Abstract
Objective(s)
To study if the GnRH agonist administration in luteal phase improves clinical pregnancy rate of fresh and frozen embryo transfer. Also, this meta-analysis compares the treatment effect of luteal GnRH agonist administration between long agonist and antagonist protocols of fresh cycles, and between two types of treatment: fresh and frozen embryo transfers.
Study design
Systematic review and meta-analysis (registration number CRD42017059152)
Results
For the overall 20 studies (5497 patients), clinical pregnancy rate significantly increased in group of GnRH agonist administration compared to control group (RR 1.24, 95% CI 1.14–1.34, p < 0.0001). Regarding the treatment effect of luteal GnRH agonist administration between long agonist and antagonist protocol fresh cycles, no significant difference was observed (RR = 1.28, 95% CI 0.98–1.67, p = 0.07). Also, in comparison between fresh and frozen embryo transfer, similar effect of GnRH agonist administration was found (RR = 0.93, 95% CI 0.74–1.16, p = 0.49).
Conclusion(s)
There is evidence that GnRH agonist administration in luteal phase improve clinical pregnancy rate in both fresh and frozen cycles. Within fresh cycles, no significant difference of clinical pregnancy rate is found between two protocols. In frozen cycles, the effect of GnRH agonist administration in enhancing clinical pregnancy rate is similar to fresh cycles.
Introduction
Successful implantation is an essential outcome in ART treatment. However, with great effort to improve, this outcome is still limited. In fact, among cases transferred with one or more embryos, less than one-third results in a live birth [[1], [2], [3]] Adequate luteal phase support is one of the acceptable solutions to improve implantation and pregnancy rates [4,5] because luteal phase in fresh or frozen cycles is deficient.
It is well established that following ovarian stimulation is an insufficient luteal phase [6,7]. LH concentrations are low during the luteal phase due to negative feedback on pituitary gland of supra-physiological serum levels of steroids which are secreted by multiple corpora lutea [6,8,9]. LH plays a substantial role to sustain corpus luteum function as well as to enhance angiogenic factors, growth factors, and cytokines that may benefit the implantation [10,11]. Inhibit LH release results in premature luteolysis or significant reduced luteal phase length [12]. Furthermore, in artificial frozen-thawed embryo transfer (FET), the current trend of assisted reproduction practice [13,14], there may be no corpus luteum. Therefore, fertility treatment with fresh or FET cycles requires adequate luteal support.
GnRH agonist (GnRHa) has been reported arguably to support the luteal phase [15,16]. On the one hand, it is believed that GnRHa with an appropriate dose may retain its stimulatory effect to preserve LH production to support the luteal phase [17]. Moreover, GnRHa may have a direct effect on early embryos in improving the implantation [16]. On the other hand, GnRHa is reported to inhibit progesterone production in human granulosa cell, and increase apoptosis in granulosa cells [18]. They cause unfavorable outcomes in IVF. Therefore, whether GnRHa administration during luteal phase is useful or not is still in debate.
Some early meta-analyses reported the positive effect of mid-luteal GnRHa administration [19,20]. Very few of them, however, reported the results of luteal phase support on ovary stimulation with GnRH antagonist protocol. Moreover, they did not compare the treatment effect among protocols or include FET cycles. With more relevant data available, this meta-analysis will update early meta-analyses, compare treatment effect of mid-luteal GnRHa administration on clinical pregnancy between two protocols, long agonist (LGA) and antagonist (AG), and two types of treatments: fresh and frozen embryo transfers.
Materials and methods
Protocol registration
This meta-analysis was performed according to PRISMA statement. The protocol was registered at the international prospective register of systematic reviews (PROSPERO) and accepted with registration number CRD42017059152.
Eligibility criteria
For fresh cycles, we include randomized control trials (RCTs), quasi-randomised control studies to assess the effects of the GnRHa in luteal phase. There were only few RCTs about luteal GnRHa in frozen transfer, so for frozen embryo transfer cycles, we include randomized control trials, quasi-randomised control studies and prospective cohort studies. Studies examining women undergoing ART treatment with fresh or frozen embryo transfer cycles (including oocyte recipient cycles) were eligible for the review. The maximum age for fresh cycles was 41 and for frozen cycles was 50. The intervention was addition of GnRHa during the luteal phase. GnRHa can be used together with long agonist (LGA) or antagonist protocol (AG) with the single dose or multi-dose. The addition of GnRHa in the luteal phase was compared with standard luteal phase support (with or without placebo). Exclusion criteria included:
-
•
Studies in which the ovarian stimulation protocols were different between the study group and the control group
-
•
The studies in which the drugs for final maturation of oocytes were different between the control group and the GnRH agonist group
-
•
The studies in which final maturation of oocytes was reported using GnRH agonist
-
•
Unpublished studies
Information sources – search strategies – selection process
A computerized literature search in PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE and RCT registries (clinicaltrials.gov) covering the period up to March 2019 was performed. Additionally, we hand-searched more potential eligible studies among references of articles we found
We used the following terms: ((in vitro fertilization) or (intracytoplasmic sperm injection) or (frozen-thawed embryo transfer) or (oocyte recipients/donor cycles/ovum donation cycles) or (embryo)) and ((agonist) or (buserelin) or (goserelin) or (leuprolide) or (nafarelin) or (triptorelin)) and (luteal) and (random* OR trial OR prospective OR cohort). There was no language or publication date restriction.
Two independent reviewers (L.T.M.C and D.K.T) screened titles and abstracts in each database, retrieved full-text articles considered to be potentially eligible and evaluated the eligibility of these studies. Our search results were checked against references listed in previous meta-analyses to ensure all relevant studies were found. The two reviewers checked the other’s retrieved titles to remove duplicates. Disagreements between two reviewers were solved by consensus.
Data collection process and items
Two reviewers also independently extracted data from included trials. With a study with multiple records, we used the most recent and most detailed publication. If more information was required, we contacted the study authors by email.
