Summary
Objective:
To identify neuroimaging and clinical biomarkers associated with a language impaired phenotype in refractory temporal lobe epilepsy (TLE).
Methods:
Eighty-five patients with TLE were characterized as language impaired (TLE-LI) or non—language-impaired (TLE-NLI) based on comprehensive neuropsychological testing. Structural MRI, diffusion tensor imaging (DTI), and fMRI were obtained in patients and forty-seven healthy controls (HC). fMRI activations and cortical thickness were calculated within language regions-of-interest and fractional anisotropy (FA) was calculated within deep white matter tracts associated with language. ANOVAs were performed to test for differences among the groups in imaging measures. Receiver operator characteristic curves were used to determine how well different clinical versus imaging measures discriminated TLE-LI from TLE-NLI.
Results:
TLE-LI patients showed significantly less activation within left superior temporal cortex compared to HC and TLE-NLI, regardless of side of seizure onset. TLE-LI also showed decreased FA in the inferior longitudinal fasciculus and arcuate fasciculus compared to HC. Cortical thickness did not differ between groups in any region. A model that included language-related fMRI activations within the superior temporal gyrus, age of onset, and demographic variables was the most predictive of language impairment (AUC of .80).
Significance:
These findings demonstrate a unique imaging signature associated with a language impaired phenotype in TLE, characterized by functional and microstructural alterations within the language network. Reduced left superior temporal activation combined with compromise to language association tracts underlie this phenotype, extending our previous work on cognitive phenotypes that could have implications for treatment-planning or cognitive progression in TLE.
Keywords: Neuroimaging, Functional Magnetic Resonance Imaging, Diffusion Tensor Imaging, Cognitive Phenotype, Neural Substrate, Clinical Biomarkers
INTRODUCTION
Temporal lobe epilepsy (TLE) is a heterogeneous disorder, characterized by an array of clinical and cognitive symptoms1. In order to explain this heterogeneity, studies are beginning to identify TLE cognitive subtypes with similar features2-4. Recently, we have identified four such “cognitive phenotypes” within TLE with unique patterns of structural and microstructural abnormalities on quantitative imaging5. Further characterization of these cognitive phenotypes could help to identify those at greatest risk for cognitive progression and/or post-operative cognitive decline.
Language impairments are a widespread comorbidity in TLE6. Among our cognitive phenotypes, 49% of patients fell within a “language impaired” group, characterized by deficits on neuropsychological tests of naming and fluency5. Although many studies report greater language impairment in patients with left TLE (LTLE) relative to right TLE (RTLE)7-9, an emerging literature has highlighted that patients with RTLE also present with language impairment10-12 and sometimes LTLE and RTLE are indistinguishable in language performance13-16. Language impairment in RTLE has often been attributed to medication effects17 or consequences of early seizure onset18,19. However, we propose that similar underlying patterns of neural abnormality in LTLE and RTLE20,21 may provide a more direct explanation of language impairment.
We examined drug-resistant TLE patients with versus without language impairment and investigate whether they differ in patterns of cortical atrophy, white matter (WM) microstructure, and functional activations in language (“perisylvian”) networks. We also test whether clinical variables, including side of seizure onset, can contribute to identification of pre-surgical language impairment. We hypothesize that shared structural and functional alterations within perisylvian networks explain much of the language impairment observed in LTLE and RTLE.
METHODS
Participants
This study was approved by the Institutional Review Boards at UC San Diego and UC San Francisco, and informed consent was collected from all participants. Eighty-five patients with medically-refractory TLE and forty-seven healthy controls (HC) met criteria for the study. Patients were recruited through referral from the UC San Diego or UC San Francisco Epilepsy Centers and were all undergoing presurgical evaluations. Inclusion criteria for patients included a 1) TLE diagnosis, 2) age 18 or older, and 3) no dual pathology or mass lesion (i.e. tumors, vascular malformations, focal cortical dysplasia, or other visible lesions on MRI). TLE diagnosis and side of seizure onset were determined by a board-certified neurologist with expertise in epileptology, in accordance with the criteria defined by the International League Against Epilepsy, based on scalp and intracranial video-EEG telemetry, seizure semiology, and neuroimaging evaluation. The presence of mesial temporal sclerosis (MTS) was determined by inspection of MRI images by a board-certified neuroradiologist with expertise in epilepsy. In 42 patients, MRI findings suggested the presence of ipsilateral MTS and the remaining patients demonstrated normal MRI. HC were included if they were between the ages of 18 and 65 and had no reported history of neurological or psychiatric disease.
