1E), they are likely to be able to form more complicated fluid architectures (e.g. a combination of serial and parallel fluid circuits) with over 60 individually controlled microactuators. The multi-MPS platform developed by the Griffith group31,32 has been used to construct 4-MPS, 7-MPS and 10-MPS systems, where they adopted more sophisticated recirculation architectures that better mimic the blood circulation and achieved differential flow distribution towards different organ models (Figure 2C).30 The configurability of the multi-MPS platform also relies on an array of individually addressable micro-pumps in-line with micro-channels that are integrated in a single device. These configurable BOC platforms are especially useful during the development stage of BOC systems, with which one can modify the collection of organ models to include and adjust fluidic parameters (e.g. flow rates, flow distribution) without reinventing the whole system. However, the configurability of these platforms may come at cost of not being able to achieve physiological relevant fluid-to-tissue ratios. For example, the volume of fluid that is recirculated in the multi-MPS systems described by Edington et al. is over 13-fold of the volume of fluid in the tissue chambers,30 which is a considerable deviation from the ratio of circulating fluid to tissue, which is 0.3 in the human body.3 This is particularly crucial if a metabolite is formed in an organ and then circulates to a second organ to alter organ function. If the ratio of liquid to cells is too high the metabolite will be diluted to a point where the effect of the metabolite cannot be observed. In addition, while the use of standardized transwell inserts to accommodate various organ models (parenchymal or barrier tissues) allows simple integration and retrieval of individual modules, it provides limited configurability on the tissue volume of each organ model. These deviations from the physiological values could seriously limit their ability to simulate human drug responses. Future development of configurable BOC platforms should focus on minimizing dead volumes in the circulation as well as to enabling incorporation of physiologically relevant volume ratios into the BOC systems.
2.1.2. Pumpless recirculating platform with unidirectional perfusion
Pumpless platforms combine gravity-driven flow and a rocking motion to create recirculation without using tubing and pumps.22,33,34 Such platforms allow designs of self-contained, highly integrated BOCs that can be constructed and maintained with relative ease and affordable cost.35 By eliminating tubing and pumps, unwanted loss of drug and its metabolites due to surface absorption is reduced. Blockage of flow due to gas bubbles is reduced significantly with removal of pumps. Initially pumpless systems results in bidirectional flow rather than unidirectional flow as experienced in the body. Bidirectional flow may alter the physiological response of shear sensitive cells, particularly the vasculature. To integrate vasculature and other shear stress-sensitive tissues into a pumpless BOC system, multiple strategies have been proposed. Lee et al. designed a fluid circuit that consist of a main channel for cell perfusion and a bypass channel for medium recirculation (Figure 3A).36 The two channels were designed to differ in hydraulic resistance by several orders of magnitude. During a 5-min “main flow mode”, the fluid level in the inlet reservoir is kept lower than the inlet of the bypass channel and fluid travels to the outlet reservoir only through the cell perfusion channel. When the platform is tilted to the opposite direction, fluid collected in the outlet reservoir then flows back to the inlet reservoir within seconds with majority through the bypass channel due to its much lower hydraulic resistance. By this design, the cell chamber can be perfused with unidirectional flow for most of the time (>99%) with a transient backflow for a few seconds. Esch et al. also utilized bypass channels in a GI tract-liver model for unidirectional perfusion (Figure 3B).27 In addition, they incorporated passive valves, where capillary forces at the air-liquid interfaces prevent fluid from flowing back driven by gravity. Thereby, fluid circulates between two reservoirs by traveling alternately through the tissue perfusion channel and the bypass channel. This design achieved unidirectional perfusion for part of the cycle and stalled flow for the rest of it.
Figure 3.
New strategies towards unidirectional perfusion on a pumpless platform. A) A pumpless platform provides unidirectional tissue perfusion for most of the time with transient backflow.36 Reproduced from A microfluidic chip with gravity-induced unidirectional flow for perfusion cell culture, Lee, D. W.; Choi, N.; Sung, J. H. Biotechnol Prog (ref 36). Copyright 2018 Wiley. B) A pumpless platform provides unidirectional tissue perfusion for a fraction of the cycle and stalled flow for the rest of it.27 Fluid travels between a pair of reservoirs alternately through the tissue perfusion channel and the bypass channel. Reproduced from Esch, M. B.; Ueno, H.; Applegate, D. R.; Shuler, M. L. Lab Chip 2016, 16, 2719–2729 (ref 27) by permission of The Royal Society of Chemistry. C) UniChip achieves continuous unidirectional perfusion with recirculation in a gravity driven flow system 37. i) An assembled UniChip device is placed on a rocker platform that flips between +18° and −18° periodically. ii) Top view of the device shows fluidic connections. Cu, perfusion channel; a1, a2, b1, and b2, Supporting channels; v1, v2, passive valves. iii) Schematic of UniChip operation. When tilted at +18°, flow in b1 is halted by the capillary force at the air–liquid interface in the passive valve v1. Flow travels from reservoir I to reservoir II through a1, a2, Cu and b2. When tilted at −18°, flow in b2 is halted by valve v2, and flow is directed from reservoir II to reservoir I through a2, a1, Cu and b1. Under either condition, the flow direction in the perfusion channel, Cu, is kept the same, shown by the green arrows. Adapted from Wang, Y. I.; Shuler, M. L. Lab Chip 2018, 18, 2563–2574 (ref 37) with permission of The Royal Society of Chemistry.
The recent development of a “UniChip” system has made breakthrough progress for the pumpless platforms (Figure 3C).37 By utilizing a set of supporting channels (a1, a2, b1, and b2) and passive valves (v1, v2), the UniChip achieves unidirectional flow in the cell perfusion channel (Cu) at both tilting directions (±18°). This is the first time that a gravity-driven flow system has achieved continuous unidirectional perfusion with recirculation. In addition, by designing the ratios of hydraulic resistance among different supporting channels, the UniChip is integrated with a backflow-proof mechanism, by which backflow in the cell perfusion channel is prevented even when those passive valves fail (e.g. no air-liquid formation due to excessive fluid in the reservoir). Vascular endothelial cells cultured on the UniChips for 5 d showed cell elongation and alignment to the flow direction, realignment of F-actin, and suppressed cell proliferation, all of which match endothelial cell responses to unidirectional laminar flows. While demonstrated with a single perfusion channel, the UniChip design is expected to be applicable to multiorgan models by expanding the cell perfusion channel into a multi-channel network. Such design allows integration of vascular cells and other shear stress-sensitive tissues (e.g. lung, kidney) into BOCs. Moreover, the use of passive fluid control in the design allows potential incorporation of circulating cells (e.g. circulating tumor cells, immune cells) into the system, which is currently still very challenging in a pump (e.g. peristaltic pumps using rollers or pneumatic actuation) driven recirculating fluid system due to shear damage of circulating cells as they pass through a mechanical pump.
2.1.3. A novel pneumatic pressure-driven recirculating BOC platform
In addition to peristatic pumping with pneumatic or electromagnetic actuation, gravity, and magnetic stirring38, novel fluid driving mechanisms are emerging in newly developed BOC systems. For example, pneumatic pressure, often used to drive peristaltic micropumps through pneumatic actuation, has recently been incorporated into a BOC platform to drive medium circulation through sequential pressurization of tissue chambers that were interconnected with microchannels in series (Figure 4A).39 The inlets were designed to be elevated to limit the amount of medium that travels back to the immediate upstream chamber (Figure 4B). The outlets were integrated with Laplace valves which stop gas phase from going through due to interfacial tension (Figure 4C). The platform has been used to construct liver-cancer 2-organ models and GI-liver-cancer-connective tissue 4-organ models. The detachable air tubing and removable lids make it easy for cell/tissue loading, medium change, and sampling. The platform is compatible with transwell inserts and chopstick electrodes for barrier tissue transepithelial/transendothelial electrical resistance (TEER) measurement (Figure 4D). The microfluidic platform achieved a two-step semi-unidirectional perfusion in the sense that the perfusion is not continuous and that while the net flow can be in one direction, there are ~8% reverse flow. The flow driven by pneumatic pressure exerts little shear stress on cells in the culture wells, which could be advantageous or disadvantageous depending on the specific tissues in culture. It is not clear whether the pressurization (4kPa) of the tissue chambers affects tissues in the culture well. Using an transwell insert may avoid potential issues due to chamber pressurization (Figure 4D). In addition, the demonstration of the platform was limited to serially connected multi-organ systems, where medium was perfused through all chambers at the same flow rate. It is not clear when the platform can adopt more complicated perfusion architectures that mimic physiological circulation.
Figure 4.
A pneumatic pressure-driven recirculating BOC system.39 A) Two-step process of medium recirculation in a 2-organ system. B) Exploded view of a culture device with 4 × 4 culture chambers and illustration of a culture chamber at X–X′ cross-section. C) Schematic of the design and function of a “Laplace valve.” D) Illustrations of the culture chamber equipped with a modified transwell insert. Adapted from Satoh, T.; Sugiura, S.; Shin, K.; Onuki-Nagasaki, R.; Ishida, S.; Kikuchi, K.; Kakiki, M.; Kanamori, T. Lab Chip 2017, 18, 115–125 (ref 39) with permission from The Royal Society of Chemistry.
2.2. From elastomers to plastics as platform materials
A major application of BOC systems is for high-content drug screening. It is crucial to minimize the amount of adsorption and absorption of drugs and their metabolites to the platform materials, which could drastically change the pharmacokinetics (PK) and pharmacodynamics (PD) profiles recreated in the BOC models. Early development of OOC devices mostly utilized elastomer materials, such as polydimethylsiloxane (PDMS), due to its low cost and ease of fabrication. Yet such materials often have significant adsorption and absorption of hydrophobic drugs and their metabolites. In the last two years, with many platforms moving beyond the initial demonstration stage, there is a clear trend that more BOC systems are steering away from elastomers and embracing plastic materials with lower adsorption and absorption rates.
A variety of biocompatible thermoplastic materials, including polymethyl methacrylate (PMMA), polycarbonate (PC), polystyrene (PS), cyclic olefin (co)polymer (COC/COP), polyetherimide (PEI), and polysulfone (PSF/PSU), have been adopted by BOC systems for building tissue culture chambers, microfluidic channels, pneumatic plate, or platform frames for mechanical clamping. These thermoplastics have much better mechanical strength than elastomers (e.g. PDMS) to maintain the original shape of microfluidic channels and chambers. Many have excellent optical transmissivity that allows in situ optical observation. Thin layers of porous PC or Polyethylene terephthalate (PET) have often been integrated into BOC systems to accommodate barrier tissues or facilitate basal side perfusion. Thin polyurethane (PU) membranes were also incorporated as actuation layers in pneumatically actuated BOC systems. Approaches used to fabricate BOC system components from these thermoplastics mainly include computer numerical control (CNC) micromachining and laser cutting, coupled with thermal or solvent-based bonding techniques. Larger scale manufacture processes, such as injection molding, are compatible with these materials, yet often not used as most current BOC systems are still at the development stage. While these thermoplastic materials are expected to have lower drug sorption rates, careful characterization for the specific drugs in test is still necessary. In addition, despite efforts to limit elastomeric materials in drug exposure areas, a complete elastomer-free fluid circuit is still challenging to build. So far, it is only achieved in irreversibly assembled BOCs.40,41 Additional strategies include surface modification and surface coating (e.g. parylene coating)33
2.3. Enabling incorporation of circulating cells into the microfluidic circuits
While a large collection of parenchymal tissues and barrier tissues have been incorporated into BOC systems, cells in the blood circulation (e.g. circulating tumor cells (CTCs) or blood immune cells) have rarely appeared in microfluidic organ models. So far, the incorporation of circulating cells has only been demonstrated in single-pass perfusion systems. Kong et al. directly incorporated CTCs into the flow streams in a four-chamber BOC system driven by a syringe pump to model organ-specific metastasis (Figure 5A).42 The introduction of CTCs into the perfusion allows the study of the interactions between CTCs and endothelial monolayers of specific organ models under flow, which are a first step in organ-specific extravasation. Xu et al. developed a lung cancer metastasis model, in which culture medium was perfused through the luminal compartment of a lung model and downstream organ models of the brain (astrocytes), bone (osteoblast cells), and liver (hepatocytes), three common sites for lung cancer metastasis (Figure 5B).43 The model allows investigation on the multi-step cascade of cancer metastasis, which involves tumor cell invasion into surrounding tissues, transport through the systemic circulation, and colonization of a secondary site. Lung cancer cells co-cultured with lung epithelial cells at the air-liquid interface were observed to gain invasive phenotype, migrate through the bronchial epithelial cell layer, travel to distant target organs, and affect the host tissues. The metastatic cascade was also recapitulated in a vascularized tumor-liver model, where 3D constructs of tumor and liver tissues were assembled in series around a perfusable vasculature constructed based on a microfabricated vascular scaffold.24 Tumor-infiltrating lymphocytes (TILs) have also recently been incorporated into perfusion streams to recapitulate the dynamic interactions between TILs and patient-derived tumor tissues that were held in the flow stream using V-shaped post arrays (Figure 5C).41 All these current models with cells in circulation have adopted single-pass microfluidic platforms. Introducing circulating cells into BOC systems with medium recirculation, which mimic the blood circulation better, remains challenging. The roots of the challenges essentially lie in the way fluids are driven in microscale systems are different from how the blood circulation is driven by the heart at macroscale. In pump-driven recirculating microfluidic platforms, there are usually periodic channel closure at the fluid driving region (e.g. near the pump head, pneumatic valves), which could expose cells in circulation to extremely high shear stress or mechanical injury. The recent development of a pumpless recirculating platform with unidirectional tissue perfusion provides promise to address the challenges by incorporating passive fluid control that is compatible with circulating cells.37
Figure 5.
