Abstract
Objective:
Empty nose syndrome (ENS) remains highly controversial, with aggressive inferior turbinate reduction (ITR) or mucocillia dysfunction frequently implicated. However, the appropriate degree of ITR is highly debatable.
Methods:
We applied individual CT based computational fluid dynamics (CFD) to 5 patients receiving relatively aggressive ITR but with no ENS symptoms, and compared them to 27 symptomatic ENS patients, who all had histories of aggressive ITRs, and 42 healthy controls. Patients’ surgical outcomes were confirmed with SNOT-22 (ITR:6.40±4.56; ENS:58.2±15.9; Healthy:13.2±14.9), NOSE scores (ITR:4.00±2.24; ENS:69.4±17.1; Healthy:11.9±12.9) and ENS6q(>=11 for ENS).
Results:
Both aggressive ITR without ENS symptoms and symptomatic ENS patients had significantly lower nasal resistance (ITR:0.059±0.020; ENS:0.052±0.015; Healthy:0.070±0.021Pa⸱s/mL) and higher cross-sectional areas surrounding the inferior turbinate (ITR:0.94±0.21; ENS:1.19±1.05; Healthy:0.42±0.22cm2) than healthy controls. The lack of significant differences among patient groups indicated similar degrees of surgeries between ITR with and without ENS symptom cohorts. However, symptomatic ENS patients have paradoxical significantly less airflow in the inferior meatus (ITR:47.7%±23.6%; ENS:25.8±17.6%; healthy: 36.5±15.9%; both p<0.01), but higher airflow around middle meatus (ITR:49.7%±22.6%; ENS:66.5±18.3%; healthy:49.9±15.1%, p<.0001) than aggressive ITR without symptoms and controls. Aggressive ITR patients have increased inferior meatus airflow as expected (p<0.05). This imbalanced airflow produced less inferior wall-shear-stress distribution among symptomatic ENS patients (ITR: 42.45 ±11.4%; ENS: 32.2±12.6; Healthy: 49.7%±9.9%). ENS patients (n=12) also had impaired nasal trigeminal function, as measured by menthol lateralization detection thresholds (ITR: 15.2 ±1.2; ENS: 10.3±3.9; Healthy: 13.8±3.09, both p<0.0001). Surprisingly, aggressive ITR patients without ENS symptoms have better menthol LDT than healthy controls.
Conclusion:
While turbinate tissue loss is linked with ENS, the degree of ITR that might distinguish post-operative patient satisfaction in their nasal breathing vs. development of ENS symptoms is unclear. Our results suggest that it may be a combination of distorted nasal aerodynamics and loss of mucosal sensory function potentially lead to ENS symptomology
Keywords: Empty nose syndrome, CFD, nasal airflow dynamics, Computer Modeling for Nasal Airflow, Nose Models, Post-Operative
Introduction
An estimated 600 thousand ambulatory nasal sinus surgeries were performed annually in the US1. The most common sinonasal surgeries among them are turbinate reduction and septoplasty, or a combination of both. The outcomes of these surgeries are variable but generally good, with short-term favorable outcomes of about 60 – 90% 1–3, however, rare complications do occur. The most controversial complication is empty nose syndrome (ENS), a rare but debilitating disease with aggressive inferior turbinate reduction (ITR) and/or mucocilia dysfunction frequently implicated in its development. ITR surgery typically results in widening of the nasal passage, yet ENS patients paradoxically report symptoms of nasal obstruction as well as nasal crusting, discharge, dryness, and nasal pain. These symptoms have been shown to have a devastating impact on the post-operative quality of the patients’ lives with reports of elevated anxiety, the constant feeling of suffocation, chronic hyperventilation, chronic fatigue, and psychological disorders4. Although the symptoms of ENS are well documented, the precise etiology of the disease is not very well understood and it still remains a highly controversial topic within the rhinology field. Its rare occurrence leads to a lack of reliable tests and metrics to diagnose the disease, which can only depend upon patients reported symptomology, with its cause remaining unknown 5.
Furthermore, most post-turbinate reduction or even post-turbinectomy patients will not go on and develop ENS. There is a need to better identify factors of ITR that results in the disease6. Altered nasal aerodynamics have been thought to be a major contributing factor in cases of ENS and 3D computational fluid dynamic (CFD) analysis of airflow through the nasal passage has been used to evaluate nasal aerodynamics among ENS patients 5,7–9. In this study we will develop CT based CDF models of 5 patients receiving relatively aggressive ITR but who have not developed ENS and compared them with two previously published data set: 27 patients with histories of ITRs who developed ENS and 42 healthy controls5. Our goal is to characterize features in nasal airflow that may separate whether or not patients will develop ENS following aggressive ITR. We also hypothesize that nasal mucosa sensory function may be another factor that contributes to the occurrence of ENS10–12 and when combined with CFD analysis may provide a better diagnostic prediction. To test these hypotheses, we quantify and compare nasal cross sectional area, peak wall shear stress, flowrate, the wall shear force and trigeminal function in all three patient populations. We aim to use these data to give better insight into the driving factors behind the development of ENS so that it may be avoided in the future.