The data extracted from each trial included: authors; country; center; funding; study period; randomization method; eligible criteria for patients; luteal phase support; number of patients in each group; age; body mass index (BMI); number of embryo transferred. For fresh embryo transfer cycles, we also extracted: ovarian stimulation protocol; triggering drug; fertilization methods; number of oocytes retrieved. For frozen embryo transfer cycles, data extracted also included endometrial preparation and endometrial thickness.
Outcomes and prioritization
Clinical pregnancy among participating women is the primary outcome. Clinical pregnancy is defined as the presence of the intrauterine gestational sac with positive heartbeat. When clinical pregnancies were not reported, we used ongoing pregnancies as primary outcome. Ongoing pregnancy was defined as pregnancy proceeding beyond the 20th gestational week.
Risk of bias in individual studies
Two reviewers independently assessed the risk of bias in individual studies: selection bias (random sequence generation, allocation concealment), performance bias, detection bias, attrition bias, reporting bias and other bias. We followed the Cochrane Collaboration’s criteria for assessment, in which each risk was classified into 3 categories: low risk, high risk or unclear. Disagreements between two reviewers were reconciled by consensus. According to the 7 risks of bias above, we classify the quality of studies into 4 grades: grade 1 included randomized control trials (RCTs) with no high risk, grade 2 included RCTs with at least one high risk, grade 3 included quasi-RCTs, grade 4 included cohort study
Data synthesis
All statistical analyses were performed using R statistical packages (release 2.15.2) [21,22]. The full analysis set from the studies was used because it is as close as possible to the intention-to-treat [ITT] principle of including all randomized patients. In this analysis, the ITT population consisted of all randomly allocated patients.
The meta-analysis used a random effects model because unclear homogeneity of baseline conditions exist. Random model generally produce a more conservative assessment, however, in case of homogeneity, the power is similar to a fixed model. Random effects model was calculated using both the restricted maximum likelihood (REML) and the DerSimonian and Laird approach [23]. Meta-regression on the ITT dataset considered pre-specified relevant covariates. Three covariates were selected: Publication year, type (LGA, AG, FET), and methodological quality was tested as a possible linear moderator. Clinical pregnancy or ongoing pregnancy (in one study when clinical pregnancy was not available) was our main endpoint. For binary variables (e.g., clinical pregnancy), the risk ratio (RR) was evaluated as the main calculation of effect size [24]. To assess the heterogeneity across the studies, we used I2 and Q-test.
The methodological quality of selected studies was evaluated using three validity domains (internal, external, and statistical validity [25,26]). Domain-based evaluations (17 items) were performed by two investigators. Any discordance between the reviewers was discussed and resolved via consensus and summarized by an Internal validity score (IVS) that included seven items, an external validity score (EVS) that included five items, and statistical validity score (SVS) that included five items. For studies with at least four inadequate items, a sensitivity analysis was conducted with and without these items and results considered reliable when the two selections provided the same conclusions. The internal and external validity of the meta-analysis were optimized by maximizing the sample size and controlling for bias. Sources of external bias were assessed to determine their possible impact on the observed effect size. The risk of publication bias was assessed using the funnel plot method.
Meta-bias
To avoid multiple-publication bias across studies, we checked for duplicates in records of eligible studies. To identify the selective reporting bias, outcomes in the protocol and in the published article were compared if study protocol was available. If not, outcomes listed in the methods section were compared with those reported in the results.
Results
Studies and patients
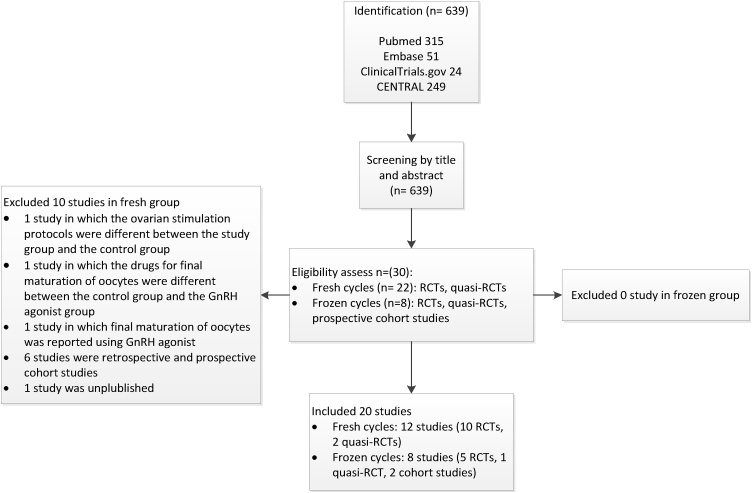
Of the 639 publications initially identified, there were 20 eligible published studies. In fresh cycle group, we have 10 RCTs and 2 quasi-RCTs. In frozen cycle group, we have 5 RCTs,1 quasi-RCTs and 2 cohort studies. All studies were published in English, except one study in French in frozen group. This study was translated into English before assessing study quality (Fig. 1).
Fig. 1.
Study flow diagram.
In total, data from 5497 patients undergoing ovarian stimulation for IVF/ICSI were available for analysis. Data for the co-primary endpoints were available. A summary of the studies (n = 20), is shown in Table 1.
Table 1.
