Abstract
Masked uncontrolled hypertension (MUCH) in treated hypertensive patients is defined as controlled automated office blood pressure (AOBP <135/85 mmHg) in clinic, but uncontrolled out-of-clinic BP by ambulatory blood pressure monitoring (ABPM; awake (daytime) readings ≥135/85 mmHg or 24-hr ≥130/80 mmHg). To determine if MUCH is attributable to antihypertensive medication non-adherence.
184 enrolled patients were confirmed to have controlled office BP, of these 167 patients were with adequate 24-hr ambulatory BP recordings. Out of 167 patients, 86 were controlled by in-clinic BP assessment, but had uncontrolled ambulatory awake BP, indicative of MUCH. The remaining 81 had controlled in-clinic and ambulatory awake BP, consistent with true controlled hypertension. After exclusion of 9 patients with missing 24-hr urine collections, antihypertensive medication adherence was determined based on detection of urinary drugs or drug metabolites by high-performance liquid chromatography-tandem mass spectrometry.
Of the 81 patients with MUCH, 69 (85.2%) were fully adherent and 12 (14.8%) patients were partially adherent (fewer medications detected than prescribed). Of the 77 patients with true controlled hypertension, 69 (89.6%) were fully adherent with prescribed antihypertensive medications and 8 (10.4%) were partially adherent. None of the patients in either group were fully non-adherent. There was no statistically significant difference in complete or partial adherence between the MUCH and true controlled groups (p =0.403).
Measurement of urinary drug and drug metabolite levels demonstrates a similarly high level of antihypertensive medication adherence in both MUCH and truly controlled hypertensive patients. These findings indicate that MUCH is not attributable to antihypertensive medication non-adherence.
Keywords: masked uncontrolled hypertension, medication adherence
Summary
Patients with MUCH have similar antihypertensive medication adherence in MUCH patients compared to true controlled hypertension.
Introduction
Masked uncontrolled hypertension (MUCH) in treated hypertensive patients is defined as controlled automated office blood pressure (AOBP < 135/85 mmHg) in clinic, but uncontrolled out-of-clinic BP by 24-hr ambulatory blood pressure monitoring (ABPM awake (daytime) ≥ 135/85 mmHg or 24 hour ≥ 130/80) 1. The prevalence of MUCH among treated hypertensive patients has been reported as 30–50% 2–5, which is higher than prevalence estimates of masked hypertension (MH) among untreated hypertensive individuals (8–20%) 2, 3, 6. According to definitions proposed in the 2017 ACC/AHA and ESH/ESC guidelines de la Sierra et al. estimated the prevalence of MUCH from the Spanish ABPM registry to be approximately 66% 1, 7, 8. The severity of clinic BP predicts the prevalence of MUCH, as higher clinic systolic BP levels are associated with higher rates of MUCH 9. Prehypertension is also associated with higher prevalence rates of MUCH than in the normotensive population 10. The prevalence of MUCH is also increased in African Americans 11, 12, the elderly 13, persons with diabetes 3, 4, 14, chronic kidney disease 4, 9, 15–18 and kidney transplant recipients 19–21. MUCH has been shown to be a precursor of sustained hypertension 22. In addition, a high prevalence of nocturnal hypertension and non-dipping BP is seen in MH patients 3, 23. Patients with obstructive sleep apnea (OSA) have also been reported to have an increased prevalence of MH 24, 25.
Patients with MH/MUCH have evidence of higher sympathetic tone compared to those with true controlled hypertension (hypertension controlled in-clinic and out-of-clinic) 15, 26. In a recent study, we reported that MUCH patients have increased out-of-clinic sympathetic tone compared to true controlled hypertensive patients 27. MUCH patients have also been shown to have higher anxiety based on Spielberger’s Strait Trait Anxiety Inventory (STAI) & Beck Depression Inventory (BDI) 28.
In the Spanish ABPM registry, MUCH has recently been shown to have greater all-cause and cardiovascular mortality compared to true controlled hypertension and treated but uncontrolled hypertension29. A meta-analysis of six studies has also reported that MUCH was associated with increased risk of cardiovascular events and all-cause mortality compared to true controlled hypertension 30.
Antihypertensive medication non-adherence is common in patients with resistant hypertension (RHTN), contributing importantly to poor BP control 31. Unknown is to what extent MUCH may simply be a consequence of poor medication adherence. The current study tested the hypothesis that MUCH is attributable to low adherence to prescribed antihypertensive agents. To test this hypothesis, we prospectively determined antihypertensive medication adherence in MUCH patients by measurement of 24-hr urinary drug or drug metabolite levels by high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS). Patients with true controlled hypertension served as controls.
Methods
Study data will be available upon request 1 year after completion of the funding grant (April 2021).
Study Population
Patients with automated office BP controlled (AOBP < 135/85 mm Hg) on antihypertensive medications were prospectively recruited from the University of Alabama at Birmingham Hypertension Clinic after having been seen by a hypertension specialist for a minimum of three follow-up visits between April 2014 and March 2019. All study patients had been evaluated for secondary causes of hypertension, including hyperaldosteronism, pheochromocytoma, and renal artery stenosis, as medically indicated. Patients with chronic kidney disease (CKD) stage 4 or 5 (eGFR <30 ml/min/1.73m2) and pregnancy were excluded. The study was approved by the UAB Institutional Review Board and written informed consent was obtained from all participants.
