Abstract
Thrombotic and bleeding complications are the major obstacle for expanding mechanical circulatory support (MCS) beyond the current use. While providing the needed hemodynamic support, those devices can induce damage to blood, particularly, platelets. In this study, we investigated device-induced alteration of three major platelet surface receptors, von Willebrand factor (VWF) and associated hemostatic functions relevant to thrombosis and bleeding. Fresh human whole blood was circulated in an extracorporeal circuit with a clinical rotary blood pump (CentriMag, Abbott) under the clinically relevant operating condition for 4 hours. Blood samples were examined every hour for glycoprotein (GP) IIb/IIIa activation and receptor loss of GPVI and GPIbα on the platelet surface with flow cytometry. Soluble P-selectin in hourly collected blood samples was measured by enzyme linked immunosorbent assay (ELISA) to characterize platelet activation. Adhesion of device-injured platelets to fibrinogen, collagen and VWF was quantified with fluorescent microscopy. Device-induced damage to VWF was characterized with western blotting. The CentriMag blood pump induced progressive platelet activation with blood circulating time. Particularly, GPIIb/IIIa activation increased from 1.1% (Base) to 11% (4 hour) and soluble P-selectin concentration increased from 14.1ng/ml (Base) to 26.5ng/ml (4 hour). Those device-activated platelets exhibited increased adhesion capacity to fibrinogen. Concurrently, the CentriMag blood pump caused progressive platelet receptor loss (GPVI and GPIbα) with blood circulating time. Specifically, MFI of the GPVI and GPIbα receptors decreased by 17.2% and 16.1% for the 4-hour sample compared to the baseline samples, respectively. The device-injured platelets exhibited reduced adhesion capacities to collagen and VWF. The high molecular weight multimers (HMWM) of VWF in the blood disappeared within the first hour of the circulation. Thereafter the multimeric patterns of VWF were stable. The change in the VWF multimeric pattern was different from the progressive structural and functional changes of platelets with the circulation time. This study suggested that the CentriMag blood pump could induce two opposite effects on platelets and associated hemostatic functions under a clinically relevant operating condition. The device-altered hemostatic function may contribute to thrombosis and bleeding simultaneously as occurring in patients supported by a rotary blood. Device-induced damage of platelets may be an important cause for bleeding in patients supported with rotary blood pump MCS systems relative to device-induced loss of HMWM-VWF.
Keywords: Continuous flow ventricular assist device, High mechanical shear stress, Platelet, VWF, Hemostasis dysfunction
Introduction
Mechanical circulatory support (MCS) has emerged as a standard therapy for patients with advanced heart failure (HF) with survival rates approaching those of heart transplant recipients. While MCS has successfully demonstrated medical benefits, the risks of clinically significant adverse events, such as thrombotic events (4 to 11%) and bleeding (30%), have not been completely eliminated and are associated with morbidity and mortality [1–3]. Thrombosis and bleeding represent the opposite extremes of the coagulation balance. It is commonly believed that thrombotic complications are initiated by artificial surfaces and non-physiological flow shear conditions of these shear-inducing mechanical devices [4–7]. A complex antithrombotic therapy including anti-coagulant with or without anti-platelet medication is often prescribed to mitigate the thrombotic risk. With every anticoagulation approach to reduce thrombosis, however, there is an accompanying risk of increasing bleeding complications [8]. Conversely, reducing bleeding complications may increase the thrombotic risk [9]. There has not been a direct link between the level of anticoagulation and bleeding events in patients with MCS [10, 11].
The rotary blood pump in a MCS system employs a high-speed rotating impeller to pump blood from the left ventricle to the aorta to provide hemodynamic support. High mechanical shear stress (HMSS) is created on the surface of impeller blades [12, 13]. The shear stress level on the blood blade tip region can be up to 600 Pa under a clinical relevant rotating speed [13]. This high level of mechanical shear stress can cause damage to blood elements, such as red blood cells, platelets and von willebrand factor (VWF). The loss of high molecular weight multimers (HMWM) of VWF (named as acquired von willebrand syndrome) was observed in almost all patients with rotary pump MCS and has been suggested to contribute to the increased events of bleeding in these patients [14–16]. Since platelets play the important role in initiating the normal hemostasis [17], HMSS-induced platelet dysfunction may also be the important factor for the hemostatic dysfunction in patients with MCS. Previous studies revealed that high shear stresses can induce platelet activation [18–23]. Holme et al. found that the high shear stress of 31.5 Pascal can activate platelets and trigger platelet microparticle formation [19]. Sheriff et al. reported that a significant increase in platelet activation state was observed beyond a stress accumulation of 1500 dyne-s/cm2 [22]. The excessive activation and aggregation of platelets may increase the risk of thrombotic complications. In a few recent studies, high shear stresses were shown to cause the loss of key platelet adhesive receptors (glycoprotein (GP) Ibα, GP VI) [24–27]. The loss of these receptors could result in defective platelet-substrate adhesion, leading to impaired physiological hemostasis and increased potential of bleeding.
When patients are placed on MCS, their blood passes through the rotating pump and tubing or graft of the MCS system repeatedly. As a result, platelets in the patient are inevitably exposed to HMSS. What happens to platelets when blood passes through the blood pump in the MCS system is critical to the hemostatic action inside the pump, which may be relevant to device thrombosis. Horobin et al. [28] found that the ADP- and collagen-induced platelet aggregation ability significantly decreased after 5 hour circulating in in-vitro loop with rotary blood pumps. The compromised hemostatic function of device-injured platelets in the complex vasculature downstream of the pump can be critical to thrombotic and bleeding complications as well. In order to investigate the relationship between device-induced platelet damage (injury) and hemostatic dysfunction relevant to thrombotic and bleeding in HF patients on MCS, an in-vitro experimental study was carried out to examine the device-induced platelet damage (injury) using the CentriMag centrifugal blood pump (Abbott), including surface key receptors and the hemostatic adhesion capacities of the device-injured platelets on biologically relevant substrates. Device-induced damage of HMWM-VWF was also characterized.
Materials and methods
Blood Collection
The study was approved by the University of Maryland’s Institutional Review Board (IRB). Informed consents from volunteers were obtained in accordance with the Declaration of Helsinki. Human blood was collected from 7 healthy adult donors who had not taken any antiplatelet medication 2 weeks before blood donation.