Summary of studies.
| Study | Number of patient in GnRH agonist group | Number of patient in control group | Center | Funding | Methodology | Patient population | Study endpoints | Results | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Fujii 2001 [15] | 161 | 158 | Single center | NR | Allocation by patient’s identification number (odd and even) | Women undergoing IVF/ICSI Age 33.6 ± 0.3 |
Implantation rate Live birth rate per embryo transferred Clinical pregnancy rate |
CPR: 44.5% vs 34.3%, p = 0.05 LBR per transferred embryos: 23.6% vs 15.7%, p < 0.05 |
| 2 | Tesarik 2006 [27] | 300 | 300 | NR | NR | Allocation by computer-generated randomization and sealed envelopes | Women undergoing ICSI. Excluded women > 40 years of age and non-obstructive azoospermia requiring testicular sperm retrieval |
Implantation rate Clinical pregnancy rate Ongoing pregnancy rate Live birth rate |
CPR 48.0% vs 39.3%, NS for long agonist protocol CPR 46.0% vs 36.0%, NS for GnRH antagonist protocol |
| 3 | Isikoglu 2007 [28] | 90 | 91 | Single center | NR | Allocation by computer-generated randomization | Women undergoing ICSI: Age 30.1 ± 4.9; BMI 24.2 ± 3.9 kg/m2 | Implantation rate Clinical pregnancy rate Live birth rate |
CPR: 44.88% vs 49.45%, p = 0.94 LBR: 37.77% vs 35.16%, p = 0.83 |
| 4 | Qublan 2008 [29] | 60 | 60 | Single center | NR | Allocation by table of random numbers available in statistics textbook Sealed, numbered opaque envelopes |
Basal FSH ≤ 10 IU/l, P ≤ 1.4 ng/ml, 19 – 36 years, BMI 19 – 29 kg/m2, presence of both ovaries, ≤ 3 prior cycles, endometrium thickness ≤ 7mm | Implantation rate Clinical pregnancy rate Live birth rate |
CPR 36.6% vs 13.3%, p < 0.01 LBR 31.6% vs 5.0%, p < 0.001 |
| 5 | Ata008 [30] | 285 | 285 | Single center | NR | Allocation by computer-generated randomization Opaque and sealed envelopes | Couples undergoing ART with their own gametes and having at least one embryo available for transfer Age 30.7 – 31.9; BMI 23.1 – 24.1 kg/m2 |
Implantation rate Clinical pregnancy rate Ongoing pregnancy rate |
CPR 42.8% vs 42.1%, p = 0.86 Ong PR 31.2% vs 29.5%, p = 0.65 |
| 6 | Isik 2009 [31] | 82 | 82 | NR | NR | Allocation by computer-generated randomization | Women undergoing ICSI Age 35.56 ± 4.46 Excluded donor, freeze-thaw cycles and surgical sperm extraction |
Implantation rate Clinical pregnancy rate Live birth rate per embryo transferred |
CPR 40.5% vs 20.0%, p = 0.0055 LBR per embryo transferred 35.1% vs 16.3%, p = 0.007 |
| 7 |
Razieh 2009 [32] |
90 | 90 | Single center | Grant from Research and Clinical Center for Infertility, Iran | Allocation by drawing a piece of paper from a bag containing equal numbers of papers assigned to each group | Women undergoing ICSI. Excluded women > 40 years old, poor responders in previous cycles | Implantation rate Clinical pregnancy rate |
CPR 25.5% vs 10.0%, p = 0.015 |
| 8 |
Inamdar 2012 [33] |
213 | 213 | Single center | NR | Allocation by computer-generated randomization | Couples undergoing ART with at least one good embryo available for transfer. Excluded women > 38 years, untreated hydrosalpinx, fibroid > 4 cm, adenomyosis, endometrium < 6mm |
Implantation rate Clinical pregnancy rate Ongoing pregnancy rate |
Ong PR 27.69% vs 26.29%, p = 0.827 CPR 36.62% vs 38.21%, p = 0.841 |
| 9 | Brigante 2013 [34] | 29 | 33 | Single center | NR | Allocation method not reported | Women undergoing IVF/ICSI | Clinical pregnancy rate Ongoing pregnancy rate Implantation rate |
CPR 51.7% vs 24.2%, p < 0.02 Ong PR 44.8% vs 18.2%, p < 0.02 |
| 10 | Yildiz 2014 [35] | 200 | 100 | Single center | NR | Allocation by computer-generated randomization | ICSI cycles without gamete donation, day 3 FSH level ≤ 10 IU/l, age 20 – 40, BMI 20 – 30 kg/m2, and presence of both ovaries | Implantation rate Clinical pregnancy rate Ongoing pregnancy rate Miscarriage rate |
Ong PR 36.0% in Group A and 42.9% in Group B, 27.4% in control group, p = 0.093 CPR 40.0% in Group A and 46.4% in Group B, 31.6% in control group, p = 0.123 |
| 11 |
Zafardoust 2015 [36] |
50 | 50 | Single center | NR | Allocation by computer-generated randomization | < 42 years old, basal FSH levels < 12mIU/ml; ≥2 previous IVF/ICSI failures | Implantation rate Clinical pregnancy rate |
CPR 14% vs 7.5%, p = 0.485 |
| 12 | Aboulghar 2015 [37] | 224 | 222 | Single center | NR | Allocation by computer-generated randomization. Dark, sealed envelopes | Women undergoing IVF/ICSI cycles. Excludes patients > 39 years, previous ≥2 failed cycles, uterine anomalies | Clinical pregnancy rate Ongoing pregnancy rate |
CPR 36.2% vs 30.6%, NS Ong PR 30.4% vs. 25.7%, NS |