BP Measurement
Unattended clinic automated office BP measurement (AOBP)
AOBP in clinic was measured after at least 5 minutes of quiet rest in a sitting position with the back supported and the arm supported at heart level 32. The AOBP was measured using the BpTRU device, which automatically obtains 6 serial BP readings, one minute apart, before displaying the average of the last 5 readings. All BpTRU assessments were unattended, i.e., unobserved in clinic 33–37. An appropriate sized cuff was used with a cuff bladder encircling at least 80% of the arm 37, 38. A BP cutoff of < 135/85 mmHg for controlled BP was used validating automated BP devices 6, 39.
Out-of-clinic 24-hr ambulatory BP monitoring (ABPM)
An automated, noninvasive, oscillometric device (Oscar 2; Suntech Medical Inc, Morrisville, NC) was used to perform 24-hr ABPM. Recordings were made every 20 minutes during the awake (daytime) and every 30 minutes during the asleep (nighttime) phases of the 24-hr period. Awake and asleep times were determined by patient self-report. Patients were counselled to take all antihypertensive medications during the ABPM period. ABPM was determined to be valid if >80% of measurements were successful 40 including at least 20 awake (daytime) and 7 asleep (nighttime) valid BP measurements 41. Uncontrolled ABPM was defined as mean awake (daytime) BP ≥ 135/85 mmHg or as mean 24-hour BP ≥ 130/80 mmHg 1, 42.
Biochemical analysis
Renal function panel
Serum electrolytes, blood urea nitrogen and creatinine were measured in a hospital laboratory using standard methods.
24-hr urine high-performance liquid chromatography-tandem mass spectrometry to detect antihypertensive medication adherence
In all study patients, 24-hour urine samples were collected. Study patients were advised to be adherent with antihypertensive medications but were not informed that medication adherence was being tested in the collected urine samples to avoid a Hawthorne effect (e.g., change in behavior when it is being observed) 43. The urine samples were stored and an aliquot was shipped at a temperature of −80° C to the National Centre for Adherence Testing (NCAT) Department of Chemical Pathology and Metabolic Medicine, University Hospitals of Leicester NHS Trust, Leicester, UK; where they were analyzed by high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) to detect antihypertensive medication adherence as previously described44. Briefly, the samples were analyzed in batches of 20. Each sample was run in dilution and after extraction. Separation was performed using Agilent technologies Zorbax Elipse column C18 2.1 × 50 mm and then the samples were introduced by electrospray ionization to an Agilent technologies 6140 tandem mass spectrometer. The analyte of interest was confirmed by its unique mass to charge ratios.
The assay provides a binary qualitative result for presence or absence of medications in the urine. Patients whose urine analysis confirmed the presence of all medications prescribed were classified as totally adherent and those with fewer medications detected than prescribed were classified as partially adherent. Patients with no detectable drug or metabolite levels were classified as totally non-adherent.
Statistical analysis
Descriptive analyses were performed to summarize the demographic and biochemical characteristics, as well as the comorbidities of study participants and antihypertensive medication adherence by classes of agents in patients with true controlled hypertension and MUCH. Two sample t-test was used to compare the continuous variables between true controlled hypertensive and MUCH patients. Chi-square test or Fisher’s exact test was used to compare the categorical variables between two study groups. Medication adherence was compared using one way ANOVA for continuous variables and using Chi-square or Fisher’s exact test for categorical variables between true controlled hypertensive and MUCH patients. according to total and partial antihypertensive medication adherence, i.e., true controlled hypertension with total medication adherence, true control hypertension with partial medication adherence, MUCH with total medication adherence and MUCH with partial medication adherence. All analyses were performed using SPSS version 25. A two-sided p-value < 0.05 was considered statistically significant.
Results
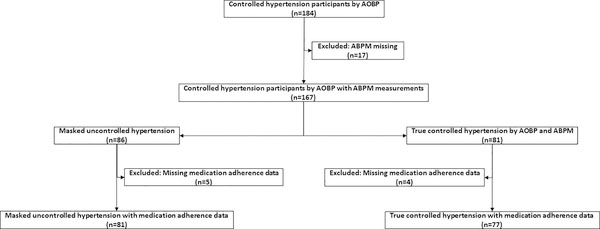
After three or more consecutive clinic visits, 184 hypertensive patients were prospectively recruited were prescribed antihypertensive medications and had controlled clinic BP in clinic (Figure 1). Of the 184 treated and controlled hypertensive patients, 167 had adequate ABPM recordings. 86 patients (51.5%) were identified as having MUCH, i.e. controlled in clinic (AOBP < 135/85 mmHg), but uncontrolled awake ambulatory (ABPM ≥ 135/85 mmHg). The remaining 81 patients (48.5%) had controlled BP in-clinic (AOBP < 135/85 mmHg) and controlled ambulatory awake BP (ABPM < 135/85 mmHg), indicative of true controlled hypertension. (Figure 1). Of the 86 MUCH patients and 81 true controlled hypertensive, 9 had missing medication adherence data such that 81 MUCH patients and 77 true controlled hypertensive were included in final analysis (Figure 1).
Figure 1.
Schematic of enrolled study participants
Patient characteristics
The mean age was 58.6±10.6 years for the MUCH patients and 60.6±10.8 years for the true controlled hypertensive (Table 1). Of the MUCH patients, 44.4% were female and 49.4% were African American compared to 45.5% female and 49.4% African American among the true controlled hypertensive patients (Table 1). The mean BMI was not statistically different in both the groups, 34.2±6.2 kg/m2 for the MUCH patients and 32.3±6.8 kg/m2 for the true controlled hypertensive (Table 1). MUCH patients had a higher prevalence of diabetes compared to the true controlled hypertensive patients (42.0% vs 23.4%, respectively; p=0.013). All other comorbidities had similar prevalence in both groups (Table 1). There were no significant differences in serum electrolytes, blood urea nitrogen and creatinine in MUCH versus true controlled hypertensive patients (Table1).