Neuropsychological measures
Tests of language ability (i.e., naming and fluency) were obtained as part of a comprehensive neuropsychological evaluation. Language tests included the Boston Naming Test (BNT)22, Auditory Naming Test (ANT)23, and Category Fluency subtest of the Delis-Kaplan Executive Function System (DKEFS)24. Naming and semantic fluency were evaluated because they are the most commonly impaired aspects of language in TLE. Conversely, language comprehension and reading are not frequently impaired in TLE25. Raw scores for all patients’ neuropsychological data were converted into z-scores based on the HC data distribution. Impairment was defined as greater than 1.5 standard deviations below the mean of the HC. Patients were determined to be impaired in the language domain if two or more of the three cognitive tests fell within the impairment range26. Patients missing only one of the three tests were included and classified as impaired if both tests fell within the impairment range. Patients missing two of the three tests were excluded from this study. Approximately 49% of patients were language impaired (Language Impaired; TLE-LI) and the remaining 51% of patients were not impaired in language (Non-Language Impaired; TLE-NLI). An estimate of nonverbal IQ (WASI Perceptual Reasoning Index)27 was also obtained to determine whether the groups differed in overall intellectual ability.
FMRI language task
During fMRI scanning, patients with TLE and HC performed a semantic judgment task in which they pressed a button when they saw a word that represented an animate noun (e.g. SHEEP). This task reliably produces strong activations within perisylvian cortex in both HCs and patients with TLE28,29. A detailed description of the task is provided in the supplementary material. Stimuli included novel object nouns (Novel Words; NW), false font (FF) stimuli, and target words (i.e., animals). The FF stimuli were alphabet-like characters, matched in size and number of characters to each NW stimulus to control for sensory but not lexical or semantic content30. The response to the target words (i.e. animate nouns) was included in the task to ensure comprehension of word meanings. However, the primary language contrast of interest was NW minus FF, excluding targets, as this contrast is not contaminated by the finger press.
Data acquisition
MRI data were collected on a General Electric (GE) Discovery MR750 3T scanner with an 8-channel phased-array head coil at UC San Diego or UC San Francisco. Image acquisitions on the 3T scanner were identical at all centers and included a conventional three-plane localizer, GE calibration scan, a T1-weighted 3D customized FSPGR structural sequence (TR = 8.08 ms, TE = 3.16 ms, TI = 600 ms, flip angle = 8°, FOV = 256 mm, matrix = 256 × 192, slice thickness = 1.0 mm), two functional T2*-sensitive echo-planar imaging (EPI) scans (TR = 3000 ms, TE = 30 ms, flip angle = 90°, FOV = 220 mm, matrix = 64 × 64, slice thickness = 2.5 mm), and for diffusion MRI, a single-shot pulse-field gradient spin-echo EPI sequence (TR = 8000 ms, TE = 82.9 ms, flip angle = 90°, FOV = 240 mm, matrix = 96 × 96m, slice thickness = 2.5 mm, echo-spacing = 588 ms). Diffusion data were acquired with b-value= 0 and 1,000 s/mm2 with 30 unique gradient directions. The fMRI and DTI scans were acquired for each individual using two different phase encoding directions to correct for geometric distortions in the EPI images31.
Image processing
Structural MRI
Images were corrected for spatial sensitivity inhomogeneities and for non-linear warping caused by non-uniform fields created by the gradient coils32. The cortical surface was reconstructed and parcellated using FreeSurfer, 5.3.033. Visual inspection was performed on all images to identify topological defects, which were subsequently edited using established software guidelines. Quantification of cortical thickness estimates was determined by measurement of the distance between the WM and the pial surfaces at each vertex. The cortical surface was then parcellated into ROIs using the Destrieux atlas34, and average thickness was calculated within each ROI. A total of 85 TLE and 47 HC had usable structural MRI data.