| 13 | Tesarik 2004 [16] | 138 | 138 | NR | NR | Allocation based on alphabetical order of the patients’ surname | Oocyte donors: age 19-37, basal FSH < 7 IU/l, AFC> 10 Oocyte recipients: age: 31-50, repeated assisted reproduction failures with their own oocytes, non-ovulating women with premature or physiological menopause. |
Implantation rate Delivery rate Miscarriage rate |
CPR: 67.4% vs 54.3% LBR: 60.9% vs. 48.6% Birth rate: 31.1% vs 21.5%, p < 0.05 |
| 14 | Check. J.H 2015 [38] | 36 | 76 | NR | NR | Allocation based on patients’ decision | Recipients receiving donor oocytes | Implantation rate Clinical pregnancy rate Live birth rate |
CPR: 63.9% vs 39.5%, p = 0.027 LBR: 52.8% vs 32.9%, p = 0.009 |
| 15 | Casanova 2015 [39] | 43 | 35 | Single center | NR | The study group was conformed according to patients’ agreement | Ovum recipients with at least one good quality embryo (Age 41.1 – 42.1) Severe male factors were excluded |
Clinical pregnancy rate Implantation rate Abortion rate |
CPR: 35.5% vs 34.4%, p = 0.809 |
| 16 | Davar 2015 [40] | 101 | 100 | Single center | Financial supporter was Research and Clinical Center for Infertility, Iran | Allocation by computer-generated randomization | Frozen-thawed embryo transfer cycles. Excluded women <18 and >40 years of age, oocyte recipients, systemic or endocrine disorders, endometriosis, submucous fibroids, intrauterine adhesions | Clinical pregnancy rate Implantation rate Ongoing pregnancy rate Abortion rate |
CPR: 26% vs 21%, p = 0.40 Ong PR: 22% vs 17%, p = 0.37 |
| 17 | Gogce 2015 [41] | 110 | 110 | Single center | NR | Randomisation with sealed opaque envelopes | Women undergoing artificial cycle of frozen-thawed embryo transfer Age 33.35 – 33.63 BMI 22.06 – 23.51 kg/m2 |
Ongoing pregnancy rate Clinical pregnancy rate Implantation rate |
CPR: 23.4% vs 17.0%, p = 0.31 Ong PR: 17.0% vs. 10.6% p = 0.29 |
| 18 |
Seikula 2016 [42] |
65 | 65 | Multi centers (2) | Funded by the University Hospital of Turku (Finland) and Turku University Foundation | Randomisation with sealed opaque envelopes | Frozen-thawed embryo transfer cycles with natural menstrual cycles. Excluded women age > 42yrs, donated cycles, uterine cavity pathologies |
Clinical pregnancy rate Live birth rate Miscarriage rate Median birth weight |
CPR: 38.5% vs 27.4%, p = 0.199 LBR: 30.8% vs 24.2%, p = 0.481 |
| 19 |
Seikula 2018 [43] |
72 | 72 | Multi centers (2) | Funded by the University Hospital of Turku (Finland), Turku University Foundation and Finnish Fertility Society | Randomisation with sealed opaque envelopes | Frozen-thawed embryo transfer cycles with artificial cycles. Excluded women age > 42yrs, donated cycles, pathologies distorting the uterine cavity |
Clinical pregnancy rate Live birth rate Miscarriage rate |
CPR: 40.3% vs 36.1%, p = 0.693 LBR: 29.2% vs 19.4%, p = 0.113 |
| 20 | Ye 2019 [44] | 434 | 434 | Single center | NR | Allocation by computer-generated randomization | Women 20-37yrs, BMI ≤ 28 kg/m2 Excluded oocyte recipients, uterine abnormalitites |
Clinical pregnancy rate Ongoing pregnancy rate Implantation rate Miscarriage rate |
CPR: 57.7% vs 58.7%, p = 0.781 Ong PR: 46.7% vs 49.3% p = 0.478 |
CPR: Clinical pregnancy rate.
Ong PR: Ongoing pregnancy rate.
LBR: Live birth rate.
NS: non-significant.
IVF: in-vitro fertilization, ICSI: intracytoplasmic sperm injection, ET: embryo transfer.
BMI: body mass index, AFC: antral follicle count.
Main endpoint (clinical pregnancy rate)
Among 20 studies, clinical pregnancy rate was reported in 19 studies and ongoing pregnancy rate was reported in 1 study (Inamdar 2012 [45]). In the study of Tesarik 2006 on fresh cycles, the author compared the luteal GnRHa administration in two types of protocols: both long agonist and antagonist. As a result, we had two studies from Tesarik 2006. Therefore, the total studies being used to calculate were 21.
The methodological quality inspection detected 11 grade 1 studies, 4 grade 2 studies, 3 grade 3 studies and 2 grade 4 studies (Table 2). These studies were not eliminated, but methodological quality (MQ) was used as a covariate in a further meta-regression to assess the influence and direction of MQ on results.
Table 2.
Methodological quality classification of studies.
| Type of studies | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Fresh cycle studies | Tesarik 2006 Isikoglu 2007 Ata 2008 Isik 2009 Razieh 2009 Inamdar 2012 Aboulghar 2015 |
Qublan 2008 Yildiz 2014 Zafardoust 2015 |
Fujii 2001 Brigante 2013 |
|
| Frozen cycle studies | Davar 2015 Gogce 2015 Seikkula 2016 Seikkula 2018 |
Ye 2019 | Tesarik 2004 | Check 2015 Casanova 2015 |
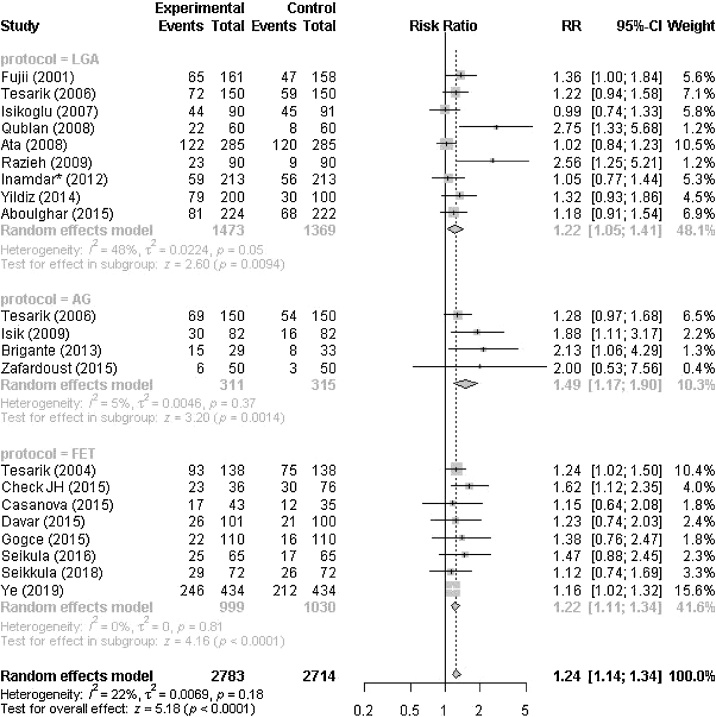
For the overall set of studies (irrespective of classification LGA-AG-FET), we found a significant benefit of using GnRHa in luteal phase for clinical pregnancy with a rate: RR 1.24 (95% CI 1.14–1.34, p < 0.0001). We detected between study heterogeneity (I2 = 22%, p = 0.18).
