Abstract
Total joint replacement is indicated to alleviate pain and disability associated with hip and knee osteoarthritis. Arthroplasty outcomes are typically reported together, or anecdotal comparisons are made between total knee arthroplasty (TKA) and total hip arthroplasty (THA) recovery. Limited data quantifies differences in recovery trajectories, especially with respect to performance-based outcomes. Seventy-nine people undergoing total knee or total hip arthroplasty were followed over six months. Functional performance was measured using the stair climb test, timed-up-and-go test, and six-minute walk test. Surgical limb isometric strength was also measured. All outcomes significantly declined one month after surgery. Participants in the total knee arthroplasty group showed greater decline in climbing stairs (P < 0.001), timed-up-and-go (P = 0.01), and six-minute walk distance (P < 0.01). Further, the total knee arthroplasty group lost more strength (P < 0.001) and were weaker than those after total hip arthroplasty (P < 0.001). Differences in postoperative outcomes between groups at 3 and 6 months were also observed. The TKA group experiences greater decline in measured outcomes than the THA group, and muscle strength and functional recovery occurred differently in each group. These findings should be considered in rehabilitation priorities after arthroplasty surgery.
Keywords: osteoarthritis, arthroplasty, muscle strength, rehabilitation, outcome measures
INTRODUCTION
Total joint arthroplasty (TJA) is often indicated to alleviate the pain and disability associated with end-stage hip and knee osteoarthritis, the most common joint disease (Buckwalter and Martin, 2006). Over 700,000 total knee arthroplasties (TKA) and 230,000 total hip arthroplasties (THA) are performed annually in the United States (Kurtz et al., 2007). Due to the aging population, the incidence of joint replacement is expected to grow to over 4,000,000 per year by 2030 (McLawhorn and Buller, 2017).
TKA and THA are successful surgeries. Patients are satisfied and report improvements in pain and quality of life (Bourne et al., 2010, Rat et al., 2010). However, postoperative deficits in strength and function are present beyond the immediate postoperative period (Bade et al., 2010, Sicard-Rosenbaum et al., 2002). Further, despite improved quality of life immediately following TJA, patients’ quality of life outcomes are poorer than adults of similar age several years after surgery (Rat et al., 2010). This phenomenon could be explained by the fact that functional performance is not optimized, potentially related to postoperative rehabilitation strategies. A comprehensive understanding of postoperative recovery and related persistent functional deficits could improve rehabilitation strategies following TJA.
Although TKA and THA surgeries are inherently different surgical procedures, many studies combine these populations to describe outcomes (Jones et al., 2005, Lau et al., 2012); further, payers do not differentiate between the surgeries for reimbursement (Belatti et al., 2014, Padegimas et al., 2016). The studies providing comparative data between TKA and THA populations focus on patient self-report, without consideration of performance-based measures of functional capacity (Bourne et al., 2010, Hamilton et al., 2012, Lau et al., 2012, Rat et al., 2010). These self-report data suggest that individuals have higher satisfaction and have their expectations met more often after THA relative to TKA (Lau et al., 2012). To date, one study has characterized performance-based outcomes in both THA and TKA; however, the postoperative time points for data collection were varied (Kennedy et al., 2006). Comparing the trajectory of functional recovery after TKA compared to THA, measured at the same postoperative time points, could better inform postoperative decision-making.
Performance-based measures and self-report measures have characterized recovery after TJA. In particular, recovery after TKA is characterized by quantifying quadriceps strength and by performance-based outcomes such as the timed-up-and-go, the six-minute walk test, five-time-sit-to-stand test, and the stair climb test (Bade et al., 2010, Kennedy et al., 2005, Stevens-Lapsley et al., 2012a, Stevens-Lapsley et al., 2012b). These have been complemented by self-report measures such as the Western Ontario and McMaster Universities Arthritis Index and The Medical Outcome Study Short Form 36 (Stevens-Lapsley et al., 2011). This battery of testing has allowed better understanding of the immediate deficits and subsequent recovery after TKA. However, this battery of outcomes assessment is not widely used after THA, and recovery after THA is not as well described.
The purpose of this study was to compare functional performance and muscle strength 1) before TKA or THA and 2) postoperative recovery after TKA or THA. We hypothesized that, following THA or TKA, participants would have similar declines and functional performance and muscle strength in the first month after surgery. Additionally, we hypothesized that strength and functional performance recovery would be similar between the two groups at one, three, and six months after surgery.