Materials and Methods
Study Population
Five patients who underwent aggressive turbinate reduction without ENS symptoms were enrolled in the study. Aggressive ITR was characterized through visual CT scan observation and clinical assessment as well as quantitative measurement of the cross sectional airway area. For the cross sectional measurement, a coronal slice was taken at 60% of the length posterior from the nostril and the airway cross sectional area for each patient is calculated. The average cross sectional airway area surrounding the inferior turbinate of aggressive ITR without ENS symptoms was 9.38e−5 cm2 ± 2.05e−5 cm2, which is comparable to that of the ENS patient group. Supplementary Figure 1 shows three cross sections of the 5 patients selected compared to those of a symptomatic ENS patient. Pre surgery, all patients presented with bilateral turbinate hypertrophy, then all underwent a submucosal resection of both inferior turbinates. We also visually examined the anterior aspect of the inferior turbinate head. One patient was missing the anterior aspect of the inferior turbinate head with another showing a slight reduction, while the rest have a relatively intact IT head. The sample population consists of 4 males and 1 female with a mean age of 46 years (range 37 – 59 years). The final post-surgery mean NOSE and SNOT scores were even lower than the healthy controls (see Table 1), indicating that they are free of sinonasal symptoms. None of the patients identified or displayed symptoms associated with ENS at the time of post-op CT and score recording. Scores were collected on average 112 days ± 90.5 days following surgery (range 52 – 270 days post-op).
TABLE 1.
Comparison of age, gender, and symptom scores between aggressive inferior turbinate reduction (ITR) patients without ENS symptoms, ENS patients, and healthy controls
| Characteristic | Aggressive ITR without ENS symptoms (n = 5) | ENS (n = 27) | Healthy (n = 42) |
|---|---|---|---|
| Age (years), mean ± SD | 46 ± 9.23 | 41.92 ± 10.39 | 31.6 ± 1120 |
| Gender, n (%) | |||
| Male | 4 (80) | 19 (70.4) | 15 (35.7) |
| Female | 1 (20) | 8 (29.6) | 27 (64.3) |
| NOSE (0–100), mean ± SD | 4.00 ± 2.24 | 69.35 ± 17.10 | 11.90 ± 12.90 |
| SNOT-22 (0–110), mean ± SD | 6.40 ± 4.56 | 58.22 ± 15.85 | 13.17 ± 14.85 |
ENS = empty nose syndrome; NOSE = Nasal Obstruction Symptom Evaluation; SD = standard deviation; SNOT-22 = 22-Item Sino-Nasal Outcome Test.
A total of 27 patients diagnosed with ENS from a previously published study5 were included as comparison group. The group consists of 8 females and 19 males with ages ranging from 25 to 67 years (mean of 41 years). Their symptoms were confirmed through a combination of ENS6Q, SNOT22, NOSE Score, and the evaluation of medical and surgical history. All of them have a history of inferior turbinate reduction surgeries that were confirmed with a CT. Two of the patients had a total inferior turbinectomy while the remaining patients either had a partial turbinectomy or mucosal preserving reduction. Out of 27 ENS patients, 7 patients also had a history of middle turbinate (MT) surgeries (5 with partial MT resection and 2 with total MT turbinectomy), while the rest had relatively intact MTs. Nineteen patients had a history of septum surgeries with 14 patients having septoplasties and 5 having rhinoplasty surgeries. Also, 8 of 27 patients underwent sinus surgery (6 with unspecified/ polpectomy, 1 with an ethmoidectomy, and 1 with a combined anthrostomy, ethmoidectomy and frontal sinusotomy). A majority of patients (20 out 27) developed ENS symptoms within one month of their surgery.
Forty-two healthy subjects from previously published study 5 were included as a control population to compare with both the symptomatic ENS and aggressive ITR without ENS symptom patients. The mean age for the control population was 31 years with a range of 21–60 years. Every healthy control subject underwent medical history screening to exclude preexisting nasal sinus disease, nasal sinus complaints, prior head trauma, and prior nasal surgery. The SNOT22 and NOSE questionnaires were also filled out by the healthy controls. The demographics and symptom score between aggressive ITR patients without ENS symptoms, symptomatic ENS patients, and healthy controls are summarized in Table 1. The current study was approved by the Institutional Review Boards of The Ohio State University.
Questionnaire
The Sino-nasal Outcome Test (SNOT-22) 13 and Nasal Obstruction Symptom Evaluation (NOSE) 14 are two commonly used validated outcome questionnaires for nasal sinus patients. The aggressive ITR patients without ENS symptoms have improved SNOT-22 (6.40 ± 4.56) and NOSE (4.00 ± 2.24) scores even compared to healthy controls (SNOT-22: 13.17 ± 14.85; NOSE: 11.90 ± 12.90), whereas ENS patients had significantly elevated SNOT-22 (58.22 ± 15.85) and NOSE scores (69.35 ± 17.1). ENS6Q15 is a recently validated specific 6-item questionnaire as an adjunct to the standard SNOT-22 questionnaire to discriminate patients with suspicion of ENS. A score of ENS6Q >11 is indicative of ENS. All of ENS patients fit this criterion with a mean score of 19.78 ± 5.42.
CT Scan and CFD Model
All patients underwent an IRB approved postoperative research CT scan which were used to construct 3-Dimensional CFD nasal airway models using methods previously described 9,16. These research CT scans are performed with a cone beam office CT scanner (3D Accuitomo 170, J. Morita USA, Inc.) at the Department of Otolaryngology at The Ohio State University School of Medicine. The scan has a radiation dosage of roughly 12% of a conventional head CT scan which significantly lowers the associated health risks.
