Highlights
-
•
Candidalysin is the first peptide toxin identified in any human fungal pathogen.
-
•
Candidalysin is critical for Candida albicans mucosal and systemic infections.
-
•
Candidalysin activates danger-response and damage-protection pathways in host cells.
-
•
Candidalysin activates the epidermal growth factor receptor in epithelial cells and the NLRP3 inflammasome in macrophages.
-
•
Candidalysin drives neutrophil recruitment and Type 17 immunity.
Abstract
Candidalysin is a cytolytic peptide toxin secreted by the invasive form of the human pathogenic fungus, Candida albicans. Candidalysin is critical for mucosal and systemic infections and is a key driver of host cell activation, neutrophil recruitment and Type 17 immunity. Candidalysin is regarded as the first true classical virulence factor of C. albicans but also triggers protective immune responses. This review will discuss how candidalysin was discovered, the mechanisms by which this peptide toxin contributes to C. albicans infections, and how its discovery has advanced our understanding of fungal pathogenesis and disease.
Current Opinion in Microbiology 2019, 52:100–109
This review comes from a themed issue on Host-microbe interactions: fungi
Edited by Chad A Rappleye and Duncan Wilson
For a complete overview see the Issue and the Editorial
Available online 6th July 2019
https://doi.org/10.1016/j.mib.2019.06.002
1369-5274/© 2019 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Deaths per annum from fungal infections are greater than the global mortality due to malaria or breast cancer and are similar to deaths due to tuberculosis or HIV [1,2]. As such, the major challenges facing medical mycology highlight the need to better understand the biological processes that promote fungal pathogenesis and host immunity, and to translate this knowledge into the development of novel immunotherapies, vaccines and diagnostics [1, 2, 3, 4]. One of the most important human fungal pathogens is Candida albicans, which causes millions of skin, mucosal (mouth, vagina, gut) and life-threatening systemic infections each year [1,2]. Recently, it was discovered that the invasive (hyphal) form of C. albicans secretes a cytolytic peptide toxin, named candidalysin [5••]. Before this, human fungal pathogens were not known to possess such toxins. This review will focus on how candidalysin was discovered and the functional roles of candidalysin during C. albicans infections, but the reader is also guided to other reviews on the general pathogenicity and immune activation mechanisms during C. albicans infections [6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16].
Epithelial activation by Candida species
The original work leading to the discovery of candidalysin was first published in 2010 when the epithelial signalling mechanisms activated by C. albicans were delineated [17•]. Upon immediate interactions with oral epithelial cells (OECs), as exemplified by a buccal epithelial cell line [18], C. albicans yeast cells modestly activated two main signalling pathways: the mitogen-activated protein kinase (MAPK), comprising ERK1/2 (extracellular signal–regulated kinase 1/2), JNK (c-Jun N-terminal kinase) and p38, and the nuclear factor κB-light-chain-enhancer of activated B cells (NF-ĸB) pathways, with c-Jun being the main MAPK transcription factor induced. By ∼30 min, this initial MAPK activation subsided and was replaced by a second, stronger and prolonged activation wave of signalling at ∼2 hour post-exposure, which coincided with hypha formation and the subsequent release of cytokines and chemokines from OECs at 24 hour. This second activation wave was predominantly comprises MAPK signalling, leading to the activation of the c-Fos transcription factor (via p38) and the MAPK phosphatase MKP1 (via ERK1/2).
Another key observation was that c-Fos/MKP1 activation, cytokine release and OEC damage (as measured by lactate dehydrogenase (LDH) release) was linked to hyphal burdens, suggesting that a threshold level of infection was required for full epithelial activation. Importantly, both c-Fos and MKP1 were upregulated in human biopsies from patients with invasive oral C. albicans infection, demonstrating the in vivo relevance of the findings. Together, the data indicated that (i) strong MAPK activation signifies a specific epithelial response to the presence of C. albicans hyphae, (ii) a threshold level of hyphal burdens are required for full epithelial activation, and (iii) NF-ĸB activation reflects a general epithelium response to the presence of C. albicans, whether in the yeast or hyphal form Ref. [17•].
This general paradigm was verified and extended to other systems. For example, c-Fos and MKP1 were activated in human vaginal epithelial cells by C. albicans [19] and by Candida species that formed true hyphae, namely C. albicans and Candida dubliniensis in oral epithelial cells [20]. Furthermore, c-Fos and MKP1 activation was independent of fungal cell wall glycosylation [21]. During this time, it was also observed that OECs respond to the damage caused by C. albicans hyphae via the phosphatidylinositol 3-kinase (PI3K) pathway [22]. Thus, MAPK activation began to be viewed as a ‘danger-response’ mechanism and PI3K activation as a ‘damage-protection’ mechanism, which together are critical for identifying when this normally commensal fungus has become pathogenic [9,10,23, 24, 25, 26, 27]. Interestingly, c-Fos and MKP1 activation was also induced by dermatophytes in skin keratinocytes [28]. Combined with similar findings in Caenorhabditis elegans following infection with C. albicans [29] and in murine intestinal epithelial cells with bacterial pathogens [30], the data suggest a common mechanism for epithelial recognition of pathogenic microbes, whereby MAPK signalling (predominantly via p38) is required to identify a microbe as ‘pathogenic’ and to initiate inflammatory responses. These studies also highlighted the instrumental role epithelial cells have in discriminating between the commensal and pathogenic states of opportunistic pathogens. However, the precise mechanism by which epithelial cells sense the ‘danger’ remained unclear.
Discovery of candidalysin
The abovementioned studies made it abundantly clear that activation of MAPK (c-Fos/MKP1) and subsequent production of proinflammatory cytokines in OECs was hypha dependent. To identify the hyphal factor responsible for these events, a library of C. albicans mutants were screened to identify strains that could form hyphae normally but were unable to induce damage, c-Fos/MKP1 or cytokines [5••]. Remarkably, the screen identified only a single mutant with these highly specific characteristics, namely a C. albicans strain deficient in ECE1 (extent of cell elongation 1). ECE1 had long been known to be a highly expressed, hypha-associated gene encoding a unique protein (Ece1p; 271 amino acids, 28.9 kDa) [31], and was identified as a core filamentation gene expressed under most hypha-inducing conditions [32]. The Moyes et al. study also found that an ECE1-deficient strain induced significantly reduced damage and immune activation in a zebrafish swimbladder model of mucosal infection and was avirulent in an immunocompromised murine model of oropharyngeal candidiasis (OPC) [5••].