Table 1:
Demographics, comorbidities, vitals and biochemistry in patients with masked uncontrolled and true controlled hypertension
| Variables | Masked uncontrolled hypertension (n=81) | True controlled hypertension (n=77) | p-value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 58.6 ± 10.6 | 60.6 ± 10.8 | 0.247 |
| Female | 36 (44.4%) | 35 (45.5%) | 0.899 |
| African American | 40 (49.4%) | 38 (49.4%) | 0.997 |
| Comorbidities | |||
| Current smoker | 10 (12.3%) | 6 (7.8%) | 0.343 |
| Dyslipidemia | 53 (65.4%) | 52 (67.5%) | 0.780 |
| Congestive heart failure | 5 (6.2%) | 4 (5.2%) | 1.000 |
| Coronary artery disease | 9 (11.1%) | 14 (18.2%) | 0.208 |
| Peripheral vascular disease | 6 (7.4%) | 4 (5.2%) | 0.568 |
| Diabetes | 34 (42.0%) | 18 (23.4%) | 0.013 |
| Prior stroke/transient ischemic attack | 13 (16.0%) | 10 (13.0%) | 0.585 |
| Body mass index (kg/m2) | 34.2 ± 6.2 | 32.3 ± 6.8 | 0.070 |
| Clinic Measurements | |||
| AOBP systolic (mmHg) | 121.1 ± 8.2 | 114.1 ± 10.4 | <0.001 |
| AOBP diastolic (mmHg) | 73.3 ± 7.7 | 70.6 ± 7.6 | 0.026 |
| AOBP heart rate (beats/minute) | 73.9 ± 11.6 | 71.7 ± 12.2 | 0.252 |
| ABPM Measurements | |||
| 24 hour (overall) systolic BP (mmHg) | 145.5 ± 11.6 | 121.5 ± 7.3 | <0.001 |
| 24 hour (overall) diastolic BP (mmHg) | 79.9 ± 8.3 | 68.8 ± 6.5 | <0.001 |
| 24 hour (overall) mean arterial pressure (mmHg) | 101.9 ± 7.9 | 86.3 ± 5.6 | <0.001 |
| 24 hour (overall) pulse pressure (mmHg) | 65.7 ± 11.4 | 52.8 ± 8.2 | <0.001 |
| 24 hour (overall) heart rate (beats/min) | 74.2 ± 11.3 | 71.1 ± 10.8 | 0.085 |
| Awake (daytime) systolic BP (mmHg) | 148.1 ± 11.2 | 123.8 ± 7.3 | <0.001 |
| Awake (daytime) diastolic BP (mmHg) | 82.1 ± 8.1 | 70.9 ± 6.9 | <0.001 |
| Awake (daytime) mean arterial pressure (mmHg) | 104.1 ± 7.5 | 88.0 ± 8.0 | <0.001 |
| Awake (daytime) pulse pressure (mmHg) | 66.0 ± 11.5 | 53.5 ± 9.3 | <0.001 |
| Awake (daytime) heart rate (beats/min) | 75.6 ± 11.4 | 72.6 ± 11.3 | 0.091 |
| Asleep (nighttime) systolic BP (mmHg) | 138.1 ± 19.2 | 114.4 ± 12.2 | <0.001 |
| Asleep (nighttime) diastolic BP (mmHg) | 72.9 ± 11.3 | 62.2 ± 7.9 | <0.001 |
| Asleep (nighttime) mean arterial pressure (mmHg) | 94.6 ± 12.7 | 79.2 ± 9.1 | <0.001 |
| Asleep (nighttime) pulse pressure (mmHg) | 65.2 ± 14.5 | 52.8 ± 10.7 | <0.001 |
| Asleep (nighttime) heart rate (beats/min) | 69.6 ± 11.7 | 66.7 ± 10.3 | 0.099 |
| Biochemistry | |||
| Sodium (mMol/L) | 137.9 ± 3.3 | 138.6 ± 2.8 | 0.213 |
| Potassium (mMol/L) | 4.0 ± 0.4 | 4.0 ± 0.4 | 0.701 |
| Bicarbonate (mMol/L) | 28.3 ± 2.8 | 27.7 ± 3.1 | 0.226 |
| Blood urea nitrogen (mg/dL) | 17.7 ± 7.1 | 18.9 ± 7.7 | 0.370 |
| Creatinine (mg/dL) | 1.0 ± 0.3 | 1.1 ± 0.5 | 0.227 |
AOBP, automated office blood pressure; ABPM, ambulatory blood pressure monitoring
BP measurements in- and out-of-clinic
The in-clinic mean AOBP readings were 121.1±8.2 / 73.3±7.7 mmHg in MUCH patients versus 114.1±10.4 / 70.6±7.6 mmHg in patients with true controlled hypertension (p < 0.001 and p = 0.026 respectively) (Table 1). The out-of-clinic awake (daytime) mean ABPM was 148.1±11.2 / 82.1±8.1 mmHg in the MUCH patients compared to 123.8±7.3 / 70.9±6.9 in true controlled hypertensive patients (both p < 0.001) (Table 1).
Antihypertensive medication adherence
Of the 81 MUCH patients, 69 (85.2%) were fully adherent and 12 (14.8%) patients were partially adherent (Table 2). Of the 77 true controlled hypertensive patients, 69 (89.6%) were fully adherent with all of the prescribed antihypertensive medications and 8 (10.4%) were partially adherent (Table 2). The number of antihypertensive medications prescribed was 3.5±1.3 in MUCH patients and 3.2±1.2 in true controlled hypertension; the number of antihypertensive medications detected by 24-hr urine LC-MS/MS was 3.3±1.2 in MUCH patients and 3.1±1.2 in true controlled hypertension. There were no significant differences in medication adherence with the different antihypertensive medication classes for the MUCH versus true controlled hypertensive groups (Table 2).