The CT scans were imported into AMIRA (Visualization Sciences Group, Hillsboro, OR, USA) software where a 3-dimensional volume was created from 2-dimensional coronal, lateral, and axial images. The volume was created following image smoothing and artifact correction before being imported into a second commercial software package, ICEM CFD (Ansys, Inc., Canonsburg, PA, USA) where the volume was meshed with tetrahedral volume elements. Volume, surface, and boundary meshing was consistent with methods used in Li et al 5 resulting in about 1.1 million to 3.6 million hybrid finite elements for each nasal geometry.
The Navier-Stokes equations were solved under steady state conditions with incompressible flow using ANSYS Fluent 16.2 (Ansys, Inc., Canonsburg, PA, USA). The nasal walls were assumed to be rigid and a no slip boundary condition was applied. A pressure drop of 15 Pa was applied between the nostrils and nasopharynx in order to simulate restful breathing, a state most relevant to patients’ symptoms during routine daily life 17,18. The numerical solutions of the continuity and momentum equations were determined using the finite-volume method. Continuous pressure and velocity fields were discretized using a second-order upwind scheme for numerical simulations. The SIMPLEC algorithm was used for pressure‐velocity coupling. The converged simulation results were determined once the residual of each variable was less than 10-5. The numerical method applied in the current study has been validated by comparing with experimental measurements 8,19.
Menthol LDTs
Nasal trigeminal sensitivity is commonly assessed using the measurement of lateralization detection thresholds (LDT) and was performed on all the aggressive ITR surgery without ENS symptom patients in our study, as well as a subset of reported symptomatic ENS and healthy controls (ITR n = 5, ENS n = 12, Healthy n = 20). Menthol was used due to its ability to activate the trigeminal TRPM8 cool sensitive pathway. Our procedure is consistent with what is reported in the literature7,10 and in brief: Menthol was diluted into mineral oil in a binary dilution series with 20 steps. The solution starts with a concentration of 0.125g/mL where each step diluted the pervious solution to one-half. The final dilution step, #20, had a dilution of 2.38e−7 g/mL. Two bottles were used for each trial, one with menthol dilution and one with mineral oil, and randomly inserted into each nostril via a nose piece. The subject simultaneously sniffed from the pair of bottles and was instructed to identify which side contained menthol. The trials were conducted based on a force-choice ascending method of limits 20 where each stimulus concentration was at most presented one time in ascending concentrations. The testing ended given the two conditions met: (1) the subject responds correctly in 4 consecutive trails and (2) the subject was confident that their last response was correct (associated with a 7/10 or higher on the confidence rating scale of 0 to 10). This was done to minimize the chances of false positives given that the probability of 4 consecutive “lucky guesses” is about 6.25%. The threshold values were tracked unilaterally and were reported as the bottle number from 1 to 20.
Data Analysis
Two-tailed independent-sample T-tests without assuming equal-variance were used for cross-group comparison (e.g. between aggress ITR without symptoms and symptomatic ENS). We used a modified power analysis (developed by Erdfelder) and confirmed that our sample size is sufficient given a Power (1-β) of 80% 21.
Results
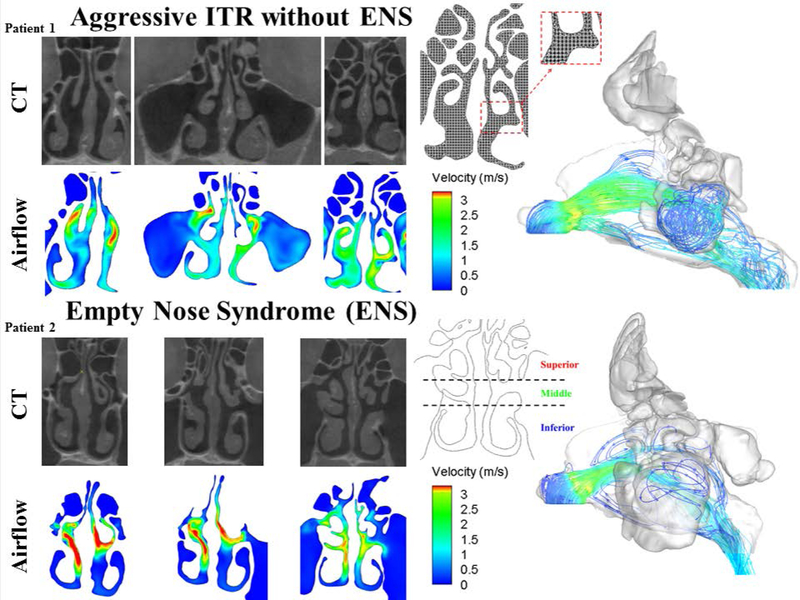
Figure 1 shows the airflow through the nasal cavity at three different cross sections for two patients who have received aggressive ITR, Patient 1, without the development of ENS symptoms and the other, Patient 2, developed ENS symptoms. In the patient without ENS symptoms, we observed that the airflow is evenly distributed throughout the superior, middle, and inferior meatus with a majority of the airflow velocity to be around 1.5 to 2.5 m/s. This is further supported by the airflow pathline plot in which we see flow dispersed throughout the nasal cavity. Conversely, in Patient 2 with symptoms of ENS, we observe that airflow is localized to the middle meatus region with higher flow velocities of 3+ m/s and with essentially no flow (blue) throughout the inferior meatus. This profile can be seen in the pathline plot in which airflow through the nasal cavity takes on a more jet stream appearance.