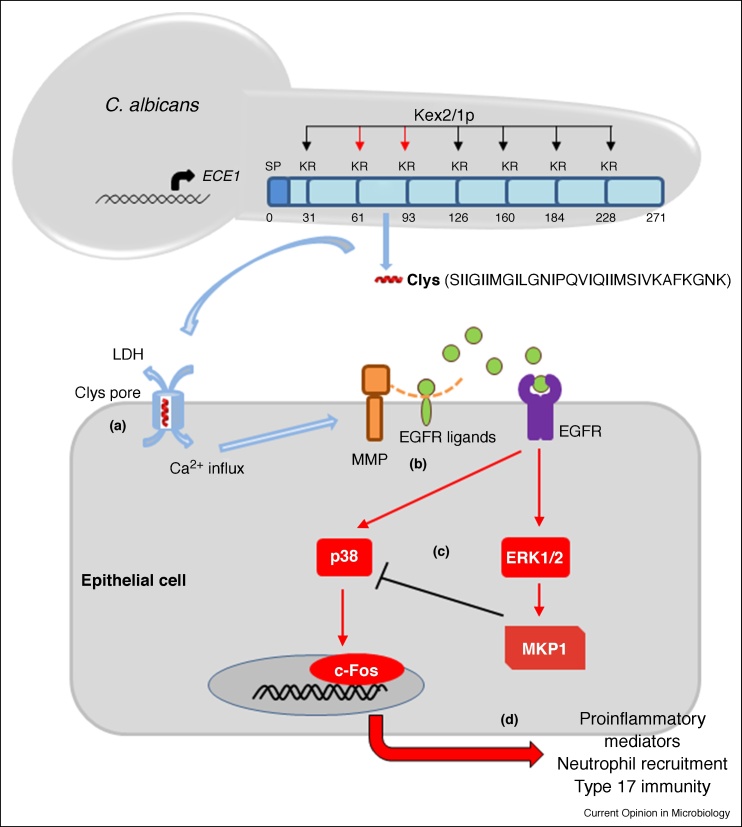
Ece1p has intriguing structural characteristics, most notably seven lysine-arginine (KR) motifs regularly dispersed throughout the full-length protein (Figure 1). These KR motifs are known processing sites for the kexin, Kex2p, suggesting that Ece1p was cleaved by Kex2p into at least eight smaller peptides and secreted [33]. Application of the eight Ece1p peptides onto OECs identified a single 32 amino acid (aa) peptide that accounted for damage induction, c-Fos/MKP1 activation and cytokine production to a similar extent as wild-type C. albicans hyphae [5••]. Further investigations demonstrated that the terminal arginine of the peptide was removed by a carboxypeptidase, Kex1p, to produce a mature 31 aa peptide, the secretion of which from C. albicans hyphae was confirmed by liquid chromatography-mass spectrometry. Site-directed mutagenesis experiments demonstrated that Kex2p-mediated proteolysis of Ece1p after Arg61 and Arg93, but not after other KR processing sites within Ece1p, was critical for peptide generation and infection in vitro and in vivo [34•]. Finally, the functional importance of the peptide was confirmed using a C. albicans strain that was deficient only in the peptide-encoding region of ECE1, which was unable to induce damage, c-Fos/MKP1 activation or cytokine production [5••].
Figure 1.
Candidalysin generation and activation of epithelial cells. C. albicans infections are initiated by increased fungal burdens with associated hypha formation. Hypha formation leads to the expression of ECE1, which encodes the Ece1p protein. Ece1p is processed by Kex2p after arginine residues at positions 61 and 93 (red arrows) to generate immature candidalysin (Clys). Immature candidalysin is further processed by Kex1p to remove the terminal Arg93 to generate mature candidalysin (SIIGIIMGILGNIPQVIQIIMSIVKAFKGNK: red α-helix) that is secreted from hyphae. (a) When accumulated at sufficient concentrations, candidalysin interacts with the cell membrane to form pore-like structures that results in membrane damage (LDH release) and calcium influx. (b) These events lead to the activation of matrix metalloproteinases and the release of epidermal growth factor receptor (EGFR) ligands, which ultimately leads to EGFR activation. (c) EGFR activation leads to induction of MAPK signalling (via p38, ERK1/2) and the activation of c-Fos. MKP1 activation (via ERK1/2) contributes to the regulation of the epithelial immune response (as it dephosphorylates p38). (d) c-Fos activation leads to chemokine and cytokine release and the subsequent recruitment of innate immune cells, including neutrophils and innate Type 17 cells (e.g. natural Th17 cells). Neutrophils phagocytose and kill the fungus and innate type 17 cells release IL-17 and IL-22. Together, these innate cells promote fungal clearance, activate epithelial tissues and improve barrier function, resulting in reduction in fungal burdens and/or clearance of the infection (commensalism).
The peptide had striking features in that it was amphipathic, α-helical and possessed two amyloidogenic regions (Figure 1). The peptide lysed red blood cells, formed lesions in synthetic membranes and induced calcium influx in OECs, confirming that it was cytolytic [5••]. Hence, the peptide was named candidalysin and is the first cytolytic peptide toxin identified in any human fungal pathogen. Importantly, the epithelial response to candidalysin is dose-dependent, supporting the concept that the host response to C. albicans during infection requires sufficient numbers of candidalysin-producing hyphae. As such, this was the first study to define the molecular link between hypha formation and pathogenicity, and correlated pathogenicity with the ability of C. albicans to damage and induce immune responses predominantly through candidalysin activity [5••] (Figure 1).
Candidalysin activates epithelial signalling via EGFR
Toxins activate epithelial cells via numerous mechanisms ranging from damage-mediated and/or receptor-mediated mechanisms [35,36]. To identify potential surface receptors activated by candidalysin, a microarray screen was undertaken, which identified the epidermal growth factor receptor (EGFR) family as being significantly affected by C. albicans infection [22]. EGFR (ErbB1/Her1) is a membrane-bound tyrosine kinase, which together with ErbB2 (Her2), ErbB3 (Her3) and ErbB4 (Her4), constitute the EGFR/ErbB family [37,38]. EGFR is distributed diversely in the body and can trigger signalling via several major pathways associated with growth, cell proliferation, survival, angiogenesis, differentiation and motility [37,39], including MAPK signalling, a key pathway activated by candidalysin [5••].
C. albicans and candidalysin were able to induce the phosphorylation of EGFR but not Her2-4 in OECs [40••]. Notably, ECE1-deficient and candidalysin-deficient strains were unable to phosphorylate EGFR in vitro or in vivo in an immune competent murine model of OPC, confirming the targeted activation of EGFR by candidalysin. Surface plasmon resonance analysis revealed that candidalysin did not interact directly with EGFR but activated EGFR via indirect mechanisms. These indirect mechanisms appeared to comprise candidalysin-induced shedding of EGFR ligands (predominantly epiregulin and epigen), activation of matrix metalloproteinase and calcium fluxes. Inhibition of EGFR strongly suppressed candidalysin-induced MAPK signalling (c-Fos/MKP1) and GM-CSF and G-CSF release [40••], which are potent neutrophil recruitment cytokines necessary for the resolution of C. albicans infections [41, 42, 43, 44]. Accordingly, in the zebrafish swimbladder model of infection, EGFR inhibition impaired neutrophil recruitment and significantly increased mortality [40••].