We then divided the whole set of studies into three categories (LGA, AG, and FET) (Fig. 2):
Fig. 2.
Forest plot with overall and separate effects of LGA, AG and FET.
Long agonist (LGA); antagonist (AG); frozen embryo transfer (FET)
In the LGA subset, a significant benefit is confirmed with RR = 1.22 (95% CI 1.05–1.41, p = 0.009) (I2 = 48%, p = 0.05). Within the AG group, a similar although slightly higher and significant effect is found (RR = 1.49 (95% CI 1.17–1.90, p = 0.001)) with a substantially low study heterogeneity (I2 = 5%, p = 0.37). In the FET sub-group, we found a significant benefit for clinical pregnancy rate with a rate: RR 1.22 (95% CI 1.11–1.34, p < 0.0001) without suspicion of heterogeneity (I2 = 0%, p = 0.81).
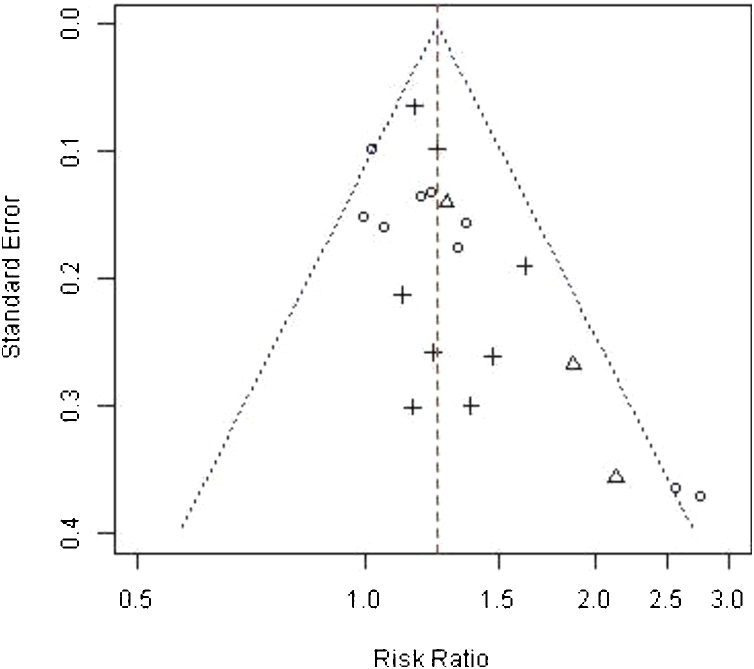
A funnel plot based on all the studies showed acceptable symmetry around the mean effect size and did not suspect between studies biases (Fig. 3).
Fig. 3.
Funnel plot showing each study distinguishing between LGA, AG and FET.
O = LGA, Δ= AG, + = FET
The following meta-regression was conducted (Table 3): we compared the benefit of LGA, AG and FET, in using LGA as reference: No significant difference was found between LGA and AG (RR = 1.28, 95% CI 0.98–1.67, p = 0.07). Similarly, no significant difference in luteal GnRHa effect was observed between fresh and frozen cycles (RR = 0.93, 95% CI 0.74–1.16, p = 0.49).
Table 3.
Meta regressions on Year (linearity assumption), GnHRa types (AG and FET compared with LGA), and methodological quality (linearity assumption).
| Coeff | 95%CI | P | ||
|---|---|---|---|---|
| Year | 1.005 | 0.989 | 1.022 | 0.540 |
| SHA compared with LGA | 1.278 | 0.981 | 1.665 | 0.070 |
| FET compared with LGA | 0.925 | 0.740 | 1.156 | 0.494 |
| Methodological quality | 1.106 | 0.996 | 1.228 | 0.060 |
We failed to find any effect of publication year (RR = 1.005/year, 95%CI 0.99–1.02, p = 0.54). By considering methodological quality as a linear covariate, we did not find any effect of MQ (RR = 1.11/category, 95%CI 0.996–1.228, p = 0.06).
Comment
There are some previous meta-analysis addressing the effect of luteal GnRH agonist (GnRHa) in fresh cycles. Nowadays, number of FET cycles increase in many ART units. The benefit of luteal GnRHa administration in the overall system (fresh and frozen embryo transfer) is needed to be evaluated. Because these two types of treatment are different, the effect of luteal GnRHa administration is analysed separately in each group of treatment: two protocols of fresh cycles and FET cycles. Our study showed that administration of GnRHa in luteal phase increased clinical pregnancy rate by 24% (95% CI 14%–34%) in the overall population including in both fresh cycles with either GnRH antagonist or GnRH agonist ovarian stimulation protocol and frozen cycles. These findings are consistent with those of previous meta-analyses [19,20,46], however, aimed at a broader target population including women seeking ART with FET cycles.
The exact mechanisms of presumed beneficial effect of GnRHa are unknown. Some theories have been proposed to explain for the success of fresh cycles, the others for FET cycles. In general, this intervention may work upon the corpus luteum, the endometrium, and the embryo. GnRHa stimulates secretion of LH from the pituitary, thus may maintain corpus luteum function. GnRH receptors are also expressed in both stroma and epithelial cells of endometrium particularly with the highest levels during the luteal phase [47,48]. LH release brings benefit on endometrium by accelerating stimulation of angiogenic factors, growth factors, and cytokines which improves implantation [10,11]. Also, GnRH receptors are expressed on human embryos [18]. GnRH seems to have the direct effect on embryo by firstly, a primary regulator of human chorionic gonadotrophin synthesis and secretion in both preimplantation embryos and in the placenta [16,49]; and secondly, successful rates of implantation, delivery of live birth [5,20]. The result of our study may support all these theories related to beneficial consequence on fresh and frozen cycles.