In-Vitro Circulation Loop
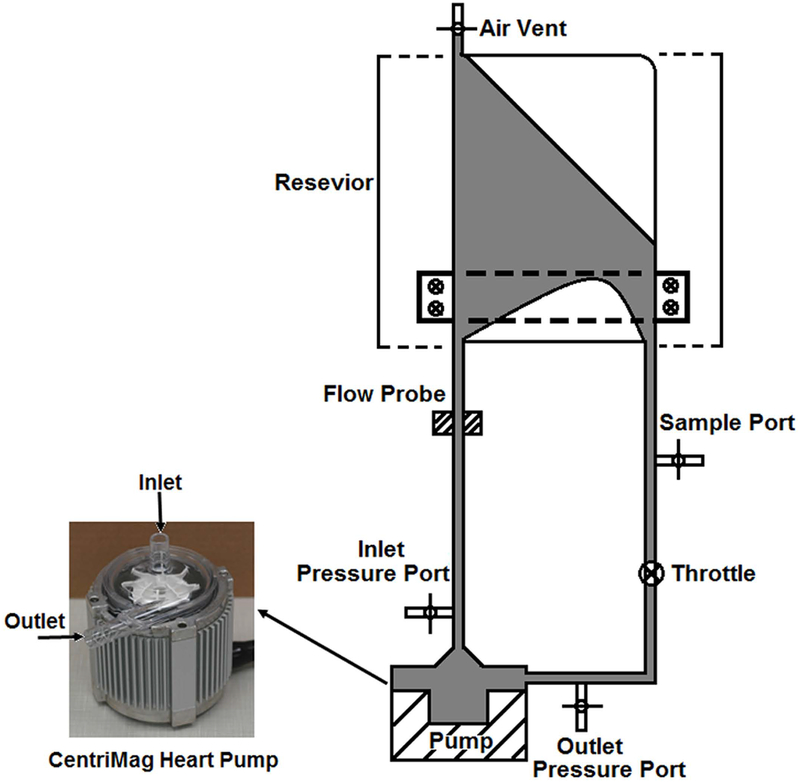
The circulatory loop with the CentriMag blood pump was constructed as depicted in Figure 1. The small-volume blood bag (reservoir) in the loop was designed with the ability to minimize resistance, promote mixing, and prevent bag collapsing [29]. Tubings (3/8”) (Medtronic, Minneapolis, MN) were used to connect the blood pump to the reservoir and the total length of tubings was 500 mm. All the blood bags and tubing used in this study were medical grade with acceptable clinical biocompatibility. A clamp was placed at the outlet tubing of the pump to adjust the pressure head (ΔP) and flow rate. The ultrasonic flow probe (Transonics, Ithaca, NY) was placed at the pump inlet tubing to measure the volumetric flow rate. Pressure transducers were placed at the pump inlet and outlet to measure the ΔP.
Figure 1.
The general experimental setup for the in-vitro circulation loop with small volume. This loop consists of the CentriMag blood pump, a special design blood reservoir, tygon tubing, throttle, ultrasonic flow probe and inlet and outlet pressure transducers.
Experimental procedure
450 mL blood was obtained from each donor and anticoagulated with sodium citrate (3.8%; 0.109mM). The hematocrit of the collected blood was adjusted to 30% using phosphate buffered saline (PBS) with 0.5% bovine serum albumin (BSA). Before filling the loop with blood, PBS was used to rinse the whole circulation loop. After PBS rinse, the blood was gently introduced into the mock loop. Then, the CentriMag blood pump was started at a low speed (500 rpm) and the speed was gradually increased to our experimental condition. The operating condition of the CentriMag blood pump was set to generate a flow rate of 4.5L with the ΔP of 150mmHg to mimic the clinical application of the CentriMag blood pump to provide temporary support for patients with heart failure. The corresponding pump speed was 2750 rpm. The blood was circulated with the CentriMag blood pump running in the loop continuously for 4 hours. A baseline blood sample was collected prior to the CentriMag blood pump operation. After the initiation of the CentriMag blood pump assisted circulation, serial blood samples were collected every 60 minutes for the analyses as described in the following sections.
Characterization of platelet activation (GPIIb/IIIa activation) and receptor loss (GPVI and GPIbα)
Anti-Human CD41a antibody labeled with V450 (CD41a-V450) and anti-human PAC-1 antibody labeled fluorescein isothiocyanate (FITC) (PAC-1 FITC) (both from BD Biosciences, San Jose, CA) were used to identify platelets and detect activated platelets, respectively. Anti-Human CD42b labeled with BV510 (CD42b-BV510) (BD Biosciences, San Jose, CA) and anti-human GPVI labeled with efluor 660 (GPVI-efluor 660) (eBioscience, San Diego, CA) were used to quantify the GPIbα and GPVI loss on the platelet surface, respectively. The antibodies for the negative controls for platelet activation and loss of GPIbα and GPVI receptors were FITC conjugated IgMK (IgMK-FITC), BV510 conjugated IgG1K (IgG1K-BV510) and efluor 660 conjugated IgG1K (IgG1K- efluor 660), respectively. For platelet activation analysis, 5μl whole blood sample (WBS) was incubated with 20μl PAC-1 FITC or IgMK-FITC, 5μl CD41a-V450 and 25 μl HEPES buffer (Corning, NY, USA). For platelet receptor loss analysis, 5μl WBS was incubated with 5μl GPVI-efluor 660 or IgG1K-eFluor 660, CD42b-BV510 or IgG1K-BV510, 5μl CD41a-V450 and 25 μl HEPES buffer. After 30 min incubation at room temperature (RT) in the dark, 1% paraformaldehyde (1ml) was used to fix the samples for 30 min at 4°C in the dark. The flow cytometric data for the blood samples were acquired by a flow cytometer with a 3-laser (488 nm, 633 nm, and 405 nm), 8-color configuration (FACSVerse, BD Biosciences, San Jose, CA) and analyzed with the FCS express software package (De Novo Software, Glendale, CA).
Enzyme-linked immunosorbent assay (ELISA) of soluble P-selectin concentration in plasma
To confirm the device-induced platelet activation, the concentrations of soluble p-selectin in plasma of the baseline and four hourly blood samples were measured [30]. According to the instruction of the manufacturer of the human P-Selectin/CD62P ELISA Kit (R&D Systems, Inc., MN, USA), the baseline and hourly blood samples were centrifuged at 1000 × g for 15 min at room temperature to obtain plasma. Sample plasma (100 μl) was added into wells of a 96-well plate. Then, diluted P-selectin conjugate (100 μl) was added into each of the wells, mixed and incubated for 1 hours. After washing the wells for three times, 100 μl of substrate was added into each well for incubation for 15 minutes. Then, 100 μl of stop solution was added into each of the wells in the same order as the substrate. The 96-well plate was read at 450 nm immediately using a SpectraMax M3 Spectrophotometer (Molecular Devices, CA, USA).