BOC systems with circulating cells. A) A four-chamber BOC system to recapitulate CTC adhesion to endothelial layers in organ-specific metastasis.42 Reproduced from Kong, J.; Luo, Y.; Jin, D.; An, F.; Zhang, W.; Liu, L.; Li, J.; Fang, S.; Li, X.; Yang, X.; Lin, B.; Liu, T. Oncotarget 2016, 7, 78421–78432 (ref 42) under the terms of the Creative Commons Attribution 3.0 License. Copyright 2016, the authors. B) A lung cancer metastasis model.43 Reprinted from Xu, Z.; Li, E.; Guo, Z.; Yu, R.; Hao, H.; Xu, Y.; Sun, Z.; Li, X.; Lyu, J.; Wang, Q. ACS Appl Mater Interfaces 2016, 8, 25840–25847 (ref 43). Copyright 2016 American Chemical Society. C) A single-pass microfluidic perfusion model to model the dynamic interactions between tumor infiltration lymphocytes (TILs) and tumor tissues.41 Adapted from Moore, N.; Doty, D.; Zielstorff, M.; Kariv, I.; Moy, L. Y.; Gimbel, A.; Chevillet, J. R.; Lowry, N.; Santos, J.; Mott, V.; Kratchman, L.; Lau, T.; Addona, G.; Chen, H.; Borenstein, J. T. Lab Chip 2018, 18, 1844–1858 (ref 41) with permission from The Royal Society of Chemistry.
2.4. Summary
In the last two years, we have seen great progress in the development of BOC microfluidic platforms. Previously developed platforms are being adapted to create new BOC models, as well as evolving towards more sophisticated versions. Configurable BOC platforms with integrated micropump on chip provide precise fluid control with reconfigurability and have yield several BOC models. The gravity-driven pumpless platform has made breakthrough development that achieves continuous unidirectional perfusion with passive fluid control approaches, which allows integration of vasculature and other shear stress sensitive tissues. Such development potentially allows integration of circulating cells into a recirculating system, demonstrated previously in single-pass BOC systems. A novel pneumatic pressure-based fluid driving mechanism has also been demonstrated. Meanwhile, more BOC systems are adopting thermoplastic materials as platform materials in replacing elastomers to reduce drug absorption and adsorption to the platform. The thermoplastic platforms may increase the throughput of BOC based drug screening by integrating multiple BOC units onto a single plate, and by being compatible with industry large scale manufacturing processes.
3. Recent advances in on-chip sensing/analysis for BOCs
The potential of organ-on-chip models to predict drug outcomes and reveal underlying disease mechanisms can be vastly improved by the type, quality and non-invasive nature of readouts obtained from these systems. End point readouts on cell viability, cell health, biomarkers, and environmental conditions provide reliable data for highly toxic compounds or for permanent cellular changes.15 Toxicity to cells, in terms of disruption of normal function, manifests in ways outside of cell death. Temporal mechanical, electrical, and biochemical readouts make the detection of such sub-lethal interactions possible. Dynamic changes to cellular health or functions at multiple timepoints over the lifecycle of the system can be captured by mechanical, electrical and biochemical readouts.23 Additionally, integrated sensors to monitor the environmental conditions in the system ensure reliability of the readouts obtained from cellular responses to stimuli. Recent advances have been made in sensing environmental conditions, cellular products, and functional outputs on multi-organ chips, with most on-chip sensors developed in single organ systems. Several multi-organ chip systems with on-chip sensing have been reported, and many of the technologies developed in single organs can be adapted for multi-organ chips.
Temperature, pH, and oxygen are three primary environmental conditions that affect cellular function, and changes in these environmental conditions may be indicative of a change in biological function. Temperature impacts metabolic rates, protein folding, and spontaneous levels of action potential frequency in electrically-active cells. Temperature sensors have been produced in which the change in resistance of temperature-sensitive metals such as Platinum is measured, and commercial sensors have also been incorporated as on-chip temperature sensors.15 Extracellular pH affects a variety of biological processes, extracellular matrix synthesis, cardiac contraction, and immune function. Perturbations in pH can be indicative of metabolic distress, loss of kidney/liver organoid function, or tumor activity. To this end, pH in multi-organ systems can be detected optically via introduction and monitoring of a pH-sensitive dye that is otherwise physiologically inert.44 Medium pH can also be monitored by voltage changes in an electrode that has been electro-deposited with a pH-sensitive material.15 Oxygen levels play a critical role in micro-biological activity. A decrease in oxygen availability can cause deficiencies in cellular metabolism while high oxygen concentrations may lead to increased generation of reactive oxygen species. Optical methods can be used to take real-time measurements of oxygen levels in integrated multi-organ systems. These can be based on the oxygen-sensitive fluorescence of a ruthenium dye, which is chosen for its high photostability and moderate brightness.7,44 Recently, an inkjet printing technique was used to produce electrochemical dissolved oxygen sensors and integrated into a microfluidic system with hepatocytes and reported oxygen gradients due to metabolism.45
Muscle tissues are responsible for generating mechanical forces to varying degrees (cardiac, skeletal muscle, smooth muscle). Cardiac dysfunctions in response to stimuli (disease, environmental, chemical compounds) can include arrhythmia, tachycardia, bradycardia, QT elongation, conduction velocity deficits, and contractile force deficits (Figure 6B). 1,15,23,25,46–49 Consequently, cardiac beat dynamics serve as one of the primary metrics for assessing cardiac health over cellular viability.23 Optical assessment combined with pixel analysis have been used to study cardiac beat dynamics for drug testing in a system containing liver, heart, and lung tissues1; a heart and liver system15; and a cardiac only platform47. This method can be used to quickly and easily identify changes in beat frequency and regularity. However, this method does not elucidate electrophysiological dynamics (such as QT duration, conduction velocity) or contractile strength and requires imaging during the assay period. Several methods of measuring contraction strength of contractile tissues have been reported for on-chip measurements. Cardiac force changes to drug response has been measured using cells grown on microscale silicon cantilevers or thin film cantilevers. The silicon cantilevers reported used an incident laser directed at the tip of the cantilever, and the deflection of the reflected beam during cellular contraction was directly correlated to force using a known transfer function in multi organ systems with heart, liver, neuron and skeletal muscle (Figure 6A).23,25 Using thin film cantilevers, the force has been determined less directly using a video capture and image analysis system to correlate bending of the thin film to force.50 Skeletal muscle contractions have also been captured under electrical stimulation by optical pixel analysis,25 and by using an optical force transducer and a computer controlled linear actuator.51 Integrated on-chip impedance electrodes were shown to measure the cardiac contractile force in a fully integrated static culture chamber 52. Additionally optical pixel methods have been employed to assess the change in contraction force and frequency dynamics,10 displacement length, and contraction frequency in neuromuscular junction models.53
Figure 6.
Four multi-organ systems used to demonstrate organ-organ interaction and toxicity to compounds using functional readouts obtained via integrated on-chip sensors. (A) Schematic overview of the microfluidic device and on-chip sensors used by Oleaga et al (2018) to demonstrate real time changes to off target cardiotoxicity due to hepatic metabolism. (organ-organ interaction). The sensors used include silicon cantilevers for cardiac force, microelectrode array for cardiac electrophysiological data. Reprinted from Biomaterials 182: 176–190, vol 182, Carlota Oleaga, Anne Riu, Sandra Rothemund, Andrea Lavado, Christopher W. McAleer, Christopher J. Long, Keisha Persaud, Narasimhan Sriram Narasimhan, My Tran, Jeffry Roles, Carlos A. Carmona-Moran, Trevor Sasserath, Daniel H. Elbrecht, Lee Kumanchik, L. Richard Bridges, Candace Martin, Mark T. Schnepper, Gail Ekman, Max Jackson, Ying I. Wang, Reine Note, Jessica Langer, Silvia Teissier, James J. Hickman, Investigation of the effect of hepatic metabolism on off-target cardiotoxicity in a multi-organ human-on-a-chip system (ref 23). Copyright 2018, with permission from Elsevier. (B) Schematic overview of system and on chip sensors used by Zhang et al (2017) to demonstrate real time drug toxicity in a heart-liver system.15 An integrated biochemical sensor module was used to measure biomarkers of interest pointing to toxicity. Reproduced from Zhang, Y. S.; Aleman, J.; Shin, S. R.; Kilic, T.; Kim, D.; Mousavi Shaegh, S. A.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; Avci, H.; Zhang, W.; Silvestri, A.; Sanati Nezhad, A.; Manbohi, A.; De Ferrari, F.; Polini, A.; Calzone, G.; Shaikh, N.; Alerasool, P., et al. Proc Natl Acad Sci U S A 2017, 114, E2293-E2302 (ref 15), with permission from proceedings of the national academy of sciences USA. (C) Schematic overview of a microfluidic system with integrated microelectrode arrays and TEER electrodes to demonstrate the effect of TNF-alpha on vascular endothelium and cardiomyocytes. Reproduced from Maoz, B. M., A. Herland, O. Y. F. Henry, W. D. Leineweber, M. Yadid, J. Doyle, R. Mannix, V. J. Kujala, E. A. FitzGerald, K. K. Parker and D. E. Ingber, Lab Chip 17(13): 2294–2302 (ref 49), with permission of The Royal Society of Chemistry. (D) Schematic overview of multiorgan microfluidic set up with integrated TEER electrodes and optical monitoring of cardiac beating in a heart-liver-lung system.17 Reproduced under the terms of the CC-BY-4.0 license. Copyright 2017, the authors.
Electrophysiological dynamics of cardiac and neuronal tissues can be assessed via their culture onto micro-electrode arrays (MEA’s) to elucidate drug effects in a heart-liver 23, vascular endothelium-heart49, and heart only52 systems. This technology enables the high-temporal-resolution measurement of cardiac action potentials with a direct electrical readout. Under the correct conditions, these measurements can be used to determine field potential duration, a QT interval analogue.49,54 When coupling with defined chemical patterns, the conduction velocity as the contraction propagates throughout the cardiac tissue can be assessed. Additionally, via electrical stimulation at one electrode, the frequency dynamics of the cardiac syncytium can be measured23,54 Patterned microelectrode arrays have been recently developed to measure cardiac response in a microfluidic system that additionally included liver and to observe changes in these measurements in response to drug compounds.23 Moutaux et al reported a MEA-integrated microfluidic device with two independent chambers to demonstrate action potential propagation between the presynaptic and postsynaptic chambers via axons growth between the chambers through microtunnels.55
Transendothelial electrical resistance (TEER) is an electrical technique implemented to quantify integrity of barrier tissues non-invasively.56 This has primarily been used in single organ systems such as the blood brain barrier (BBB),34,49,51 GI tract,27,32 skin models.57,58 This method is versatile, with a variety of electrode materials, electrode configurations, and measurement conditions (such as input frequency) being reported. Cells release and uptake biomolecules as part of routine maintenance, metabolic activities as well as in response to external stimuli. The release of biomarkers in response to stimuli can be immediate or delayed. Monitoring the presence of these biomolecules over time provides a description of cell function including efficient working or disruption of cellular pathways and sheds light on mechanism of drug actions.59 Zhang et al. reported an integrated biochemical sensing platform in a multi organ system composing heart and liver. Electrochemical biosensors that operate by an enzymatic catalysis reaction to generate an electric current was employed for on-chip detection of albumin, glutathione A transferase for monitoring liver toxicity and creatine kinase to assess cardiac viability.15 Misun et al. developed an integrated multi-analyte biosensor system for the monitoring of glucose and lactate to assess cellular metabolism of cancer microtissues, in which the working electrodes were functionalized with enzymes and detection was achieved through amperometry.59 Bavli et al. reported on-chip microprobes to measure glucose and lactate for monitoring the metabolic pathways in a liver on chip model.60 Curto et al used an on-chip organic electrochemical transistor to report oxygen measurements in a microfluidic system with canine kidney cells 61.
The most exciting advancement in on-chip sensing in BOC systems has been the development of systems incorporating multiple on-chip sensors for non-invasive measurements of several tissue systems simultaneously. Oleaga et al. combined MEAs for electrical measurements and silicon cantilevers for mechanical measurements in a system with cardiac and liver components, and used the system for measuring off-target cardiac toxicity of compounds which undergo hepatic metabolism, demonstrating organ-organ interactions.23 Specifically, in this multi-organ system, the liver reduced the cardiotoxicity of terfenadine, as measured by electrical and mechanical function, as the liver metabolized the cardiotoxic parent to non-toxic metabolites. Additionally, the liver increased the cardiotoxicity of cyclophosphamide as the liver metabolized the non-toxic parent to toxic metabolites such as acrolein. The varying dose effects of doxorubicin a chemotherapeutic to different organ systems were shown in a 4-organ BOC system comprising liver, heart, skeletal muscle and neurons using readouts for cardiac, skeletal muscle contraction force and neuron electrophysiological data.25 Zhang et al showed cardiotoxicity in a heart-liver system to acetaminophen with real time on-chip monitoring of biomarkers and cardiac beat frequency.15 Skardal et al. demonstrated an assortment of bioengineered tissue organoids and tissue constructs that are integrated in a closed circulatory perfusion system, facilitating inter-organ responses between the liver, heart, and lung, using integrated TEER electrodes and optical monitoring (Figure 6D).17
Recent advances in techniques for on-chip analysis of electrical, mechanical, and chemical activity of tissues have contributed to the improvement in the performance of recent BOC models, as well as easier and more in-depth validation of the BOC models. As noted earlier, further improvements in the resolution of the detection, as well as non-invasive, real-time, and multiplexed detection in more devices will contribute to a more physiologically-relevant BOC models.