Previously, the C. albicans adhesin Als3p was found to interact with Her2, which induces EGFR/Her2 heterodimerisation and the subsequent endocytosis of C. albicans [45]. However, this EGFR/Her2/Als3p interaction complex does not activate c-Fos/MKP1 signalling or cytokine release [46]. The data indicate that EGFR plays a central role in mucosal C. albicans infections, with candidalysin-mediated activation of EGFR driving MAPK-based immune activation and EGFR/Her2/Als3p interactions promoting fungal endocytosis. Given that Mucorales fungi also activate EGFR signalling to induce fungal uptake into airway epithelial cells [47], collectively these studies demonstrate the critical importance of EGFR in fungal infections. Together with the potential exploitation of other fungal epithelial receptors, such as E-cadherin [48], AhR (aryl hydrocarbon receptor) [49] and EphA2 (ephrin type-A receptor 2) [50], these EGFR functions may be pivotal for the balance between commensalism, disease and restoration of health in the context of mucosal fungal infections (Figure 2).
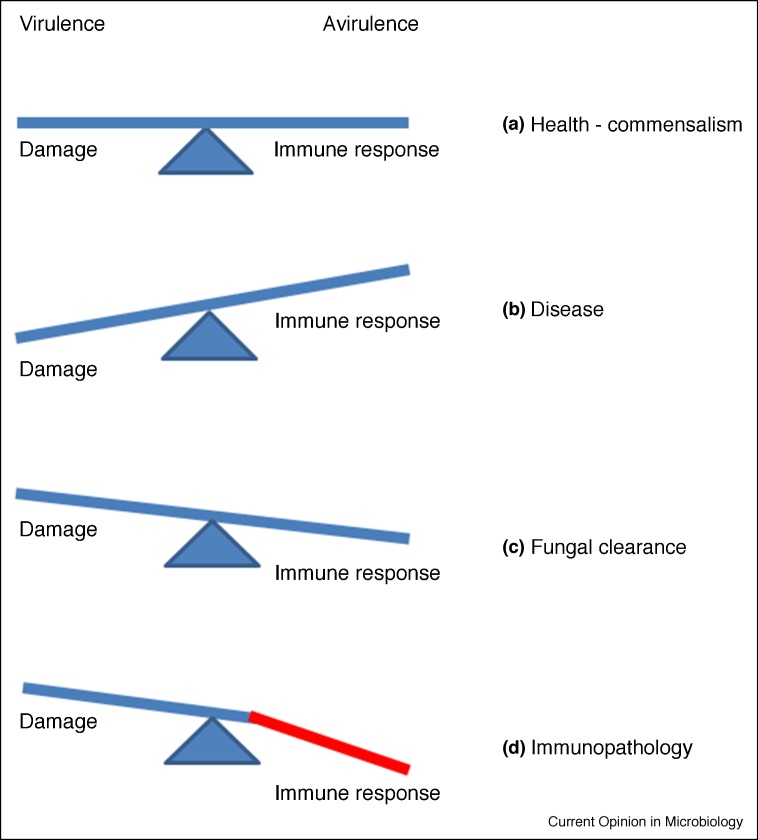
Figure 2.
Conceptual aspects of the dual function of candidalysin: virulence and avirulence.
(a) In health, C. albicans acts as a commensal typified by asymptomatic carriage, producing low levels of candidalysin required for an efficient lifestyle. (b) Under conditions permitting C. albicans proliferation, increased candidalysin levels lead to damage of host cells and tissues (disease). (c) Concomitantly, increased candidalysin levels lead to the activation of protective innate responses via neutrophil recruitment and Type 17 immunity, resulting in fungal clearance. (d) In certain infections (i.e. vaginal) and when the immune response is dysregulated, increased candidalysin levels can lead to an overreaction of the immune response (immunopathology).
Candidalysin is critical for mucosal immune activation in vivo
A defining feature of the oral immune response to C. albicans is the activation of Type 17 immunity, characterised by secretion of interleukin (IL)-17A, IL-17F and IL-22, first demonstrated using mice lacking IL-23 or the IL-17 receptor [51•] and verified in subsequent studies (reviewed in Refs. [24,52,53]. Given the rapid kinetics of fungal clearance during murine OPC, innate production of IL-17 is a key event in this protection. While IL-17 may originate from several different innate cellular sources, including CD4+ ‘natural’ TCRαβ+ Th17 cells (nTh17), γδ T cells [54] and innate lymphoid cells [55], C. albicans oral infection only induces the proliferation of nTh17 cells. This contrasts with dermal candidiasis where γδ-T cells predominate [56]. The precise nature of these nTh17 cells is not fully understood but they exhibit high TCR diversity and do not exhibit active TCR signalling upon encounter with C. albicans [54,57••], so they are not antigen-specific which confirms the innate nature of these cells. In probing how these nTh17 cells are activated, it was unexpected to find that nTh17 proliferation, induction of IL-17 and clearance of C. albicans did not require classic fungal pattern recognition receptors such as Dectin-1, CARD9 (Caspase Recruitment Domain Family Member 9) or TLR2 (Toll-like receptor 2) [57••,58]. Rather, nTh17 cell proliferation was driven by candidalysin [57••]. Moreover, candidalysin signalled synergistically with IL-17 on OECs, augmenting expression of proinflammatory mediators including multiple IL-1 cytokines [57••]. Indeed, candidalysin-induced nTh17 activation required both IL-1α/β and IL-36, since Il1r1−/− [57••] and Il36r−/− [59•] mice were susceptible to oral C. albicans infection. Thus, IL-17 and candidalysin amplify inflammation in a self-reinforcing, feed-forward loop that is initiated only when candidalysin-induced signals trigger production of IL-1-family cytokines and cell damage. An analogous pathway was observed in cutaneous bacterial infections, where the PSMα toxin from Staphylococcus aureus drives keratinocyte cell damage and IL-1/IL-36 cytokines from innate Type 17 cells [60,61].
The neutrophil response is essential for immunity to mucosal candidiasis [62,63]. IL-17 is a potent activator of neutrophils, acting indirectly through induction of CXC chemokines and G-CSF on non-hematopoietic cells [64]. Both IL-17 and non-IL-17 signals drive neutrophil activation in oral candidiasis [51•,65, 66, 67]. Given that neutrophil recruitment to oral sites requires both candidalysin [5••,57••,59•] and IL-17 signalling [51•,65,66], these studies collectively reveal that candidalysin plays a critical role in driving protective innate immunity via Th17 cells and neutrophils during OPC (Table 1).