Platelet adhesion on hemostasis related proteins (Fibrinogen, Collagen and VWF)
Glass capillary tubes (0.2mm × 2mm × 25mm, VitroCom, Mountain Lakes, NJ) were initially coated with fibrinogen (1mg/ml, EMD Millipore, Billerica, MA), collagen (1mg/ml, Chrono-log, Havertown, PA), and VWF (100μg/ml, EMD Millipore, Billerica, MA), individually. After overnight incubation at 4°C in a humidified box, the glass tubes were blocked with 5% BSA in PBS at 20°C (room temperature) for 2 hours and then rinsed with PBS. Platelets in the baseline blood sample and the hourly collected blood samples were fluorescently labeled with mepacrine (10 μM) (Sigma, St. Louis, MO) for 20 minutes. Previous studies have shown that platelet function is not affected when a final concentration of mepacrine <20 μM is used [31, 32]. Then the blood samples were perfused through the protein coated glass tubes at a controlled flow rate (0.4 ml/min, shear rate of 500 s−1) for 5 minutes in the dark using a syringe pump (PHD2000, Harvard Apparatus, MA, USA). Platelet adhesion was visualized by a fluorescent microscope (IX71, Olympus) equipped with an Olympus DP80 digital camera. Digital images were captured on the hard drive of a personal computer using the Olympus cellSens software (Olympus, Tokyo, Japan). Ten images along the central axis of each tube with 2 mm spacing were acquired and used to calculate the area covered by adhered platelets using a custom-written program in Matlab (Natick, MA, USA).
Western blot analysis of the loss of HMWM-VWF
The loss of the HMWM-VWF in the blood samples at the baseline and 4 consecutive hours from the device-assisted circulation loop was analyzed by western blotting. 4 ml blood samples were spun at 160 g for 15 minutes at 20°C. The supernate was then centrifuged at 14000 rpm for 10 minutes at 4°C. Platelet poor plasma was collected for analysis. Each plasma sample was mixed 1:20 with laemmli sample buffer (Bio-Rad, Hercules, CA). Agarose gels (0.6%) were used to perform the electrophoresis for separating VWF multimers. After electrophoresis, the protein ladders were transferred to a nitrocellulose 0.45 μm membrane (Thermo Fisher Scientific, Waltham, MA). The polyclonal rabbit anti-human VWF antibody conjugated with Horseradish Peroxidase (1:3000 dilution) (Agilent Dako, Santa Clara, CA) was used to detect VWF. Blots were developed with Clarity™ Western ECL Blotting Substrates (BIO-RAD, Hercules, CA) and scanned with a ChemiDoc™ Imaging System (BIO-RAD, Hercules, CA). The optical density of each band was quantitated with UN-SCAN-IT Gel 6.1 analysis software (Silk Scientific, Orem, UT). The normalized value of optical density of HMWM-VWF was calculated as the following equation [33]:
Ei is the optical density of HMWM-VWF bands of interest, Emin and Emax is the minimum and maximum optical densities for the HMWM-VWF bands in the image, respectively. The details of the method for using western blot to analyze the loss of HMWM-VWF can be found in the work of Krizek et al. [34].
Statistical analysis
The data are presented as mean ± SE (standard Error of Mean). The comparisons of the repeated measured values of platelet activation, platelet adhesion and HMWM-VWF over the circulation time were carried out using the one-way ANOVA (Prism, GraphPad Software, San Diego, CA). The Tukey’s multiple comparisons test was then performed for the comparison between the measured values in the baseline and hourly collected blood samples. A P-value less than 0.05 was considered to be statistically significant.
Results
CentriMag blood pump induced platelet activation, resulting in increased adhesion of platelets to fibrinogen
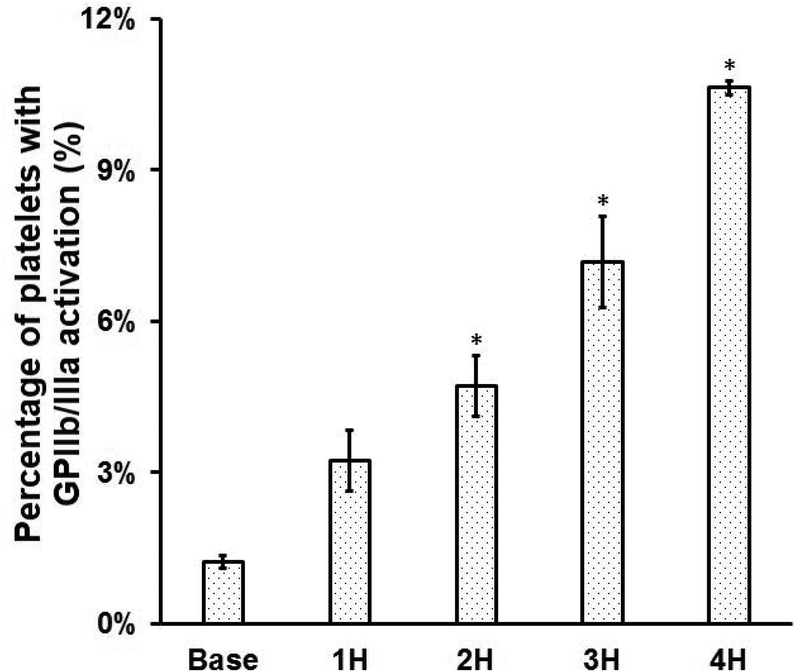
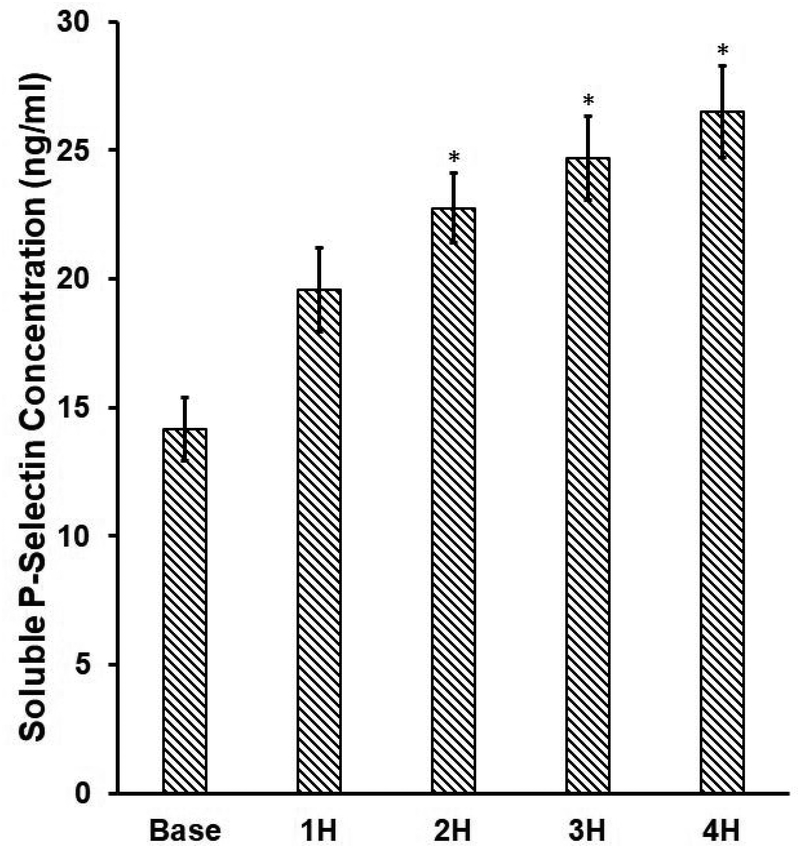