Figure 1.
Velocity plots of three coronal sections of the nasal cavity and their corresponding CT images (left) for a patient with ITR and no ENS symptoms (top) and a patient who developed ENS symptoms (bottom). (Right) A three-dimensional model of the nasal cavity and the corresponding airflow pathline patterns ENS = Empty Nose Syndrome, CT = computed tomography, ITR = Inferior Turbinate Reduction.
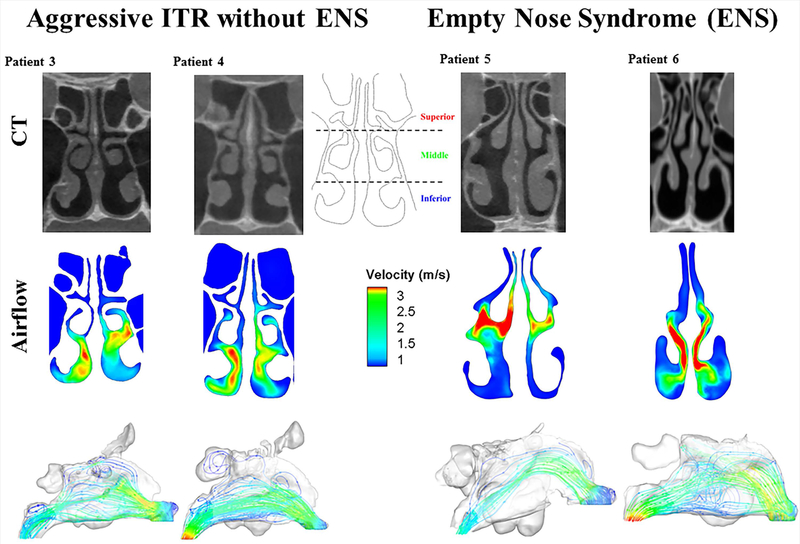
In Figure 2, we compared four more patients with and without ENS symptoms and found a similar pattern. Here, we see that in Patient 3 and Patient 4, both without ENS symptoms, airflow is evenly distributed through the inferior and middle meatus. The flow stream profiles also indicate a more dispersed and spread out flow profile through the nasal cavity. For the symptomatic ENS patients, Patient 5 and 6, the airflow is concentrated again through the middle meatus with velocities over 3 m/s, and with little flow reaching the inferior meatus. Visually inspecting the CT scans of all of the patients (see supplementary figure 1), the degree of ITR is not much different between aggressive ITR without ENS symptoms vs aggressive ITR with ENS.
Figure 2.
A coronal section of the nasal cavity, 2D airflow, and airflow pathline through the nasal cavity in 3D for two ITR patients without ENS symptoms and two patients with ITR surgery who developed ENS symptoms. ENS = Empty Nose Syndrome, ITR = Inferior Turbinate Reduction.
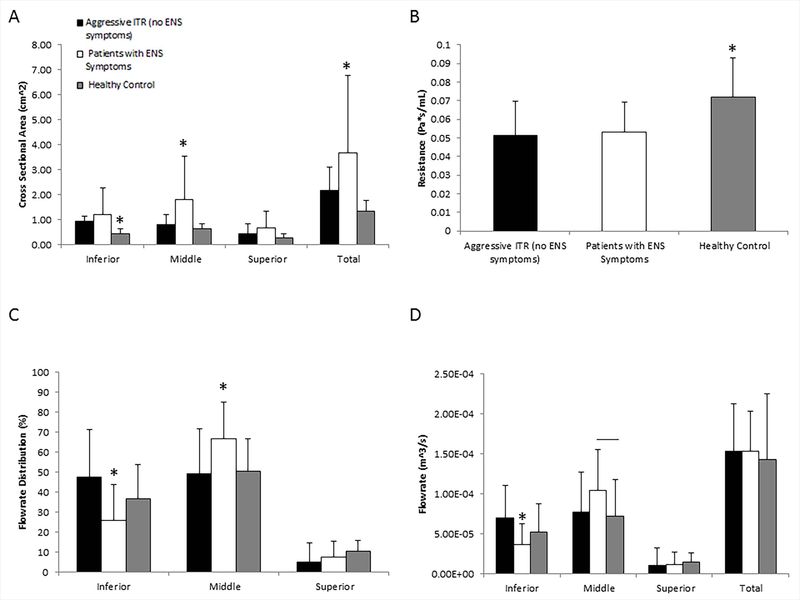
Figures 3A, 3C and 3D quantify the cross sectional area and flow rates for all subject groups. Both aggressive ITR patients without ENS symptoms and symptomatic ENS patients had significantly higher cross-sectional areas surrounding the inferior turbinates than healthy controls (ITR: 0.94±0.21; ENS: 1.19±1.05; Healthy: 0.42±0.22 cm2, two tailed t-test p < 0.05), with no statistically significant difference between aggressive ITR without ENS symptoms and symptomatic ENS groups. Both aggressive ITR without ENS symptom patients and symptomatic ENS patients also had significantly lower nasal resistance then healthy controls (Figure 3B, ITR: 0.051±0.020; ENS: 0.052±0.015; Healthy: 0.070±0.021 Pa∙s/mL), but again with no statistical difference between them. The lack of statistically significant differences in both inferior airway areas and nasal resistances implicate a comparable degree of turbinate reduction in surgery between the aggressive ITR without ENS symptom and symptomatic ENS patients. Furthermore, symptomatic ENS patients have significantly higher superior and middle cross-sectional airway areas than those of the healthy control (ENS superior/middle: 0.66 cm2 /1.77 cm2; healthy control: 0.29 cm2 /0.64 cm2; and ITR: 0.42 cm2/ 0.81cm2, p < 0.05), and symptomatic ENS patients also had significantly higher cross sectional area surrounding the middle turbiante region than aggressive ITR without ENS symptom patients. As the result, symptomatic ENS patients have the largest total cross sectional area of about 3.62 ± 3.04 cm2 when compared to the average of 1.34 ± 0.44 and 2.18 ± 0.91 cm2 in healthy controls and aggressive ITR patients without ENS symptoms, respectively.