Functional MRI
The fMRI data analysis was carried out using Analysis of Functional NeuroImages (AFNI version 17.3.00)35, SUMA36, and Matlab programs (MathWorks, Natick, MA). Co-registration of functional and structural MRI datasets was obtained according to established methods31, head motion between scans was removed using AFNI’s 3dvolreg, and time series were aligned using AFNI’s 3dTshift. EPI datasets were spatially aligned and then resampled to 2.5 mm3 isotropic voxels. Cortical parcellations were applied to the EPI images using SUMA’s @SUMA_Make_Spec_FS. Time series data were analyzed based on the general linear model using AFNI’s 3dDeconvolve with the BLOCK function. Additional regressors were used to model motion residuals and baseline drifts were modeled using quadratic polynomials. The regression coefficients that formed the response function were then used in general linear tests, for the main language contrast (NW-FF). A total of 67 TLE and 39 HC had usable fMRI data. In total, 18 patients and 8 controls were excluded from fMRI inclusion in the study due to motion artifacts.
fMRI Cortical Activation Maps:
Whole brain fMRI cortical activation maps of NW-FF were generated using the mixed effects model in AFNI’s 3dANOVA2. To ensure that cortical map differences between groups were not driven by strict threshold effects, for display purposes these cluster-based surface maps were liberally thresholded and corrected for multiple comparisons by using a combined significance level of p < .05 and a cluster size ≥ 20.
fMRI and MRI ROI analysis:
The cortical surface was parcellated into ROIs based on the Destrieux atlas34. The number of activated voxels were extracted from six left-hemisphere regions most frequently implicated in neuro-anatomical models of visual language lexical-semantic processing: 1) ventral temporal: fusiform, 2) lateral temporal: middle temporal gyrus (MTG), superior temporal gyrus (STG), superior temporal sulcus (STS), and 3) frontal: pars opercularis and pars triangularis37,38. For use in the ROI analysis, fMRI voxel activations were corrected for multiple comparisons by using a significance level of p < .01 and a cluster size ≥20, for a corrected α of .05 as determined by 3dClustSim. To probe possible network reorganization differences between groups, language laterality indices were calculated (LI= [L-R]/[L+R])39 using a lateral temporal ROI6,29.
DTI data
Preprocessing of the diffusion data included corrections for distortions due to magnetic susceptibility (B0), eddy currents, and head motion, followed by registration of the diffusion image to the T1-weighted structural image. A detailed description of the DTI image processing is provided elsewhere40. DTI-derived fractional anisotropy (FA) was calculated based on a tensor fit to the b = 1,000 data. A total of 78 TLE and 45 HC had usable DTI data. In total, 7 patients and 2 controls were excluded from DTI inclusion due to imaging artifacts.
Fiber Tracts of Interest
Mean FA was extracted for two fiber tracts of interest: the arcuate fasciculus (ARC) and the inferior longitudinal fasciculus (ILF), as these two fibers represent the main dorsal and ventral language pathways respectively41. Fiber tract FA was derived using an automated probabilistic diffusion tensor atlas (i.e., AtlastTrack) that was developed in house and has been validated in both HCs and patients with TLE. A full description of the atlas and methodology is provided in Hagler et al.42.
Statistical Analysis
Independent t-tests and Fisher’s tests were used to test for differences in demographic variables between patients and HC, and to test for differences in clinical variables between TLE-LI and TLE-NLI. Analyses of variance (ANOVAs) were conducted to compare fMRI activations, mean cortical thickness, and mean tract FA across HC, TLE-LI, and TLE-NLI within each ROI. Pairwise comparisons were performed when the ANOVA was significant. Follow-up 2 (TLE-LI vs TLE-NLI) x 2 (LTLE vs RTLE) ANOVAs were also conducted to determine whether structural and/or functional differences between the TLE-LI and TLE-NLI groups differed as a function of side of seizure onset. This follow-up 2-x-2 ANOVA included 73 DTI subjects (5 bilateral subjects excluded) and 63 fMRI subjects (4 bilateral subjects excluded).