Table 1.
Chronological milestones in candidalysin discovery and function in Candida albicans infections
| Key findings | References |
|---|---|
| Original discovery and identification of the C. albicans ECE1 gene | [31] |
| Original identification of the MAPK ‘danger-response’ c-Fos/MKP1 pathway in oral epithelial cells activated by C. albicans hyphae. | [17•] |
| MAPK ‘danger-response’ c-Fos/MKP1 pathway activated in vaginal epithelial cells by C. albicans hyphae. | [19] |
| MAPK ‘danger-response’ c-Fos/MKP1 pathway activated in oral epithelial cells only by Candida species that form true hyphae. | [20] |
| Identification of the PI3K ‘damage-protection’ pathway in oral epithelial cells activated by C. albicans hyphae. | [22] |
| Discovery of candidalysin as the activator of the MAPK ‘danger-response’ c-Fos/MKP1 pathway in oral epithelial cells. Candidalysin activity critical for oral infection in vivo. First cytolytic peptide toxin identified in any human fungal pathogen. | [5••] |
| Candidalysin induces nTh17 cell expansion via IL-1α/β release in vitro and in vivo, and signals synergistically with IL-17 on oral epithelial cells. | [57••] |
| Candidalysin induces IL-36 release from oral epithelial cells leading to protective oral immunity in vivo. | [59•] |
| Ece1p processing critical for candidalysin generation and pathogenicity in vitro and in vivo. | [34•] |
| Candidalysin drives neutrophil-mediated immunopathology during vaginal candidiasis in vivo. | [70] |
| C. albicans translocation via the transcellular route requires candidalysin-induced epithelial damage. | [74] |
| Candidalysin activates the NLRP3 inflammasome in human and mouse primary monocyte-derived macrophages and dendric cells. | [79••] |
| Candidalysin activates the NLRP3 inflammasome in primary macrophages. | [84•] |
| Candidalysin induces FGF-2 secretion from human endothelial cells and drives angiogenesis during murine systemic infections. | [88] |
| Candidalysin activates the MAPK ‘danger-response’ c-Fos/MKP1 pathway in oral epithelial cells via EGFR. | [40••] |
| Candidalysin induces IL-1β and CXCL1 secretion from CARD9+ microglial cells in a p38/c-Fos-dependent manner to recruit CXCR2-expressing neutrophils to the brain to control C. albicans infection. | [87••] |
Unlike oral candidiasis, vulvovaginal candidiasis is a disease of otherwise healthy individuals and does not seem to strongly involve an IL-17 response [68]. However, robust recruitment of neutrophils is a hallmark of vaginal candidiasis and appears to exacerbate disease rather than clear the infection [69,70]. Notably, neutrophil-driven immunopathology was recently shown to be mediated by candidalysin, as mice intravaginally challenged with ECE1-deficient and candidalysin-deficient C. albicans strains showed significant decreases in neutrophil recruitment, damage, and pro-inflammatory cytokine expression [70]. The study was the first to link vaginitis immunopathogenesis with the capacity of candidalysin to damage the vaginal mucosa.
The role of candidalysin during C. albicans gut infections is less clear. Several studies ascribe the major source of systemic candidiasis to the commensal C. albicans gut population [71, 72, 73]. In vitro data indicate that C. albicans translocation is a dynamic fungal-driven process initiated by invasion (active penetration) and followed by cellular damage and loss of epithelial integrity [74]. C. albicans translocation via the transcellular route required candidalysin-induced epithelial damage, but low-level fungal translocation occurred via a paracellular route in a candidalysin-independent manner. While the requirement of candidalysin for C. albicans gut translocation needs to be confirmed in vivo, this study showed that a peptide toxin can drive translocation of a human pathogenic fungus across the intestinal barrier.
Candidalysin is critical for systemic infection and immune activation in vivo
Phagocytes such as macrophages and dendritic cells are critically important for efficient clearance of C. albicans infections and initiation of inflammatory responses [75]. Once phagocytosed, C. albicans forms hyphae, resulting in inflammasome activation, cell lysis and escape. Inflammasome activation is a two-step process, requiring an initial priming step and a second, inflammasome-activating step [76, 77, 78]. Using human and mouse primary monocyte-derived macrophages and dendric cells, candidalysin was shown to provide the second signal to activate the NLRP3 inflammasome, resulting in caspase-1-dependent maturation and secretion of IL-1β [79••]. However, candidalysin-induced cytolysis occurred independently of inflammasome activation and pyroptosis. Thus, the study identified candidalysin-induced cell damage as an additional mechanism of C. albicans-mediated cell death in addition to pyroptosis [80, 81, 82] and the growth of glucose-consuming hyphae [83]. NLRP3 inflammasome activation by candidalysin was recently confirmed using primary macrophages [84•]. NLRP3 inflammasome activation also promotes the immunopathogenesis of vulvovaginal candidiasis [85] but a role for candidalysin has not yet been formally demonstrated.
Candidalysin also drives C. albicans systemic infections. The C-type lectin receptor/Syk adaptor CARD9 is known to facilitate protective antifungal immunity within the central nervous system (CNS) through neutrophil recruitment [86]. Recently, candidalysin was shown to induce IL-1β and CXCL1 secretion from CARD9+ microglial cells in a p38/c-Fos-dependent manner, and that they function to recruit CXCR2-expressing neutrophils to the brain to control the infection [87••]. The work revealed an intricate network of host–pathogen interactions that promotes CNS antifungal immunity via candidalysin activity and provided novel mechanistic insights into how human CARD9-deficiency is associated with CNS fungal disease.
Finally, candidalysin also induces FGF-2 secretion from human endothelial cells and drives angiogenesis during murine systemic infections [88]. As to why candidalysin promotes angiogenesis is intriguing but it is notable that candidalysin also activates EGFR signalling [40••], which is associated with angiogenesis [37,39].
Conceptual aspects of candidalysin production
Why does C. albicans produce candidalysin? Evidence so far points towards a dual role for candidalysin in C. albicans pathogenesis. One on hand, candidalysin suits the description of a classical virulence factor in that it directly damages host cells [89]. On the other hand, candidalysin is an immunomodulatory molecule that is sensed by the host to initiate a protective response (via neutrophils and Type 17 immunity); such molecules have been termed ‘avirulence factors’ [90,91]. The balance of this virulence/avirulence encounter, namely damage induction versus immune protection, dictates the outcome of infection. This is elegantly addressed in the damage-response framework [92], which was recently utilised to conclude that C. albicans infections fit all six classifications of the framework [93]. Given that candidalysin is critical for driving damage and immunity/immunopathology in all infection models tested, candidalysin is probably a pivotal factor in the outcome of this virulence/avirulence encounter.
