Abstract
Background:
Mesonephric adenocarcinomas are rare neoplasms most commonly occurring in the lateral cervix and vagina. Tumors with similar morphologic, immunophenotypic and molecular characteristics have been recently described in the uterine corpus and ovary. We sought to characterize the cytomorphologic features of adenocarcinomas exhibiting mesonephric-like differentiation (AMDs) arising in the upper gynecologic tract.
Methods:
Institutional databases were retrospectively queried for tumors of the upper gynecologic tract described as “tumor of Wolffian origin” or “with mesonephric features” between 2007 and 2017. All available cytologic material was reviewed. Cytomorphologic characteristics were detailed by three pathologists.
Results:
Our cohort consisted of eight cases from seven patients. Primary sites included ovary (n=3), endometrium (n=4) and pelvis, not otherwise specified (n=1). All cases showed tight 3-dimensional clusters of overlapping cells. Additional architectural features included tubular (5/8, 63%) and papillary (3/8, 38%) formations. Cells were small with scant (7/8, 88%) to moderate (1/8, 12%) cytoplasm. Three cases (3/8, 38%) showed extracellular hyaline globules. Nuclei were uniform in size (6/8, 75%) or showed mild anisonucleosis (2/8, 25%). Nuclear grooves and indentations were seen in all cases. Mitoses (5/8, 63%) and apoptotic bodies (4/8, 50%) when present, were rare. No necrosis was seen.
Conclusions:
AMD shows a monotonous population of small cells with scant to moderate cytoplasm and abundant nuclear grooves arranged in tight, overlapping 3-dimensional clusters. Occasionally papillary or tubular architecture, as well as extracellular hyaline globules, can be seen. These features should prompt further testing (e.g. immunohistochemistry) to confirm the diagnosis and to exclude potential mimics.
Keywords: Adenocarcinoma, Cytology, Gynecologic cancer, Mesonephric-like differentiation, Ovary, Uterus
Precis for use in the Table of Contents:
We describe the cytopathologic features of adenocarcinoma with mesonephric-like differentiation arising in the upper gynecologic tract, a newly described and rare entity.
INTRODUCTION
Mesonephric adenocarcinoma of the female genital tract is a rare malignant neoplasm thought to arise from the embryonal remnants of mesonephric or Wolffian ducts. Most commonly, these tumors arise in the lower gynecologic tract, particularly in the lateral cervix and vagina1,2. Examples in the upper gynecologic tract, such as in the uterine corpus and ovary3–6, are rarely reported. In this context, they have been referred to in the literature as “mesonephric-like adenocarcinoma”3,6,7, as their association with mesonephric remnants has not been firmly established. However, similarities in immunohistochemical and molecular signature argue in favor of mesonephric differentiation7,8. Given the exceptional rarity of these tumors, they may be mistaken for other, more common tumors.
Histologically, mesonephric and mesonephric-like adenocarcinomas are characterized by variable architectural patterns including tubular, ductal/glandular, retiform/slit-like, sex cord-like, solid, or papillary, and may exhibit a spindle cell component9–12 Eosinophilic material is frequently encountered within tubular lumens. Nuclei tend to be uniform and hyperchromatic with coarse to vesicular chromatin10,13. These tumors frequently express cytokeratins (AE1/AE3, CAM 5.2, CK7), EMA, CD10, GATA-3, PAX-8, HMGA2, vimentin, calretinin, and CA-125,1,4,10,13 and are negative for hormone receptors and WT13,13. Frequent mutations in KRAS, as well as mutations in NRAS, ARID1A, ARID1B and SMARCA4 have been reported in mesonephric-like adenocarcinomas7,13.
Here, we describe the cytomorphological features of a cohort of adenocarcinomas exhibiting mesonephric-like differentiation (AMDs) arising in the upper gynecologic tract.
METHODS
This study was performed in accordance with institutional research guidelines (protocol no. 16–1684). The institutional databases at Memorial Sloan Kettering Cancer center were queried for upper gynecologic tract tumors reported as “tumor of Wolffian origin” or “with mesonephric features” between 2007–2017. Cases with concurrent cytologic specimens were identified. One additional case was prospectively identified.