Then one practical question was raised is that which protocol or treatment regime will have the most benefit from GnRHa administration during the luteal phase. To answer this question, the whole population of the study was divided into three subgroups: LGA, AG, and FET.
For fresh cycles, two common protocols for ovarian stimulation in in-vitro fertilization (IVF) are long down and short antagonist. AG is a friendly protocol with shorter duration of stimulation, lower dosage of using; and especially safer by reducing rate of ovarian hyperstimulation syndrome (OHSS) [[50], [51], [52]]. Recently, antagonist protocol combined with GnRH agonist trigger has been introduced as an innovation strategy to reduce OHSS effectively [53,54]. Also, GnRH antagonist, the expression of tissue inhibitors of matrix metalloproteinases in stroma tissue, was reported as a benefit factor to improve implantation of embryos [55]. Therefore, if luteal support with GnRHa adds more benefit, the more advantage to apply antagonist protocol for patients. Oliveira et al. 2010, included 5 RCTs, showed that the clinical pregnancy rate and ongoing pregnancy rate were not significantly different between study group and control group in long protocol, but statistically significantly higher clinical pregnancy rate and ongoing pregnancy rate in study group in GnRH antagonist protocol [19]. In our study the benefit of administration of GnRHa in luteal phase to women using AG protocol (RR = 1.49 (95% CI 1.17–1.90, p = 0.001)) is numerically larger than that of using LGA protocol (RR = 1.22 (95% CI 1.05–1.41, p = 0.009)) but the difference is not significant (RR = 1,28 (95% CI 0.98–1.67, p = 0.07). A larger population of women using antagonist protocol is needed to get the definitive answer. Notably, in our study, heterogeneity within antagonist group (I2 = 5%) is much smaller compared to agonist group (I2 = 48%)
Owning to advantages of effectively preventing ovarian hyperstimulation syndrome [53] as well as enhancing IVF outcomes [56,57], FET cycles in IVF become popular. Improving success by administration of GnRHa in luteal phase may put more advantage for FET treatment. From the first report showing an increasing pregnancy rates after the mid-luteal administration of GnRHa in ovum donation [16], some similar papers on FET cycles were published. However, conflictive results [16,38,[40], [41], [42],58] and small sample sizes prevent getting a definite conclusion. Our study, including all relevant studies published, found an increase of clinical pregnancy rate of 22% (95% CI from 11% to 34%) by adding GnRHa in luteal phase with no evidence of heterogeneity (I2 = 0%, p = 0.81). This result is an encouring data in clinical practice especially during the time in which the tendency of FET cycles in IVF clinics increases [13,14]. However, within the subgroup, two out of eight studies are not randomized. In more details, the comparison between fresh and frozen embryo transfer was analysed. However, no different effect of GnRH agonist administration was found (RR = 0.93, 95% CI 0.74–1.16, p = 0.49).
In brief, GnRHa administration in luteal phase improves clinical pregnancy rate regardless fresh or FET cycles. No statistically significant difference was found between AG and LGA, as well as between fresh and frozen embryo transfer cycles.
This meta-analysis has involved in large numbers of studies with large participants. However, not all studies are RCTs and some studies have methodological flaws (Table 2). Nevertheless, methodological quality was not found to affect the outcome (p = 0.06). Moreover, there was no significant difference regarding publication year (p = 0.54). So, to a certain extent, our study provided an encouraging result.
Since there may be a direct effect of GnRHa on early embryonic development, long-term follow up of children born after GnRHa administration are needed.
Conclusions
Our meta analysis has large sample size including FET cycles, and important biases are controlled. The study provides evidence that GnRHa administration in luteal phase improve clinical pregnancy rate in both fresh and frozen cycles. Within fresh cycles, no significant difference of clinical pregnancy rate is found between two protocols. In frozen cycles, the effect of GnRH agonist administration in enhancing clinical pregnancy rate is similar to fresh cycles. More studies of antagonist protocol and more RCTs for FET cycles are needed to confirm the outcome.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgements
We would like to thank Dr Claudio Maurizio Brigante and Dr Baris Ata who kindly provided additional information regarding their studies. This study was not received any specific grant from funding organisations.
Contributor Information
Le Thi Minh Chau, Email: dr.lethiminhchau@gmail.com.
Duong Khue Tu, Email: duongkhuetu@gmail.com.
Philippe Lehert, Email: philippe.lehert@gmail.com.
Do Van Dung, Email: dvdung@ump.edu.vn.
Le Quang Thanh, Email: quangthanhbvtd@yahoo.com.
Vo Minh Tuan, Email: vominhtuan@ump.edu.vn.