Another conceptual aspect is whether candidalysin also acts as a commensal factor. C. albicans is adapted to life in the host, which is typified by asymptomatic commensal carriage. Indeed, gene expression analysis directly from patient samples indicated that both yeast and hyphal morphologies are present during asymptomatic colonisation of human mucosal surfaces [94, 95, 96]. Intriguingly, in a murine gastrointestinal colonisation model, competitive infection experiments revealed that commensal fitness may inversely correlate with the gene network associated with morphogenesis [97••]. This apparent antagonism between commensalism and hyphal growth is supported by the observation that serial passage of C. albicans through the murine gastrointestinal tract resulted in the loss of hypha-forming ability in the absence of a competitive microbiota [98••]. Furthermore, gut-evolved C. albicans strains that lost the ability to form hyphae exhibited reduced virulence in vitro and in vivo. Given this, it may be unsurprising that commensal fitness inversely correlates with morphogenesis, since hypha formation will lead to candidalysin secretion, damage and immune activation, which will ultimately lead to fungal clearance or immunopathology. Therefore, it may not be in the fungus’ interests to secrete high levels of candidalysin when colonising host surfaces. This is supported by data showing that a threshold level of candidalysin activity is required to damage epithelial cells and drive immune responses [5••,17•,19,70]. Hence, the commensal lifestyle of C. albicans may be promoted by reduced hypha formation accompanied by low levels of candidalysin secretion, which may function to acquire nutrients from intracellular sources (through non-damaging pore formation) or by promoting colonisation through direct antimicrobial activity on the local microbiota. On the other hand, a pathogenic lifestyle may be promoted when C. albicans hyphal burdens increase accompanied by high levels of candidalysin secretion, or in immunocompromised individuals that exhibit defective anti-Candida immunity. These conceptual aspects for a role of candidalysin in C. albicans infections will no doubt be addressed more fully in the coming years.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was supported by grants from the Wellcome Trust (214229_Z_18_Z), Biotechnology & Biological Sciences Research Council (BB/N014677/1), King’s Health Partners Challenge Fund (R170501) and the NIH Research at Guys and St. Thomas’s NHS Foundation Trust and the King’s College London Biomedical Research Centre (IS-BRC-1215-20006) to J.R.N; by the National Institutes of Health (R37-DE022550) to S.L.G. and J.R.N; and by the Deutsche Forschungsgemeinschaft CRC/TR “FungiNet” Project C1 and the H2020–H2020–Marie Skłodowska‐Curie Actions–European Training Networks–Marie Sklodowska‐Curie grant agreements no 642095 — “OPATHY” and no 812969 – “FunHoMic” to B.H.
References
- 1.Brown G.D., Denning D.W., Gow N.A., Levitz S.M., Netea M.G., White T.C. Hidden killers: human fungal infections. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Brown G.D., Denning D.W., Levitz S.M. Tackling human fungal infections. Science. 2012;336:647. doi: 10.1126/science.1222236. [DOI] [PubMed] [Google Scholar]
- 3.Netea M.G., Brown G.D. Fungal infections: the next challenge. Curr Opin Microbiol. 2012;15:403–405. doi: 10.1016/j.mib.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Gow N.A.R., Amin T., McArdle K., Brown A.J.P., Brown G.D., Warris A., The Wtsa-Mmfi C Strategic research funding: a success story for medical mycology. Trends Microbiol. 2018;10:811–813. doi: 10.1016/j.tim.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 5••.Moyes D.L., Wilson D., Richardson J.P., Mogavero S., Tang S.X., Wernecke J., Höfs S., Gratacap R.L., Robbins J., Runglall M. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. doi: 10.1038/nature17625. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovery of candidalysin, the first cytolytic peptide toxin identified in any human fungal pathogen.
- 6.Naglik J.R., Moyes D.L., Wachtler B., Hube B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect. 2011;13:963–976. doi: 10.1016/j.micinf.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobsen I.D., Wilson D., Wachtler B., Brunke S., Naglik J.R., Hube B. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther. 2012;10:85–93. doi: 10.1586/eri.11.152. [DOI] [PubMed] [Google Scholar]
- 8.Hebecker B., Naglik J.R., Hube B., Jacobsen I.D. Pathogenicity mechanisms and host response during oral Candida albicans infections. Expert Rev Anti Infect Ther. 2014;12:867–879. doi: 10.1586/14787210.2014.916210. [DOI] [PubMed] [Google Scholar]
- 9.Wilson D., Naglik J.R., Hube B. The missing link between Candida albicans hyphal morphogenesis and host cell damage. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naglik J.R., Konig A., Hube B., Gaffen S.L. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr Opin Microbiol. 2017;40:104–112. doi: 10.1016/j.mib.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu W., Filler S.G. Interactions of Candida albicans with epithelial cells. Cell Microbiol. 2010;12:273–282. doi: 10.1111/j.1462-5822.2009.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filler S.G. Can host receptors for fungi be targeted for treatment of fungal infections? Trends Microbiol. 2013;21:389–396. doi: 10.1016/j.tim.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheppard D.C., Filler S.G. Host cell invasion by medically important fungi. Cold Spring Harb Perspect Med. 2014;5 doi: 10.1101/cshperspect.a019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swidergall M., Filler S.G. Oropharyngeal Candidiasis: fungal Invasion and epithelial cell responses. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma A., Gaffen S.L., Swidergall M. Innate immunity to mucosal Candida infections. J Fungi (Basel) 2017;3 doi: 10.3390/jof3040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer F.L., Wilson D., Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Moyes D.L., Runglall M., Murciano C., Shen C., Nayar D., Thavaraj S., Kohli A., Islam A., Mora-Montes H., Challacombe S.J. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8:225–235. doi: 10.1016/j.chom.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identified the MAPK-based danger response pathway and how epithelial cells respond to C. albicans hyphae (subsequently candidalysin – Ref. 5).