4. Recent advances in multi-organ models
The human body is composed of multiple organs, connected via circulatory networks. The organs in the human body function in an inter-dependent manner, which is orchestrated by complex, yet largely unknown biological mechanisms. In addition to the organs comprising the body, the vascular networks play an important role of supplying nutrients and removing wastes from organ tissues. Vascular networks enable communications between different organs and tissues, via soluble signals such as cytokines and hormones. Mimicking the biological mechanisms of the human body requires development of a model system that emulates such complex multi-organ interactions. Current in vitro model systems are generally based on static single organ or tissue systems and are incapable of capturing such mechanisms. There exist some microplate well-based in vitro model systems for modeling interactions between different organs or different cells, by co-culturing cells or transferring media in between different cell cultures.62,63 However, these systems do not capture the real-time dynamics of multi-organ interaction and consequently may miss essential underlying mechanisms.
The importance of real-time dynamics of multi-organ interaction can be manifested with several examples. Xenobiotics entering the body may undergo a series of transformations involving multiple organs. For example, the fate of an orally administered drug is largely determined by interactions between the gut, liver, kidney, and even the microbiota in the gut.64 How the drugs interact with these organs profoundly affect the pharmacokinetics (PK) of the drugs (that is, absorption, distribution, metabolism and elimination (ADME) characteristics), as well as the toxicity of the drugs, which are often observed in organs other than the target sites. Maintaining homeostasis in the body requires organs interacting with one another via several different mechanisms, soluble signaling being one of them. For example, the glucagon and insulin are endocrine hormones that control the balance in glucose metabolism.65 Cytokines regulate the immune response to foreign invaders.66 Many of metabolic diseases, such as diabetes mellitus, progress as the interaction between multiple organs goes awry.67 Organs and tissues often interact with one another through more direct mechanisms, such as cell migration or cell to cell communication via direct contact. For example, immune cells can migrate into a tissue with inflammation as part of the body’s immune response to foreign invaders. Cancer cell metastasis occurs via malignant cells transmigrating the vascular endothelium and invading tissues at distant site. Many of the biological phenomena described above are difficult to recapitulate with current in vitro models. For example, recirculation of immune cells between organs is difficult due to shear damage. In this section, we start with an in vitro model of a single organ primarily focusing on the models of the liver and the skin as organs representing a solid organ with metabolic functions and a barrier tissue, respectively. Then we expand our discussion to a more complicated model of multiple organ interactions.
4.1. In vitro models of a single organ with multiple cell types
4.1.1. Liver models
A single organ or tissue usually consists of multiple cell types. The liver tissue consists of parenchymal cells, hepatocytes, the major functional cell type, which coexist with non-parenchymal cells such as sinusoidal endothelial cells, Kupffer cells, and hepatic stellate cells.68 The heterotypic interaction between the parenchymal and nonparenchymal cells is important in maintaining the complete function and survival of the liver tissue. The most basic approach is to simply co-culture these different cell types in a mixture in a single well to improve the liver-specific functions of hepatocytes.69, 70 Although this type of approach enables interaction between different cell types, it may fail to capture some important aspects of the interaction, because the three-dimensional organization of different cell types is often critical to tissue response. For example, improvement in the liver-specific functions were observed when hepatocytes were co-cultured with fibroblasts in specific patterns, rather than in simple mixture.71 The specific organization of the tissue may play an important role in determining function.
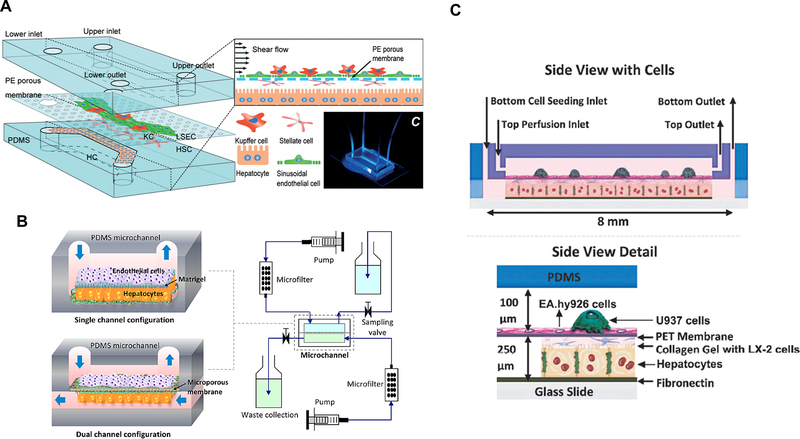
The capillaries within the liver tissue are called sinusoids. The crosstalk between the sinusoid endothelium and the hepatocytes often plays an important role in the normal functions of the liver as well as in liver diseases. Du et al, attempted to replicate the key structures and configurations of the liver tissue by co-culturing the four major cell types of the liver tissue in a microfluidic chip (Figure 7A).72 Two adjacent channels separated by a porous membrane were intended to mimic the structure of the liver sinusoid. The authors observed enhancement in several liver-specific functions, which they attributed to the signaling between different cell types and the mechanical stimulation from the shear flow. In a different approach, bovine aortic endothelial cells were cultured in a chip with primary rat hepatocytes, separated with an extracellular matrix (ECM) protein layer or a microporous membrane73 (Figure 7B). Transformed endothelial cells were cultured with immortalized hepatic stellate cells and monocyte cells, and primary hepatocytes (Figure 7C).74 In a more recent study, two collagen layers containing HepG2 and HUVEC were formed. HUVECs in collagen formed a monolayer, creating a structure resembling the liver endothelium.75 In this work, formation of sinusoids-like structure was based on the laminar flow inside the chip and self-assembly of endothelial cells. The viability, albumin secretion, and urea synthesis of the liver sinusoids on chip were evaluated for seven days, and the response to acetaminophen was also examine.
Figure 7.
(A) A liver sinusoid model containing hepatocytes, Kupffer cells, stellate cells and sinusoid endothelial cells. Reproduced from Du, Y.; Li, N.; Yang, H.; Luo, C.; Gong, Y.; Tong, C.; Gao, Y.; Lü, S.; Long, M. Lab Chip, 17, 782–794 (ref 72), with permission of The Royal Society of Chemistry. (B) A microfluidic chip with single and dual channel configurations, mimicking the liver sinusoid structure. Reproduced from Liver sinusoid on a chip: Long-term layered co-culture of primary rat hepatocytes and endothelial cells in microfluidic platforms, Kang, Y.B.; Sodunke, T.R.; Lamontagne, J.; Cirillo, J.; Rajiv, C.; Bouchard, M.J..; Noh, M. Biotech. Bioeng., Vol. 112, Issue 12 (ref 73). Copyright 2016 Wiley (C) A two-chambered microfluidic device containing four cell types (hepatocytes, endothelial, stellate, and Kupffer cell lines). Reproduced from Long-term maintenance of a microfluidic 3D human liver sinusoid, Prodanov, L.; Jindal, R.; Bale, S.S.; Hegde, M.; McCarty, W.J.; Golberg, I.; Bhushan, A.; Yarmush, M.L.; Usta, O.B. Biotech. Bioeng., Vol. 113, Issue 1 (ref 74). Copyright 2016 Wiley.
Since the liver plays a central role in the metabolism of xenobiotics, as well as maintenance of homeostasis within the body, the development of the liver model has been the area of intense research.76–80 Recent advances in engineered liver models show a great promise in developing physiologically-relevant in vitro liver models, but the liver is an extremely complex organ with diverse functions, and several challenges remain. For example, two critical issues are maintaining functional primary hepatocytes for an extended period of time. Currently, on-chip cultures of hepatocytes for several weeks, for example 30 days by Kang et al.,73 have been reported. Enabling longer culture time, for example, for several months, especially in co-existence with cells from other organs, would be ideal. Also, including and maintaining non-parenchymal cells is important, such as Kupffer cells and macrophages, that may play important roles in fibrosis and inflammation. In particular, the diverse nature of the liver organ functions makes it an important organ in several different phases of a drug development process, and it may be necessary to use different liver models depending on the requirements for the model.80
4.1.2. Skin models
The skin tissue consists of different cell types, organized in multiple layers. The epidermis is the outermost layer consisting mainly of differentiated keratinocytes. Beneath the epidermis, the dermis layer contains connective tissue, hair follicles, and sweat glands. The vascular networks are also located in the dermis layer. The deepest layer, subcutaneous layer consists of fat and connective tissues. It has been known that the different layers of the skin communicate with each other in several ways, including soluble signals an direct contact.81 Conventionally, in vitro models of the skin have been constructed by co-culturing different cells of the skin tissue. The most conventional method is to culture keratinocytes, epithelial cells dominant in the epidermis, with fibroblasts, which are dominant cell types in the dermis layer. 82,83 Fibroblasts are often encapsulated within a hydrogel matrix, usually collagen, to mimic the extracellular matrix (ECM) of the dermis layer. These co-culture models are maintained in transwells, where the dermis layer is placed on top of a porous membrane, immersed within the cell culture media in the bottom chamber. Although such models provide a basic form of an in vitro skin model, it does not capture many of the essential functions of the skin tissue. For example, commonly seen skin conditions, such as acne, psoriasis, and dermatitis, involve the responses of immune cells, which are not included in such models. Co-culture of immune cells such as dendritic cells or macrophages with skin cells has been attempted,84,85 but such models are still far from a complete model of the immune system in the skin.
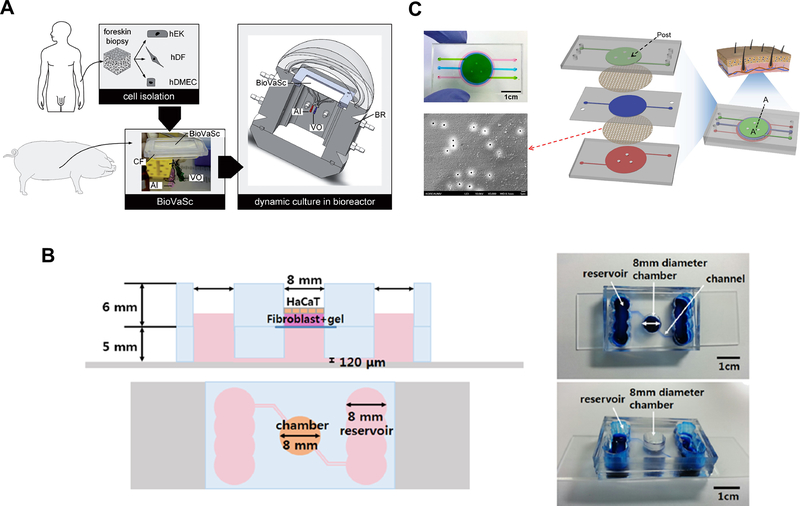
Several research groups reported development of a microfluidic chip as an improved model of the skin tissue. One of the advantages of using a microfluidic chip instead of traditional transwell is the presence of mechanical stimulus provided by the dynamic flow.58 Another advantage is that microfluidic devices can accommodate the vascular structure more easily than static, transwell system, and the fluid dynamics in the vasculature can be controlled. Vascular endothelial cells were co-cultured with skin-constituting cells within a microfluidic chip to mimic the vascular structure within skin dermis. Groeber et al. developed a vascularized skin model by combining a biological vascularized scaffold (BioVaSc) based on decellularized segment of porcine jejunum and a bioreactor system (Figure 8A).86 Human fibroblasts, keratinocytes, and human microvascular endothelial cells were seeded into the BioVaSc system and cultured for 14 days. A successful differentiation was verified by hematoxylin & eosin (H&E) and immunohistochemical staining, revealing the dermis and epidermis structure. The formation of vascular wall by endothelial cells was also confirmed. More recently, Lee et al., developed a two-layer microfluidic chip for a skin model with vasculature, by forming a 3D skin model with human primary keratinocytes and dermal fibroblasts in collagen on one side and HUVEC on the other side (Figure 8B).87 The H&E and immunohistochemical staining confirmed the formation of dermal and epidermal layers of the skin. A mathematical model of the skin chip was used to confirm that the presence of medium perfusion was essential for the delivery of nutrients to a sufficient level to support the survival of the 3D skin tissue.
Figure 8.
(A) Construction of vascularized skin model using a decellularized segment of porcine jejunum and keratinocytes, fibroblasts, and endothelial cells. Reproduced from Groeber, F.; Engelhardt, L.; Lange, J.; Kurdyn, S.; Schmid, F.F.; Rücker, C.; Mielke, S.; Walles, H.; Hansmann, J., ALTEX, 33, 415–422 (ref 86), under the terms of the Creative Commons Attribution 4.0 International License. Copyright 2016, the authors. (B) A two-layer microfluidic skin chip containing human primary keratinocytes, fibroblasts and HUVEC. Reprinted by permission from Lee, S.; Jin, S.P.; Kim, Y.K.; Sung, G.Y.; Chung, J.H.; Sung, J.H., Biomed Microdev 2017, 19, 22 (ref 87). (C) A microfluidic skin chip with HaCaT, fibroblasts, and HUVEC. Reprinted from Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B. J.; Choi, T. H.; Lee, S. Sci Rep 2016, 6, 37471 (ref 88), under the terms of the Creative Commons Attribution 4.0 International License. Copyright 2016, the authors.