Table 2:
Antihypertensive medication adherence in patients with masked uncontrolled and true controlled hypertension
| Variables | Masked uncontrolled hypertension (n=81) | True controlled hypertension (n=77) | p-value |
|---|---|---|---|
| Total medication adherence | 69 (85.2%) | 69 (89.6%) | 0.403 |
| Partial medication adherence | 12 (14.8%) | 8 (10.4%) | 0.403 |
| Total antihypertensive medications prescribed | 3.5 ± 1.3 | 3.2 ± 1.2 | 0.072 |
| Total antihypertensive medications detected | 3.3 ± 1.2 | 3.1 ± 1.2 | 0.184 |
| Antihypertensive medication classes | |||
| Angiotensin converting enzyme inhibitors (benazepril, fosinopril, lisinopril, quinapril, ramipril) | 30 (90.9%) | 34 (97.1%) | 0.349 |
| Angiotensin II receptor blockers (azilsartan, candesartan, irbesartan, losartan, olmesartan, valsartan) | 31 (93.9%) | 32 (100.0%) | 0.492 |
| Calcium channel blockers (amlodipine, diltiazem, felodipine, nifedipine, verapamil) | 59 (95.2%) | 48 (98.0%) | 0.629 |
| Thiazide diuretics (chlorthalidone, hydrochlorothiazide) | 61 (95.3%) | 54 (98.2%) | 0.623 |
| Loop diuretics (furosemide, torsemide) | 4 (100.0%) | 1 (50.0%) | 0.333 |
| Epithelial sodium channel blockers (triamterene) | 2 (100.0%) | 2 (100.0%) | -- |
| Mineralocorticoid receptor antagonists (eplerenone, spironolactone) | 27 (93.1%) | 29 (96.7%) | 0.612 |
| α blockers (doxazosin) | 5 (100.0%) | 2 (100.0%) | -- |
| β Blockers (acebutalol, atenolol, bisoprolol, metoprolol, nebivolol) | 16 (94.1%) | 17 (85.0%) | 0.609 |
| αβ blockers (carvedilol, labetalol) | 18 (90.0%) | 9 (100%) | 1.000 |
| α2 agonists (clonidine, guanfacine) | 11 (91.7%) | 7 (100.0%) | 1.000 |
| Nitric oxide vasodilators (hydralazine) | 3 (100.0%) | 1 (100.0%) | -- |
| Potassium channel openers (minoxidil) | 2 (100.0%) | -- | |
The number of antihypertensive medications prescribed was 3.4±1.2 in MUCH patients with total adherence and 4.5±1.4 in MUCH patients with partial adherence versus 3.1±1.2 in true controlled hypertension with total adherence and 3.9±1.0 in true controlled hypertension with partial adherence. Patients with partial adherence missed on average one prescribed medication in both the MUCH and true controlled groups; The number of antihypertensive medications detected by 24-hr urine HP LC-MS/MS was 3.4±1.3 in MUCH patients with total adherence and 3.1±1.2 in MUCH patients with partial adherence versus 3.1±1.2 in true controlled hypertension with total adherence and 2.8±1.0 in true controlled hypertension with partial adherence (Table 3). Patients with full medication adherence were significantly more adherent to angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, thiazide diuretics, mineralocorticoid receptor antagonists and β blockers than were patients with partial medication adherence, both in the MUCH and true controlled hypertensive groups.
Table 3:
Antihypertensive medication adherence in patients with masked uncontrolled and true controlled hypertension subdivided by total and partial antihypertensive medication adherence
| Variables | Masked uncontrolled hypertension |
True controlled hypertension |
p-value | ||
|---|---|---|---|---|---|
| Total medication adherence (n=69) | Partial medication adherence (n=12) | Total medication adherence (n=69) | Partial medication adherence (n=8) | ||
| Total antihypertensive medications prescribed | 3.4 ± 1.2 | 4.5 ± 1.4 | 3.1 ± 1.2 | 3.9 ± 1.0 | 0.002 |
| Total antihypertensive medications detected | 3.4 ± 1.3 | 3.1 ± 1.2 | 3.1 ± 1.2 | 2.8 ± 1.0 | 0.406 |
| Antihypertensive medication classes | |||||
| Angiotensin converting enzyme inhibitors (benazepril, fosinopril, lisinopril, quinapril, ramipril) | 29 (100.0%) | 1 (25.0%) | 30 (100.0%) | 4 (80.0%) | <0.001 |
| Angiotensin II receptor blockers (azilsartan, candesartan, irbesartan, losartan, olmesartan, valsartan) | 26 (100%) | 5 (71.4%) | 30 (100%) | 2 (100.0%) | 0.017 |
| Calcium channel blockers (amlodipine, diltiazem, felodipine, nifedipine, verapamil) | 52 (100.0%) | 7 (70.0%) | 43 (100.0%) | 5 (83.3%) | <0.001 |
| Thiazide diuretics (chlorthalidone, hydrochlorothiazide) | 52 (100.0%) | 9 (75.0%) | 49 (100.0%) | 5 (83.3%) | <0.001 |
| Loop diuretics (furosemide, torsemide) | 4 (100.0%) | 1 (100.0%) | 0 | 0.333 | |
| Epithelial sodium channel blockers (triamterene) | 1 (100.0%) | 1 (100.0%) | 2 (100%) | -- | |
| Mineralocorticoid receptor antagonists (eplerenone, spironolactone) | 23 (100.0%) | 4 (66.7%) | 25 (100.0%) | 4 (80.0%) | 0.005 |
| α blockers (doxazosin) | 5 (100%) | 2 (100%) | -- | ||
| β blockers (acebutalol, atenolol, bisoprolol, metoprolol, nebivolol) | 15 (100.0%) | 1 (50.0%) | 17 (100.0%) | 0 | <0.001 |
| αβ blockers (carvedilol, labetalol) | 13 (100.0%) | 5 (71.4%) | 8 (100%) | 1 (100.0%) | 0.121 |
| α2 agonists (clonidine, guanfacine) | 8 (100%) | 3 (75.0%) | 6 (100%) | 1 (100.0%) | 0.263 |
| Nitric oxide vasodilators (hydralazine) | 3 (100.0%) | 1 (100%) | -- | ||
| Potassium channel openers (minoxidil) | 1 (100%) | 1 (100%) | -- | ||
In addition, analyzing MUCH and true controlled hypertensive patients based on 24hr ABPM cutoff of 130/80 mmHg showed similar antihypertensive medication adherence in MUCH patients, with 86.7% of total adherence and 13.3% patients were partially adherent. In true controlled hypertensive patients, 88.2% were fully adherent with all of the prescribed antihypertensive medications and 11.8% were partially adherent (p=0.483). In addition, based on an ABPM asleep (nighttime) cutoff value of 120/70 mmHg, the medication adherence rates were not different between MUCH and true controlled patients, with 89.4% of the former being fully and 10.6% being partially versus 84.4% of the latter being fully and 15.6% being partially adherent (p=0.246).