METHODS
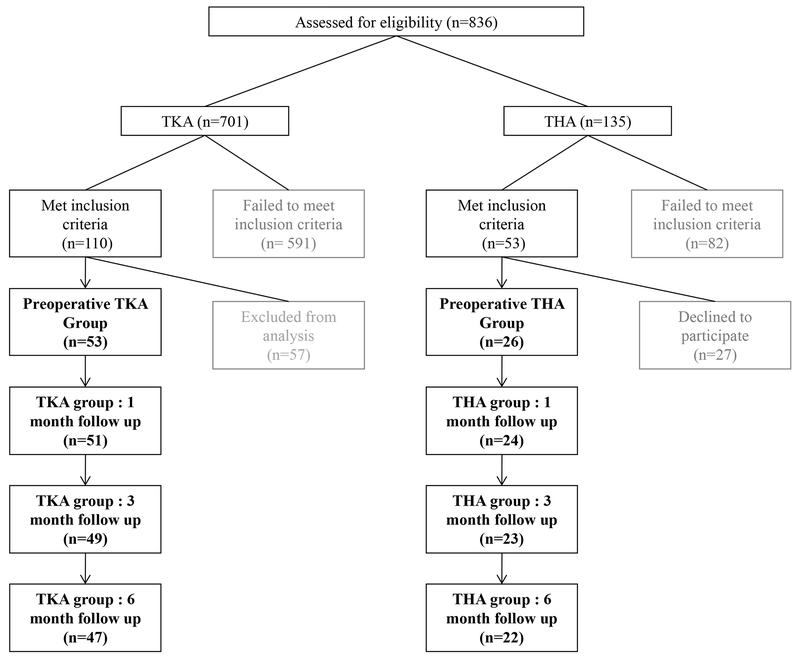
A convenience sample of 79 individuals, including 53 individuals undergoing TKA and 26 individuals undergoing THA, were studied for this analysis (figure 1). Participants with TKA were control group subjects from two recently completed randomized controlled trials (Stevens-Lapsley et al., 2012a, Stevens-Lapsley et al., 2012b). They participated in the control group study arm and were recruited from June, 2006 to January, 2011. Participants with THA were recruited from a separate ongoing observational study from June, 2010 to August, 2011, and were not receiving specific postoperative intervention. Participants were considered eligible if they were age 45-80 and scheduled for a primary, unilateral TKA or THA. Participants were excluded if they had uncontrolled hypertension, uncontrolled diabetes, BMI >40 kg/m2, or additional orthopedic or neurologic disorders that impaired daily function. Participants with TKA underwent a tri-compartmental, cemented TKA with a medial parapatellar surgical approach. Participants with THA underwent a posterolateral approach, non-cemented THA. All participants discharged home and participated in home health and outpatient physical therapy. Following TKA, participants received five visits of home physical therapy and attended 10-12 visits of outpatient physical therapy (Stevens-Lapsley et al., 2012a, Stevens-Lapsley et al., 2012b). After THA, participants attended physical therapy as prescribed by their surgeon, which was not controlled due to the observational nature of the ongoing project. Participants received an average of four visits of home physical therapy and two visits of outpatient physical therapy after THA (Judd et al., 2013). Participants in both cohorts received written informed consent, and all three parent studies were approved by the Colorado Multiple Institutional Review Board.
Figure 1.
Recruitment, enrollment and flow of study participants.
Outcome measures
All outcomes were assessed preoperatively and one, three, and six months after surgery in both the TKA and THA groups.
Functional Performance Measures
We measured functional performance using the timed-up-and-go test, stair climb test, and six-minute walk test. The timed-up-and-go test measures the time to rise from a chair, walk three meters, turn around, and return to sitting in the same chair (Podsiadlo and Richardson, 1991). It is reliable (ICC=0.75) and responsive to change in individuals after TJA (Kennedy et al., 2005). Time to ascend and descend 12 stairs was measured in the stair climb test; it has shown excellent reliability (ICC=0.90) and is responsive to change in individuals after TJA (Kennedy et al., 2005). The six-minute walk test (Steffen et al., 2002) assesses how far a person can walk in six minutes, and has been used extensively to measure endurance in several populations; it has shown excellent reliability (ICC=0.94) in individuals after joint arthroplasty (Kennedy et al., 2005).