To examine the platelet activation in the blood in the device-assisted circulation with the CentriMag blood pump under the clinical operating condition, we measured the percentage of activated platelets with GPIIb/IIIa activation and the concentration of plasma soluble P-selectin in the blood samples at the baseline and four circulation time points https://www.baidu.com/link?url=qNsDyUwQ57GkB8gOhrlq3lXNV8zgrki4z9XTpXyES2qqzdvki3LYsWttmRfzxdpDfW15KjpHLST7XuXEXqn1VfApnpgOEJzmVYJW5BAzaNC&wd=&eqid=ef15d644000155a1000000035a3c087f. Figure 2A shows that the levels of platelet activation in the baseline and four hourly collected blood samples. Figure 2B shows that the change of soluble P-selectin concentration in plasma of the baseline and four hourly collected blood samples. As shown in Figure 2A, the percentage of activated platelets (indicated by the GPIIb/IIIa activation) increased from 1.1% for the baseline blood sample to 3.3%, 4.9%, 7.3% and 11% for four consecutive hourly blood samples of the device-assisted circulation. A Tukey’s post hoc test revealed that the levels of the platelet GPIIb/IIIa activation were significantly higher (P<0.05) in the blood samples collected two hours after the device-assisted circulation compared to that in the baseline blood sample. The difference in platelet activation as indicated by the GPIIb/IIIa activation was not significant in the blood samples collected at baseline and after the first hour of circulation. Consistent with the increasing number of activated platelets with the circulation time, the concentration of the soluble P-selectin in plasma also increased with the circulation time. As shown in Figure 2B, the soluble P-selectin concentration in plasma increased from 14.1ng/ml for the baseline blood sample to 19.6 ng/ml, 22.7 ng/ml, 24.7 ng/ml and 26.5 ng/ml for the 1st, 2nd, 3rd and 4th hour blood samples of the device-assisted circulation. The increase in the concentration of P-selectin in plasma of the blood sample became statistically significant after two hours of the device-assisted circulation compared to that in the baseline blood sample (P<0.05).
Figure 2.
A) The percentage of platelets with GPIIb/IIIa activation (indicated by PAC-1 FITC) and B) the concentrations of plasma soluble P-selectin in the baseline and consecutive 4 hourly samples collected from the CentriMag blood pump-assisted circulation loop (n=7, *P<0.05 for baseline vs post-circulation).
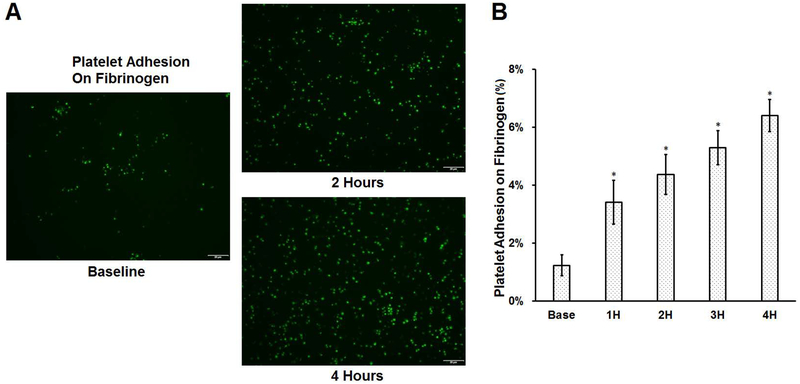
The impact of the device-assisted in-vitro circulation on platelet adhesion to fibrinogen is shown in Figure 3. Typical images of platelets adhered to fibrinogen for the baseline and two hourly collected blood samples (the 2nd and 4th hour) are shown in Figure 3A. The calculated percentages of area coverage over fibrinogen by platelets for the baseline and four hourly collected blood samples are shown in Figure 3B. Significantly more platelets were observed to adhere to fibrinogen after the blood was circulated in the loop. The area covered by platelets increased progressively from 1.12% for the baseline blood sample to 3.41%, 4.37%, 5.29%, and 6.40% for the four consecutive hourly blood samples of the CentriMag blood pump-assisted circulation. As shown in Figures 2 and 3, it is noticed that the more platelets became activated in the blood samples at each increased hour of the device-assisted circulation, the more platelets adhered to the fibrinogen-coated surface. The Pearson’s correlation coefficient between the mean percentage of activated platelets in the platelet population and the mean area coverage by platelets is 0.95.
Figure 3.
The comparison of platelet adhesion on fibrinogen for baseline and 4 consecutive hourly samples from the CentriMag blood pump-assisted circulation loop. A) Typical fluorescence adhesion images of baseline and two hourly samples (magnification, X400); B) The quantitative comparison of the area coverage of adherent platelets on fibrinogen (n=7, *P<0.05 for baseline vs post-circulation).