Available cytologic material (smears, ThinPrep and cell block preparations) were reviewed by three pathologists (BK, SM, and RM) with particular emphasis on architecture of cell groups, cytoplasmic quality and volume, nuclear shape, contour, and size variation, presence of nuclear molding, chromatin pattern, number, size, and location of nucleoli, background quality, and presence of mitoses, apoptosis and necrosis. Consensus was reached for each parameter in all cases. Available surgical pathology material was also reviewed.
RESULTS
Eight cases of AMDs from seven patients (with two samples from one patient) were reviewed. Primary sites included ovary (n=3), endometrium (n=4) and pelvis, not otherwise specified (n=1). Details of the patients and the cytologic preparations reviewed are provided in Table 1.
Table 1.
Clinical findings and cytologic specimens
| Sample ID | Primary Site | Age at Diagnosis | TNM Stage at Presentation | Specimen Source | Specimen type | Preparations reviewed |
|---|---|---|---|---|---|---|
| Case 1 | Endometrium | 65 | IA | Abdominal wall soft tissue | Touch preparation** | Smears, Thinprep, Cell block |
| Case 2 | Ovary, right | 36 | III | Peritoneum | Peritoneal fluid | Thinprep, Cell block |
| Case 3* | Ovary, left | 45 | IIIA | Abdominal mass | Touch preparation** | Smears, Thinprep, Cell block |
| Case 4* | Ovary, left | 45 | Not known | Peritoneum | Pelvic washing | Thinprep, Cell block |
| Case 5 | Endometrium | 58 | IA | Iliac lymph node | Touch preparation** | Smears, Cell block |
| Case 6 | Endometrium | 77 | IB | Peritoneum | Pelvic washing | ThinPrep |
| Case 7 | Pelvic mass | 62 | III | Pelvic nodule | Fine needle aspiration biopsy | Smears, Thinprep, Cell block |
| Case 8 | Endometrium | 56 | IIIB | Omentum | Touch preparation** | Smears, Cell block |
Separate specimens taken from the same patient
Touch preparation samples in this study were performed at the time of diagnostic core biopsy to assess the adequacy of the core biopsy samples.
Cytologic findings
The cytologic findings are summarized in Table 2. All 8 samples showed tight 3-dimensional clusters of overlapping cells (Figs. 1–5). Tubular architecture was seen in 5/8 (63%) cases (Figs. 2–4) and 3/8 (38%) cases exhibited papillary structures (Fig. 5). These morphologies were not mutually exclusive, with 2/8 (25%) cases showing both tubular and papillary structures. Dispersed, single tumor cells were seen in 6 cases (75%). The cells were small and contained scant (7/8, 88%) to moderate (1/8, 12%) amounts of pale cytoplasm (Figs. 1B, 1C, 2B, 2C). The nuclei were round to oval in all cases, and in addition, one case showed occasional fusiform nuclei (Figs. 1B, 3C, 4C). The nuclei were uniform in size (6/8, 75%) or showed mild anisonucleosis (2/8, 25%). Nuclear molding was seen in four (50%) cases (Fig. 3C). Nuclear grooves and indentations were readily identified, and the nuclear chromatin ranged from coarse to vesicular. Nucleoli were either not seen (1/8, 12%), inconspicuous (3/8, 38%) or prominent (4/8, 50%). Nucleoli tended to be multiple and peripherally located (5/7, 71%). Luminal hyaline globules were seen in three cases (3/8, 38%) (Figs. 3B, 4B). Mitoses were seen in five cases (63%) and were generally rare. Rare apoptotic bodies were identified in four (50%) cases. All cases showed a clean background with scant inflammation; no necrosis was seen.
Table 2.