Duration between BP measurements and 24-hour urine collection
All the patients completed in-clinic AOBP measurements, out-of-clinic 24-hour ABPM and 24-hour urine collection for antihypertensive medication adherence during a one week period without any change in any antihypertensive medications. The mean duration between the BP measurements and 24-hour urine collection was 1.5±2.9 days (range 2–7).
Post-hoc power analysis
Sample sizes of 81 in the masked uncontrolled hypertension group and 77 in the true controlled hypertension group resulted in a 78% power to detect equivalence. The margin of equivalence, given in terms of the difference, extended from −20% to 10.4% with an actual difference of −4.4% (85.2% vs. 89.6%) using Z test with a significance level of 0.05.
Discussion
This prospective study identified equal antihypertensive medication adherence between patients with MUCH and true controlled hypertension. Precision measurement of drug metabolites in the urine using 24-hr urine LC-MS/MS provided an unbiased assessment of medication adherence. Based on these data, we conclude that MUCH is not attributable to non-adherence.
Multiple assessments of medication adherence in general hypertensive cohorts with use of LC-MS/MS have demonstrated non-adherence (i.e. absence of 1 or more antihypertensive medications) rates of 25–65% among patients with uncontrolled HTN 44, 45. For example, Gupta et al. found that 30–40% of a cohort of 1348 hypertensive patients were non-adherent with their prescribed antihypertensive medications. Female gender, younger age, higher number of antihypertensive medications and use of certain antihypertensive medication classes i.e. diuretics were associated with greater degrees of non-adherence46. In another study of 238 hypertensive patients, serial determinations of medication adherence and subsequent discussion of poor adherence with appropriate patients improved adherence rates from 33% to 100% and lowered systolic and diastolic BP by ~19.5 and 7.5 mmHg 47.
Medication adherence rates have also been determined in patients with RHTN by LC-MS/MS analysis. Jung et al., Strauch et al and Lawson et al. have reported antihypertensive medication non-adherence rates of 47–53% in cohorts of patients with RHTN48–50. Schmieder et al. also reported high rates of non-adherence to antihypertensive medications among 79 patients with RHTN undergoing renal denervation. Medication non-adherence was 44% at baseline and 34% six months after renal denervation 51. Brinker et al. reported that informing patients with RHTN of documented low medication adherence improved systolic and diastolic BP by 46±10 / 26±14 mm Hg in non-adherent group, 12±17 / 7±7 mm Hg in adherent group and 11±4 / 4±2 mm Hg in the untested group (p<0.01) without treatment intensification while no differences in the number of antihypertensive medications were found (5.3±0.7 vs. 4.2±0.4 vs. 3.7±0.2 drugs, respectively, p>0.05) 52.
In the current study, antihypertensive medication adherence was measured by detecting urinary drug and drug metabolite levels using LC-MS/MS in MUCH patients versus patients with confirmed controlled hypertension. We found that medication adherence was high in both MUCH and true controlled hypertensive groups (85.2 vs. 89.6%) with no statistically significant difference between the two groups. These findings allow us to exclude reduced medication adherence as a cause of MUCH. Further, there was no significant difference in the total number or classes of antihypertensive agents detected in the MUCH versus true controlled hypertensive groups, suggesting that under treatment was also not contributing to development of MUCH. Patients in both groups i.e. MUCH and true controlled hypertension who were partially adherent were being treated with a higher number of prescribed antihypertensive medications (4.5±1.4 in MUCH, 3.9±1.0 in true controlled) compared to those who were total adherence (3.4±1.2 in MUCH, 3.1±1.2 in true controlled).
As this prospective study was started prior to release of the updated 2017 Hypertension guidelines, we reanalyzed the data with application of the lower BP cutoff value of 130/80mmHg 8. Based on an out-of-clinic ABPM awake (daytime) cutoff value of 130/80mmHg 8, we found similar antihypertensive medication adherence levels in MUCH and true controlled patients, with 88.9% of the MUCH patients being totally and 11.1% being partially adherent compared to 88.4% of the true controlled patients being totally and 11.6% partially adherent (p=0.579). In addition, based on newer guidelines out-of-clinic 24hr ABPM cutoff of 125/75 mmHg 8, MUCH patients had similar antihypertensive medication adherence levels compared to true controlled patients (90.1% of MUCH patients were totally and 9.9% partially adherent, while 85.3% of true controlled patients were totally and 14.7% were partially adherent; p=0.326).