Figure 3.
(A) Mean cross sectional area +/− SD along the nasal cavity divided into three different regions (inferior, middle, and superior), (B) Mean nasal resistance +/− SD for the three different patient populations, (C) Mean flow rate percentage distribution +/− SD through the three subdivided regions of the nasal cavity, D) Mean flowrate +/− SD along the nasal cavity divided into three different regions (inferior, middle, and superior) All symbols represent statistical significance with p < 0.05 using a two tailed t-test. SD = Standard deviation, ENS = Empty Nose Syndrome, Middle = Middle Turbinate, Inferior = Inferior Turbinate, Superior = Superior Turbinate.
For the airflow rates (Figures 3C and 3D), symptomatic ENS patients have significantly lower inferior airflow rates than healthy controls and aggressive ITR patients without ENS symptoms (ITR: 6.96e−5m3/s ± 4.12e−5m3/s; ENS: 3.69e−5m3/s ±2.58e−5m3/s; healthy: 5.21e−5m3/s ±3.60e−5m3/s; p < 0.05). This lower flowrate corresponds with less airflow distribution in the inferior meatus (ITR: 47.7%±23.6%; ENS: 25.8±17.6%; healthy: 36.5±15.9%; both p < 0.01) and significantly more airflow distribution around the middle meatus (ITR: 49.1%±10.6%; ENS: 66.5±18.3%; healthy: 49.9±15.1%, p<0.05). These results are paradoxical, as both aggressive ITR patients without ENS symptoms and symptomatic ENS patients have similar degree of expansion of inferior airway. Also, we observe in aggressive ITR patients without ENS symptomsan increased inferior meatus airflow of about 17.6% (p < 0.05, two tailed t-test), yet ENS patients have a reduction of flow distribution in the inferior region, even compared to healthy controls. This outcome is contradictive to the purpose of ITR which serves to expand the inferior airway and allow more flow through the airway surrounding the inferior turbinate. However this is not the case in ENS patients where an increase in flow through the middle region is observed.
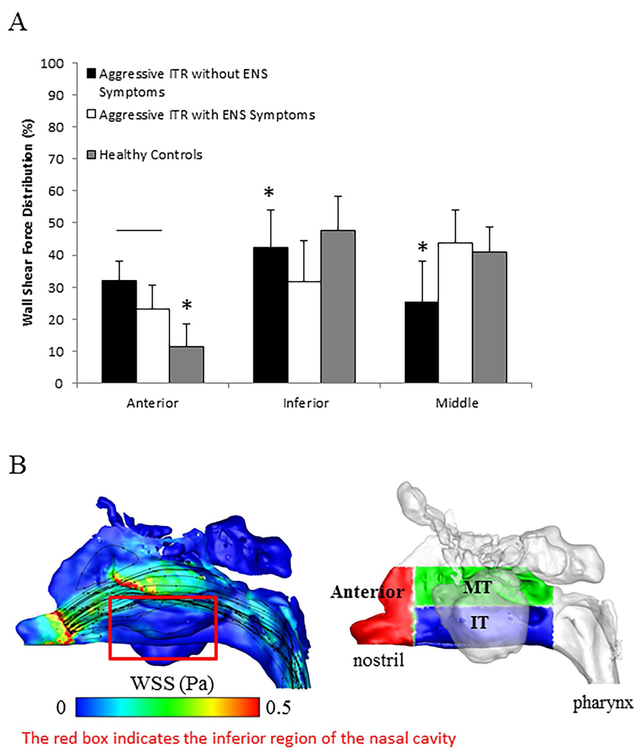
Figure 4A quantifies the regional wall shear force distribution among all groups. Wall shear force is the amount of force exerted onto a region of nasal mucosa by airflow, which is an indicator for airflow-mucosa interactions. Since total wall shear force varies as a function of nasal resistance, here, we focus on the percentage of regional force distribution. Both aggressive ITR patients without ENS symptoms and symptomatic ENS patient groups showed a significantly higher wall shear force distribution in the anterior region compared to the healthy control group, with ENS patients displaying a significantly lower wall shear force distribution in the inferior meatus (32.24% ± 12.64%) compared to both aggressive ITR without ENS symptom patients and healthy patients (p < 0.05, two tailed t-test). Interestingly, aggressive ITR patients without ENS symptoms have a significantly lower middle meatus shear force (25.3% ± 12.74%) than the healthy controls (39.88% ± 6.96%) and ENS patients (43.82% ± 10.2%). Figure 4B plots the color contour of wall shear stresses in a symptomatic ENS patient, with the lack of shear forces in the inferior meatus as seen (red box), as well as the definition of different mucosal regions used for Figure 4A.