Strength Measures
Maximal voluntary isometric contractions of the participants’ surgical limb knee extensors and flexors were measured using an electromechanical dynamometer (HUMAC NORM; CSMI Solutions, Stoughton, MA, USA). Participants were seated and stabilized on the dynamometer while positioned in 85° of hip flexion and 60° of knee flexion (Stevens-Lapsley et al., 2010). Two sub-maximal contractions were performed for warm up and task familiarity, then maximal, voluntary isometric contractions were performed twice. The trial with the highest torque (Nm) was used for analysis. Data were sampled using a Biopac Data Acquisition System at a sampling frequency of 2000Hz (Biodex Medical Systems, Inc., Shirley, NY) then analyzed using AcqKnowledge software (Biodex Medical Systems).
Data analysis
To characterize the pattern of recovery, preoperative and one-, three-, and six-month postoperative measures were evaluated using maximum likelihood estimation in a multivariate repeated measures model using all available data. In the model for each outcome, we controlled for the baseline value of the outcome, age, and sex. We used linear contrasts to estimate the differences between TKA and THA at each time point, and within-group differences from baseline at three and six months after surgery. All analysis was done using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and alpha was set for P-value 0.05.
RESULTS
Participants
There were no differences between the TKA and THA groups for most outcomes preoperatively. However, the TKA group had a significantly higher BMI and was significantly slower on the stair climb test (table 1).
Table 1.
Baseline characteristics of study participants. Data are mean (SD). Sample size includes percentage of female participants in the group. BMI= body mass index, TUG= Timed Up and Go, SCT= Stair Climb Test, 6MWT= Six-Minute Walk Test
| Outcome | TKA | THA | p-value |
|---|---|---|---|
| N (% female) | 53 (49%) | 26 (69%) | -- |
| Age | 64.4 (7.9) | 61.4 (8.1) | 0.26 |
| BMI (kg/m2) | 31.2 (4.5) | 27.9 (5.1) | 0.01 |
| TUG (s) | 9.34 (3.56) | 8.79 (3.26) | 0.76 |
| SCT (s) | 19.91 (14.39) | 14.84 (6.35) | 0.03 |
| 6MWT (m) | 434.17 (119.83) | 461.10 (123.39) | 0.57 |
| Normalized Hamstrings Strength (Nm/kg) | 0.75 (0.25) | 0.77 (0.19) | 0.87 |
| Normalized Quadriceps Strength (Nm/kg) | 1.34 (0.52) | 1.44 (0.28) | 0.89 |
Functional Performance
One month after surgery, both groups were significantly slower during the timed-up-and-go test and stair climb test, and walked less distance during the six-minute walk test compared to preoperative values (table 2). Between the preoperative and three-month time points, there was no difference in timed-up-and-go (both groups), stair climb (THA group), or six-minute walk (TKA group). At the same time point, the TKA group was slower on stairs compared to preoperative performance, and the THA group walked farther during the six-minute walk test and climbed stairs faster compared to their preoperative performance. Six months after surgery, both groups were faster during the timed-up-and-go test and walked further on the six-minute walk test compared to preoperative values.
Table 2.
Change in Functional Performance and Muscle Strength Outcomes at 1, 3, and 6 months postoperatively. Data are mean (95% CI).