- 18.Rupniak H.T., Rowlatt C., Lane E.B., Steele J.G., Trejdosiewicz Lk, Laskiewicz B., Povey S., Hill B.T. Characteristics of four new human cell lines derived from squamous cell carcinomas of the head and neck. J Natl Cancer Inst. 1985;75:621–635. [PubMed] [Google Scholar]
- 19.Moyes D.L., Murciano C., Runglall M., Islam A., Thavaraj S., Naglik J.R. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moyes D.L., Murciano C., Runglall M., Kohli A., Islam A., Naglik J.R. Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med Microbiol Immunol. 2012;201:93–101. doi: 10.1007/s00430-011-0209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murciano C., Moyes D.L., Runglall M., Islam A., Mille C., Fradin C., Poulain D., Gow N.A., Naglik J.R. Candida albicans cell wall glycosylation may be indirectly required for activation of epithelial cell proinflammatory responses. Infect Immun. 2011;79:4902–4911. doi: 10.1128/IAI.05591-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moyes D.L., Shen C., Murciano C., Runglall M., Richardson J.P., Arno M., Aldecoa-Otalora E., Naglik J.R. Protection against epithelial damage during Candida albicans infection is mediated by PI3K/Akt and mammalian target of rapamycin signaling. J Infect Dis. 2014;209:1816–1826. doi: 10.1093/infdis/jit824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson J.P., Ho J., Naglik J.R. Candida-epithelial interactions. J Fungi (Basel) 2018;4 doi: 10.3390/jof4010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson J.P., Moyes D.L., Ho J., Naglik J.R. Candida innate immunity at the mucosa. Semin Cell Dev Biol. 2019;89:58–70. doi: 10.1016/j.semcdb.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 25.Tang S.X., Moyes D.L., Richardson J.P., Blagojevic M., Naglik J.R. Epithelial discrimination of commensal and pathogenic Candida albicans. Oral Dis. 2016;22:114–119. doi: 10.1111/odi.12395. [DOI] [PubMed] [Google Scholar]
- 26.Moyes D.L., Richardson J.P., Naglik J.R. Candida albicans-epithelial interactions and pathogenicity mechanisms: scratching the surface. Virulence. 2015;6:338–346. doi: 10.1080/21505594.2015.1012981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naglik J.R., Richardson J.P., Moyes D.L. Candida albicans pathogenicity and epithelial immunity. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Achterman R.R., Moyes D.L., Thavaraj S., Smith A.R., Blair K.M., White T.C., Naglik J.R. Dermatophytes activate skin keratinocytes via MAPK signaling and induce immune responses. Infect Immun. 2015;4:1705–1714. doi: 10.1128/IAI.02776-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pukkila-Worley R., Ausubel F.M., Mylonakis E. Candida albicans infection of Caenorhabditis elegans induces antifungal immune defenses. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guma M., Stepniak D., Shaked H., Spehlmann M.E., Shenouda S., Cheroutre H., Vicente-Suarez I., Eckmann L., Kagnoff M.F., Karin M. Constitutive intestinal NF-{kappa}B does not trigger destructive inflammation unless accompanied by MAPK activation. J Exp Med. 2011;208:1889–1900. doi: 10.1084/jem.20110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birse C.E., Irwin M.Y., Fonzi W.A., Sypherd P.S. Cloning and characterization of ECE1, a gene expressed in association with cell elongation of the dimorphic pathogen Candida albicans. Infect Immun. 1993;61:3648–3655. doi: 10.1128/iai.61.9.3648-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin R., Albrecht-Eckardt D., Brunke S., Hube B., Hunniger K., Kurzai O. A core filamentation response network in Candida albicans is restricted to eight genes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bader O., Krauke Y., Hube B. Processing of predicted substrates of fungal Kex2 proteinases from Candida albicans, C. glabrata, Saccharomyces cerevisiae and Pichia pastoris. BMC Microbiol. 2008;8:116. doi: 10.1186/1471-2180-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Richardson J.P., Mogavero S., Moyes D.L., Blagojevic M., Kruger T., Verma A.H., Coleman B.M., De La Cruz Diaz J., Schulz D., Ponde N.O. Processing of Candida albicans Ece1p Is critical for candidalysin maturation and fungal virulence. mBio. 2018;9 doi: 10.1128/mBio.02178-17. e02178-e02117. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identified that Ece1p processing was critical for candidalysin generation and C. albicans virulence.
- 35.Brito C., Cabanes D., Sarmento Mesquita F., Sousa S. Mechanisms protecting host cells against bacterial pore-forming toxins. Cell Mol Life Sci. 2019;76:1319–1339. doi: 10.1007/s00018-018-2992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratner A.J., Hippe K.R., Aguilar J.L., Bender M.H., Nelson A.L., Weiser J.N. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J Biol Chem. 2006;281:12994–12998. doi: 10.1074/jbc.M511431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho J., Moyes D.L., Tavassoli M., Naglik J.R. The role of ErbB receptors in infection. Trends Microbiol. 2017;25:942–952. doi: 10.1016/j.tim.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roskoski R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Chakraborty S., Li L., Puliyappadamba V.T., Guo G., Hatanpaa K.J., Mickey B., Souza R.F., Vo P., Herz J., Chen M.R. Constitutive and ligand-induced EGFR signalling triggers distinct and mutually exclusive downstream signalling networks. Nat Commun. 2014;5 doi: 10.1038/ncomms6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Ho J., Yang X., Nikou S.A., Kichik N., Donkin A., Ponde N.O., Richardson J.P., Gratacap R.L., Archambault L.S., Zwirner C.P. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat Commun. 2019;10:2297. doi: 10.1038/s41467-019-09915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovered that candidalysin activates the MAPK-based danger response pathway and neutrophil recruiting cytokines via EGFR.
- 41.Richardson M.D., Brownlie C.E., Shankland G.S. Enhanced phagocytosis and intracellular killing of Candida albicans by GM-CSF-activated human neutrophils. J Med Vet Mycol. 1992;30:433–441. [PubMed] [Google Scholar]
- 42.Yamamoto Y., Klein T.W., Friedman H., Kimura S., Yamaguchi H. Granulocyte colony-stimulating factor potentiates anti-Candida albicans growth inhibitory activity of polymorphonuclear cells. FEMS Immunol Med Microbiol. 1993;7:15–22. doi: 10.1111/j.1574-695X.1993.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 43.Gaviria J.M., van Burik J.A., Dale D.C., Root R.K., Liles W.C. Modulation of neutrophil-mediated activity against the pseudohyphal form of Candida albicans by granulocyte colony-stimulating factor (G-CSF) administered in vivo. J Infect Dis. 1999;179:1301–1304. doi: 10.1086/314728. [DOI] [PubMed] [Google Scholar]
- 44.Liles W.C., Huang J.E., van Burik J.A., Bowden R.A., Dale D.C. Granulocyte colony-stimulating factor administered in vivo augments neutrophil-mediated activity against opportunistic fungal pathogens. J Infect Dis. 1997;175:1012–1015. doi: 10.1086/513961. [DOI] [PubMed] [Google Scholar]
- 45.Zhu W., Phan Q.T., Boontheung P., Solis N.V., Loo J.A., Filler S.G. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc Natl Acad Sci U S A. 2012;109:14194–14199. doi: 10.1073/pnas.1117676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murciano C., Moyes D.L., Runglall M., Tobouti P., Islam A., Hoyer L.L., Naglik J.R. Evaluation of the role of Candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watkins T.N., Gebremariam T., Swidergall M., Shetty A.C., Graf K.T., Alqarihi A., Alkhazraji S., Alsaadi A.I., Edwards V.L., Filler S.G. Inhibition of EGFR signaling protects from mucormycosis. mBio. 2018;9 doi: 10.1128/mBio.01384-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phan Q.T., Myers C.L., Fu Y., Sheppard D.C., Yeaman M.R., Welch W.H., Ibrahim A.S., Edwards J.E., Filler S.G. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5 doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solis N.V., Swidergall M., Bruno V.M., Gaffen S.L., Filler S.G. The aryl hydrocarbon receptor governs epithelial cell invasion during oropharyngeal candidiasis. mBio. 2017;8 doi: 10.1128/mBio.00025-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swidergall M., Solis N.V., Lionakis M.S., Filler S.G. EphA2 is an epithelial cell pattern recognition receptor for fungal beta-glucans. Nat Microbiol. 2018;3:53–61. doi: 10.1038/s41564-017-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Conti H.R., Shen F., Nayyar N., Stocum E., Sun J.N., Lindemann M.J., Ho A.W., Hai J.H., Yu J.J., Jung J.W. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]; Key finding describing the critical nature of Th17 and IL-17 in mediating epithelial host defence against C. albicans.