Cytologic findings
| Architectural Features | Cytoplasmic Quantity | Hyaline Globules | Nuclear Size Variation | Nuclear Shape | Chromatin Pattern | Nuclear Contours | Nucleoli – Number | Nucleoli – Size | Nucleoli - Location | Mitoses | Apoptosis | Necrosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 3D, T, P, S | S | − | M | R | C | G, M | 1 | P | C | + | + | − |
| Case 2 | 3D | S | − | M | R | V | G, M | >1 | I | P | − | − | − |
| Case 3/4 | 3D, T, S | S | + | P | R | C | G, M | >1 | I | P | + | + | − |
| Case 5 | 3D, T, S | S | + | M | R | NA | G | >1 | I | P | + | + | − |
| Case 6 | 3D | S | − | M | R | V | G, M | >1 | P | P | − | − | − |
| Case 7 | 3D, T, P, S | S | + | M | R, F | C | G | − | − | − | + | − | − |
| Case 8 | 3D, P, S | M | − | P | R | NA | G | 1 | P | C | − | − | − |
Architectural Features: 3D = 3D Overlapping Clusters; T = Tubular Formations; P = Papillary Formations; SC = Cellular Discohesion/Single Cells
Cytoplasmic Quantity: S = Scant; M = Moderate
= present
= absent
NA = not assessable
Nuclear Size Variation: M = monomorphic; P = moderately pleomorphic (2x size variation)
Nuclear Shape: R = round/oval; F = fusiform
Chromatin Pattern (when assessable): C = coarse; V = vesicular
Nuclear Contours: G = grooves/indentations; M = molding
Nucleoli – Number: - = not seen; 1 = single; >1 = multiple
Nucleoli – Size (when present): I = inconspicuous; P = prominent
Nucleoli - Location (when present): C = central; P = peripheral
Figure 1.
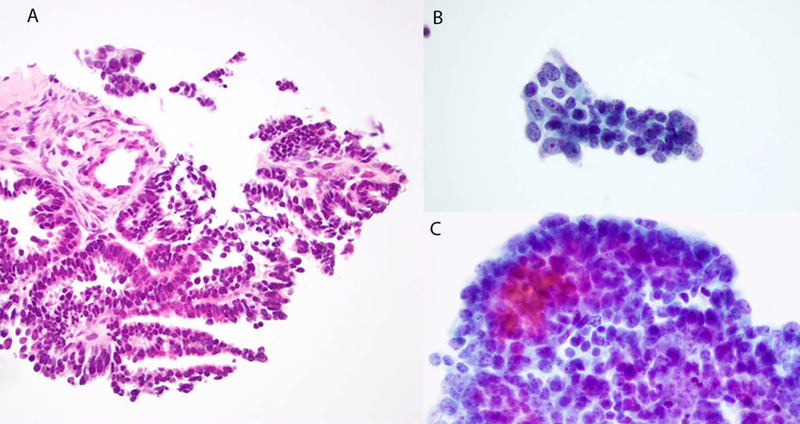
Case 1. Histology shows a low-grade tubulopapillary neoplasm (A). Cytology: papillary and glandular groups composed of mildly pleomorphic small cells with small central nucleoli (B and C).
Figure 5.
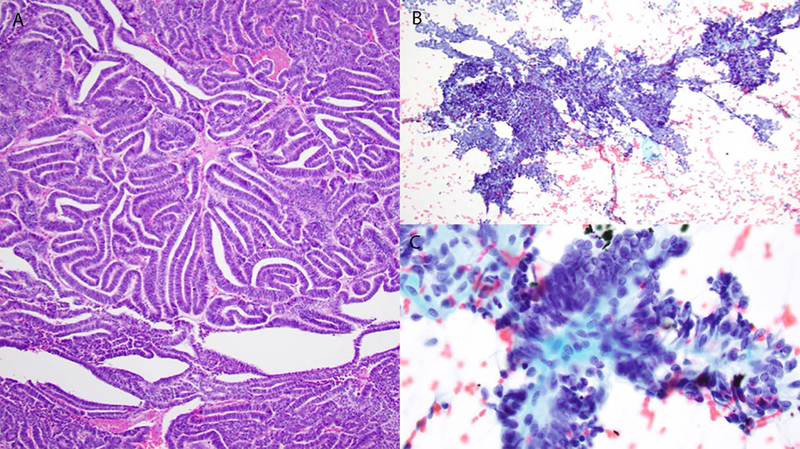
Case 7. Histologic specimen shows a low-grade adenocarcinoma with tubulo-papillary architecture (A). Cytology: Papilliform fragments (B) and true papillary fragments with fibrovascular cores (C), lined by uniform tumor cells (C).
Figure 2.