| TKA | THA | Difference in change between TKA and THA | |
|---|---|---|---|
| Timed-up-and-go (s) | |||
| Change from baseline (1 month) | 3.65 (2.96, 4.40)* | 2.25 (1.21, 3.29)* | 1.40 (0.12-2.68)† |
| Change from baseline (3 months) | −0.23 (−1.02, 0.57) | −0.92 (−2.09, 0.25) | 0.69 (−0.72, 2.11) |
| Change from baseline (6 months) | −0.97 (−1.78, −0.15)* | −1.35 (−2.56, −0.13)* | 0.38 (−1.08, 1.84) |
| Stair climb test (s) | |||
| Change from baseline (1 month) | 16.05 (13.3, 18.74)* | 7.51 (3.69, 11.33)* | 8.54 (3.87, 13.21)† |
| Change from baseline (3 months) | −2.85 (−5.68, −0.03)* | −2.29 (−6.41, 1.84) | −0.57 (−5.57, 4.43) |
| Change from baseline (6 months) | −4.64 (−7.50, −1.78)* | −3.16 (−7.37, 1.04) | −1.48 (−6.56, 3.60) |
| Six-minute walk test (m) | |||
| Change from baseline (1 month) | −415.28 (−474.51, −356.05)* | −158.32 (−237.23, −79.41)* | −256.96 (−355.62, −158.29)† |
| Change from baseline (3 months) | 18.12 (−48.19, 84.42) | 170.76 (74.83, 266.69)* | −152.64 (−355.62, −158.29)† |
| Change from baseline (6 months) | 117.48 (47.32, 187.63)* | 230.26 (128.02, 332.49)* | −112.78 (−236.75, 11.19) |
| Normalized Hamstrings Strength (Nm/kg) | |||
| Change from baseline (1 month) | −0.32 (−0.36, −0.28)* | −0.12 (−0.18, −0.06)* | −0.20 (−0.27, −0.13)† |
| Change from baseline (3 months) | −0.04 (−0.09, 0.01) | 0.00 (−0.08, 0.07) | −0.04 (−0.12, 0.05) |
| Change from baseline (6 months) | 0.00 (−0.06, 0.05) | 0.07 (−0.01, 0.15) | −0.07 (−0.17, 0.02) |
| Normalized Quadriceps Strength (Nm/kg) | |||
| Change from baseline (1 month) | −0.67 (−0.76, −0.58)* | −0.21 (−0.34, −0.08)* | −0.46 (−0.62, −0.30)† |
| Change from baseline (3 months) | −0.21 (−0.31, −0.11)* | −0.05 (−0.20, 0.10) | −0.16 (−0.62, −0.30)† |
| Change from baseline (6 months) | 0.01 (−0.10, 0.11) | 0.16 (0.00, 0.32)* | −0.16 (−0.35, 0.04) |
significant difference from preoperative assessment
significant difference in change between TKA and THA groups
At the one-month time point, the TKA group lost more time on the timed-up-and-go test and the stair climb test, and lost more distance on the six-minute walk test compared to the THA group and their respective preoperative levels (table 2). These measures, transformed to relative change, were also significantly different across all three outcomes, suggesting the TKA group experienced a greater decline in these measures. Three months after surgery, participants after TKA continued to perform worse on the timed-up-and-go test and the six-minute walk test compared to participants after THA, and there was no difference in the change in stair climbing time between groups. Six months after surgery, there were no differences between groups in stair climb test or timed-up-and-go test performance; however, the THA group walked a greater distance during the six-minute walk than the TKA group.
Muscle Strength
Both the TKA and THA groups were significantly weaker in the knee flexor and knee extensor muscles one month after surgery (table 2). By three months, there were no differences in strength from preoperative values in either muscle group in either surgical group. Additionally, both groups’ strength remained similar to preoperative values at six months postoperative.
The TKA group lost more strength in both muscle groups compared with the THA group one month after surgery. When transformed to relative change values, the groups were also significantly different across all three outcomes, suggesting the TKA group experienced more strength loss. Three months after surgery, the TKA group remained weaker in the knee extensors compared to preoperative levels, and still demonstrated greater strength loss in these muscles compared to the THA group. By the six-month time point, knee extensor strength was not significantly different from preoperative values in the TKA group and was greater than preoperative values in the THA group.
DISCUSSION
This study provides a longitudinal comparison of performance-based outcomes after TKA and THA to highlight the differences in the trajectory of recovery. Participants lose significant amounts of strength and functional capacity one month after both surgeries, but participants after TKA decline more in the first month. Further, three months after TKA, participants performed worse and had less muscle strength than participants after THA. Despite the initial decline in functional performance and strength, participants after both TKA and THA recovered strength and function to preoperative levels after six months of recovery.
To our knowledge, this is the first study that directly compares prospective, performance-based measures of strength and function between TKA and THA both before and after surgery, highlighting the first six months of recovery. The importance of this comparison is twofold. First, these populations are often considered one cohort when reporting outcomes following surgery, and second, previous investigations have primarily relied on patient self-report to examine post-surgical outcomes (Bourne et al., 2010, Hamilton et al., 2012, Jones et al., 2005, Lau et al., 2012, Rat et al., 2010, Wylde et al., 2009). The current investigation highlights the differences in postoperative functional recovery after TKA and THA.