CentriMag blood pump caused platelet receptor (GPVI and GPIbα) loss, resulting in reduced adhesion of platelets to collagen and VWF
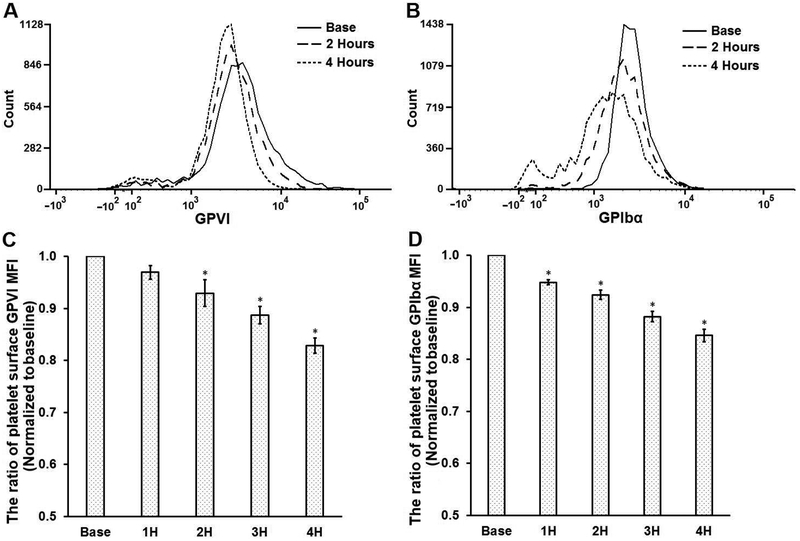
To investigate the loss of platelet GPVI and GPIbα receptors induced by the CentriMag blood pump in the circulation loop, the levels of the two receptors on the platelet surface in the baseline and four hourly blood samples were characterized with flow cytometry assays. Figures 4A and 4B show typical histograms of flow cytometric fluorescence intensity (FI) of GPVI and GPIbα expression on the platelet surface at the baseline, two and four hours after the device-assisted circulation. Since the FI is proportional to the intensity of the fluorescent light emitting from the fluorophores-conjugated to the antibody bound to the available receptors on platelet surface, the FI can represent the relative quantity of the receptors of GPVI and GPIbα on the platelet surface. As shown in Figure 4A and 4B, the FI corresponding to the GPVI and GPIbα expression in the platelets from the samples at 2 and 4 hours shifted to the left (low FI) compared to those in the baseline samples. This indicates that the expression of GPVI and GPIbα on the platelet surface decreased with the time of the blood circulation. Correspondingly, the mean fluorescence intensity (MFI) levels (GPVI and GPIbα) relative to those at the baseline decreased as the circulation time increased (Figure 4C and 4D). The decreases of GPIbα MFI are 4.7%, 8.3%, 13.1% and 16.1% for the blood samples at 1, 2, 3 and 4 hours compared to that in the baseline samples. For GPVI, the decreases are 3.5%, 7.5%,11.9% and 17.2% for the blood samples at 1, 2, 3 and 4 hours compared to baseline samples. There was a statistically significant difference in the platelet GPIbα and GPVI expression in the blood samples collected at baseline and hourly after the circulation as determined by one-way ANOVA (P<0.05). A Tukey post hoc test indicated that the reduction in the MFI levels for the GPIbα expression was significant in the four hourly samples compared to that in the baseline sample while the reduction in the MFI for the platelet GPVI expression was significant two hours after the CentriMag blood pump-assisted circulation.
Figure 4.
The comparison of platelet receptor loss (GPVI and GPIbα) for baseline and 4 consecutive hourly samples from the CentriMag blood pump-assisted circulation loop. Typical flow cytometric histograms of GPVI (A) and GPIbα (B) surface expression of baseline and two samples collected at the 2nd and the 4th hours during loop circulation. The quantification of GPVI (C) and GPIbα (D) surface expression. The ratio of mean fluorescence intensity (MFI) was obtained by normalizing the MFI of sheared samples to that of the baseline sample (non-sheared) (n=7, *P<0.05 for baseline vs post-circulation).
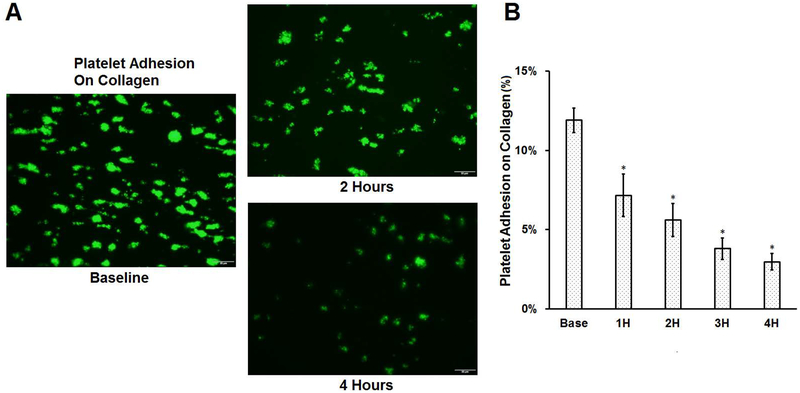
To examine the effect of the device-assisted circulation on platelet adhesion on collagen and VWF, the blood samples collected at baseline and hourly after the device-assisted circulation were perfused through the glass tubes coated with collagen and VWF. Figure 5A shows the typical images of platelets adhered to collagen for the baseline blood sample and two hourly collected blood samples (the 2nd and 4th hour) from the circulatory loop. Figure 5B shows the calculated percentage of area coverage on collagen by platelets from the baseline and hourly collected blood samples. As shown in the images, less platelets adhered to collagen as the circulation time increased in the loop. As shown in Figure 5B, the percentage of the covered areas on collagen by platelets decreased progressively from 11.41% for the baseline blood sample to 7.16%, 5.61%, 3.79% and 2.96% for the blood samples collected at each increased hour of the device-assisted circulation. A Tukey post hoc test revealed that the reduction in the platelet coverage on collagen was statistically significant for all the hourly samples of the CentriMag blood pump-assisted circulation compared to the baseline sample (P<0.05).
Figure 5.
Platelet adhesion to collagen for baseline and 4 consecutive hourly samples from the CentriMag bloop pump-assisted circulation loop. A) Typical fluorescent images of platelet adhesion to collagen of the baseline and two hourly samples collected at two and four hours after the pump-assisted circulation (magnification, X400); B) The quantitative comparison of the area coverage of adherent platelets on collagen (n=7, *P<0.05 for baseline vs post-circulation).
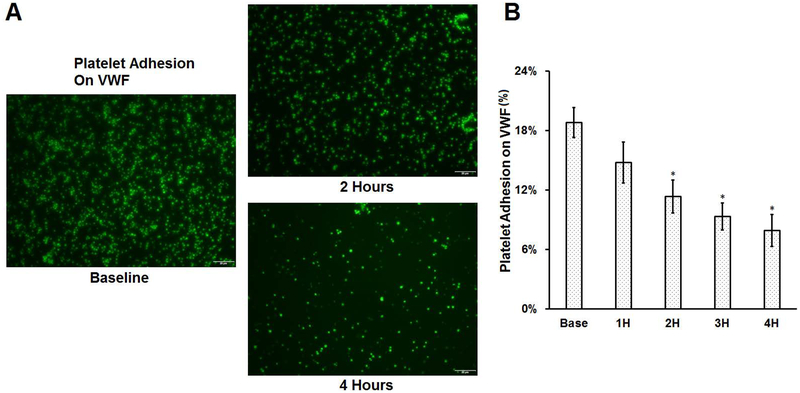
Figure 6A shows the typical images of platelets adhered to VWF for the baseline blood sample and two hourly collected samples (the 2nd and the 4th hour) from the circulatory loop. Figure 6b shows the percentage of the area coverage on VWF by platelets for the baseline and the following hourly blood samples of the CentriMag blood pump assisted circulation. Similar to the observation for collagen, significantly less platelets adhered to VWF as the circulation time increased. The percentage of platelet adhesion to VWF decreased from 19.19% for the baseline blood sample to 14.78%, 11.34%, 9.33% and 7.92% for the blood samples collected at each increased hour of the CentriMag-assisted circulation. A Tukey’s post hoc test indicated that the reduction in the platelet coverage on VWF for the blood samples collected two hours after the CentriMag blood pump-assisted circulation was statistically significant.