Figure 4.
(A) Mean wall shear force distribution +/− SD along the nasal cavity divided into three different regions (anterior, inferior, and middle), (B) Example of WSS distribution and flow streamlines for a symptomatic ENS patient as well as a schematic depicting the anterior, middle and inferior regions. The red box in 4B indicates the inferior region of the nasal cavity and is used to highlight the lack of shear forces and flow. All symbols represent statistical significance with p < 0.05 using a two tailed t-test.
Figure 5A summarizes the menthol LDT results for all groups. In this figure, each box represents the range between the first and third quartiles with the median LDT value indicated by a horizontal line. The whiskers extend to the maximum and minimum values for each group with the exception of outliers which are plotted as individual points. Our data indicate that ENS patients (10.2 ± 3.87, p < 0.0001, two-tailed t-test) have significantly lower menthol LDT than both aggressive ITR without ENS symptoms (15.2 ± 1.23) and healthy controls (14.8 ± 1.59). And surprisingly, aggressive ITR without ENS symptoms had a slightly but significantly better menthol LDT than healthy controls (p<0.05).
Figure 5.
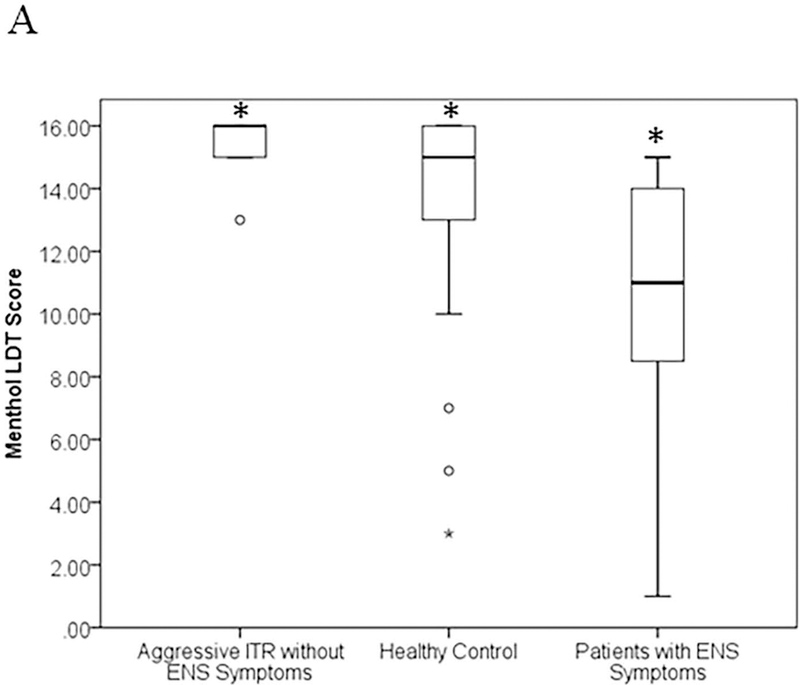
Comparison of menthol lateralization detection thresholds (LDT) between ITR patient populations (without symptoms of ENS and with symptoms of ENS) and healthy controls. The box represents the first and third quartiles with the mean value inside the box in bold. The whiskers extend to the minimum and maximum values except for in the case of outliers. Outliers are plotted as individual points. All symbols represent statistical significance with p < 0.05 using a two tailed t-test.
Discussion
ENS is a rare and debilitating disease that may occur after ITR and is characterized by symptoms of paradoxical nasal obstruction, discharge, dryness and nasal pain. These symptoms can often lead to devastating effects for patients such as the constant feeling of suffocation, chronic hyperventilation, increased anxiety, and other psychological disorders. Its underlying pathogenesis has been attributed to surgery, poor mucosal wound healing, altered nasal aerodynamics, as well as impaired nasal sensory function7. Yet, not all patients who underwent aggressive turbinate reduction or even a turbinectomy will develop symptoms of ENS. This study examined the alteration of the nasal geometries and aerodynamics of patients who have undergone aggressive ITR without symptoms of ENS and compared them to published data sets of patients with ENS symptoms and healthy patient populations.
What we found is that there are significant differences in airflow patterns comparing aggressive ITR patients without ENS symptoms and healthy controls to symptomatic ENS patients. In both the aggressive ITR patients without ENS symptoms and healthy controls, nasal airflow is dispersed throughout the inferior and middle regions of the nasal cavity. However, the airflow of the patients with symptoms of ENS displays a concentrated jet of air that is centralized around the middle region with little to no flow reaching the inferior region (Figures 1 and 2). We further compared the cross sectional areas of each patient population to determine if that could explain the discrepancy. Figure 3A indicates that the inferior meatus area of symptomatic ENS patients, which is comparable to that of asymptomatic ITR patients, is significantly higher than that of the healthy control group. Furthermore the nasal resistance (Figure 3B) for both patients receiving aggressive ITR is significantly lower than the healthy control. This indicates that the surgical impact which increases the inferior region of the nasal airway as well as decreases the overall nasal resistance is similar between the two groups. So what is driving the change in flow patterns between the two ITR patient populations?