Because previous literature often considers outcomes after TKA and THA together, we hypothesized that both groups would experience similar declines in strength and function early after surgery. However, the data suggests that individuals after TKA experience a greater decline in functional performance and strength than individuals after THA one month after surgery. The dramatic postoperative decline after TKA has been well described (Bade et al., 2010, Mizner et al., 2005, Stevens-Lapsley et al., 2010), so the decline observed in this cohort was not surprising. Declines in strength and function after THA have also been described, suggesting a similar postoperative experience (Judd et al., 2013). However, direct comparison between these two groups suggest that individuals actually have different early postoperative experience after TKA compared with after THA. Participants following THA are more satisfied after surgery, more willing to undergo surgery again (Bourne et al., 2010, Hamilton et al., 2012), and report higher levels of functional performance several years after surgery compared to individuals following TKA (Wylde et al., 2009). This phenomenon may be explained by the fact that the immediate THA postoperative period may be relatively easier due the less dramatic decline in strength and functional performance in the first few weeks after THA compared to after TKA. Conversely, higher levels of self-reported function, and therefore satisfaction, could be a result of individuals overestimating their functional capacity early after THA due to significant pain relief follow THA (Dayton et al., 2016).
The interpretation of these findings should not be taken to mean that individuals after THA might recover without formal rehabilitation. Although it appears immediate postoperative period appears easier for individuals after THA compared to TKA based on the battery outcome measures used in this study, the complete picture of deficits and rehabilitative needs is not reflected. A more in-depth analysis, included a biomechanical analysis would clarify the effects of THA surgery on function, and may be more valuable than, or at least complimentary to, the outcome measures presented here, considering the current literature that suggests movement compensation is a persistent problem following THA (Kolk, 2014). Further, other studies suggest quality of life measures begin to decline as early as 18 months following THA (Ng et al., 2007). Individuals after THA also struggle to regain and sustain hip muscle strength (Rasch et al., 2010), and have difficulty restoring gait mechanics (Beaulieu et al., 2010) and activities of daily living (Kolk et al., 2014). The THA group studied for this analysis participated in PT ranging from 0-8 visits, with an average of three visits of home-based PT and two visits of outpatient PT (Judd et al., 2013), which is representative of standard of care in our area. Although recovery to preoperative levels did occur over the study period for both groups, participants after THA hit a plateau in their recovery after three months, while participants after TKA continued to improve from the three to six-month time points, as they were continuing their rehabilitation. Further, rehabilitation should aim to improve functional performance outcomes beyond preoperative levels, since, despite rehabilitation efforts, individuals in both groups experience difficulties with muscle strength and functional performance compared to their peers who have not have had joint replacement (Bade et al., 2010, Nilsdotter and Isaksson, 2010).
We also hypothesized that participants in each group would demonstrate similar strength and functional performance capacity after the acute postoperative timeframe, at the three- and six-month time points. However, participants with TKA took longer to recover strength and function compared to participants with THA. At three months, although both groups were statistically similar to their respective preoperative values, the THA group more closely approached their preoperative levels of strength and function. This finding is similar to results presented by Kennedy et al. (Kennedy et al., 2006) in which a THA group outperformed a TKA group with a more rapid trajectory of recovery over the first three months. Individuals after TKA may require a relatively longer recovery time, which could be achieved with increased rehabilitation utilization to achieve the desired outcomes.
Study Limitations
We utilized the same muscle strength and functional performance outcomes to examine recovery in both populations. While this was a logical extension of current practices in TKA research, these outcomes may not be ideal for the THA population because the functional tasks represented by the outcome measures selected rely heavily on the strength of the quadriceps muscle. Although participants after THA experienced quadriceps strength loss, the TKA group remains more deficient, perhaps more greatly affecting their performance on some of these measures. Individuals after THA are also impaired by muscle weakness in the gluteal muscles, movement compensations, and the relationship of those outcomes to each other (Perron et al., 2000, Rasch et al., 2010, Kolk et al., 2014). An analysis of movement and mechanics may aid in better understanding of the effects of THA. Secondly, the THA cohort had a significantly lower BMI and significantly faster SCT time than the TKA group at the start of the study (table 1). Both findings could represent a bias in favor of the THA group and related to the higher level of functional performance in that group. Additionally, we omitted measures of patient self-report, which may add a dimension to the understanding of the differences in recovery. Finally, we used a sample of convenience from previous studies in our laboratory that had exclusion criteria eliminating a substantial number of individuals from the overall screened population. This may have left a higher functioning cohort in each group, which might affect generalizability of these findings.