References
- 1.Calhaz-Jorge C. Assisted reproductive technology in Europe, 2012: results generated from European registers by ESHRE. Hum Reprod. 2016;31(8):1638–1652. doi: 10.1093/humrep/dew151. [DOI] [PubMed] [Google Scholar]
- 2.Chambers G.M. Assisted reproductive technology in Australia and New Zealand: cumulative live birth rates as measures of success. Med J Aust. 2017;207(3):114–118. doi: 10.5694/mja16.01435. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Chronic Disease Prevention and Health Promotion . 2014. Assisted reproductive technology national summary report.https://www.cdc.gov/art/pdf/2014-report/ART-2014-National-Summary-Report.pdf 2016 [cited 2017; Available from: [Google Scholar]
- 4.Humaidan P., Engmann L., Benadiva C. Luteal phase supplementation after gonadotropin-releasing hormone agonist trigger in fresh embryo transfer: the American versus European approaches. Fertil Steril. 2015;103(4):879–885. doi: 10.1016/j.fertnstert.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 5.van der Linden M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015;(7) doi: 10.1002/14651858.CD009154.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavaniotou A. Comparison of LH concentrations in the early and mid-luteal phase in IVF cycles after treatment with HMG alone or in association with the GnRH antagonist Cetrorelix. Hum Reprod. 2001;16(4):663–667. doi: 10.1093/humrep/16.4.663. [DOI] [PubMed] [Google Scholar]
- 7.Hubayter Z.R., Muasher S.J. Luteal supplementation in in vitro fertilization: more questions than answers. Fertil Steril. 2008;89(4):749–758. doi: 10.1016/j.fertnstert.2008.02.095. [DOI] [PubMed] [Google Scholar]
- 8.Fatemi H.M. The luteal phase after 3 decades of IVF: what do we know? Reprod Biomed Online. 2009;19:1–13. [PubMed] [Google Scholar]
- 9.Fauser B.C.J.M., Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;14(5):236–242. doi: 10.1016/s1043-2760(03)00075-4. [DOI] [PubMed] [Google Scholar]
- 10.Sugino N. Expression of vascular endothelial growth factor and its receptors in the human Corpus luteum during the menstrual cycle and in early Pregnancy1. J Clin Endocrinol Metab. 2000;85(10):3919–3924. doi: 10.1210/jcem.85.10.6888. [DOI] [PubMed] [Google Scholar]
- 11.Wang T.-H. Human chorionic gonadotropin-induced ovarian hyperstimulation syndrome is associated with up-regulation of vascular endothelial growth factor. J Clin Endocrinol Metab. 2002;87(7):3300–3308. doi: 10.1210/jcem.87.7.8651. [DOI] [PubMed] [Google Scholar]
- 12.Macklon N.S. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27(2):170–207. doi: 10.1210/er.2005-0015. [DOI] [PubMed] [Google Scholar]
- 13.Kushnir V.A. Systematic review of worldwide trends in assisted reproductive technology 2004–2013. Reprod Biol Endocrinol. 2017;15(1):6. doi: 10.1186/s12958-016-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans J. Fresh versus frozen embryo transfer: backing clinical decisions with scientific and clinical evidence. Hum Reprod Update. 2014;20(6):808–821. doi: 10.1093/humupd/dmu027. [DOI] [PubMed] [Google Scholar]
- 15.Fujii S. Continuous administration of gonadotrophin-releasing hormone agonist during the luteal phase in IVF. Hum Reprod. 2001;16(8):1671–1675. doi: 10.1093/humrep/16.8.1671. [DOI] [PubMed] [Google Scholar]
- 16.Tesarik J., Hazout A., Mendoza C. Enhancement of embryo developmental potential by a single administration of GnRH agonist at the time of implantation. Hum Reprod. 2004;19(5):1176–1180. doi: 10.1093/humrep/deh235. [DOI] [PubMed] [Google Scholar]
- 17.Pirard C., Donnez J., Loumaye E. GnRH agonist as novel luteal support: results of a randomized, parallel group, feasibility study using intranasal administration of buserelin*. Hum Reprod. 2005;20(7):1798–1804. doi: 10.1093/humrep/deh830. [DOI] [PubMed] [Google Scholar]
- 18.Metallinou C. Gonadotropin-releasing hormone in the ovary. Reprod Sci. 2007;14(8):737–749. doi: 10.1177/1933719107310707. [DOI] [PubMed] [Google Scholar]
- 19.Oliveira J.B.A. Administration of single-dose GnRH agonist in the luteal phase in ICSI cycles: a meta-analysis. Reprod Biol Endocr RB&E. 2010;8:107. doi: 10.1186/1477-7827-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyrou D. Increased live birth rates with GnRH agonist addition for luteal support in ICSI/IVF cycles: a systematic review and meta-analysis. Hum Reprod Update. 2011;17(6):734–740. doi: 10.1093/humupd/dmr029. [DOI] [PubMed] [Google Scholar]
- 21.Viechtbauer W. 2010. Metafor: meta-analysis package for r.https://CRAN.R-project.org/package=metafor [cited 2018; Available from: [Google Scholar]
- 22.Viechtbauer W. Conducting meta-analyses in r with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 23.Raudenbush S.W. Russell Sage Foundation; New York: 2009. Analyzing effect sizes: random effects models.THe handbook of research synthesis and meta-analysis. [Google Scholar]
- 24.Akobeng A. Communicating the benefits and harms of treatments. Arch Dis Child. 2008;93:710–713. doi: 10.1136/adc.2008.137083. [DOI] [PubMed] [Google Scholar]
- 25.Collaboration T.C. 2011. Cochrane handbook for systematic reviews of interventions version 5.1.0. [updated March 2011] [Google Scholar]
- 26.Higgins J.P.T., Altman D.G. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesarik J. Beneficial effect of luteal-phase GnRH agonist administration on embryo implantation after ICSI in both GnRH agonist- and antagonist-treated ovarian stimulation cycles. Hum Reprod. 2006;21(10):2572–2579. doi: 10.1093/humrep/del173. [DOI] [PubMed] [Google Scholar]
- 28.Isikoglu M., Ozgur K., Oehninger S. Extension of GnRH agonist through the luteal phase to improve the outcome of intracytoplasmic sperm injection. J Reprod Med. 2007;52:639–644. [PubMed] [Google Scholar]
- 29.Qublan H. Luteal phase support with GnRH-a improves implantation and pregnancy rates in IVF cycles with endometrium of ≤7 mm on day of egg retrieval. Hum Fertil. 2008;11(1):43–47. doi: 10.1080/14647270701704768. [DOI] [PubMed] [Google Scholar]
- 30.Ata B. GnRH agonist protocol administration in the luteal phase in ICSI–ET cycles stimulated with the long GnRH agonistprotocol: a randomized, controlled double blind study. Hum Reprod. 2008;23(3):668–673. doi: 10.1093/humrep/dem421. [DOI] [PubMed] [Google Scholar]