Figure 6.
Platelet adhesion on VWF for baseline and consecutive hourly samples from the CentriMag blood pump-assisted circulation loop. A) Typical fluorescent images of platelet adhesion on VWF of the baseline and two hourly samples collected at two and four hours after the pump-assisted circulation (magnification, X400); B) The quantitative comparison of the area coverage of adherent platelets on VWF (n=7, *P<0.05 for baseline vs post-circulation).
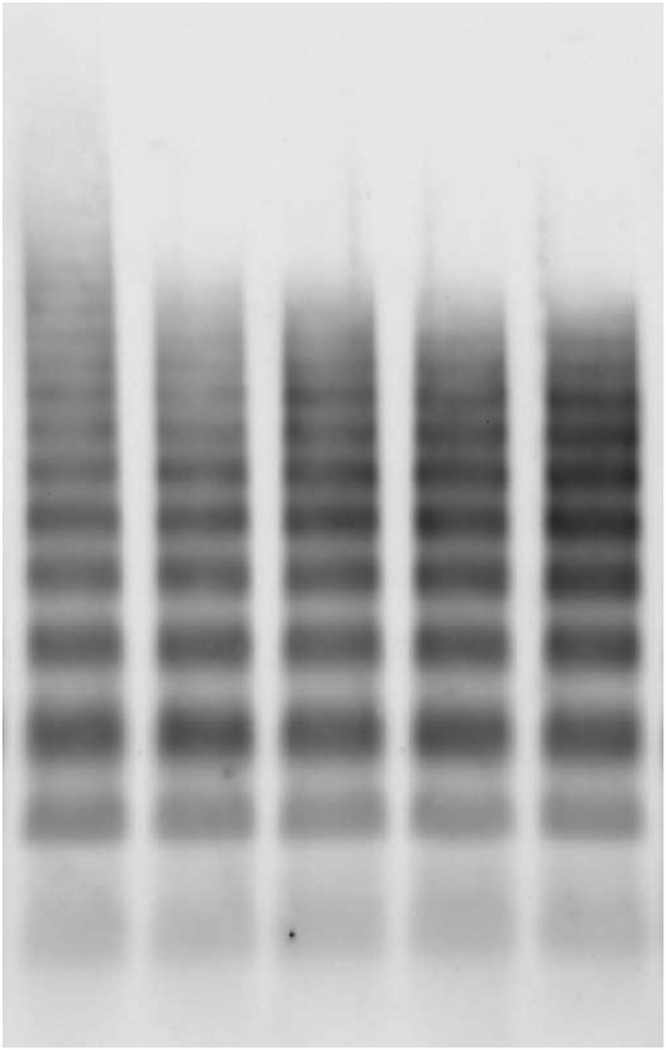
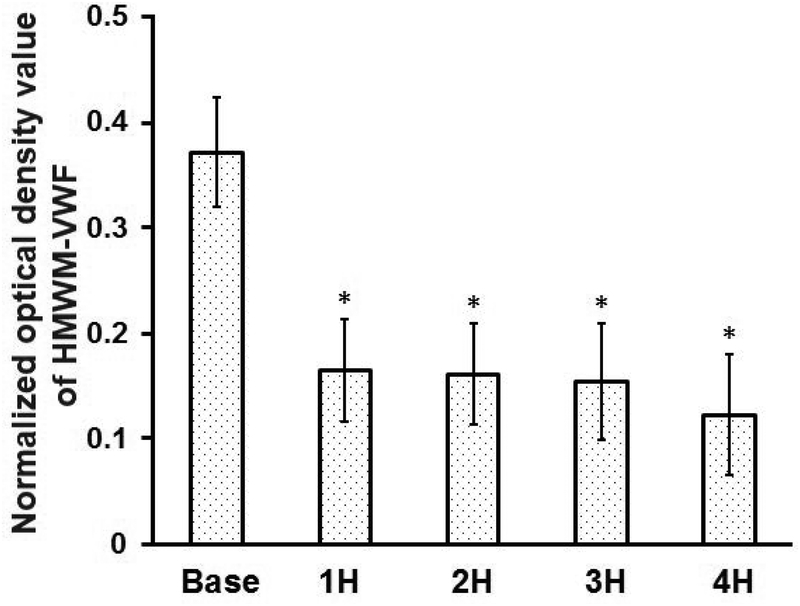
CentriMag blood pump induced the loss of HMWM-VWF
Figure 7 shows the typical image of the western blots of VWF multimers (Figure 7A) and the quantitative comparison of the normalized optical densities of HMWM-VWF of the baseline and four hourly blood samples after the CentriMag blood pump-assisted circulation (Figure 7B). As shown in Figure 7, the CentriMag blood pump-assisted circulation significantly altered the HMWM-VWF in all the hourly collected samples compared to that in the baseline blood sample. It was interesting to note that HMWM-VWF disappeared within the first hour of the circulation and thereafter the VWF multimeric pattern was relatively stable.
Figure 7.
CentriMag blood pump-induced loss of HMWM-VWF in the circulation loop. A) Typical image of plasma VWF multimer distribution for the baseline and 4 consecutive hourly samples from the CentriMag blood pump-assisted circulation loop. B) Quantitative comparison of normalized optical density value of the plasma HMWM-VWF (n=7, *P<0.05 for baseline vs post-circulation).
Discussion
Contemporary MCS systems are all based on the rotary blood pump technology. The high speed rotation of their impellers inevitably creates regions of HMSS inside rotary blood pumps. The HMSS can cause damage to blood cells. Because of the important role of platelets in physiological hemostasis and pathological thrombosis, HMSS-induced platelet damage can affect normal hemostatic function contributing to thrombosis and bleeding complications associated with the use of the MCS systems. In this study, we found that the CentriMag blood pump-assisted circulation could cause platelet activation and subsequently heightened the adhesion of platelets to fibrinogen. The number of activated platelets as well as platelet adhesion on fibrinogen increased with the time of the blood circulation in the loop. Since the activated GPIIb/IIIa on the platelet surface has high affinity with fibrinogen, the increase in activated platelets could be one of the important factors to enhance the adhesion of platelets to fibrinogen. Meanwhile, we also found that the CentriMag blood pump-assisted circulation could induce the loss of platelet GPVI and GPIbα receptors and subsequently resulted in the reduced adhesion of platelets to collagen and VWF. The loss of these receptors and reduction of platelet adhesion to collagen and VWF increased with the blood circulation time in the loop. The loss of the GPVI and GPIbα receptors on the platelet surface is consistent with the reduction of platelet adhesion on collagen and VWF. Since the interactions of the platelet GPVI receptor with collagen and the platelet GPIbα with VWF are the key steps for hemostasis initiation, the loss of GPIbα and GPVI receptors on the platelet surface may increase the risk of bleeding due to the weakened platelet adhesion capacity. Hu et al., Arthur et al. and Nieswandt et al. [35–37] have previously reported that the loss of platelet receptor GPIbα and GPVI are associated with bleeding.