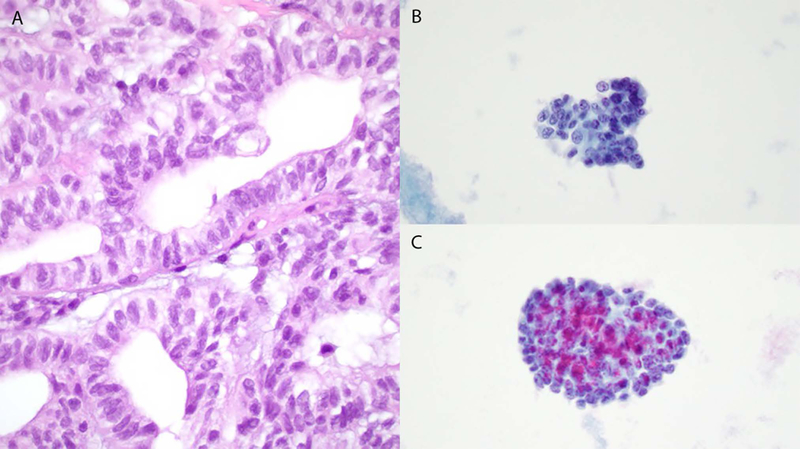
Case 2. Histology shows a low-grade glandular architecture composed of cells exhibiting relatively uniform nuclei with nuclear grooves, and small amounts of pale cytoplasm (A). Cytology: 3-dimensional groups composed of mildly pleomorphic small cells with scant cytoplasm and small nucleoli (B and C), and nuclear grooves (B).
Figure 4.
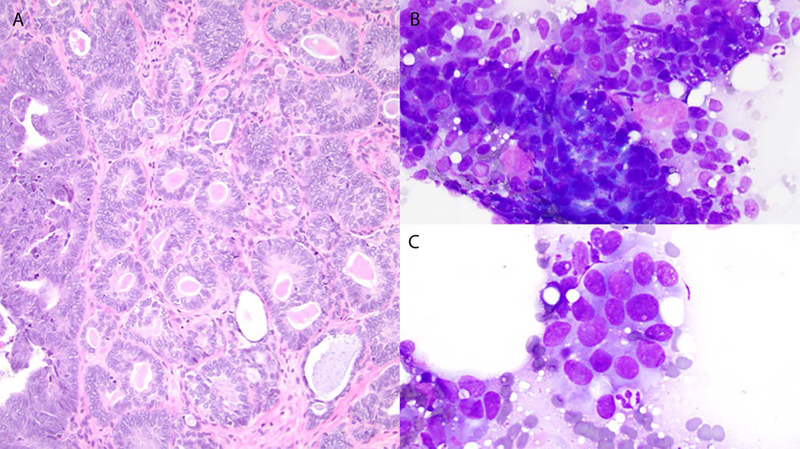
Case 5. Histology shows a low-grade adenocarcinoma containing numerous hyaline globules (A). Cytology: Mildly pleomorphic cells with round-to-oval nuclei (B and C) and hyaline globules (B).
Figure 3.
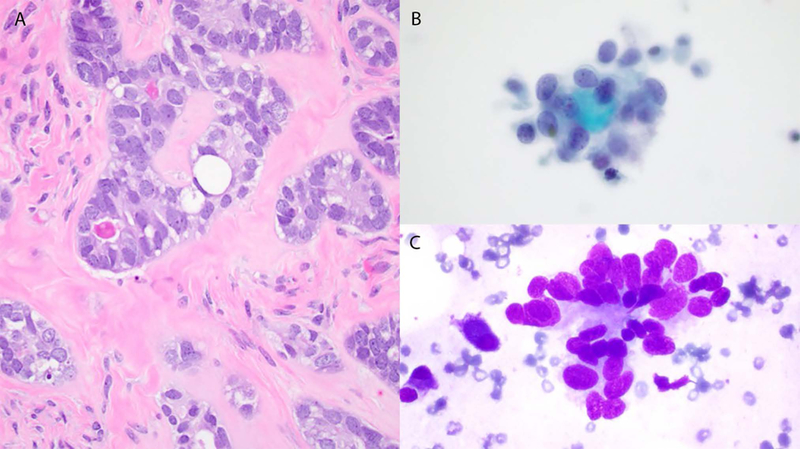
Cases 3 and 4. Histology shows a low-grade adenocarcinoma with tubular architecture composed of cells exhibiting relatively uniform nuclei with small nucleoli, and occasional hyaline globules (A). Cytology: Mildly pleomorphic small cells with scant cytoplasm, oval nuclei and nuclear molding (B and C), and hyaline globules (B).