- 52.Conti H.R., Gaffen S.L. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J Immunol. 2015;195:780–788. doi: 10.4049/jimmunol.1500909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drummond R.A., Lionakis M.S. Organ-specific mechanisms linking innate and adaptive antifungal immunity. Semin Cell Dev Biol. 2019;89:78–90. doi: 10.1016/j.semcdb.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conti H.R., Peterson A.C., Brane L., Huppler A.R., Hernandez-Santos N., Whibley N., Garg A.V., Simpson-Abelson M.R., Gibson G.A., Mamo A.J. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211:2075–2084. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gladiator A., Wangler N., Trautwein-Weidner K., LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 56.Kashem S.W., Igyarto B.Z., Gerami-Nejad M., Kumamoto Y., Mohammed J.A., Jarrett E., Drummond R.A., Zurawski S.M., Zurawski G., Berman J. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity. 2015;42:356–366. doi: 10.1016/j.immuni.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Verma A.H., Richardson J.P., Zhou C., Coleman B.M., Moyes D.L., Ho J., Huppler A.R., Ramani K., McGeachy M.J., Mufazalov I.A. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor Candidalysin. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aam8834. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identified candidalysin as the driver of protective Th17 and IL-17 responses against oral C. albicans infection via the induction of IL-1 family members from epithelial cells.
- 58.Bishu S., Hernandez-Santos N., Simpson-Abelson M.R., Huppler A.R., Conti H.R., Ghilardi N., Mamo A.J., Gaffen S.L. The adaptor CARD9 is required for adaptive but not innate immunity to oral mucosal Candida albicans infections. Infect Immun. 2014;82:1173–1180. doi: 10.1128/IAI.01335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Verma A.H., Zafar H., Ponde N.O., Hepworth O.W., Sihra D., Aggor F.E.Y., Ainscough J.S., Ho J., Richardson J.P., Coleman B.M. IL-36 and IL-1/IL-17 drive immunity to oral candidiasis via parallel mechanisms. J Immunol. 2018;201:627–634. doi: 10.4049/jimmunol.1800515. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identified candidalysin as the driver of protective immune responses against oral C. albicans infection via the induction of IL-36 family members from epithelial cells.
- 60.Liu H., Archer N.K., Dillen C.A., Wang Y., Ashbaugh A.G., Ortines R.V., Kao T., Lee S.K., Cai S.S., Miller R.J. Staphylococcus aureus epicutaneous exposure drives skin inflammation via IL-36-mediated T cell responses. Cell Host Microbe. 2017;22:653–666.e655. doi: 10.1016/j.chom.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakagawa S., Matsumoto M., Katayama Y., Oguma R., Wakabayashi S., Nygaard T., Saijo S., Inohara N., Otto M., Matsue H. Staphylococcus aureus virulent PSMalpha peptides induce keratinocyte alarmin release to orchestrate IL-17-dependent skin inflammation. Cell Host Microbe. 2017;22:667–677.e665. doi: 10.1016/j.chom.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swerdloff J.N., Filler S.G., Edwards J.E., Jr. Severe candidal infections in neutropenic patients. Clin Infect Dis. 1993;17(Suppl. 2):S457–S467. doi: 10.1093/clinids/17.supplement_2.s457. [DOI] [PubMed] [Google Scholar]
- 63.Romani L., Mencacci A., Cenci E., Puccetti P., Bistoni F. Neutrophils and the adaptive immune response to Candida albicans. Res Immunol. 1996;147:512–518. doi: 10.1016/s0923-2494(97)85216-9. [DOI] [PubMed] [Google Scholar]
- 64.Gaffen S.L., Jain R., Garg A.V., Cua D.J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huppler A.R., Conti H.R., Hernandez-Santos N., Darville T., Biswas P.S., Gaffen S.L. Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J Immunol. 2014;192:1745–1752. doi: 10.4049/jimmunol.1302265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conti H.R., Bruno V.M., Childs E.E., Daugherty S., Hunter J.P., Mengesha B.G., Saevig D.L., Hendricks M.R., Coleman B.M., Brane L. IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis. Cell Host Microbe. 2016;20:606–617. doi: 10.1016/j.chom.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altmeier S., Toska A., Sparber F., Teijeira A., Halin C., LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yano J., Kolls J.K., Happel K.I., Wormley F., Wozniak K.L., Fidel P.L., Jr. The acute neutrophil response mediated by S100 alarmins during vaginal candida infections is independent of the Th17-pathway. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fidel P.L., Jr., Barousse M., Espinosa T., Ficarra M., Sturtevant J., Martin D.H., Quayle A.J., Dunlap K. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun. 2004;72:2939–2946. doi: 10.1128/IAI.72.5.2939-2946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richardson J.P., Willems H.M.E., Moyes D.L., Shoaie S., Barker K.S., Tan S.L., Palmer G.E., Hube B., Naglik J.R., Peters B.M. Candidalysin drives epithelial signaling, neutrophil recruitment, and immunopathology at the vaginal mucosa. Infect Immun. 2018;86 doi: 10.1128/IAI.00645-17. e00645-00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nucci M., Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis. 2001;33:1959–1967. doi: 10.1086/323759. [DOI] [PubMed] [Google Scholar]
- 72.Miranda L.N., van der Heijden I.M., Costa S.F., Sousa A.P., Sienra R.A., Gobara S., Santos C.R., Lobo R.D., Pessoa V.P., Jr., Levin A.S. Candida colonisation as a source for candidaemia. J Hosp Infect. 2009;72:9–16. doi: 10.1016/j.jhin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 73.Eggimann P., Garbino J., Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis. 2003;3:685–702. doi: 10.1016/s1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 74.Allert S., Forster T.M., Svensson C.M., Richardson J.P., Pawlik T., Hebecker B., Rudolphi S., Juraschitz M., Schaller M., Blagojevic M. Candida albicans-induced epithelial damage mediates translocation through intestinal barriers. mBio. 2018;9:e00915–e00918. doi: 10.1128/mBio.00915-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lionakis M.S. New insights into innate immune control of systemic candidiasis. Med Mycol. 2014;52:555–564. doi: 10.1093/mmy/myu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gross O., Poeck H., Bscheider M., Dostert C., Hannesschlager N., Endres S., Hartmann G., Tardivel A., Schweighoffer E., Tybulewicz V. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 77.Franchi L., Munoz-Planillo R., Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22:28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79••.Kasper L., Konig A., Koenig P.A., Gresnigt M.S., Westman J., Drummond R.A., Lionakis M.S., Gross O., Ruland J., Naglik J.R. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun. 2018;9 doi: 10.1038/s41467-018-06607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identified candidalysin as providing the second signal to activate the NLRP3 inflammasome in mononuclear phagocytes.