As implicated by our results, the CentriMag blood pump can cause structural alteration resulting in two opposite effects on the platelet hemostatic function. One is to induce the platelet activation and enhance the adhesion of platelets to fibrinogen, which can lead to an elevated risk of thrombosis. The other is to cause the loss of two platelet adhesive receptors (GPVI and GPIbα) and reduction of the platelet adhesion capacities on related proteins (collagen and VWF), which can lead to hemostasis dysfunction and elevated propensity of bleeding. When a HF patient supported with a MCS system, the blood passes through the high shear blood pump continuously once the pump started. The effect of the device-induced platelet dysfunction (activation and receptor loss) can be accumulated gradually as the blood passes through the pump and the number of damaged platelets will increase. When activated platelets circulate in a HF patient’s vasculature, it is easier for them to aggregate on the artificial surface of the MCS system with deposited fibrinogen or in low shear regions of vasculature or at sites of vascular injury. On the other side, when platelets with less receptor GPIbα and GPVI due to shear-induced shedding circulate in the HF patient’s vasculature, it is more likely to lead to the weakened binding of platelets with collagen and VWF at sites of vascular injury. However, the relative strength of the enhanced binding of activated GPIIb/IIIa and other integrins compared to the weakened binding of GPVI and GPIbα to collagen and VWF due to shedding is unknown. If the balance between the two competing forces is broken, device-induced biochemical alterations may have a net effect to reduce adhesion. The reduced platelet adhesion capacity and device-induced damage of HMWM-VWF may result in the attenuated hemostasis function leading to high risk of bleeding. Moreover, part or all of already aggregated platelets may be easier to detach from the device surface or injury sites of blood vessels during the hemostatic process. The detached aggregated platelets could block small arteries when they flow downstream of the device or through the circulation system. This may explain why bleeding and stroke occur simultaneously in HF patients on MCS.
The damage of HMWM-VWF has been suggested to be one of the causes of the increased bleeding events in patients on MCS [14–16]. The loss of HMWM-VWF induced by the CentriMag Blood pump in our in-vitro flow loop was also observed in the present study. HMWM-VWF disappeared within the first hour after the CentriMag blood pump-assisted circulation was initiated. However, the multimeric pattern of VWF did not alter in the subsequent blood samples collected at the second, third and fourth hours compared to that of the first hour (Figure 7). As implied by our results, the damage of HMWM-VWF may appear in patients at a short period of time after MCS system implantation. Thereafter the other relatively low molecular weight VWF may remain stable. In contrast to the device-induced damage to VWF, our data showed that the device-induced damage to platelets as well as the comprised platelet adhesion capacities on fibrinogen, VWF and collagen continued after the first hour of the CentriMag blood pump assisted-circulation. The continued platelet damage induced by a MCS device in patients can affect the normal hemostasis function contributing to thrombotic and bleeding complications. This may underscore the importance of device-induced platelet dysfunction relative to the loss of HMWM-VWF for bleeding in patients on MCS.
Different with previous studies which focused on blood pump-induced damage of red blood cell (hemolysis) [38], this study focused on blood pump-induced damage to platelets and HMWM-VWF, and relevant hemostatic dysfunction. For the first time, this in-vitro loop study demonstrated that the device-induced platelet injury altered the platelet interaction with fibrinogen, collagen and VWF, which may be responsible for the increased risks of thrombotic and bleeding complications in HF patients on MCS.
It should be mentioned that the real clinical scenario for patients supported by a CentriMag blood pump is very complicated. Many possible factors can cause thrombosis and bleeding for these patients [39]. Our data suggest that device-induced platelet hemostasis dysfunction may be one of these important factors for causing thrombosis and bleeding in patients with a CentriMag blood pump. More comprehensive studies need to be performed to investigate the relationship of device-induced platelet dysfunction with thrombosis and bleeding complications.
Conclusion
Contemporary rotary blood pump-based MCS systems can induce opposite effects on platelet hemostatic function that can lead to the concurrent propensity for both thrombotic and bleeding complications. On one hand, the rotary blood pump of the MCS systems can induce platelet activation and enhance the platelet adhesion on fibrinogen, which increases the risk of thrombosis. On the other hand, the rotary blood pump can induce the loss of hemostasis related receptors (GPVI and GPIbα), resulting in the reduction of the platelet adhesion capabilities to collagen and VWF, which compromises platelet hemostasis function and increases the risk of bleeding. The device-induced damage accumulation of platelets and altered hemostatic function may be another important cause for bleeding in patients on MCS in addition to device-induced loss of HMWM-VWF. The data from this study support the emerging concept that thrombosis and bleeding may occur simultaneously in patients on MCS.
Acknowledgments
This work was funded by the National Institutes of Health (Grant numbers: R01HL124170 and R01HL131750).
Footnotes
Declaration of Interest statement
All authors declared no conflicts of interests.