Emerging evidence suggests that increase sympathetic tone may play a role in the pathogenesis of MUCH. We have recently observed that MUCH patients have evidence of higher out-of-clinic sympathetic tone assessed by plasma and urinary catecholamine and metanephrine levels and BP and heart rate variability in- and out-of-clinic compared to true controlled hypertensive patients27. Other investigators have also reported that MUCH patients have higher anxiety levels as indexed by the Spielberger’s Strait Trait Anxiety Inventory (STAI) compared with RHTN after renal denervation 28. Further, risk of MH has been shown to be increased in patients with OSA who are not receiving antihypertensive medications, suggesting that OSA-related oxygen desaturation, heightened sympathetic tone, nocturnal hypertension, and non-dipping BP may contribute to development of MH 24, 25.
Strengths of the current study include: prospective design; inclusion of a diverse cohort of well characterized patients; rigorous confirmation of MUCH and true controlled hypertension; comparison of MUCH patients to a comparator group of true controlled hypertension; medication adherence tested on uninformed patients to avoid change in behavior (i.e., Hawthorne effect); and detection of antihypertensive medications by 24-hr urine LC-MS/MS, the current recommended method for determination of medication adherence.
Study weaknesses include binary determination of drug and drug metabolite levels as opposed to a quantitative assessment. In addition, the time duration between ABPM and drug metabolite testing could have introduced some variation in the detection of the urinary drug metabolites by qualitative analysis. These limitations preclude a more nuanced interpretation of drug exposure, such as potential variation in drug levels related to once versus multiple daily dosing in individual patients.
Patients with MUCH have similar levels of antihypertensive medication adherence compared to patients with hypertension controlled both in the office and in the clinic. These findings suggest that poor adherence to antihypertensive medication is not a cause of MUCH.
Perspectives
Patients with MUCH have similar levels of antihypertensive medication adherence compared to patients with true controlled hypertension. These findings suggest that poor adherence to antihypertensive medication is not a cause of MUCH.
Supplementary Material
Novelty and Significance.
What is new: This is the first study to evaluate if masked uncontrolled hypertension (MUCH) attributed to antihypertensive medication non-adherence.
What is relevant: This study shows there is similar antihypertensive medication adherence in MUCH patients compared to true controlled hypertension. In a large diverse cohort of hypertensive patients subdivided into MUCH and true controlled hypertension as controls around 85+ % of the patients were adherent to antihypertensive medication in both the groups. This eliminates medication non-adherence as one of the possible causes of MUCH.
Acknowledgments
Sources of funding
The National Institutes of Health (NIH R01 HL113004 and 2T32HL007457-36A1) and the American Heart Association Strategically Focused Research Network (AHA 5SFRN2390002) supported this research.
Footnotes
Disclosures: None
References
- 1.O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y and European Society of Hypertension Working Group on Blood Pressure M. European Society of Hypertension position paper on ambulatory blood pressure monitoring. Journal of hypertension. 2013;31:1731–68. [DOI] [PubMed] [Google Scholar]
- 2.Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H and Imai Y. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46:508–15. [DOI] [PubMed] [Google Scholar]
- 3.Banegas JR, Ruilope LM, de la Sierra A, de la Cruz JJ, Gorostidi M, Segura J, Martell N, Garcia-Puig J, Deanfield J and Williams B. High prevalence of masked uncontrolled hypertension in people with treated hypertension. European heart journal. 2014;35:3304–12. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann MV, Zeymer U, Dechend R, Kaiser E, Hagedorn I, Deeg E, Senges J and Schmieder RE. Ambulatory blood pressure monitoring: is it mandatory for blood pressure control in treated hypertensive patients?: prospective observational study. Int J Cardiol. 2013;168:2255–63. [DOI] [PubMed] [Google Scholar]
- 5.Bobrie G, Clerson P, Menard J, Postel-Vinay N, Chatellier G and Plouin PF. Masked hypertension: a systematic review. Journal of hypertension. 2008;26:1715–25. [DOI] [PubMed] [Google Scholar]
- 6.Wohlfahrt P, Cifkova R, Movsisyan N, Kunzova S, Lesovsky J, Homolka M, Soska V, Bauerova H, Lopez-Jimenez F and Sochor O. Threshold for diagnosing hypertension by automated office blood pressure using random sample population data. Journal of hypertension. 2016;34:2180–6. [DOI] [PubMed] [Google Scholar]
- 7.de la Sierra A, Banegas JR, Vinyoles E, Segura J, Gorostidi M, de la Cruz JJ and Ruilope LM. Prevalence of Masked Hypertension in Untreated and Treated Patients With Office Blood Pressure Below 130/80 mm Hg. Circulation. 2018;137:2651–2653. [DOI] [PubMed] [Google Scholar]
- 8.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Pappas MK and Sinha AD. Masked Uncontrolled Hypertension in CKD. Journal of the American Society of Nephrology : JASN. 2016;27:924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin SS and Wong ND. The complexity of masked hypertension: diagnostic and management challenges. Curr Hypertens Rep. 2014;16:474. [DOI] [PubMed] [Google Scholar]