To understand what is driving the change in flow, we compared the airway ratios of the inferior and middle regions. Using the healthy controls as our baseline, the ratio of airway cross sectional areas between the inferior and middle meatus is about 0.66, which corresponds to a 1.06 ratio of airflow rates between inferior and middle regions and can be considered as baseline of a rather even distribution between the two regions. For aggressive ITR patients without ENS symptoms, the airway ratio between the inferior and middle meatus increases to 1.34, as a result of the turbinate reduction, with a matching shift in the airflow ratio to 1.70. However, for symptomatic ENS patients, this balance is completely disrupted with an increased airway ratio to 0.82 but a decreased airflow ratio to 0.55. This suggests that while there is an increase in airway cross section ratio compared to the healthy controls, this relative increase in ratio doesn’t result in more distributed airflow through the inferior region in patients who developed ENS, but rather results in significant decrease.
The imbalance of nasal airflow will affect regional wall shear stress distribution within the middle and inferior regions of the nasal passage. Here, we found that all ITR surgeries were successful in decreasing the nasal resistance (Figure 3B) compared to healthy controls and increasing the airflow into the nasal cavity to values similar to healthy controls (Figure 3D). We also observed that shear forces in the anterior region are significantly higher for both ITR patient populations when compared to the healthy controls in Figure 4. However, in patients who developed ENS symptoms, there is a significantly decreased flowrate through the inferior region of the nasal cavity that resulted in decreased wall shear forces. In animal models mechanoreceptors have been identified in the nasal mucosa and distributed at the ethmoidal nerve area. It has been suggested that stress on the mucosa can lead to stretching and simulation of these nerve endings 22,23 and suggests that they serve as a feedback mechanism to inform the body of air volume inhaled. While the similar volume of air is able to flow through the nasal cavity for aggressive ITR patients both with and without symptoms of ENS, the competing reduction of resistances between the inferior and middle meatus results in a decrease or elimination of flow to the inferior region only in the ENS group. This reduction in flowrate then results in a significant decrease in wall shear forces within the inferior portion of the nasal cavity (Figure 4A and 4B). The reduction in wall shear force may lead to a lack of stimulation of mechanoreceptors or cooling receptors within the nasal passage thus giving patients the feeling of suffocation or obstruction despite sufficient volumes of inspired air.
Nasal neurosensory impairment due to wound healing or loss of sensory nerve fibers has also been thought to contribute to ENS. To explore whether or not post-surgery mucosal sensory impairment may contribute in ENS after aggressive ITR, we conducted menthol LDT tests. Figure 5A shows that the LDT results of the ENS patients are significantly lower (worse) than that of both healthy controls and the aggressive ITR group without ENS symptoms. Furthermore, the LDT results of aggressive ITR patients without ENS symptoms are even significantly better than those of the healthy controls. This suggests that one potential contributing cause of ENS could be that the perception of airflow requires the activation of nasal trigeminal cool receptors when cool ambient air is inspired10,24. Menthol has also been shown to mimic the feeling of increased airflow through activation of the TRPM8 trigeminal cool receptors25. It is possible that mucosa trigeminal sensory function is more disrupted due to surgery or poor wound healing in some patient populations than others. But it is also likely that less only 25% of the airflow is traveling through the inferior region among symptomatic ENS patients, the menthol may be unable to be successfully transported and activate the TRPM8 pathway26,27. This is a first study to investigate the difference between patients with aggressive ITR (with no ENS symptoms) and patients with ENS symptoms. However, due to low sample size, we are unable to pinpoint whether the imbalanced nasal aerodynamics or the impaired trigeminal sensory function are more direct contributors to their different symptomatology among these cohort of patients. In addition to the low sample size for this pilot study, other potential limitation includes the heterogeneous nature of nasal sinus disease. Pre-surgery, two patients had a history of past turbinate procedures (one with turbinate cauterization and another with submucosal resection), and three had an additional diagnosis of chronic rhinosinusitis and one with allergic rhinitis. Hopefully in future, given a larger patient base we can begin to investigate trends within each patient sub-group.
Conclusion
While inferior (and sometimes middle) turbinate tissue loss is linked with ENS, the degree of ITR that might distinguish post-operative patient satisfaction in their nasal breathing vs. development of ENS symptoms is unclear. Our results suggest that it may be a combination of distorted nasal aerodynamics and also loss of mucosal sensory function potentially lead to ENS symptomology. The findings indicate that CFD and Menthol testing may be used as a potential objective diagnosis of ENS in the future.
Supplementary Material
Acknowledgments
Funding: NIH NIDCD R01 DC013626 to KZ
Footnotes
Conflict of Interest: There are no conflicts of interest to disclose.