Histologic findings
The histologic findings are summarized in Table 3, and in part A of each of Figs 1–5. Histologic material from the seven patients in large part mirrored the cytologic findings, and confirmed previous descriptions in the literature9–12. Glandular (7/7, 100%), papillary (7/7, 100%), sex cord-like (6/7, 86%), solid (3/7, 43%), spindled (2/7, 29%) and retiform (1/7, 14%) patterns were seen. Mitoses, apoptotic bodies, and necrosis were identified in 6 (86%), 6 (86%) and 2 (29%) cases, respectively. Hyaline globules were identified in 5 (71%) surgical specimens (Figs. 3A, 4A); they were seen in 3 of the corresponding cytology specimens.
Table 3.
Histologic findings
| Patterns | Hyaline Globules | Psammoma Bodies | Mitoses | Necrosis | Apoptosis | |
|---|---|---|---|---|---|---|
| Case 1 | G,P | Absent | Absent | Absent* | Absent | Absent |
| Case 2 | G, P, Se, So, Sp | Absent | Absent | Present | Absent | Present |
| Case 3/4 | G, P, R, Se, So | Present | Present | Rare* | Focal | Present |
| Case 5 | G, P, Se, So, Sp | Present | Absent | Frequent | Absent | Present |
| Case 6 | G, P, Se | Present | Present | Present* | Focal | Present |
| Case 7 | G, P, Se | Present | Present | Present | Ischemic-type | Present |
| Case 8 | G, P, Se | Present | Absent | Present | Absent | Present |
G=Glandular, P=Papillary, R=Retiform, Se=Sex-Cord-Like, So=Solid, Sp=Spindled
Ki-67 index in cases 1, 3 and 6 was 30%, 50% and 20%, respectively.
Immunohistochemical Features
Immunohistochemical stains were performed on histologic specimens as needed on clinical grounds, and therefore an exhaustive immunohistochemical profile was not performed in all cases. Cases were positive for PAX8, GATA3 and cytokeratins. One case showed p53 overexpression, and a TP53 mutation was confirmed by molecular analysis. Full results of immunohistochemical studies are provided in Table 4.
Table 4.
Immunohistochemical findings
| Number Positive | N performed | % | |
|---|---|---|---|
| PAX8 | 3 | 3 | 100% |
| GATA3 | 4 | 4 | 100% |
| TTF-1 | 1 | 3 | 33% |
| CD10 | 2 | 5 | 40% |
| Calretinin | 3 | 6 | 50% |
| ER | 1 | 7 | 14% |
| PR | 0 | 5 | 0% |
| HNF-1b | 2 | 5 | 40% |
| AE1/AE3 | 2 | 2 | 100% |
| CK7 | 2 | 3 | 67% |
| EMA | 2 | 2 | 100% |
| WT-1 | 0 | 3 | 0% |
| p53 (overexpressed) | 0* | 4 | 0% |
| p16 (diffuse overexpression) | 0 | 3 | 0% |
| Mismatch Repair Proteins (retained) | 2 | 2 | 100% |
| PTEN (retained) | 2 | 2 | 100% |
| ARID1A (retained) | 2 | 2 | 100% |
| S100 | 1 | 1 | 100% |
| A103 | 0 | 1 | 0% |
| SOX10 | 0 | 1 | 0% |
| AR | 0 | 1 | 0% |
| FOXL2 | 1 | 4 | 25% |
| Inhibin | 1 | 2 | 50% |
| SF1 | 0 | 1 | 0% |
| SALL4 | 0 | 1 | 0% |
| Glypican | 0 | 1 | 0% |
| AFP | 1 | 1 | 100% |
| Thyroglobulin | 0 | 1 | 0% |
| Ki-67 proliferation index | Range 20–50% in 3 cases (1, 3 and 6) | ||
Case 1 - immunochemistry for P53 on the corresponding histologic specimen showed heterogenous expression (wild-type pattern). However, a subsequent recurrence one year later showed high-grade carcinoma which showed aberrant P53 overexpression. Review of the earlier histologic specimen at this time showed small foci of high-grade carcinoma morphologically similar to the recurrence.