As noted earlier, the human skin is a barrier tissue and complex immunological responses are involved in skin irritation and toxicity, as well as skin diseases. The inflammatory response of the skin tissue has been investigated by co-culturing keratinocyte cell line, HaCaT, fibroblasts, and HUVEC (Human Umbilical Vein Endothelial Cells), and challenging them with TNF-α (Figure 8C).88 These cells were separated by porous membranes within the device, while allowing interlayer communications. Skin inflammation was induced by applying tumor necrosis factor alpha and measuring the levels of proinflammatory cytokines. Such models suggest the possibility that they may be used for recapitulating more complex mechanisms of dermal diseases. This recent progress carries a significant potential for developing therapeutics for dermal diseases or testing drugs for dermal toxicity, because a large part of the mechanisms underlying the dermal toxicity,89 as well as dermal diseases such as atopic dermatitis are still unknown.90.
4.1.3. Vascularization of tissues
Every tissue in the body contains vasculature for nutrient supply and waste removal. Since blood perfusion is an essential element in maintaining the tissue functions, there have been many attempts to integrate a vascular structure with OOC systems.91 These ‘vascularized OOC systems’ can potentially provide additional physiological functionalities to better mimic the physiology and pathology of the organ tissue. Microfluidic chips for the liver sinusoids and the skin tissue with vasculature have been discussed above. Other OOC systems have also been vascularized to recapitulate their essential physiological functions. For example, vascular endothelium is an essential part of the blood-brain barrier (BBB) model. Several attempts have been made to create the neurovascular model of BBB and to test the permeability of the BBB platform.92–94 It is also interesting to note that these systems incorporated different cell types constituting the brain, such as neurons, astrocytes and pericytes. The lung requires highly vascularized structures due to its gas-exchange functions. The basic unit of the lung is the alveolar-capillary interface, which consists of extremely thin membrane to allow efficient gas exchange. An in vitro lung model containing the vasculature was proposed by Huh et al., where human alveolar epithelial cells and human pulmonary microvascular endothelial cells were cultured on opposing sides of a porous membrane.95,96
While we have summarized examples of development of multi-cellular single organ models in this section, our focus is on how we create multiorgan models to capture inter-organ interactions. Multiorgan models are dependent on having appropriate single-organ modules, but interactions between organs is often necessary to predict both efficacy and toxicity (or side effects) of drugs.
4.2. Multi-organ tumor models
Multi-organ models have important implications in cancer metastasis, as migration of cancer cells to remote locations involve multiple tissues and organs, as well as circulatory systems. Several on-chip devices have been developed to study the process of metastasis.97 These devices usually focus on a specific aspect of metastasis. For example, the migration of tumor cells through an endothelial layer has been observed in a microfluidic device, which was then used to evaluate the antimetastatic effect of several natural products.98 In another example, extravasation and invasion of breast cancer cells to bone-like matrix was evaluated in a microfluidic chip consisting of human osteo-differentiated bone marrow-derived mesenchymal stem cells and endothelial cells.99 In a more recent study, a 3D bone-on-a-chip, containing mature osteoblastic tissue co-cultured with metastatic breast cancer cells was used to examine the process of bone colonization by the cancer cells.100 These approaches may prove useful for screening anti-cancer drugs or studying the mechanism of metastasis, but they do not provide a full picture of the metastasis, from the primary site to the metastatic site. Development of an in vitro model that can encompass the entire process of metastasis is still challenging but should prove very useful.
More recently, a microfluidic device with recirculating fluid flow was used to track the migration of tumor cells from a colon organoid to a liver organoid.101 The authors observed that metastatic cancer cells were able to disseminate out of the colon organoid into the circulation and invade the downstream liver organoid. Conversely, a non-metastatic cancer cells proliferated at the primary site but did not show a metastatic property in the system. This approach can potentially provide a more complete picture of the metastasis, although it is still a highly simplified system. Wu et al, developed a multi-organ microfluidic chip containing the ‘upstream lung’ compartment, and three ‘downstream distant organs’.43 Bronchial epithelial, lung cancer, microvascular endothelial, mononuclear, and fibroblast cells were grown in the lung compartment, while astrocytes, osteocytes, and hepatocytes were grown in distant compartments to mimic lung cancer cell metastasis to the brain, bone, and liver. The authors reported observation of epithelial-mesenchymal transition of tumor cells, and indications of damaged tissues in all of the ‘distant organs’.
Development and remodeling of vascular structures are important processes in both normal and diseased tissues. The process of new vessels branching out to distal regions, known as angiogenesis, plays a crucial role in the embryonic development, as well as in the pathology of several diseases including cancer. Several angiogenesis assay systems have been developed to examine the mechanism of crosstalk between different tissues and vasculature and to screen potent drugs that can inhibit angiogenesis.102 Zheng et al. developed a microfluidic model to study angiogenesis induced by liquid tumor such as leukemia.103 Endothelial cells, leukemic cells, and bone marrow stromal fibroblasts were co-cultured in a microfluidic platform to provide biomimetic environment to study bone marrow angiogenesis. A biomimetic tumor model for studying paracrine signaling between tumor cells and fibroblasts was developed.104 In this study, morphological changes of tumor cells and angiogenesis induced by tumor-stromal interaction were observed, in a microfluidic system where endothelial cells and cancer cells were cultured in close proximity by controlled patterning 105. In this study, formation of vessels was observed by natural morphogenic process. It was demonstrated that the newly formed vessels were perfusable and have well-defined openings toward inlet and outlet channels. These studies demonstrate that microfluidic systems incorporating multiple cell types involved in pathogenic process of angiogenesis are potentially useful platforms for studying such processes and screening potential drugs.
4.3. BOC systems for simulating ADME processes
The importance of MPS technology in pharmaceutical industry has been well recognized.3,5 One of the fundamental issues in drug development is that drugs, once administered, go through a series of complex interactions with organs in the body. The major processes that the drugs go through are termed absorption, distribution, metabolism and elimination (ADME), which depend on many factors, such as the chemical and physical properties of drugs, genetic background of the patient, and the route of administration. Prediction of ADME characteristics for new drug candidates is a major step in the drug development process. BOC systems can be an ideal in vitro platform for predicting the ADME of drugs, by providing functions of key organs and connecting them in a physiological manner. A series proof-of-concept studies demonstrated that the bioconversion and bioaccumulation of chemicals or drugs can be observed in a BOC system, and subsequent effects of the drugs evaluated.8,19,106 Based on these initial findings, more advanced BOC systems have been developed.26,27,35,37
The liver plays a central role in determining the ADME properties of drugs, as many of them go through hepatic conversion before reaching the target site. Therefore, there has been a huge effort to reproduce the functions of the liver in vitro (see section 4.1.1).80 Diverse in vitro models exist, with varying degrees of complexity and physiological relevance to humans. While established transformed hepatocytes cell lines, such as HepG2, Hep3B, HepaRG, provide relatively high-throughput platform at reduced cost, their physiological relevance is often severely limited. Combination of primary human hepatocytes and OOC technology to enable more accurate and more stable in vitro liver platforms has led to a considerable improvement in the ability to predict hepatic conversion of drugs.79 In addition to the liver metabolism, the absorption in the gut is another major determining step of the bioavailability of an orally taken drug. When oral drugs are ingested, they are absorbed through the gut wall and are transported to the liver via portal vein, where they undergo hepatic metabolism before reaching the systemic circulation through the hepatic vein. Simply co-culturing the gut and liver cells can mimic this process in a primitive form,107 but it does not capture the real-time dynamics of the gut-liver interaction.
BOC systems offer the possibility to mimic such processes in a more physiologically relevant manner, by culturing the gut and the liver in separate compartments, which can be connected via microfluidic channels or separated by a porous membrane. The transport dynamics within the device between different compartments can be controlled, and using a simple two-layer design, a gut-liver chip for recapitulating the first-pass metabolism of drugs has been developed.21,108 Combination of the gut-liver chip with a corresponding mathematical transport model and a PK model revealed several important design considerations when simulating the first-pass metabolism of drugs, such as relative sizes of each organ and the design of the architectures of channel and cell culture chambers.
Leclerc et al. published a series of papers aimed at reproducing the first-pass metabolism of drugs in a microfluidic chip (Figure 9A).109–111 The gut and liver cells were co-cultured in a microfluidic device, and concentrations of a drug and its metabolites in the circulating media were measured. Based on the measurements, and by combining the in vitro experimental platform with mathematical models of the device, pharmacokinetic parameters were deduced and compared with in vivo parameters. Fujii et al., described an on-chip small intestine-liver coupled model.38 This model was designed based on several physiological parameters, such as the structure of internal circulation, volume ratios of each organ, and blood flow ratio of the portal vein to the hepatic artery. Anticancer drugs (epirubicine, irinotecan, and cyclophosphamide) were tested, and the activities of the drugs were observed. This work demonstrates that the first-pass metabolism of drugs can be tested at a basic level, although quantitative PK analysis was lacking in this study.
Figure 9.
(A) A schematic diagram of gut-liver platform for studying PK of drugs. Reproduced from First pass intestinal and liver metabolism of paracetamol in a microfluidic platform coupled with a mathematical modeling as a means of evaluating ADME processes in humans, Prot J. M.; Maciel L.; Bricks T.; Merlier F.; Cotton J.; Paullier P.; Bois F. Y.; Leclerc E. Biotech. Bioeng., Vol. 111, Issue 10 (ref 111). Copyright 2014 Wiley. (B) A microfluidic 4-organ platform and fluid dynamics simulation showing the levels of shear stress in each compartment. Reprinted from Oleaga, C.; Bernabini, C.; Smith, A. S.; Srinivasan, B.; Jackson, M.; McLamb, W.; Platt V.; Bridges, R.; Cai, Y.; Santhanam, N.; Berry B.; Najjar, S.; Akanda, N.; Guo, X.; Martin, C.;, Ekman, G.; Esch, M. B.; Langer, J.; Ouedraogo, G.; Cotovio, J.; Breton, L.; Shuler, M. L.; Hickman, J. J., Sci Rep 2016, 6, 20030 (ref 25), under the terms of the Creative Commons Attribution 4.0 International License. Copyright 2016, the authors. (C) A microfluidic 4-organ system (left), showing (1) intestine, (2) liver, (3) skin, and (4) kidney, and top view of the chip layout (right). Reproduced from Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; Sambo, N.S.; Sonntag, F.; Lauster, R.; Marx, U., Lab Chip 2015, 15, 2688–99 (ref 116), published by The Royal Society of Chemistry, under the terms of the Creative Commons Attribution 3.0 Unported Licence.
Quantitative in vitro PK studies of diclofenac and hydrocortisone were demonstrated using integrated gut-liver microphysiological system.32 In this study, mechanistic model-based analysis of experimental data allowed the authors to derive several intrinsic PK parameters, such as intestinal permeability and metabolic clearance. The authors made sure that pharmacologically relevant concentrations of model drugs were achieved within the system. Operational characteristics of the system, for example, flow rates and volumes, were considered when developing the mechanistic PK model. ADME processes of model drugs, propranolol, thiopentone and pentobarbital were simulated using a BOC with gut (Caco-2 cell line), liver (primary hepatocytes), blood (HUVEC), tumor (MCF-7 cell line), heart, lung, and adipose (primary cells).112 Changes in the concentration of model drugs as they interact with these cells, and pharmacokinetic parameters were deduced.
Varnetti et al., reported a ‘functional coupling’ of four human micro physiological system models.51 The intestine, liver, proximal tubule kidney, and BBB/neurovascular unit were selected, as they represent major organs involved in ADME of drugs. This approach was realized by transferring media between six institutions with different MPS units for subsequent experiment and analysis, rather than direct coupling of these units. This way the authors could circumvent some of the important challenges when trying to integrate multiple organ MPS units. For example, implementing a universal medium that can support growth and differentiation of all organ tissues, as well as proper scaling of different MPS models to accurately reproduce organ interactions in the human body. The major drawback of this approach is that the real-time dynamic interaction between different organs was not faithfully reproduced, which can lead to missing some of the essential dynamics.