Work Presented: Oral Presentation – October 05 – 06: American Rhinology Society 2018 Annual Meeting in Atlanta, Georgia, United States of America
References
- 1.Bhattacharyya N Ambulatory sinus and nasal surgery in the United States: demographics and perioperative outcomes. Laryngoscope 2010;120(3):635–638. [DOI] [PubMed] [Google Scholar]
- 2.Ho WK, Yuen AP, Tang KC, Wei WI, Lam PK. Time course in the relief of nasal blockage after septal and turbinate surgery: a prospective study. Arch Otolaryngol Head Neck Surg 2004;130(3):324–328. [DOI] [PubMed] [Google Scholar]
- 3.Stewart MG, Smith TL, Weaver EM, et al. Outcomes after nasal septoplasty: results from the Nasal Obstruction Septoplasty Effectiveness (NOSE) study. Otolaryngol Head Neck Surg 2004;130(3):283–290. [DOI] [PubMed] [Google Scholar]
- 4.Lee TJ, Fu CH, Wu CL, et al. Evaluation of depression and anxiety in empty nose syndrome after surgical treatment. Laryngoscope 2016;126(6):1284–1289. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Farag AA, Maza G, et al. Investigation of the abnormal nasal aerodynamics and trigeminal functions among empty nose syndrome patients. Int Forum Allergy Rhinol 2018;8(3):444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dayal A, Rhee JS, Garcia GJ. Impact of Middle versus Inferior Total Turbinectomy on Nasal Aerodynamics. Otolaryngol Head Neck Surg 2016;155(3):518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Farag AA, Leach J, et al. Computational fluid dynamics and trigeminal sensory examinations of empty nose syndrome patients. Laryngoscope 2017;127(6):E176–E184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Jiang J, Dong H, Zhao K. Computational modeling and validation of human nasal airflow under various breathing conditions. Journal of Biomechanics 2017. [DOI] [PMC free article] [PubMed]
- 9.Otto BA, Li C, Farag AA, et al. Computational fluid dynamics evaluation of posterior septectomy as a viable treatment option for large septal perforations. International Forum of Allergy & Rhinology 2017;7(7):718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao K, Blacker K, Luo Y, Bryant B, Jiang J. Perceiving nasal patency through mucosal cooling rather than air temperature or nasal resistance. PLoS One 2011;6(10):e24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao K, Jiang J, Blacker K, et al. Regional peak mucosal cooling predicts the perception of nasal patency. Laryngoscope 2014;124(3):589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konstantinidis I, Tsakiropoulou E, Chatziavramidis A, Ikonomidis C, Markou K. Intranasal trigeminal function in patients with empty nose syndrome. Laryngoscope 2017;127(6):1263–1267. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins C, Gillett S, Slack R, Lund V, Browne J. Psychometric validity of the 22‐item Sinonasal Outcome Test. Clinical otolaryngology 2009;34(5):447–454. [DOI] [PubMed] [Google Scholar]
- 14.Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngology—Head and Neck Surgery 2004;130(2):157–163. [DOI] [PubMed] [Google Scholar]
- 15.Velasquez N, Thamboo A, Habib AR, Huang Z, Nayak JV. The Empty Nose Syndrome 6-Item Questionnaire (ENS6Q): a validated 6-item questionnaire as a diagnostic aid for empty nose syndrome patients. Int Forum Allergy Rhinol 2017;7(1):64–71. [DOI] [PubMed] [Google Scholar]
- 16.Zhao K, Scherer PW, Hajiloo SA, Dalton P. Effect of anatomy on human nasal air flow and odorant transport patterns: implications for olfaction. Chemical senses 2004;29(5):365–379. [DOI] [PubMed] [Google Scholar]
- 17.Keyhani K, Scherer P, Mozell M. Numerical simulation of airflow in the human nasal cavity. Journal of Biomechanical Engineering 1995;117:429–441. [DOI] [PubMed] [Google Scholar]
- 18.Zhao K, Jiang J. What is normal nasal airflow? A computational study of 22 healthy adults. International forum of allergy & rhinology 2014;4(6):435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di MY, Jiang Z, Gao ZQ, Li Z, An YR, Lv W. Numerical simulation of airflow fields in two typical nasal structures of empty nose syndrome: a computational fluid dynamics study. PLoS One 2013;8(12):e84243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(ATSM) ASfTaM. Standard Practice for Determination of Odor and Taste Thresholds by a Forced-choice Ascending Concentration Series Method of Limits In. Philidelphia, PA: 2011. [Google Scholar]
- 21.Erdfelder E Zur Bedeutung und Kontrolle des !B-Fehlers bei der inferenzstatistischen Prüfung log-linearer Modelle [The significance and control of the !B-error during the inference-statistical examination of the log-linear models]. In. Vol 15: Zeitschrift für Sozialpsychologie; 1984:18–32. [Google Scholar]
- 22.Tsubone H Nasal ‘flow’ receptors of the rat. Respir Physiol 1989;75(1):51–64. [DOI] [PubMed] [Google Scholar]
- 23.Clarke RW, Jones AS. Nasal airflow receptors: the relative importance of temperature and tactile stimulation. Clin Otolaryngol Allied Sci 1992;17(5):388–392. [DOI] [PubMed] [Google Scholar]
- 24.Eccles R Nasal airflow in health and disease. Acta Otolaryngol 2000;120(5):580–595. [DOI] [PubMed] [Google Scholar]
- 25.Bautista DM, Siemens J, Glazer JM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007;448(7150):204–208. [DOI] [PubMed] [Google Scholar]
- 26.Clarke RW, Jones AS. The distribution of nasal airflow sensitivity in normal subjects. J Laryngol Otol 1994;108(12):1045–1047. [DOI] [PubMed] [Google Scholar]
- 27.Wrobel BB, Bien AG, Holbrook EH, et al. Decreased nasal mucosal sensitivity in older subjects. Am J Rhinol 2006;20(3):364–368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.