References
- 1.Strueber M, O’Driscoll G, Jansz P, et al. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol 2011;57(12):1375–82. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein D, John R, Salerno C. Increase in left ventricular assist device thrombosis. N Engl J Med 2014;370(15):1465–6. [DOI] [PubMed] [Google Scholar]
- 3.Najjar SS, Slaughter MS, Pagani FD. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2014;33(1): 23–34. [DOI] [PubMed] [Google Scholar]
- 4.Blitz Arie. Pump thrombosis—A riddle wrapped in a mystery inside an enigma. Ann Cardiothorac Surg. 2014. September; 3(5): 450–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frazier OH, Delgado RM 3rd, Kar B, Patel V, Gregoric ID, Myers TJ First clinical use of the redesigned HeartMate II left ventricular assist system in the United States: a case report. Tex Heart Inst J. 2004;31(2):157–9. [PMC free article] [PubMed] [Google Scholar]
- 6.John R, Panch S, Hrabe J, et al. Activation of endothelial and coagulation systems in left ventricular assist device recipients. Ann Thorac Surg. 2009;88(4):1171–1179 [DOI] [PubMed] [Google Scholar]
- 7.Selmi M, Chiu WC, Chivukula VK, Melisurgo G, Beckman JA, Mahr C, Aliseda A, Votta E, Redaelli A, Slepian MJ, Bluestein D, Pappalardo F, Consolo F. Blood damage in Left Ventricular Assist Devices: Pump thrombosis or system thrombosis? Int J Artif Organs. 2018. October 24:391398818806162. [DOI] [PubMed] [Google Scholar]
- 8.Nassif ME, LaRue SJ, Raymer DS, Novak E, Vader JM, Ewald GA1, Gage BF. Relationship Between Anticoagulation Intensity and Thrombotic or Bleeding Outcomes Among Outpatients With Continuous-Flow Left Ventricular Assist Devices. Circ Heart Fail. 2016. May;9(5). pii: e002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adatya S, Bennett MK. Anticoagulation management in mechanical circulatory support. J Thorac Dis 2015. December;7(12):2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrode CW, Draper KV, Huang RJ, et al. Significantly higher rates of gastrointestinal bleeding and thromboembolic events with left ventricular assist devices. Clin Gastroenterol Hepatol 2014;12(9):1461–7. [DOI] [PubMed] [Google Scholar]
- 11.Rossi M, Serraino GF, Jiritano F, Renzulli A. What is the optimal anticoagulation in patients with a left ventricular assist device? Interact Cardiovasc Thorac Surg 2012;15(4):733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser KH, Zhang T, Taskin ME, Griffith BP, Wu ZJ. A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. J Biomech Eng. 2012;134(8):081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Jena SK, Giridharan GA, et al. Flow features and device-induced blood trauma in CF-VADs under a pulsatile blood flow condition: A CFD comparative study. Int J Numer Method Biomed Eng 2017;31(2). doi: 10.1002/cnm.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg 2010; 90:1263–9. [DOI] [PubMed] [Google Scholar]
- 15.Meyer AL, Malehsa D, Bara C, et al. Acquired von Willebrand syndrome inpatients with an axial flow left ventricular assist device. Circ HeartFail 2010;3:675–81. [DOI] [PubMed] [Google Scholar]
- 16.Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol 2010;56:1207–13. [DOI] [PubMed] [Google Scholar]
- 17.Koliopoulou A, McKellar SH, Rondina M, Selzman CH. Bleeding and thrombosis in chronic ventricular assist device therapy: focus on platelets. Curr Opin Cardiol 2016; 31(3):299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shankaran H, Alexandridis P, Neelamegham S. Aspects of hydrodynamic shear regulating shear-induced platelet activation and self-association of von Willebrand factor in suspension. Blood 2003:101(7):2637–45. [DOI] [PubMed] [Google Scholar]
- 19.Holme PA, Orvim U, Hamers MJ, et al. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler Thromb Vasc Biol 1997;17(4):646–53. [DOI] [PubMed] [Google Scholar]
- 20.Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Wu ZJ. Paradoxical Effect of Nonphysiological Shear Stress on Platelets and von Willebrand Factor. Artif Organs 2016;40(7):659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slepian MJ, Sheriff J, Hutchinson M, et al. Shear-mediated platelet activation in the free flow: Perspectives on the emerging spectrum of cell mechanobiological mechanisms mediating cardiovascular implant thrombosis. J Biomech 2017;50:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheriff J, Tran PL, Hutchinson M, et al. Repetitive Hypershear Activates and Sensitizes Platelets in a Dose-Dependent Manner. Artif Organs 2016; 40(6):586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheriff J, Soares JS, Xenos M, Jesty J, Slepian MJ, Bluestein D. Evaluation of shear-induced platelet activation models under constant and dynamic shear stress loading conditions relevant to devices. Ann Biomed Eng. 2013;41(6):1279–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Mondal NK, Ding J, Gao J, Griffith BP, Wu ZJ. Shear-induced platelet receptor shedding by non-physiological high shear stress with short exposure time: glycoprotein Ibα and glycoprotein VI. Thromb Res 2015;135(4):692–8; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Mondal NK, Ding J, et al. Activation and shedding of platelet glycoprotein IIb/IIIa under non-physiological shear stress. Mol Cell Biochem 2015;409(1–2):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Tamimi M, Tan CW, Qiao J, et al. Pathologic shear triggers shedding of vascular receptors: a novel mechanism for down-regulation of platelet glycoprotein VI in stenosed coronary vessels. Blood 2012;119(18):4311–20. [DOI] [PubMed] [Google Scholar]
- 27.Cheng H, Yan R, Li S, et al. Shear-induced interaction of platelets with von Willebrand factor results in glycoprotein Ibalpha shedding. Am J Physiol Heart Circ Physiol 2009;297(6):2128–35. [DOI] [PubMed] [Google Scholar]
- 28.Horobin JT, Simmonds MJ, Nandakumar D, et al. Speed Modulation of the HeartWare HVAD to Assess In Vitro Hemocompatibility of Pulsatile and Continuous Flow Regimes in a Rotary Blood Pump. Artif Organs. 2018;42(9):879–890. [DOI] [PubMed] [Google Scholar]
- 29.Olia SE, Herbertson LH, Malinauskas RA, Kameneva MV. A Reusable, Compliant, Small Volume Blood Reservoir for In Vitro Hemolysis Testing. Artif Organs 2017;41(2):175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kostelijk EH, Fijnheer R, Nieuwenhuis HK, Gouwerok CW, de Korte D. Soluble P-selectin as parameter for platelet activation during storage. Thromb Haemost. 1996;76(6):1086–9. [PubMed] [Google Scholar]
- 31.Savage B, Saldívar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996. January 26;84(2):289–97. [DOI] [PubMed] [Google Scholar]
- 32.Jamiolkowski MA et al. , Real time visualization and characterization of platelet deposition under flow onto clinically relevant opaque surfaces. J Biomed Mater Res A. 2015;103(4):1303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arabnia HR, Tran QN. Emerging Trends in Applications and Infrastructures for Computational Biology, Bioinformatics, and Systems Biology: Systems and Applications. 1st ed. Burlington, MA: Morgan Kaufmann;2016 [Google Scholar]
- 34.Krizek DR, Rick ME. A rapid method to visualize von willebrand factor multimers by using agarose gel electrophoresis, immunolocalization and luminographic detection. Thromb Res. 2000;15;97(6):457–62. [DOI] [PubMed] [Google Scholar]
- 35.Hu J, Mondal NK, Sorensen EN, et al. Platelet glycoprotein Ibα ectodomain shedding and non-surgical bleeding in heart failure patients supported by continuous-flow left ventricular assist devices.J Heart Lung Transplant. 2014;33(1):71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arthur JF, Dunkley S, Andrews RK. Platelet glycoprotein VI-related clinical defects. Br J Haematol. 2007; 139(3):363–72. [DOI] [PubMed] [Google Scholar]
- 37.Nieswandt B, Schulte V, Bergmeier W, et al. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J Exp Med. 2001. February 19;193(4):459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akin S, Soliman OI, Constantinescu AA, et al. Haemolysis as a first sign of thromboembolic event and acute pump thrombosis in patients with the continuous-flow left ventricular assist device HeartMate II. Neth Heart J 2016;24(2):134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125(24):3038–47. [DOI] [PubMed] [Google Scholar]