- 11.Veerabhadrappa P, Diaz KM, Feairheller DL, Sturgeon KM, Williamson ST, Crabbe DL, Kashem AM and Brown MD. Endothelial-dependent flow-mediated dilation in African Americans with masked-hypertension. American journal of hypertension. 2011;24:1102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mezick EJ, Hall M and Matthews KA. Sleep duration and ambulatory blood pressure in black and white adolescents. Hypertension. 2012;59:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alwan H, Pruijm M, Ponte B, Ackermann D, Guessous I, Ehret G, Staessen JA, Asayama K, Vuistiner P, Younes SE, Paccaud F, Wuerzner G, Pechere-Bertschi A, Mohaupt M, Vogt B, Martin PY, Burnier M and Bochud M. Epidemiology of masked and white-coat hypertension: the family-based SKIPOGH study. PloS one. 2014;9:e92522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin SS, Thijs L, Li Y, Hansen TW, Boggia J, Liu Y, Asayama K, Bjorklund-Bodegard K, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Filipovsky J, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA and International Database on Ambulatory blood pressure in Relation to Cardiovascular Outcomes I. Masked hypertension in diabetes mellitus: treatment implications for clinical practice. Hypertension. 2013;61:964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal R and Pappas MK. Delayed systolic blood pressure recovery following exercise as a mechanism of masked uncontrolled hypertension in chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2016. [DOI] [PubMed] [Google Scholar]
- 16.Cha RH, Lee H, Lee JP, Kang E, Song YR, Kim YS and Kim SG. Changes of blood pressure patterns and target organ damage in patients with chronic kidney disease: results of the APrODiTe-2 study. Journal of hypertension. 2017;35:593–601. [DOI] [PubMed] [Google Scholar]
- 17.Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, Rostand S, Hiremath L, Sika M, Kendrick C, Hu B, Greene T, Appel L, Phillips RA, African American Study of Kidney D and Hypertension Collaborative Research G. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53:20–7. [DOI] [PubMed] [Google Scholar]
- 18.Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, Deo R, Fischer MJ, He J, Hsu CY, Huan Y, Keane MG, Kusek JW, Makos GK, Miller ER 3rd, Soliman EZ, Steigerwalt SP, Taliercio JJ, Townsend RR, Weir MR, Wright JT Jr., Xie D, Rahman M and Chronic Renal Insufficiency Cohort Study I. Masked Hypertension and Elevated Nighttime Blood Pressure in CKD: Prevalence and Association with Target Organ Damage. Clinical journal of the American Society of Nephrology : CJASN. 2016;11:642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano U, Matteucci MC, Calzolari A, Turchetta A, Rizzoni G and Alpert BS. Ambulatory blood pressure monitoring in children with aortic coarctation and kidney transplantation. J Pediatr. 2000;136:520–3. [DOI] [PubMed] [Google Scholar]
- 20.Ferraris JR, Ghezzi L, Waisman G and Krmar RT. ABPM vs office blood pressure to define blood pressure control in treated hypertensive paediatric renal transplant recipients. Pediatr Transplant. 2007;11:24–30. [DOI] [PubMed] [Google Scholar]
- 21.Sberro-Soussan R, Rabant M, Snanoudj R, Zuber J, Bererhi L, Mamzer MF, Legendre C and Thervet E. Home and office blood pressure monitoring in renal transplant recipients. J Transplant. 2012;2012:702316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lurbe E, Thijs L, Torro MI, Alvarez J, Staessen JA and Redon J. Sexual dimorphism in the transition from masked to sustained hypertension in healthy youths. Hypertension. 2013;62:410–4. [DOI] [PubMed] [Google Scholar]
- 23.Lima NK, Moriguti JC and Ferriolli E. Uncontrolled hypertension in older patients: markers and associated factors to masked and white-coat effect. J Geriatr Cardiol. 2016;13:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baguet JP, Levy P, Barone-Rochette G, Tamisier R, Pierre H, Peeters M, Mallion JM and Pepin JL. Masked hypertension in obstructive sleep apnea syndrome. Journal of hypertension. 2008;26:885–92. [DOI] [PubMed] [Google Scholar]
- 25.Drager LF, Diegues-Silva L, Diniz PM, Bortolotto LA, Pedrosa RP, Couto RB, Marcondes B, Giorgi DM, Lorenzi-Filho G and Krieger EM. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. American journal of hypertension. 2010;23:249–54. [DOI] [PubMed] [Google Scholar]
- 26.Grassi G, Seravalle G, Trevano FQ, Dell’oro R, Bolla G, Cuspidi C, Arenare F and Mancia G. Neurogenic abnormalities in masked hypertension. Hypertension. 2007;50:537–42. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui M, Judd EK, Jaeger BC, Bhatt H, Dudenbostel T, Zhang B, Edwards LJ, Oparil S and Calhoun DA. Out-of-Clinic Sympathetic Activity Is Increased in Patients With Masked Uncontrolled Hypertension. Hypertension. 2019;73:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert GW, Hering D, Marusic P, Thorp A, Sata Y, Lee R, Duval J, Hammond L, Head GA, Esler MD, Lambert EA, Dixon JB, Dhar AK, Barton DA and Schlaich MP. Health-related quality of life and blood pressure 12 months after renal denervation. Journal of hypertension. 2015;33:2350–8. [DOI] [PubMed] [Google Scholar]
- 29.Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, Ruiz-Hurtado G, Segura J, Rodriguez-Artalejo F and Williams B. Relationship between Clinic and Ambulatory Blood-Pressure Measurements and Mortality. N Engl J Med. 2018;378:1509–1520. [DOI] [PubMed] [Google Scholar]