A three-tissue BOC system, comprised of liver, heart, and lung organoids was developed and used for observation of inter-organ responses to drug administration.17 Each organ module was constructed using various biofabrication methods to meet specific individual requirements of different organ tissues. Spherical liver organoids were formed with primary human hepatocytes, stellate cells, and Kupffer cells. Spherical cardiac organoids were formed using induced pluripotent stem (iPS) cells. A bioprinting technique was employed to insert these organoids into the microreactor devices using hydrogel matrices. The lung module was constructed by seeding lung fibroblasts, epithelial, and endothelial cells on a semi-porous membrane in layers. Each module was connected via circulatory perfusion system driven by micro-peristaltic pump. This work demonstrates direct coupling of different organoids with high physiological relevance. Using this system, organ-interactive screening of drugs was demonstrated. In a heart-liver two-tissue setting, the effect of epinephrine and propranolol on the heart organoids varied, depending on the presence of the liver organoids. In a heart-liver-lung three-tissue setting, bleomycin was administered to induce its known lung toxicity. Interestingly, unanticipated toxic side-effect on the heart organoids was observed, which was not observed in the heart-only system. Through a series of control experiments, the authors concluded that bleomycin-induced production of IL-1β by the lung epithelium contributed to the observed cardiac toxicity, which is consistent with previous literature reports. Such results are in line with previously reported observation using BOC systems, that is, BOC systems can recapitulate the interaction between organs and enable observation of biological responses that cannot be observed with conventional in vitro models.19,25,108,113
More recently, a BOC system, termed by the authors as interconnected microphysiological systems, containing either 4, 7, or 10 organs have been reported.30 The 4-way interconnected MPS comprised of the liver/immune, lung, gut/immune, and endometrium. Three more organ modules (brain, heart and pancreas) were added to make a 7-way MPS, which was used to quantify PK of diclofenac. A 10-way MPS platform further included kidney, skin and skeletal muscle. It was reported that the 10-way MPS successfully maintained phenotypic functionality for 4 weeks. In their device, recirculation of media and distribution into each organ module was achieved by pneumatically-driven pump with independently programmable flow rates. In case of a 4-way MPS platform, metrics of tissue functions were measured for each organ. Trans-epithelial electrical resistance (TEER) for gut and lung, albumin for liver, and IGFBP-1 for endometrium. All metrics showed relatively stable values during the two-week culture, and similar results were obtained for 7-way and 10-way MPS platforms as well. Distribution of endogenous (albumin) and exogenous (diclofenac) molecules in the MPS was examined by measuring the concentrations in each module. A physiologically-based pharmacokinetic (PBPK) model for diclofenac in the MPS was developed and used to predict the concentration changes of the drug, which showed a good agreement with experimentally measured values, with some of the PBPK parameters fitted to experimental values.
The notable trend towards the use of more physiologically relevant cells, for example, primary cells, stem cells, and organoids, will make BOC systems an increasingly useful platform for predicting and analyzing PK profiles of drugs. One important consideration in analyzing PK profiles is that quantitative aspect is very important, since PK deals with time-dependent concentrations of drugs in the body. Correctly scaling different organs and connecting them in correct configurations will be particularly important for BOC systems for PK analysis.108
4.4. BOC systems for modeling normal and diseased organs
The homeostasis between different organs plays a crucial role in maintaining a healthy body. In the event of diseases occurring, complex interactions between organs in the body play important roles in progression of the diseases. In this section, we highlight recent development in BOC systems for modeling the co-existence of multiple organs while maintaining homeostasis, and for modeling diseased state when such homeostasis goes wrong.
Marx et al. published a series of papers on BOC systems (termed as multi-organ chip by the authors) containing two, three, or four organs.11,114–116. In their papers, the authors successfully demonstrated that the basic physiological functions of each organ were maintained by measuring key metabolites or markers. Although this does not guarantee the maintenance of fully functional homeostasis between the cultured organs in the system, it is notable that the basic functionalities were maintained for two to four weeks. While BOC systems comprising two to four organ modules have been extensively developed and reported, the human body consists of more organs, and there have been efforts to include a larger number of organs in a BOC system. Design of 14-compartment BOC system was demonstrated recently.26 This 14-chamber device could accommodate 13 different organ compartments of barrier (skin, GI tract, and lung) and non-barrier (fat, kidney, heart, adrenal glands, liver, spleen, pancreas, bone marrow, brain, and muscle) types. The volumes of each ‘organ’ were determined by scaling down proportionately from the average human organ volumes by a factor of 40,000. Non-barrier tissues were encapsulated in a hydrogel matrix and inserted into a chamber made by placing a membrane sandwiched between silicone gaskets to create a chamber with 400 μm depth. Barrier tissues were made by seeding appropriate numbers of cells onto a membrane bonded to a silicone gasket. After attaching the cells, they were incubated for 20 days to form a barrier layer before assembly. As a proof of concept study, five types of cell lines were maintained in the device for 7 days while maintaining reasonable viability. Physiological functional markers were also examined, such as urea and albumin synthesis, P450 enzyme activity, production of surfactant and expression of tight junction proteins. This work demonstrates that it is possible to construct a physiologically realistic model with many compartments that can operate for an extended period.
The interaction between organs in the progression of diseases can be important in some cases. For example, the central role of gut-liver axis in several diseases, including nonalcoholic fatty liver disease (NAFLD), has well been documented.117,118 NAFLD is a pathological condition where excessive fat is accumulated in the liver due to causes other than alcohol uptake. It can sometime progress to nonalcoholic steatohepatitis (NASH) and further to liver cirrhosis and hepatic fibrosis, ultimately leading to hepatic carcinoma. Progression of this disease is associated with impairment of intestinal barrier function (leaky gut) caused by disruption of bacterial flora, and the release of toxic factors (pathogen-associated molecular patterns, PAMP) from intestinal bacteria to the liver due to the increased permeability in the gut. In response to the PAMPs, cells in the liver release inflammatory cytokines. The possibility of using microfluidic gut-liver chip as a model platform to evaluate the progression of NAFLD was explored in a recent study.113 Authors examined the process of lipid accumulation in the liver, caused by excessive uptake of fatty acids in the gut. Administration of anti-steatotic compound inhibited accumulation of lipid in the liver as expected. Interestingly, the tested anti-steatotic compounds showed efficacy via different mechanisms, where butyrate reduced lipid accumulation in the liver by inhibiting lipid uptake in the gut, whereas α-lipoic acid reduced lipid accumulation in the liver by acting directly on the liver. These results suggest that the gut-liver chip platform can be used to examine the gut-liver communication in the context of the fatty liver disease.
The gut-liver crosstalk was examined in a multi-organ platform consisting of the liver (hepatocytes and Kupffer cells) and the intestine (enterocytes, goblet cells, and dendritic cells) to model endotoxemia.31 Gene expression patterns in the gut and liver cells when they were cultured alone or together were compared. Interestingly, significant changes in expression of several genes were noted. Of particular interests was the bile acid metabolism, which is mediated by CYP7A1. Suppression of CYP7A1 was observed, thought to be caused by FGF19 in enterohepatic communication. Chen et al. examined gut-liver crosstalk in inflammatory context as well. A condition of endotoxemia, characterized by increased concentration of circulating lipopolysaccharide (LPS), one of major PAMPs, was simulated. Genes involved in immune response and interferon signaling were upregulated in both gut and liver cells, and genes related to hepatic metabolism were suppressed. Measuring the levels of cytokines revealed non-linear modulation of cytokine production as the result of gut-liver crosstalk, meaning that the measured cytokine levels of integrated gut-liver system deviated from the linear sum of cytokine levels of isolated gut and liver modules.
Since the initial proof-of-concept study of BOC systems containing three to four organs,7,8,106 the recent reports of a BOC systems with a larger number of organs show a great promise of these systems being utilized in clinical settings or drug development process. Although these models are not complete models of the whole body, they may potentially start proving to be useful in specific circumstances or specific drug development phases, such as predicting organ-specific toxicity of drug candidates arising from multi-organ interactions, as described in the next section.
4.5. BOC platforms for drug testing and toxicity screening
How multiple organs in the body respond to administration of drugs is an important issue in drug development process, which can affect the efficacy and the toxicity of drug candidates. Various organ-specific toxicity models are available. But in many cases, drugs interact with multiple tissues/organs in the body, resulting in complex whole-body response. A proof-of-concept study demonstrated this concept earlier, as the toxicity of naphthalene in the lung was caused by the metabolism in the liver.8 In another example, the efficacy and toxicity of anti-cancer agents, 5-fluorouracil (5-FU) and Tegafur, were tested in a BOC system 106. Tegafur is a pro-drug of 5-FU, is inactive by itself, but becomes active after being metabolized in the liver to 5-FU to exert anti-cancer effect. Using a three-organ BOC device containing the liver, colon cancer, and the bone marrow cell line, anti-cancer effects of 5-FU and Tegafur were tested. In line with a known mechanism of action, 5-FU exerted anti-cancer effect on tumor cells regardless of the liver cells, but Tegafur exerted anti-cancer effect only when the liver cells were present in the system, indicating that the presence of hepatic metabolism was required for the anti-cancer effect of Tegafur.
Oleaga et al. demonstrated multi-organ toxicity using a four-organ system of cardiac, muscle, neuronal and liver (Figure 9B)25. Measurement of functional markers for each organ module, albumin and urea production by the liver cells, spontaneous contractile activity of cardiomyocytes, skeletal muscle contractility, and action potentials in neuron cells, verified that the cells were active and viable for 14 days. Cardiotoxicity and hepatotoxicity were observed when doxorubicin was administered, due to the metabolism and oxidative stress generation in the liver. Myotoxic effects were observed for atorvastatin and hepatotoxicity was observed for valproic acid and acetaminophen. These toxic reactions are consistent with known toxic reactions to doxorubicin in literature reports. A negative control, N-acetyl-m-aminophenol caused no significant toxic reaction in the four-organ BOC system, which was also consistent with literature reports. In a more recent study, Oleaga et al., developed a BOC system containing iPSC-derived cardiomyocytes and primary hepatocytes with perfusion of serum-free media to study hepatic metabolism-dependent cardiotoxicity of drugs.23 The contractile force and electrical conductivity were measured to examine the cardiac function. The authors observed that the cardiotoxic effect of cyclophosphamide was induced by the presence of hepatic metabolism, whereas the effect of terfenadine was reduced due to the hepatic metabolism. In addition, hepatotoxicity was also examined by measuring liver-relevant markers and metabolites.
Marx et al. published a series of papers on BOC systems containing two to four organ modules on a chip, primarily aimed at long-term substance testing (Figure 9C)119–121. A liver-skin on-chip model was developed for the long-term cultivation of cells and assessment of drug toxicity.11 Toxicity of troglitazone as an antidiabetic drug was tested and dose-dependent responses were observed. A similar platform was used to enable liver-intestine and liver-skin interactions on a chip.115 Gene expression levels in the liver and intestine cells were measured in response to a daily administration of troglitazone. One of the areas of pharmaceuticals that show a wide gap between the animal and human models is neuroprotective drugs, due to the phylogenetic distance between humans and laboratory animals. A multi-organ chip containing a human liver microtissue and a human neurospheres were maintained for two weeks while being exposed to fluid flow.122 A stable steady-state co-culture was established after six days of co-cultivation, indicated by the measurement of lactate production and glucose consumption. Examination of neural markers indicated that the neurospheres remained differentiated. A two-week toxicity assay with repeated exposure to a neurotoxin 2,5-hexanedione was observed to induce apoptosis within both the neurospheres and liver microtissues. A similar co-culture platform was used to test co-culture of liver-skin and liver-intestine modules.115 In this study, the intestine and the skin were chosen as models of human barrier organs. Human endothelial cells were added to the system to mimic the vasculature. Co-culture in the platform for 14 days and immunostaining results confirmed that the intestinal and dermal epithelial cells, as well as endothelial cells maintained differentiated state. Oral and systemic exposure of a model toxicant, troglitazone, were tested using this system. A four-organ BOC system, containing the liver, skin, intestine, and the kidney, was maintained for 28 days and the maintenance of physiological functions of each organ module was examined.116 Examination of differentiation markers by immunostaining and RT-PCR verified that the tissues remained viable and physiologically functional during the co-culture period. These promising observations by Marx et al. indicates that it may be possible to achieve a significantly long-term co-culture of multiple organ modules while maintaining homeostasis, making chronic toxicity study possible. It would be interesting to see an expansion of their work to a larger number of organs.
One important issue in drug efficacy and toxicity screening is the high-throughput (or even medium-throughput) implementation.123 It is quite a challenging work to realize multi-organ interaction in a high-throughput format. As the number of organ modules in the BOC increase, the complexity of the system increases greatly, as well as the difficulty of high-throughput assay. Several primitive BOC systems with a relatively simple design having two or three organ modules have been reported, which may potentially lead to high-throughput platforms in the future. A liver-immune co-culture array was used to study skin sensitization.124 Hepatocyte spheroids and U937 myeloid cells were co-cultured in concentric micro-chambers, connected by a microchannel network. Model drugs known to cause cutaneous reactions (carbamazepine, phenytoin, and allopurinol) were metabolized by the liver spheroids and activate U937 cells in the immune compartment. The immune reactions were verified by measuring the levels of cytokines in response to the drugs. However, this liver-immune array is a rather incomplete model because it did not contain the skin tissue, and cutaneous reaction could not be directly observed. A heart-liver-vascular multi-organ platform, termed as HeLiVa platform, containing microtissues derived from human pluripotent stem cells was reported.125 A modular design and array-type microfluidic device may potentially allow high-throughput screening of multi-organ interaction at a primitive level. A multi-throughput MOC was developed using a pneumatic pressure-driven medium recirculation.39 An eight-throughput two-organ system and four-throughput four-organ systems containing the intestine, liver, cancer and connective tissues were demonstrated. A microfluidic immune-tumor model containing 12 separate tumor biopsies interacting with tumor-infiltrating lymphocytes was developed.41 This system allows culture of tumor and immune cells for multiple days while allowing real-time analysis of the interaction. Such systems, although in primitive form, demonstrate the possibility of high-throughput implementation while allowing real-time observation of multi-organ interactions.
5. Remaining challenges and future directions
Despite the considerable advances on BOC systems made during the past decade, several aspects remain to be improved. Overall, BOC systems should be able to provide reproducible results that have significant physiological relevance to human data. Validation of these in vitro models that can substitute or complement current animal models will be critical to development of this technology.