- 31.Isik A.Z. Single-dose GnRH agonist administration in the luteal phase of GnRH antagonist cycles: a prospective randomized study. Reprod Biomed Online. 2009;19(4):472–477. doi: 10.1016/j.rbmo.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Razieh D.F., Maryam A.R., Nasim T. Beneficial effect of luteal-phase gonadotropin-releasing hormone agonist administration on implantation rate after intracytoplasmic sperm injection. Taiwan J Obstet Gynecol. 2009;48(3):245–248. doi: 10.1016/S1028-4559(09)60297-7. [DOI] [PubMed] [Google Scholar]
- 33.Inamdar D.B., Majumdar A. Evaluation of the impact of gonadotropin-releasing hormone agonist as an adjuvant in luteal-phase support on IVF outcome. J Hum Reprod Sci. 2012;5(3):279–284. doi: 10.4103/0974-1208.106341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brigante C.M.M. Efficacy of luteal phase support with GnRH agonists: a preliminary comparative study. Fertil Steril. 2013;100(3):S299. [Google Scholar]
- 35.Yıldız G.A. The addition of gonadotrophin releasing hormone agonist to routine luteal phase support in intracytoplasmic sperm injection and embryo transfer cycles: a randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2014;182(Supplement C):66–70. doi: 10.1016/j.ejogrb.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 36.Zafardoust S. Effect of administration of single dose GnRH agonist in Luteal Phase on outcome of ICSI-ET cycles in women with previous history of IVF/ICSI failure: a randomized controlled trial. J Reprod Infertil. 2015;16(2):96–101. [PMC free article] [PubMed] [Google Scholar]
- 37.Aboulghar M.A. GnRH agonist plus vaginal progesterone for luteal phase support in ICSI cycles: a randomized study. Reprod Biomed Online. 2015;30(1):52–56. doi: 10.1016/j.rbmo.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Check J.H., Wilson C., Levine K., Cohen R., Corley D. Improved implantation and live delivered pregnancy rates following transfer of embryos derived from donor oocytes by single injection of leuprolide in mid-luteal phase. Clin Exp Obstet Gynecol. 2015;42(4):429–430. [PubMed] [Google Scholar]
- 39.Casanova P. The addition of GNRH agonist for luteal phase support in ovum donation cycles. Fertil Steril. 2015;104(3):e346. [Google Scholar]
- 40.Davar R., Farid Mojtahedi M., Miraj S. Effects of single dose GnRH agonist as luteal support on pregnancy outcome in frozen-thawed embryo transfer cycles: an RCT. Iran J Reprod Med. 2015;13(8):483–488. [PMC free article] [PubMed] [Google Scholar]
- 41.Gogce M. Administering GnRH agonists in the luteal phase of artificial cycle frozen-thawed embryo transfers. a prospective randomized study. Gynecol Obstet Fertil. 2015;43(11):728–734. doi: 10.1016/j.gyobfe.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Seikkula J. Effect of mid-luteal phase GnRH agonist on frozen-thawed embryo transfers during natural menstrual cycles: a randomised clinical pilot study. Gynecol Endocrinol. 2016;32(12):961–964. doi: 10.1080/09513590.2016.1196176. [DOI] [PubMed] [Google Scholar]
- 43.Seikkula J. Mid-luteal phase gonadotropin-releasing hormone agonist support in frozen-thawed embryo transfers during artificial cycles: a prospective interventional pilot study. J Gynecol Obstet Hum Reprod. 2018;47(8):391–395. doi: 10.1016/j.jogoh.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Ye H. The addition of single dose GnRH agonist to luteal phase support in artificial cycle frozen embryo transfer: a randomized clinical trial. Gynecol Endocrinol. 2019:1–5. doi: 10.1080/09513590.2018.1563888. [DOI] [PubMed] [Google Scholar]
- 45.Inamdar D.B., Majumdar A. Evaluation of the impact of gonadotropin-releasing hormone agonist as an adjuvant in luteal-phase support on IVF outcome. J Hum Reprod Sci. 2012;5(3):279–284. doi: 10.4103/0974-1208.106341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martins W.P. GnRH agonist during luteal phase in women undergoing assisted reproductive techniques: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2016;47(2):144–151. doi: 10.1002/uog.14874. [DOI] [PubMed] [Google Scholar]
- 47.Reshef E. The presence of gonadotropin receptors in nonpregnant human uterus, human placenta, fetal membranes, and decidua*. J Clin Endocrinol Metab. 1990;70(2):421–430. doi: 10.1210/jcem-70-2-421. [DOI] [PubMed] [Google Scholar]
- 48.Raga F. Quantitative gonadotropin-releasing hormone gene expression and immunohistochemical localization in human endometrium throughout the menstrual cycle. Biol Reprod. 1998;59(3):661–669. doi: 10.1095/biolreprod59.3.661. [DOI] [PubMed] [Google Scholar]
- 49.Prager D., Weber M.M., Herman-Bonert V. Placental growth factors and releasing/inhibiting peptides. Semin Reprod Endocrinol. 1992;(10):83–91. [Google Scholar]
- 50.Toftager M. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod. 2016;31(6):1253–1264. doi: 10.1093/humrep/dew051. [DOI] [PubMed] [Google Scholar]
- 51.Al-Inany H.G. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4 doi: 10.1002/14651858.CD001750.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang R. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: a systematic review and meta-analysis. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0175985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mourad S., Brown J., Farquhar C. Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;1 doi: 10.1002/14651858.CD012103.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dosouto C., Haahr T., Humaidan P. Gonadotropin-releasing hormone agonist (GnRHa) trigger – state of the art. Reprod Biol. 2017;17(1):1–8. doi: 10.1016/j.repbio.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Raga F. Independent regulation of matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 (TIMP-1), and TIMP-3 in human endometrial stromal cells by gonadotropin-releasing hormone: implications in early human implantation1. J Clin Endocrinol Metab. 1999;84:636–642. doi: 10.1210/jcem.84.2.5464. [DOI] [PubMed] [Google Scholar]
- 56.Roque M. Freeze-all policy: fresh vs. Frozen-thawed embryo transfer. Fertil Steril. 2015;103(5):1190–1193. doi: 10.1016/j.fertnstert.2015.01.045. [DOI] [PubMed] [Google Scholar]
- 57.Shapiro B.S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–348. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 58.Casanova P. The addition og GnRH agonist for luteal phase support in ovum donation cycles. ASRM Abstracts. 2015;104(3, Supplement):e346. [Google Scholar]