- 30.Pierdomenico SD, Pierdomenico AM, Coccina F, Clement DL, De Buyzere ML, De Bacquer DA, Ben-Dov IZ, Vongpatanasin W, Banegas JR, Ruilope LM, Thijs L and Staessen JA. Prognostic Value of Masked Uncontrolled Hypertension. Hypertension. 2018;72:862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grigoryan L, Pavlik VN and Hyman DJ. Characteristics, drug combinations and dosages of primary care patients with uncontrolled ambulatory blood pressure and high medication adherence. J Am Soc Hypertens. 2013;7:471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terent A and Breig-Asberg E. Epidemiological perspective of body position and arm level in blood pressure measurement. Blood pressure. 1994;3:156–63. [DOI] [PubMed] [Google Scholar]
- 33.Beckett L and Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC cardiovascular disorders. 2005;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright JM, Mattu GS, Perry TL Jr, Gelferc ME, Strange KD, Zorn A and Chen Y. Validation of a new algorithm for the BPM-100 electronic oscillometric office blood pressure monitor. Blood pressure monitoring. 2001;6:161–5. [DOI] [PubMed] [Google Scholar]
- 35.Mattu GS, Heran BS and Wright JM. Overall accuracy of the BpTRU--an automated electronic blood pressure device. Blood pressure monitoring. 2004;9:47–52. [DOI] [PubMed] [Google Scholar]
- 36.Culleton BF, McKay DW and Campbell NR. Performance of the automated BpTRU measurement device in the assessment of white-coat hypertension and white-coat effect. Blood pressure monitoring. 2006;11:37–42. [DOI] [PubMed] [Google Scholar]
- 37.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG and Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 38.Manning DM, Kuchirka C and Kaminski J. Miscuffing: inappropriate blood pressure cuff application. Circulation. 1983;68:763–6. [DOI] [PubMed] [Google Scholar]
- 39.Myers MG, Kaczorowski J, Paterson JM, Dolovich L and Tu K. Thresholds for Diagnosing Hypertension Based on Automated Office Blood Pressure Measurements and Cardiovascular Risk. Hypertension. 2015;66:489–95. [DOI] [PubMed] [Google Scholar]
- 40.de la Sierra A, Redon J, Banegas JR, Segura J, Parati G, Gorostidi M, de la Cruz JJ, Sobrino J, Llisterri JL, Alonso J, Vinyoles E, Pallares V, Sarria A, Aranda P, Ruilope LM and Spanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry I. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466–72. [DOI] [PubMed] [Google Scholar]
- 41.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR, Urbina EM, Viera AJ, White WB and Wright JT, Jr. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension. 2019;73:e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pickering T Recommendations for the use of home (self) and ambulatory blood pressure monitoring. American Society of Hypertension Ad Hoc Panel. American journal of hypertension. 1996;9:1–11. [DOI] [PubMed] [Google Scholar]
- 43.McCambridge J, Witton J and Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomaszewski M, White C, Patel P, Masca N, Damani R, Hepworth J, Samani NJ, Gupta P, Madira W, Stanley A and Williams B. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceral J, Habrdova V, Vorisek V, Bima M, Pelouch R and Solar M. Difficult-to-control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non-responsiveness from non-adherence to recommended therapy. Hypertension research : official journal of the Japanese Society of Hypertension. 2011;34:87–90. [DOI] [PubMed] [Google Scholar]
- 46.Gupta P, Patel P, Strauch B, Lai FY, Akbarov A, Maresova V, White CMJ, Petrak O, Gulsin GS, Patel V, Rosa J, Cole R, Zelinka T, Holaj R, Kinnell A, Smith PR, Thompson JR, Squire I, Widimsky J Jr., Samani NJ, Williams B and Tomaszewski M Risk Factors for Nonadherence to Antihypertensive Treatment. Hypertension. 2017;69:1113–1120. [DOI] [PubMed] [Google Scholar]
- 47.Gupta P, Patel P, Strauch B, Lai FY, Akbarov A, Gulsin GS, Beech A, Maresova V, Topham PS, Stanley A, Thurston H, Smith PR, Horne R, Widimsky J, Keavney B, Heagerty A, Samani NJ, Williams B and Tomaszewski M. Biochemical Screening for Nonadherence Is Associated With Blood Pressure Reduction and Improvement in Adherence. Hypertension. 2017;70:1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H and Toennes SW. Resistant hypertension? Assessment of adherence by toxicological urine analysis. Journal of hypertension. 2013;31:766–74. [DOI] [PubMed] [Google Scholar]
- 49.Strauch B, Petrak O, Zelinka T, Rosa J, Somloova Z, Indra T, Chytil L, Maresova V, Kurcova I, Holaj R, Wichterle D and Widimsky J Jr. Precise assessment of noncompliance with the antihypertensive therapy in patients with resistant hypertension using toxicological serum analysis. Journal of hypertension. 2013;31:2455–61. [DOI] [PubMed] [Google Scholar]
- 50.Lawson AJ, Shipman KE, George S and Dasgupta I. A Novel ‘Dilute-and-Shoot’ Liquid Chromatography-Tandem Mass Spectrometry Method for the Screening of Antihypertensive Drugs in Urine. J Anal Toxicol. 2016;40:17–27. [DOI] [PubMed] [Google Scholar]
- 51.Schmieder RE, Ott C, Schmid A, Friedrich S, Kistner I, Ditting T, Veelken R, Uder M and Toennes SW. Adherence to Antihypertensive Medication in Treatment-Resistant Hypertension Undergoing Renal Denervation. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brinker S, Pandey A, Ayers C, Price A, Raheja P, Arbique D, Das SR, Halm EA, Kaplan NM and Vongpatanasin W. Therapeutic drug monitoring facilitates blood pressure control in resistant hypertension. J Am Coll Cardiol. 2014;63:834–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.