First, the development of a blood surrogate, a common medium for multiple cell types, is essential for advancing to more complete BOC systems. Currently, different cell types may require different nutrients and growth factors, which makes it challenging to co-culture these cells in the same systems for an extended time. This problem becomes more serious as there is often a desire to use stem cells and primary cells in BOC systems, as they often require specialized media. A modular approach, where each organ module can be integrated or detached as needed, can be a partial solution to this issue, because cells usually require specialized medium during the differentiation phase, and it is often possible to maintain a short-term co-culture of multiple cell types with basal medium.126 In some cases, mixing different media at specific ratios has been used with some success.15 However, these strategies are only partial solutions since it will be more difficult with increasing number of organs, and obviously, the development of a universal, as well as chemically well-defined media that can support growth and differentiation of all cell types would be a more complete solution. Hickman et al. demonstrated using a serum-free medium for supporting co-culture of muscle cells derived from human stem cells and sensory neurons,127 and co-culture of motoneurons and oligodendrocyte,128 as well as in their 4-organ systems,25 which show great promise for a co-culture systems with a larger number of organs.25
Secondly, development of biomaterials that meet specifications of different cell types is also needed to further improve current BOC models. Although current researchers are equipped with diverse families of natural and synthetic biomaterials,129 chemical and physical interaction of cells with surrounding 3D environment is an important factor in the cellular microenvironment, and fine-tuning of this microenvironment is needed for different cell types. Another importance aspect of using appropriate materials in OOC technology is the issue of unwanted absorption and adsorption of chemicals to the inner surface of MPS devices, which can distort the experimental observation. Since different materials have widely varying degrees of interaction with different molecules, choice of materials in developing MPS devices should be made with caution. In general, stainless-steel, glass, Teflon, and polystyrene are considered to be inert to most of chemicals,130 whereas PDMS (polydimethylsiloxane) is known for its hydrophobic surface and tendency to both adsorb and absorb many molecules 131. Coating of PDMS surface with various materials such as extracellular matrix proteins or paraffin has shown to be helpful.22,132,133 Using thermoplastic materials such as polystyrene, polymethyl methacrylate (PMMA), and polycarbonate offers advantages in both aspects of mass production and relatively low adsorption and absorption, but require more complicated fabrication techniques.
Thirdly, construction of physiologically realistic fluidic microenvironment is important. The role of fluidic shear stress on cell morphology, alignment, and cytoskeleton organization of vascular endothelial cells is well known,134 and microfluidic devices have been extensively used in investigating the underlying mechanisms.135 The fluid dynamics of circulating medium may also carry important implications for circulating cells, such as circulating tumor cells (CTC) and immune cells, as a high level of shear stress can damage the cells in the device.136
Finally, correctly scaling the BOC systems is essential to achieve maximum physiological relevance. In allometric scaling principles, it is known that the sizes of different organs scale differently between animals of varying sizes.137 Basal metabolic rates of different organisms are also known to follow a three quarter power relationship with the masses of organisms.138 This issue of scaling becomes serious since BOC systems are generally highly miniaturized system of the human or animal body. Maintaining correct ratios of organ sizes, as well as transport rates between the organs is essential for replicating physiological responses, for example, pharmacokinetic response to drug administration 22,108. Several strategies of scaling and designing BOC systems have been suggested, but a more wide consensus needs to be formed.137,139,140 Establishing physiologically realistic ratios of liquid to cell volumes is also an important issue. In most cases, biochemical reactions are concentration dependent, which means that the ratio of liquid to cells can alter considerably the rates of reactions. In a traditional microwell plate-setting, cells are usually immersed in excess amount of liquid, resulting in the dilution of molecules. Comparing the liquid-to-cell ratios of various BOC systems revealed that the values of liquid per cells were considerably smaller than traditional macroscale systems, but still larger than human physiological values.3 However, attempts to meet the physiological level of liquid-to-cell ratio poses additional engineering problem, such as need to achieve sufficient supply of circulating medium, and analytical techniques to detect molecules with small quantities of sample.
6. Concluding remarks
As described, MPS and particularly BOC systems have been developing rapidly. The primary focus has been on using such systems in preclinical drug development to select which drug candidates are most likely to enter human clinical trials and become approved drugs. The focus has been on BOC systems using human cells to potentially predict efficacy and off-target toxicity during preclinical drug development studies. Almost all major pharmaceutical companies are exploring this technology, because they recognize its potential to improve the success rate of drug candidates in clinical trials. Even a modest improvement in success rate would provide more useful drugs to society and at a potentially reduced cost.
The acceptance of such new technology by the pharmaceutical industry will be involved with acceptance by regulatory bodies. Fortunately, FDA and its European counterpart, EMA, have been involved in six workshops with academic and industrial leaders on this technology.141 These workshops have begun a dialog among technology developers, regulators, and potential users on approaches to best bring this technology forward. In cases where the safety of a drug is already established, it is possible to use only in vitro data to extend its use to new applications.
While pharmaceutical firms are exploring this new technology, it is critical for MPS and BOC systems to reach the point where they are adopted as a standard component of the drug development process. The fact that it has been demonstrated that BOC systems could have predicted the failure of drugs in clinical trials or even those drugs that had to be withdrawn from the market is encouraging. As companies develop more confidence from tests of their own proprietary compounds, they will become more willing to adopt this technology in preclinical testing.
Of course, further improvement in MPS and BOC technologies is required for wide spread adoption of this technology. A primary constraint will be to reduce the cost of those systems, increase throughput and maintain relevant predictions. Many companies are working on methods to reduce cost significantly and increase throughput. Self-contained multiorgan systems with embedded sensors may be ideal. Additionally, advanced manufacturing techniques should reduce per unit costs significantly. A critical issue, and often a bottleneck, is development of biological organ modules that are robust, work in a common medium, and replicate key aspects of organ physiology. For example, iPS cell technology is improving rapidly. If one could derive all the relevant organs from a single genotype, it would make testing more reproducible. It would also open doors to use in precision medicine to find the best treatment option for individuals. Also, many of the drugs that fail in the market do so because a subpopulation responds adversely to the drug. One can imagine the use in drug development of iPS cells derived from a spectrum of subpopulations. These subpopulations can differ from one and another in many ways; for an example, they may differ in certain cytochrome P450 enzymes, which would profoundly affect drug metabolism. You could picture using perhaps 10 or 12 models from humans from diverse geographic locations or highly inbred populations. From such studies you might decide that a drug would be ideal for one subpopulation, but not useful or potentially toxic for another.
While this review has focused on preclinical drug development, those systems have the ability to test for the potential toxicity of chemicals, particularly in cosmetics and foods. Indeed, some firms in the cosmetic industry have invested significantly in MPS and BOC technology to predict human response to chemical additives. Ultimately, we believe that these systems are likely to reduce significantly the reliance on animal testing and to improve predictions concerning potential toxicity.
Overall, we believe that this technology is beginning to mature and to approach commercial adoption. There still remains much to do to increase reliability and decrease cost, but the large number of academic groups and companies involve suggest that creative solutions to any problems will be found.
Acknowledgments
The preparation of this review was supported by the National Institutes of Health (R44 TR001326, U01CA214300, and 1R44AG058330), Korea Institute for Advancement of Technology (Establishment of Infrastructure for Industrialization of Korean Useful Microbes, R0004073), National Research Foundation of Korea (2016R1D1A1B03934710), and Hongik University Research Fund.
Biographies
Jong Hwan Sung studied Chemical and Biological Engineering at Seoul National University (Korea), where he received his BS and MS degrees in 2000 and 2003, respectively. He studied Chemical and Biomolecular Engineering at Cornell University (USA), where he received his Ph.D. degree in 2009. He worked for one year as a postdoctoral researcher in Biological and Environmental Engineering Department at Cornell University until 2010. From 2010 to 2016 he has been working in Department of Chemical Engineering at Hongik University (Korea) as an Assistant Professor, and since 2016 he has been working as an Associate Professor. His current research area is in bioengineering, including development of body-on-a-chip systems for studying the first-pass metabolism and pharmacokinetics of drugs, and diseases related to obesity and fatty liver diseases.
Ying I. Wang received her Diploma and Master’s degrees in Biomedical Engineering at Zhejiang University (China) in 2005 and 2007, respectively. Wang continued her research at the University of California at Davis, where she studied the inflammatory basis of dietary lipids using in vitro vascular mimetic models and received her PhD degree in Biomedical Engineering in 2013. Wang then joined Dr. Michael Shuler’s lab at Cornell University as a postdoctoral research fellow. Wang’s current research focuses on the development of organ-on-a-chip and body-on-a-chip microphysiological systems for disease modeling and drug screening.
Narasimhan NS is a design and development engineer at Hesperos, Inc, the human-on-a-chip company based out of Orlando, FL, USA. He received his MS in bioengineering from the University of Illinois at Chicago in 2016 focusing on computational mechanics. He obtained his Bachelor of Technology in Biomedical engineering from Vellore institute of technology, India in 2014. His current research interests include PKPD modeling, computational mechanics, scientific computing and electronics design for electrophysiological recordings.
Max Jackson graduated with a degree in Psychology from the University of Central Florida in 2011. He worked at the Institute for Simulation and Training for 3 years before joining the Hybrid Systems Lab in 2014, finally joining Hesperos in 2017 where he works as a data analyst. His current research interests include computational neuroscience, brain-computer interfacing, and analytical automation. He has contributed to 3 peer reviewed publications in the field of drug delivery and pharmacokinetics.
Christopher J. Long attended Auburn University (USA) where he received his B.Mtl.E degree in Physics and Materials Science and Engineering in 2004, and the University of Florida (USA) where he received his M.S. and Ph.D. degrees in Materials Science and Engineering, in 2007 and 2009, respectively. His postdoctoral work at the University of Central Florida (USA) from 2010 through 2013 focused on bioMEMS systems and microfluidics for in vitro systems. As a Lead Development Engineer at Plasmonics, Inc. (Orlando, Florida) from 2013 to 2015, he led development of technologies for infrared sensors. As of 2015, he currently serves as Senior Staff Engineer at Hesperos, Inc. developing platforms and technologies for multi-organ human-on-a-chip systems.
James J. Hickman is the Founding Director of the NanoScience Technology Center and a Professor of Nanoscience Technology, Chemistry, Biomolecular Science, Material Science and Electrical Engineering at the University of Central Florida. Previously, he held the position of the Hunter Endowed Chair in the Bioengineering Department at Clemson University. Dr. Hickman has a Ph.D. from the Massachusetts Institute of Technology in Chemistry. For the past twenty-five years, he has been studying the interaction of biological species with modified surfaces, first in industry and in the latter years in academia. While in industry he established one of the first bioelectronics labs in the country that focused on cell-based sensors and their integration with electronic devices and MEMS devices. He is interested in creating hybrid systems for biosensor and biological computation applications and the creation of functional in vitro systems for human body-on-a-chip applications. He has worked at NSF and DARPA in the area of biological computation. He is also the founder and current Chief Scientist of a biotechnology company, Hesperos, which is focusing on cell-based systems for drug discovery and toxicity. He has 134 publications and 20 book chapters, in addition to 21 issued patents out of 44 total patent applications. He is a Fellow of both the American Institute of Medical and Biomedical Engineers (AIMBE) (2004) and the American Vacuum Society (AVS) (2007). He was a Board Member for AIMBE from 2009–2013 and Co-Chaired 6 AIMBE/NIH Workshops on “Validation and Qualification of New In Vitro Tools and Models for The Pre-clinical Drug Discovery Process” held at the NIH Campus, Bethesda, MD (2012 – 2017). He was also a Charter Member, NIH Bioengineering of Neuroscience, Vision and Low Vision Technologies (BNVT) Study Section. Dr. Hickman along with Dr. Michael Shuler, won the Lush Prize, in the Science Category, which Supports Animal Free Testing in 2015.
Michael L. Shuler is the Eckert Professor of Engineering, Emeritus in the Meinig Department of Biomedical Engineering and in the Smith School of Chemical and Biomolecular Engineering at Cornell University, and was director of Cornell’s Nanobiotechnology Center. Shuler has degrees in chemical engineering (BS, Notre Dame, 1969 and Ph.D., Minnesota, 1973) and has been a faculty member at Cornell University since 1974. Shuler’s research includes development of “Body-on-a-Chip” for testing pharmaceuticals for toxicity and efficacy, creation of production systems for useful compounds, such as paclitaxel from plant cell cultures, and construction of whole cell models relating genome to physiology. Shuler is CEO and President of Hesperos, a company founded to implement the “Body-on-a-Chip” system. Shuler, Kangi and Delisa have authored a popular textbook, “Bioprocess Engineering; Basic Concepts” now in its third edition. He has an honorary doctorate from the University of Notre Dame. Shuler has been elected to the National Academy of Engineering and the American Academy of Arts and Science and is a fellow of numerous professional societies.
Footnotes
Disclosure of Potential Conflict of Interest
The authors confirm that competing financial interests exist but there has been no financial support for this work that could have influenced its outcome. However, JJH and MLS have a potential competing financial interest, in that a company has been formed to market services for types of cells like this in body-on-a-chip devices.