Conclusions
We compared performance-based outcomes in muscle strength and functional performance over the first six months of recovery after TKA and THA. These results are an important complement to existing self-report data comparing these surgeries. Our data suggest that individuals after TKA experience more decline in strength and functional performance compared to individuals after THA early after surgery, and the recovery trajectory is different in each population. This should be considered in both the outcomes chosen to measure recovery and the rehabilitation strategies adopted after surgery. Future directions should include identifying the most appropriate outcome measures to capture recovery following THA, as well as identifying the most efficacious rehabilitation strategies to optimize strength and functional capacity in both populations.
Acknowledgements:
We would like to acknowledge and thank Carol Baym, PT, DPT, PhD for review of this manuscript, and the study participants for their valuable contribution.
Funding: This work was supported by the National Institute on Aging Grant: NIH K23AG029978 (JSL); American College of Rheumatology REF/Abbot Health Professional Graduate Student Research Preceptorship Award (DLJ).
Footnotes
Declaration of Interest Statement: The authors report no conflicts of interest.
REFERENCES
- BADE MJ, KOHRT WM & STEVENS-LAPSLEY JE 2010. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther, 40, 559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAULIEU ML, LAMONTAGNE M & BEAULE PE 2010. Lower limb biomechanics during gait do not return to normal following total hip arthroplasty. Gait Posture, 32, 269–73. [DOI] [PubMed] [Google Scholar]
- BELATTI DA, PUGELY AJ, PHISITKUL P, AMENDOLA A & CALLAGHAN JJ 2014. Total joint arthroplasty: trends in Medicare reimbursement and implant prices. The Journal of arthroplasty, 29, 1539–1544. [DOI] [PubMed] [Google Scholar]
- BOURNE RB, CHESWORTH B, DAVIS A, MAHOMED N & CHARRON K 2010. Comparing patient outcomes after THA and TKA: is there a difference? Clin Orthop Relat Res, 468, 542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCKWALTER JA & MARTIN JA 2006. Osteoarthritis. Adv Drug Deliv Rev, 58, 150–67. [DOI] [PubMed] [Google Scholar]
- DAYTON MR, JUDD DL, HOGAN CA & STEVENS-LAPSLEY JE 2016. Performance-Based Versus Self-Reported Outcomes Using the Hip Disability and Osteoarthritis Outcome Score After Total Hip Arthroplasty. Am J Phys Med Rehabil, 95, 132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON D, HENDERSON GR, GASTON P, MACDONALD D, HOWIE C & SIMPSON AH 2012. Comparative outcomes of total hip and knee arthroplasty: a prospective cohort study. Postgrad Med J, 88, 627–31. [DOI] [PubMed] [Google Scholar]
- JONES CA, BEAUPRE LA, JOHNSTON DW & SUAREZ-ALMAZOR ME 2005. Total joint arthroplasties: current concepts of patient outcomes after surgery. Clin Geriatr Med, 21, 527–41, vi. [DOI] [PubMed] [Google Scholar]
- JUDD DL, DENNIS DA, THOMAS AC, WOLFE P, DAYTON MR & STEVENS-LAPSLEY JE 2013. Muscle Strength and Functional Recovery During the First Year After THA. Clin Orthop Relat Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY DM, STRATFORD PW, HANNA SE, WESSEL J & GOLLISH JD 2006. Modeling early recovery of physical function following hip and knee arthroplasty. BMC Musculoskelet Disord, 7, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY DM, STRATFORD PW, WESSEL J, GOLLISH JD & PENNEY D 2005. Assessing stability and change of four performance measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskelet Disord, 6, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLK S, MINTEN MJ, VAN BON GE, RIJNEN WH, GEURTS AC, VERDONSCHOT N & WEERDESTEYN V 2014. Gait and gait-related activities of daily living after total hip arthroplasty: a systematic review. Clin Biomech (Bristol, Avon), 29, 705–18. [DOI] [PubMed] [Google Scholar]