- 80.Uwamahoro N., Verma-Gaur J., Shen H.H., Qu Y., Lewis R., Lu J., Bambery K., Masters S.L., Vince J.E., Naderer T. The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. mBio. 2014;5:e00003–e00014. doi: 10.1128/mBio.00003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wellington M., Koselny K., Krysan D.J. Candida albicans morphogenesis is not required for macrophage interleukin 1beta production. mBio. 2012;4 doi: 10.1128/mBio.00433-12. e00433-00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wellington M., Koselny K., Sutterwala F.S., Krysan D.J. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell. 2014;2:329–340. doi: 10.1128/EC.00336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tucey T.M., Verma J., Harrison P.F., Snelgrove S.L., Lo T.L., Scherer A.K., Barugahare A.A., Powell D.R., Wheeler R.T., Hickey M.J. Glucose homeostasis is important for immune cell viability during Candida challenge and host survival of systemic fungal infection. Cell Metab. 2018;27:988–1006. doi: 10.1016/j.cmet.2018.03.019. e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84•.Rogiers O., Frising U.C., Kucharikova S., Jabra-Rizk M.A., van Loo G., Van Dijck P., Wullaert A. Candidalysin crucially contributes to Nlrp3 inflammasome activation by Candida albicans hyphae. mBio. 2019;10 doi: 10.1128/mBio.02221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]; Confirmatory publication supporting the role of candidalysin as activating the NLRP3 inflammasome in macrophages.
- 85.Bruno V.M., Shetty A.C., Yano J., Fidel P.L., Jr., Noverr M.C., Peters B.M. Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome. mBio. 2015;6 doi: 10.1128/mBio.00182-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Drummond R.A., Collar A.L., Swamydas M., Rodriguez C.A., Lim J.K., Mendez L.M., Fink D.L., Hsu A.P., Zhai B., Karauzum H. CARD9-dependent neutrophil recruitment protects against fungal invasion of the central nervous system. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87••.Drummond R.A., Swamydas M., Oikonomou V., Zhai B., Dambuza I.M., Schaefer B.C., Bohrer A.C., Mayer-Barber K.D., Lira S.A., Iwakura Y. CARD9(+) microglia promote antifungal immunity via IL-1beta- and CXCL1-mediated neutrophil recruitment. Nat Immunol. 2019;20:559–570. doi: 10.1038/s41590-019-0377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identified candidalysin as the activator of microglial cells and the inducer of neutrophil recruitment in C. albicans CNS infections.
- 88.Vellanki S., Huh E.Y., Saville S.P., Lee S.C. Candida albicans morphology-dependent host FGF-2 response as a potential therapeutic target. J Fungi (Basel) 2019;5 doi: 10.3390/jof5010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Casadevall A., Pirofski L.A. Microbiology: ditch the term pathogen. Nature. 2014;516:165–166. doi: 10.1038/516165a. [DOI] [PubMed] [Google Scholar]
- 90.Medzhitov R., Schneider D.S., Soares M.P. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.White F.F., Yang B., Johnson L.B. Prospects for understanding avirulence gene function. Curr Opin Plant Biol. 2000;3:291–298. doi: 10.1016/s1369-5266(00)00082-0. [DOI] [PubMed] [Google Scholar]
- 92.Casadevall A., Pirofski L.A. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jabra-Rizk M.A., Kong E.F., Tsui C., Nguyen M.H., Clancy C.J., Fidel P.L., Jr., Noverr M. Candida albicans pathogenesis: fitting within the host-microbe damage response framework. Infect Immun. 2016;84:2724–2739. doi: 10.1128/IAI.00469-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Naglik J.R., Newport G., White T.C., Fernandes-Naglik L.L., Greenspan J.S., Greenspan D., Sweet S.P., Challacombe S.J., Agabian N. In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. Infect Immun. 1999;67:2482–2490. doi: 10.1128/iai.67.5.2482-2490.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Naglik J.R., Rodgers C.A., Shirlaw P.J., Dobbie J.L., Fernandes-Naglik L.L., Greenspan D., Agabian N., Challacombe S.J. Differential expression of Candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J Infect Dis. 2003;188:469–479. doi: 10.1086/376536. [DOI] [PubMed] [Google Scholar]
- 96.Naglik J.R., Fostira F., Ruprai J., Staab J.F., Challacombe S.J., Sundstrom P. Candida albicans HWP1 gene expression and host antibody responses in colonization and disease. J Med Microbiol. 2006;55:1323–1327. doi: 10.1099/jmm.0.46737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97••.Witchley J.N., Penumetcha P., Abon N.V., Woolford C.A., Mitchell A.P., Noble S.M. Candida albicans morphogenesis programs control the balance between gut commensalism and invasive infection. Cell Host Microbe. 2019;25:432–443.e436. doi: 10.1016/j.chom.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Commensal fitness in the gut may inversely correlate with the gene network associated with morphogenesis.
- 98••.Tso G.H.W., Reales-Calderon J.A., Tan A.S.M., Sem X., Le G.T.T., Tan T.G., Lai G.C., Srinivasan K.G., Yurieva M., Liao W. Experimental evolution of a fungal pathogen into a gut symbiont. Science. 2018;362:589–595. doi: 10.1126/science.aat0537. [DOI] [PubMed] [Google Scholar]; Antagonism between commensalism and hyphal growth results in the loss of hypha-forming ability in the absence of a competitive microbiota.