References
- (1).Skardal A; Shupe T; Atala A Drug discovery today 2016, 21, 1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Greek R; Menache A International journal of medical sciences 2013, 10, 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wang YI; Carmona C; Hickman JJ; Shuler ML Adv Healthc Mater 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ishida S Drug Metab Pharmacokinet 2018, 33, 49–54. [DOI] [PubMed] [Google Scholar]
- (5).Lee SH; Sung JH Adv Healthc Mater 2018, 7. [DOI] [PubMed] [Google Scholar]
- (6).Sung JH; Wang YI; Kim JH; Lee JM; Shuler ML AIChE Journal 2018, 64, 4351–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sin A; Chin KC; Jamil MF; Kostov Y; Rao G; Shuler ML Biotechnol Prog 2004, 20, 338–345. [DOI] [PubMed] [Google Scholar]
- (8).Viravaidya K; Sin A; Shuler ML Biotechnol Prog 2004, 20, 316–323. [DOI] [PubMed] [Google Scholar]
- (9).Viravaidya K; Shuler ML Biotechnol Prog 2004, 20, 590–597. [DOI] [PubMed] [Google Scholar]
- (10).Santhanam N; Kumanchik L; Guo X; Sommerhage F; Cai Y; Jackson M; Martin C; Saad G; McAleer CW; Wang Y; Lavado A; Long CJ; Hickman JJ Biomaterials 2018, 166, 64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wagner I; Materne EM; Brincker S; Sussbier U; Fradrich C; Busek M; Sonntag F; Sakharov DA; Trushkin EV; Tonevitsky AG; Lauster R; Marx U Lab Chip 2013, 13, 3538–3547. [DOI] [PubMed] [Google Scholar]
- (12).Bauer S; Wennberg Huldt C; Kanebratt KP; Durieux I; Gunne D; Andersson S; Ewart L; Haynes WG; Maschmeyer I; Winter A; Ammala C; Marx U; Andersson TB Sci Rep 2017, 7, 14620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hubner J; Raschke M; Rutschle I; Grassle S; Hasenberg T; Schirrmann K; Lorenz A; Schnurre S; Lauster R; Maschmeyer I; Steger-Hartmann T; Marx U Sci Rep 2018, 8, 15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Coppeta JR; Mescher MJ; Isenberg BC; Spencer AJ; Kim ES; Lever AR; Mulhern TJ; Prantil-Baun R; Comolli JC; Borenstein JT Lab Chip 2016, 17, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zhang YS; Aleman J; Shin SR; Kilic T; Kim D; Mousavi Shaegh SA; Massa S; Riahi R; Chae S; Hu N; Avci H; Zhang W; Silvestri A; Sanati Nezhad A; Manbohi A; De Ferrari F; Polini A; Calzone G; Shaikh N; Alerasool P, et al. Proc Natl Acad Sci U S A 2017, 114, E2293–E2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Maoz BM; Herland A; FitzGerald EA; Grevesse T; Vidoudez C; Pacheco AR; Sheehy SP; Park TE; Dauth S; Mannix R; Budnik N; Shores K; Cho A; Nawroth JC; Segre D; Budnik B; Ingber DE; Parker KK Nat Biotechnol 2018, 36, 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Skardal A; Murphy SV; Devarasetty M; Mead I; Kang HW; Seol YJ; Shrike Zhang Y; Shin SR; Zhao L; Aleman J; Hall AR; Shupe TD; Kleensang A; Dokmeci MR; Jin Lee S; Jackson JD; Yoo JJ; Hartung T; Khademhosseini A; Soker S, et al. Sci Rep 2017, 7, 8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Xiao S; Coppeta JR; Rogers HB; Isenberg BC; Zhu J; Olalekan SA; McKinnon KE; Dokic D; Rashedi AS; Haisenleder DJ; Malpani SS; Arnold-Murray CA; Chen K; Jiang M; Bai L; Nguyen CT; Zhang J; Laronda MM; Hope TJ; Maniar KP, et al. Nat Commun 2017, 8, 14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Sung JH; Kam C; Shuler ML Lab Chip 2010, 10, 446–455. [DOI] [PubMed] [Google Scholar]
- (20).Chen HJ; Miller P; Shuler ML Lab Chip 2018, 18, 2036–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Choe A; Ha SK; Choi I; Choi N; Sung JH Biomed Microdevices 2017, 19, 4. [DOI] [PubMed] [Google Scholar]
- (22).Lee H; Kim DS; Ha SK; Choi I; Lee JM; Sung JH Biotechnol Bioeng 2017, 114, 432–443. [DOI] [PubMed] [Google Scholar]
- (23).Oleaga C; Riu A; Rothemund S; Lavado A; McAleer CW; Long CJ; Persaud K; Narasimhan NS; Tran M; Roles J; Carmona-Moran CA; Sasserath T; Elbrecht DH; Kumanchik L; Bridges LR; Martin C; Schnepper MT; Ekman G; Jackson M; Wang YI, et al. Biomaterials 2018, 182, 176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lai BFL; Huyer LD; Lu RXZ; Drecun S; Radisic M; Zhang BY Adv Funct Mater 2017, 27. [Google Scholar]
- (25).Oleaga C; Bernabini C; Smith AS; Srinivasan B; Jackson M; McLamb W; Platt V; Bridges R; Cai Y; Santhanam N; Berry B; Najjar S; Akanda N; Guo X; Martin C; Ekman G; Esch MB; Langer J; Ouedraogo G; Cotovio J, et al. Sci Rep 2016, 6, 20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Miller PG; Shuler ML Biotechnol Bioeng 2016, 113, 2213–2227. [DOI] [PubMed] [Google Scholar]
- (27).Esch MB; Ueno H; Applegate DR; Shuler ML Lab Chip 2016, 16, 2719–2729. [DOI] [PubMed] [Google Scholar]
- (28).Loskill P; Marcus SG; Mathur A; Reese WM; Healy KE PLoS One 2015, 10, e0139587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Frey O; Misun PM; Fluri DA; Hengstler JG; Hierlemann A Nat Commun 2014, 5, 4250. [DOI] [PubMed] [Google Scholar]
- (30).Edington CD; Chen WLK; Geishecker E; Kassis T; Soenksen LR; Bhushan BM; Freake D; Kirschner J; Maass C; Tsamandouras N; Valdez J; Cook CD; Parent T; Snyder S; Yu J; Suter E; Shockley M; Velazquez J; Velazquez JJ; Stockdale L, et al. Sci Rep 2018, 8, 4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Chen WLK; Edington C; Suter E; Yu J; Velazquez JJ; Velazquez JG; Shockley M; Large EM; Venkataramanan R; Hughes DJ; Stokes CL; Trumper DL; Carrier RL; Cirit M; Griffith LG; Lauffenburger DA Biotechnol Bioeng 2017, 114, 2648–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Tsamandouras N; Chen WLK; Edington CD; Stokes CL; Griffith LG; Cirit M AAPS J 2017, 19, 1499–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Esch MB; Prot JM; Wang YI; Miller P; Llamas-Vidales JR; Naughton BA; Applegate DR; Shuler ML Lab Chip 2015, 15, 2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wang YI; Abaci HE; Shuler ML Biotechnol Bioeng 2017, 114, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wang YI; Oleaga C; Long CJ; Esch MB; McAleer CW; Miller PG; Hickman JJ; Shuler ML Exp Biol Med (Maywood) 2017, 242, 1701–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Lee DW; Choi N; Sung JH Biotechnol Prog 2018. [DOI] [PubMed] [Google Scholar]
- (37).Wang YI; Shuler ML Lab Chip 2018, 18, 2563–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kimura H; Ikeda T; Nakayama H; Sakai Y; Fujii T Journal of laboratory automation 2015, 20, 265–273. [DOI] [PubMed] [Google Scholar]
- (39).Satoh T; Sugiura S; Shin K; Onuki-Nagasaki R; Ishida S; Kikuchi K; Kakiki M; Kanamori T Lab Chip 2017, 18, 115–125. [DOI] [PubMed] [Google Scholar]
- (40).Theobald J; Ghanem A; Wallisch P; Banaeiyan AA; Andrade-Navarro MA; Taškova K; Haltmeier M; Kurtz A; Becker H; Reuter S; Mrowka R; Cheng X; Wölfl S ACS Biomaterials Science & Engineering 2017, 4, 78–89. [DOI] [PubMed] [Google Scholar]
- (41).Moore N; Doty D; Zielstorff M; Kariv I; Moy LY; Gimbel A; Chevillet JR; Lowry N; Santos J; Mott V; Kratchman L; Lau T; Addona G; Chen H; Borenstein JT Lab Chip 2018, 18, 1844–1858. [DOI] [PubMed] [Google Scholar]
- (42).Kong J; Luo Y; Jin D; An F; Zhang W; Liu L; Li J; Fang S; Li X; Yang X; Lin B; Liu T Oncotarget 2016, 7, 78421–78432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Xu Z; Li E; Guo Z; Yu R; Hao H; Xu Y; Sun Z; Li X; Lyu J; Wang Q ACS Appl Mater Interfaces 2016, 8, 25840–25847. [DOI] [PubMed] [Google Scholar]
- (44).Mousavi Shaegh SA; De Ferrari F; Zhang YS; Nabavinia M; Binth Mohammad N; Ryan J; Pourmand A; Laukaitis E; Banan Sadeghian R; Nadhman A; Shin SR; Nezhad AS; Khademhosseini A; Dokmeci MR Biomicrofluidics 2016, 10, 044111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Moya A; Ortega-Ribera M; Guimerà X; Sowade E; Zea M; Illa X; Ramon E; Villa R; Gracia-Sancho J; Gabriel G Lab on a Chip 2018, 18, 2023–2035. [DOI] [PubMed] [Google Scholar]
- (46).Nunes SS; Miklas JW; Liu J; Aschar-Sobbi R; Xiao Y; Zhang B; Jiang J; Masse S; Gagliardi M; Hsieh A; Thavandiran N; Laflamme MA; Nanthakumar K; Gross GJ; Backx PH; Keller G; Radisic M Nature methods 2013, 10, 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Mannhardt I; Breckwoldt K; Letuffe-Breniere D; Schaaf S; Schulz H; Neuber C; Benzin A; Werner T; Eder A; Schulze T; Klampe B; Christ T; Hirt MN; Huebner N; Moretti A; Eschenhagen T; Hansen A Stem cell reports 2016, 7, 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Marsano A; Conficconi C; Lemme M; Occhetta P; Gaudiello E; Votta E; Cerino G; Redaelli A; Rasponi M Lab Chip 2016, 16, 599–610. [DOI] [PubMed] [Google Scholar]
- (49).Maoz BM; Herland A; Henry OYF; Leineweber WD; Yadid M; Doyle J; Mannix R; Kujala VJ; FitzGerald EA; Parker KK; Ingber DE Lab Chip 2017, 17, 2294–2302. [DOI] [PubMed] [Google Scholar]
- (50).Nawroth JC; Scudder LL; Halvorson RT; Tresback J; Ferrier JP; Sheehy SP; Cho A; Kannan S; Sunyovszki I; Goss JA; Campbell PH; Parker KK Biofabrication 2018, 10, 025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Vernetti L; Gough A; Baetz N; Blutt S; Broughman JR; Brown JA; Foulke-Abel J; Hasan N; In J; Kelly E; Kovbasnjuk O; Repper J; Senutovitch N; Stabb J; Yeung C; Zachos NC; Donowitz M; Estes M; Himmelfarb J; Truskey G, et al. Sci Rep 2017, 7, 42296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Qian F; Huang C; Lin YD; Ivanovskaya AN; O’Hara TJ; Booth RH; Creek CJ; Enright HA; Soscia DA; Belle AM; Liao R; Lightstone FC; Kulp KS; Wheeler EK Lab Chip 2017, 17, 1732–1739. [DOI] [PubMed] [Google Scholar]
- (53).Steinbeck JA; Jaiswal MK; Calder EL; Kishinevsky S; Weishaupt A; Toyka KV; Goldstein PA; Studer L Cell stem cell 2016, 18, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Stancescu M; Molnar P; McAleer CW; McLamb W; Long CJ; Oleaga C; Prot JM; Hickman JJ Biomaterials 2015, 60, 20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Moutaux E; Charlot B; Genoux A; Saudou F; Cazorla M Lab on a Chip 2018, 18, 3425–3435. [DOI] [PubMed] [Google Scholar]
- (56).Elbrecht DH; Long CJ; Hickman JJ J Rare Dis Res Treat. 2016, 1, 46–52. [Google Scholar]
- (57).Alexander FA; Eggert S; Wiest J Genes 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Sriram G; Massimo A; Dancik Y; Wu B; Wu R; Feng Z; Ramasamy S; Bigliardi P; Bigliardi-Qi M; Wang Z Materials Today 2018, 21, 326–340. [Google Scholar]
- (59).Misun PM; Rothe J; Schmid YRF; Hiermelann A; Frey O Microsystems & Nanoengineering 2016, 2, 160222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Bavli D; Prill S; Ezra E; Levy G; Cohen M; Vinken M; Vanfleteren J; Jaeger M; Nahmias Y Proc Natl Acad Sci U S A 2016, 113, E2231–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Curto VF; Marchiori B; Hama A; Pappa A-M; Ferro MP; Braendlein M; Rivnay J; Fiocchi M; Malliaras GG; Ramuz M; Owens RM Microsystems &Amp; Nanoengineering 2017, 3, 17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Towfigh S; Heisler T; Rigberg DA; Hines OJ; Chu J; McFadden DW; Chandler C The Journal of surgical research 2000, 88, 160–164. [DOI] [PubMed] [Google Scholar]
- (63).Lozoya-Agullo I; Araujo F; Gonzalez-Alvarez I; Merino-Sanjuan M; Gonzalez-Alvarez M; Bermejo M; Sarmento B Molecular pharmaceutics 2017, 14, 1264–1270. [DOI] [PubMed] [Google Scholar]
- (64).Agoram BM; Martin SW; van der Graaf PH Drug discovery today 2007, 12, 1018–1024. [DOI] [PubMed] [Google Scholar]
- (65).Bergman RN Diabetes 2007, 56, 1489–1501. [DOI] [PubMed] [Google Scholar]