DISCUSSION
While mesonephric adenocarcinomas are uncommonly encountered in the cervix and vagina, AMDs are exceptionally rare in the upper gynecologic tract2. The purpose of this study was to describe the appearances of these rare tumors in cytologic specimens, and to identify features which should prompt pathologists to consider these tumors in the differential diagnosis. While no single cytologic characteristic was pathognomonic for the diagnosis, all cases in this cohort consistently showed relative cellular and nuclear monotony, scant cytoplasm, round to oval nuclei and conspicuous nuclear grooves and indentations. Mitotic figures and apoptotic bodies were rare, and necrosis was not seen. Nuclear molding and hyaline globules were identified in 50% and 38% of cases, respectively. Although not all cases were subjected to a full panel of immunohistochemical stains, the tumors were frequently positive for PAX8, GATA3, less often expressed TTF-1, CD10 and calretinin, and were largely negative for ER, PR and WT1, typical of the immunoprofile of AMDs reported in the literature3,13.
In the context of a low-grade adenocarcinoma of gynecologic origin, the differential diagnosis includes low-grade endometrioid adenocarcinoma, low-grade serous neoplasms and endocervical adenocarcinomas, in addition to AMD.
Low grade (FIGO grade 1 or 2) endometrioid adenocarcinoma usually exhibits tubular or glandular configurations, and by virtue of their low-grade nuclear features and relatively low mitotic index, may mimic AMDs. However, endometrioid adenocarcinomas are often characterized by columnar cells and squamous or mucinous metaplasia, and lack the characteristic cellular monotony, nuclear features and hyaline globules seen in AMDs14. In challenging cases, these entities should be distinguished using immunohistochemistry. Low-grade endometroid adenocarcinomas express ER and PR, and are negative for GATA-3 with rare exceptions, contrary to AMDs3,13.
Low-grade serous neoplasia may enter into differential diagnostic consideration in cases demonstrating papillary architecture, which represented 38% of the cases in our series. Low grade serous neoplasms usually demonstrate a low mitotic index and lack of pleomorphism15 on par with AMD. Serous neoplasms are immunoreactive for WT1 and negative for GATA-3, while AMDs will have the opposite staining pattern3. In general, high-grade serous carcinomas are unlikely to be mistaken for AMDs, as they are characterized by high-grade features and show a typical immunoprofile (positive for WT1 and aberrant expression of p53).
Usual type endocervical adenocarcinoma (UEA) associated with high risk human papillomavirus (HPV) infection is not an uncommon neoplasm, which can be seen in specimens from the upper gynecologic tract in metastatic or widely locally invasive disease. Similar to AMD, these tumors can show a glandular or tubular architecture. Unlike AMDs, however, they exhibit more conspicuous pleomorphism, high mitotic activity (including apical mitoses) and numerous easily identifiable apoptotic bodies. Due to their association with HPV-p16 is diffusely and strongly positive, and HPV is detected sequences by in situ hybridization (ISH)16,17. In contrast, p16 staining has been shown to be patchy or focal18, and since these tumors are not HPV-driven, they should be negative for HPV ISH4.
In tumors occurring in the ovary, a papillary carcinoma of thyroid-type (arising in a background of teratoma/struma ovarii) may be considered, since similar to AMDs, they can exhibit relatively uniform nuclei with nuclear grooves and TTF-1 expression3,4. However, unlike AMDs, papillary thyroid-type carcinomas also express thyroglobulin, and lack expression of GATA3.
CONCLUSION
AMDs of the upper gynecologic tract show a wide variety of architectural variability, with relatively consistent cytologic features. In cytologic specimens, tumor cells are characteristically small and monotonous, with nuclear grooves and molding, and occasional hyaline globules. However, the cytologic features alone are not diagnostic, and in the presence of these suggestive cytomorphologic features, immunohistochemistry to confirm the diagnosis (PAX8, GATA3, CD10, TTF-1, ER, PR) and to exclude potential mimics (Table 5) will aid correct diagnosis. It should be stressed that this is an observational morphological study, and no conclusions regarding the sensitivity and specificity of the cytomorphologic features can be inferred, although it would be interesting to explore this in future studies.