- KURTZ S, ONG K, LAU E, MOWAT F & HALPERN M 2007. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am, 89, 780–5. [DOI] [PubMed] [Google Scholar]
- LAU RL, GANDHI R, MAHOMED S & MAHOMED N 2012. Patient satisfaction after total knee and hip arthroplasty. Clin Geriatr Med, 28, 349–65. [DOI] [PubMed] [Google Scholar]
- MCLAWHORN AS & BULLER LT 2017. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Current reviews in musculoskeletal medicine, 10, 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIZNER RL, PETTERSON SC & SNYDER-MACKLER L 2005. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther, 35, 424–36. [DOI] [PubMed] [Google Scholar]
- NG CY, BALLANTYNE JA & BRENKEL IJ 2007. Quality of life and functional outcome after primary total hip replacement. A five-year follow-up. J Bone Joint Surg Br, 89, 868–73. [DOI] [PubMed] [Google Scholar]
- NILSDOTTER AK & ISAKSSON F 2010. Patient relevant outcome 7 years after total hip replacement for OA - a prospective study. BMC Musculoskelet Disord, 11, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PADEGIMAS EM, VERMA K, ZMISTOWSKI B, ROTHMAN RH, PURTILL JJ & HOWLEY M 2016. Medicare reimbursement for total joint arthroplasty: the driving forces. JBJS, 98, 1007–1013. [DOI] [PubMed] [Google Scholar]
- PERRON M, MALOUIN F, MOFFET H & MCFADYEN BJ 2000. Three-dimensional gait analysis in women with a total hip arthroplasty. Clin Biomech (Bristol, Avon), 15, 504–15. [DOI] [PubMed] [Google Scholar]
- PODSIADLO D & RICHARDSON S 1991. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc, 39, 142–8. [DOI] [PubMed] [Google Scholar]
- RASCH A, DALEN N & BERG HE 2010. Muscle strength, gait, and balance in 20 patients with hip osteoarthritis followed for 2 years after THA. Acta Orthop, 81, 183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAT AC, GUILLEMIN F, OSNOWYCZ G, DELAGOUTTE JP, CUNY C, MAINARD D & BAUMANN C 2010. Total hip or knee replacement for osteoarthritis: mid- and long-term quality of life. Arthritis Care Res (Hoboken), 62, 54–62. [DOI] [PubMed] [Google Scholar]
- SICARD-ROSENBAUM L, LIGHT KE & BEHRMAN AL 2002. Gait, lower extremity strength, and self-assessed mobility after hip arthroplasty. J Gerontol A Biol Sci Med Sci, 57, M47–51. [DOI] [PubMed] [Google Scholar]
- STEFFEN TM, HACKER TA & MOLLINGER L 2002. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther, 82, 128–37. [DOI] [PubMed] [Google Scholar]
- STEVENS-LAPSLEY JE, BADE MJ, SHULMAN BC, KOHRT WM & DAYTON MR 2012a. Minimally invasive total knee arthroplasty improves early knee strength but not functional performance: a randomized controlled trial. J Arthroplasty, 27, 1812–1819 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEVENS-LAPSLEY JE, BALTER JE, KOHRT WM & ECKHOFF DG 2010. Quadriceps and hamstrings muscle dysfunction after total knee arthroplasty. Clin Orthop Relat Res, 468, 2460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEVENS-LAPSLEY JE, BALTER JE, WOLFE P, ECKHOFF DG & KOHRT WM 2012b. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther, 92, 210–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEVENS-LAPSLEY JE, SCHENKMAN ML & DAYTON MR 2011. Comparison of self-reported knee injury and osteoarthritis outcome score to performance measures in patients after total knee arthroplasty. PM R, 3, 541–9; quiz 549. [DOI] [PubMed] [Google Scholar]
- WYLDE V, BLOM AW, WHITEHOUSE SL, TAYLOR AH, PATTISON GT & BANNISTER GC 2009. Patient-reported outcomes after total hip and knee arthroplasty: comparison of midterm results. J Arthroplasty, 24, 210–6. [DOI] [PubMed] [Google Scholar]