- (66).Schett G; Elewaut D; McInnes IB; Dayer JM; Neurath MF Nature medicine 2013, 19, 822–824. [DOI] [PubMed] [Google Scholar]
- (67).Cedersund G; Stralfors P European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences 2009, 36, 91–104. [DOI] [PubMed] [Google Scholar]
- (68).Dubiak-Szepietowska M; Karczmarczyk A; Jonsson-Niedziolka M; Winckler T; Feller KH Toxicology and applied pharmacology 2016, 294, 78–85. [DOI] [PubMed] [Google Scholar]
- (69).Rojkind M; Novikoff PM; Greenwel P; Rubin J; Rojas-Valencia L; de Carvalho AC; Stockert R; Spray D; Hertzberg EL; Wolkoff AW The American journal of pathology 1995, 146, 1508–1520. [PMC free article] [PubMed] [Google Scholar]
- (70).Begue JM; Guguen-Guillouzo C; Pasdeloup N; Guillouzo A Hepatology 1984, 4, 839–842. [DOI] [PubMed] [Google Scholar]
- (71).Khetani SR; Bhatia SN Nat Biotechnol 2008, 26, 120–126. [DOI] [PubMed] [Google Scholar]
- (72).Du Y; Li N; Yang H; Luo C; Gong Y; Tong C; Gao Y; Lu S; Long M Lab Chip 2017, 17, 782–794. [DOI] [PubMed] [Google Scholar]
- (73).Kang YB; Sodunke TR; Lamontagne J; Cirillo J; Rajiv C; Bouchard MJ; Noh M Biotechnol Bioeng 2015, 112, 2571–2582. [DOI] [PubMed] [Google Scholar]
- (74).Prodanov L; Jindal R; Bale SS; Hegde M; McCarty WJ; Golberg I; Bhushan A; Yarmush ML; Usta OB Biotechnol Bioeng 2016, 113, 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Mi S; Yi X; Du Z; Xu Y; Sun W Biofabrication 2018, 10, 025010. [DOI] [PubMed] [Google Scholar]
- (76).van Grunsven LA Advanced drug delivery reviews 2017, 121, 133–146. [DOI] [PubMed] [Google Scholar]
- (77).Farghali H; Kgalalelo Kemelo M; Wojnarova L; Kutinova Canova N Physiological research 2016, 65, S417–S425. [DOI] [PubMed] [Google Scholar]
- (78).Gural N; Mancio-Silva L; He J; Bhatia SN Cellular and molecular gastroenterology and hepatology 2018, 5, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Khetani SR; Berger DR; Ballinger KR; Davidson MD; Lin C; Ware BR Journal of laboratory automation 2015, 20, 216–250. [DOI] [PubMed] [Google Scholar]
- (80).Ware BR; Khetani SR Trends in biotechnology 2017, 35, 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Wojtowicz AM; Oliveira S; Carlson MW; Zawadzka A; Rousseau CF; Baksh D Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 2014, 22, 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Carlson MW; Alt-Holland A; Egles C; Garlick JA Current protocols in cell biology 2008, Chapter 19, Unit 19 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Hill DS; Robinson ND; Caley MP; Chen M; O’Toole EA; Armstrong JL; Przyborski S; Lovat PE Molecular cancer therapeutics 2015, 14, 2665–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Chau DY; Johnson C; MacNeil S; Haycock JW; Ghaemmaghami AM Biofabrication 2013, 5, 035011. [DOI] [PubMed] [Google Scholar]
- (85).Pupovac A; Senturk B; Griffoni C; Maniura-Weber K; Rottmar M; McArthur SL Adv Healthc Mater 2018, 7, e1701405. [DOI] [PubMed] [Google Scholar]
- (86).Groeber F; Engelhardt L; Lange J; Kurdyn S; Schmid FF; Rucker C; Mielke S; Walles H; Hansmann J Altex 2016, 33, 415–422. [DOI] [PubMed] [Google Scholar]
- (87).Lee S; Jin SP; Kim YK; Sung GY; Chung JH; Sung JH Biomed Microdevices 2017, 19, 22. [DOI] [PubMed] [Google Scholar]
- (88).Wufuer M; Lee G; Hur W; Jeon B; Kim BJ; Choi TH; Lee S Sci Rep 2016, 6, 37471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Kovacic P; Somanathan R Reviews of environmental contamination and toxicology 2010, 203, 119–138. [DOI] [PubMed] [Google Scholar]
- (90).Martel BC; Lovato P; Baumer W; Olivry T The Yale journal of biology and medicine 2017, 90, 389–402. [PMC free article] [PubMed] [Google Scholar]
- (91).Lee S; Ko J; Park D; Lee SR; Chung M; Lee Y; Jeon NL Lab Chip 2018, 18, 2686–2709. [DOI] [PubMed] [Google Scholar]
- (92).Herland A; van der Meer AD; FitzGerald EA; Park TE; Sleeboom JJ; Ingber DE PLoS One 2016, 11, e0150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Cho H; Seo JH; Wong KH; Terasaki Y; Park J; Bong K; Arai K; Lo EH; Irimia D Sci Rep 2015, 5, 15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Adriani G; Ma D; Pavesi A; Kamm RD; Goh EL Lab Chip 2017, 17, 448–459. [DOI] [PubMed] [Google Scholar]
- (95).Huh D; Leslie DC; Matthews BD; Fraser JP; Jurek S; Hamilton GA; Thorneloe KS; McAlexander MA; Ingber DE Science translational medicine 2012, 4, 159ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Huh D; Matthews BD; Mammoto A; Montoya-Zavala M; Hsin HY; Ingber DE Science 2010, 328, 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Bersini S; Jeon JS; Moretti M; Kamm RD Drug discovery today 2014, 19, 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Niu Y; Bai J; Kamm RD; Wang Y; Wang C Molecular pharmaceutics 2014, 11, 2022–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Bersini S; Jeon JS; Dubini G; Arrigoni C; Chung S; Charest JL; Moretti M; Kamm RD Biomaterials 2014, 35, 2454–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Hao S; Ha L; Cheng G; Wan Y; Xia Y; Sosnoski DM; Mastro AM; Zheng SY Small 2018, 14, e1702787. [DOI] [PubMed] [Google Scholar]
- (101).Skardal A; Devarasetty M; Forsythe S; Atala A; Soker S Biotechnol Bioeng 2016, 113, 2020–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Akbari E; Spychalski GB; Song JW Microcirculation 2017, 24. [DOI] [PubMed] [Google Scholar]
- (103).Zheng Y; Sun Y; Yu X; Shao Y; Zhang P; Dai G; Fu J Adv Healthc Mater 2016, 5, 1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Chung M; Ahn J; Son K; Kim S; Jeon NL Adv Healthc Mater 2017, 6. [DOI] [PubMed] [Google Scholar]
- (105).Lee H; Park W; Ryu H; Jeon NL Biomicrofluidics 2014, 8, 054102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Sung JH; Shuler ML Lab Chip 2009, 9, 1385–1394. [DOI] [PubMed] [Google Scholar]
- (107).Lau YY; Chen YH; Liu TT; Li C; Cui X; White RE; Cheng KC Drug metabolism and disposition: the biological fate of chemicals 2004, 32, 937–942. [PubMed] [Google Scholar]
- (108).Lee DW; Ha SK; Choi I; Sung JH Biomed Microdevices 2017, 19, 100. [DOI] [PubMed] [Google Scholar]
- (109).Bricks T; Hamon J; Fleury MJ; Jellali R; Merlier F; Herpe YE; Seyer A; Regimbeau JM; Bois F; Leclerc E Biopharmaceutics & drug disposition 2015, 36, 275–293. [DOI] [PubMed] [Google Scholar]
- (110).Jellali R; Bricks T; Jacques S; Fleury MJ; Paullier P; Merlier F; Leclerc E Biopharmaceutics & drug disposition 2016, 37, 264–275. [DOI] [PubMed] [Google Scholar]
- (111).Prot JM; Maciel L; Bricks T; Merlier F; Cotton J; Paullier P; Bois FY; Leclerc E Biotechnol Bioeng 2014, 111, 2027–2040. [DOI] [PubMed] [Google Scholar]
- (112).An F; Qu Y; Luo Y; Fang N; Liu Y; Gao Z; Zhao W; Lin B Sci Rep 2016, 6, 25022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Lee SY; Sung JH Biotechnology and bioengineering 2018. [DOI] [PubMed] [Google Scholar]
- (114).Marx U; Andersson TB; Bahinski A; Beilmann M; Beken S; Cassee FR; Cirit M; Daneshian M; Fitzpatrick S; Frey O; Gaertner C; Giese C; Griffith L; Hartung T; Heringa MB; Hoeng J; de Jong WH; Kojima H; Kuehnl J; Leist M, et al. Altex 2016, 33, 272–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Maschmeyer I; Hasenberg T; Jaenicke A; Lindner M; Lorenz AK; Zech J; Garbe LA; Sonntag F; Hayden P; Ayehunie S; Lauster R; Marx U; Materne EM European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V 2015, 95, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Maschmeyer I; Lorenz AK; Schimek K; Hasenberg T; Ramme AP; Hubner J; Lindner M; Drewell C; Bauer S; Thomas A; Sambo NS; Sonntag F; Lauster R; Marx U Lab Chip 2015, 15, 2688–2699. [DOI] [PubMed] [Google Scholar]
- (117).Konrad D; Wueest S Physiology 2014, 29, 304–313. [DOI] [PubMed] [Google Scholar]
- (118).Federico A; Dallio M; Godos J; Loguercio C; Salomone F Translational research : the journal of laboratory and clinical medicine 2016, 167, 116–124. [DOI] [PubMed] [Google Scholar]
- (119).Dehne EM; Hasenberg T; Marx U Future Sci OA 2017, 3, FSO185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Ewart L; Dehne EM; Fabre K; Gibbs S; Hickman J; Hornberg E; Ingelman-Sundberg M; Jang KJ; Jones DR; Lauschke VM; Marx U; Mettetal JT; Pointon A; Williams D; Zimmermann WH; Newham P Annu Rev Pharmacol Toxicol 2018, 58, 65–82. [DOI] [PubMed] [Google Scholar]
- (121).Maschmeyer I; Lorenz AK; Lauster R; Marx U Drug Metabolism and Pharmacokinetics 2017, 32, S25–S26. [Google Scholar]
- (122).Materne EM; Ramme AP; Terrasso AP; Serra M; Alves PM; Brito C; Sakharov DA; Tonevitsky AG; Lauster R; Marx U Journal of biotechnology 2015, 205, 36–46. [DOI] [PubMed] [Google Scholar]
- (123).Du G; Fang Q; den Toonder JM Analytica chimica acta 2016, 903, 36–50. [DOI] [PubMed] [Google Scholar]
- (124).Chong LH; Li H; Wetzel I; Cho H; Toh YC Lab on a chip 2018. [DOI] [PubMed] [Google Scholar]
- (125).Vunjak-Novakovic G; Bhatia S; Chen C; Hirschi K Stem cell research & therapy 2013, 4 Suppl 1, S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Li AP; Bode C; Sakai Y Chemico-biological interactions 2004, 150, 129–136. [DOI] [PubMed] [Google Scholar]
- (127).Guo X; Colon A; Akanda N; Spradling S; Stancescu M; Martin C; Hickman JJ Biomaterials 2017, 122, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Davis H; Gonzalez M; Stancescu M; Love R; Hickman JJ; Lambert S Biomaterials 2014, 35, 8840–8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Lee SH; Shim KY; Kim B; Sung JH Biotechnol Prog 2017, 33, 580–589. [DOI] [PubMed] [Google Scholar]
- (130).Sweeney LM; Shuler ML; Babish JG; Ghanem A Toxicol In Vitro 1995, 9, 307–316. [DOI] [PubMed] [Google Scholar]
- (131).van Midwoud PM; Janse A; Merema MT; Groothuis GM; Verpoorte E Analytical chemistry 2012, 84, 3938–3944. [DOI] [PubMed] [Google Scholar]
- (132).Xu H; Shuler ML Biotechnol Prog 2009, 25, 543–551. [DOI] [PubMed] [Google Scholar]
- (133).van Meer BJ; de Vries H; Firth KSA; van Weerd J; Tertoolen LGJ; Karperien HBJ; Jonkheijm P; Denning C; AP IJ; Mummery CL Biochemical and biophysical research communications 2017, 482, 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Wang C; Baker BM; Chen CS; Schwartz MA Arteriosclerosis, thrombosis, and vascular biology 2013, 33, 2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Bogorad MI; DeStefano J; Karlsson J; Wong AD; Gerecht S; Searson PC Lab Chip 2015, 15, 4242–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Regmi S; Fu A; Luo KQ Sci Rep 2017, 7, 39975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Wikswo JP; Curtis EL; Eagleton ZE; Evans BC; Kole A; Hofmeister LH; Matloff WJ Lab Chip 2013, 13, 3496–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Ahluwalia A Sci Rep 2017, 7, 42113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Abaci HE; Shuler ML Integrative biology : quantitative biosciences from nano to macro 2015, 7, 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Moraes C; Labuz JM; Leung BM; Inoue M; Chun TH; Takayama S Integrative biology : quantitative biosciences from nano to macro 2013, 5, 1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).A series of 6 workshops by AIMBE/NIH on “Validation and Qualification of New In Vitro Tools and Models for the Pre-clinical Drug Discovery Process” between March 19, 2012 and May 25–26, 2017.