Table 5.
Immunohistochemistry in differential diagnosis of mesonephric-like adenocarcinoma
| GATA-3 | ER | WT-1 | p16 | HPV | |
|---|---|---|---|---|---|
| Mesonephric-like adenocarcinoma | + | − | − | Patchy | − |
| Endometrioid Adenocarcinoma | − | + | − | Patchy | − |
| Low-grade serous neoplasms | − | + | + | Patchy | − |
| Usual-type Endocervical Adenocarcinoma | − | −/+ | − | Strong | + |
Acknowledgments
Funding: This work was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of interest: The authors have no conflicts of interest.
REFERENCES
- 1.Howitt BE et al. GATA3 is a sensitive and specific marker of benign and malignant mesonephric lesions in the lower female genital tract. Am. J. Surg. Pathol 39, 1411–1419 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Howitt BE & Nucci MR Mesonephric proliferations of the female genital tract. Pathology 50, 141–150 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Mcfarland M, Quick CM & McCluggage WG Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: Report of a series of mesonephric-like adenocarcinomas. Histopathology 68, 1013–1020 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Kenny SL, McBride HA, Jamison J & Glenn McCluggage W Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-?? Am. J. Surg. Pathol 36, 799–807 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Wu H, Zhang L, Cao W, Hu Y & Liu Y Mesonephric adenocarcinoma of the uterine corpus. Int. J. Clin. Exp. Pathol 7, 7012–7019 (2014). [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J, Liu C, Qi J & Qu P Mesonephric carcinoma of the uterine corpus: A report of two cases. Oncol. Lett 11, 2–4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirkovic J et al. Targeted genomic profiling reveals recurrent KRAS mutations in mesonephric-like adenocarcinomas of the female genital tract. Am. J. Surg. Pathol 42, 227–233 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Ordi J et al. Mesonephric adenocarcinoma of the uterine corpus: CD10 expression as evidence of mesonephric differentiation. Am. J. Surg. Pathol 25, 1540–1545 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Clement PB, Young RH, Keh P, Ostor AG & Scully RE Malignant Mesonephric Neoplasms of the Uterine Cervix. Am. J. Surg. Pathol 19, 1158–1171 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Silver SA, Devouassoux-Shisheboran M, Mezzetti TP & Tavassoli FA Mesonephric adenocarcinomas of the uterine cervix: A study of 11 cases with immunohistochemical findings. Am. J. Surg. Pathol 25, 379–387 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Bagué S, Rodríguez IM & Prat J Malignant Mesonephric Tumors of the Female Genital Tract: A Clinicopathologic Study of 9 Cases. Am. J. Surg. Pathol 28, 601–607 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Hart WR & Norris HJ Mesonephric Adenocarcinomas of the Cervix 11, 2238–2246 (1976). [DOI] [PubMed] [Google Scholar]
- 13.Na K & Kim HS Clinicopathologic and molecular characteristics of mesonephric adenocarcinoma arising from the uterine body. Am. J. Surg. Pathol 43, 12–25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mills AM & Longacre TA Atypical Endometrial Hyperplasia and Well Differentiated Endometrioid Adenocarcinoma of the Uterine Corpus. Surg. Pathol. Clin 4, 149–198 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Prat J Pathology of borderline and invasive cancers. Best Pract Res Clin Obstet Gynaecol 41, 15–30 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Walboomers JMM et al. Human Papillomavirus Is a Necessary Cause. J. Pathol 19, 12–19 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Poulter MD, Dirks DC, Mills AM, Stoler MH & Mills SE HR-HPV E6/E7 mRNA In Situ Hybridization. Am. J. Surg. Pathol 41, 607–615 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Goyal A & Yang B Differential patterns of PAX8, p16,and ER immunostains in mesonephric lesions and adenocarcinomas of the cervix. Int. J. Gynecol. Pathol 33, 613–619 (2014). [DOI] [PubMed] [Google Scholar]