Logistic regression was used to determine whether imaging variables offer unique information for classification of TLE-LI versus TLE-NLI above what is accounted for by demographic and clinical information. Model performance was determined by comparing the area under the curve (AUC) for models with demographic/clinical information only (handedness, side of seizure onset, age of seizure onset, MTS status, seizure frequency, number of AEDs, and treatment with zonisamide or topiramate) to models that also included significant imaging variables. Significant differences between model AUC were tested by creating a 95% confidence interval with 10,000 bootstrapped samples. A model was considered superior if the AUC was above the 95% confidence interval of the model to which it was being compared.
Results
Demographics and patient clinical variables
Table 1 provides descriptive data and statistical comparisons on the HC and TLE (TLE-LI and TLE-NLI) samples. Between HC and TLE, there were no differences in age or sex distribution (all p>.05); However, HC had more years of education (p<.001).
Table 1:
Demographics and clinical variables
| TLE | HC | |||
|---|---|---|---|---|
| N | 85 | 47 | ||
| Age | 36.20 (13.32) | 36.26 (13.99) | t(130) = −.02 | p = .98 |
| Education | 13.51 (2.12) | 15.89 (2.38) | t(130) = −5.92 | p < .001 |
| Sex: M/F | 38/47 | 19/28 | Fisher’s Exact = .23 | p = .72 |
| Language Impaired | Not Language Impaired |
|||
| N | 43 | 42 | ||
| Age (years) | 34.47 (14.02) | 37.98 (12.48) | t(83) = −1.22, | p = .23 |
| Education (Years) | 13.27 (2.31) | 13.73 (1.90) | t(83)= −.99 | p = .32 |
| Sex: M/F | 18/25 | 20/22 | Fisher’s Exact = .29 | p = .67 |
| Handedness: L/R/A | 4/38/1** | 4/37/1 | Fisher’s Exact = .002 | p = 1.0 |
| MTS: Yes/No | 22/21 | 20/22 | Fisher’s Exact = .11 | p =.83 |
| Side: L/R/Bilateral | 22/16/5 | 20/22/0 | Fisher’s Exact=.85# | p =.38 |
| Age of Onset | 16.26 (12.39) | 26.17 (15.07) | t(83) = −3.32 | p < .001 |
| Duration (years) | 18.10 (15.35) | 11.87 (13.34) | t(83) = 1.997 | p = .049 |
| Number of AEDs | 2.24 (.943) | 2.36 (.906) | t(82)= 1.08 | p = .30 |
| Seizure frequency | 8.87 (16.21) | 6.02 (6.95) | t(81)= −.56 | p = .58 |
| # of patients on topiramate | 3 (6.97%) | 9 (21.43%) | Fisher’s Exact=3.66 | p =.068 |
| # of patients on zonisamide | 5 (11.63%) | 4 (9.52%) | Fisher’s Exact=.099 | p =1.00 |
| fMRI Language Laterality: L/R/Bilateral | 22/9/3 | 26/¾ | Fisher’s Exact=3.4 | p=.21 |
| Neuropsychological Measures | ||||
| Mean z-score | Mean z-score | |||
| Boston Naming Test | −5.67 (3.28) | −1.05 (1.415) | t(75)= −7.795 | p<0.001 |
| Auditory Naming Test | −2.59 (2.64) | −.136 (.666) | t(75)= −5.504 | p<0.001 |
| D-KEFS Category | −2.01 (.892) | −.892 (.843) | t(75)= −5.763 | p<0.001 |
| Perceptual Reasoning IQ* | 92.52 (17.63) | 98.47 (14.51) | t(61) = −1.47 | p = .15 |
TLE: temporal lobe epilepsy; F: females; M: males; L: left; R: right; A: ambidextrous; MTS: mesial temporal sclerosis; AEDs: antiepileptic drugs; standard deviations are presented inside the parentheses
Two-subtest IQ based on performance on WASI Matrix Reasoning and Block Design Subtests
Test Reported is for a Left/Right Fisher’s test
All left-handed TLE-LI patients were LTLE
Between TLE-LI and TLE-NLI, there were no differences in age, education, sex distribution, handedness, seizure frequency (calculated as the total number of focal seizures with impaired awareness and secondary generalized seizures per month), number of antiepileptic drugs (AEDs), MTS status or nonverbal IQ (all p>.05). There were no differences in side of seizure onset (LTLE vs RTLE) between the TLE-LI and TLE-NLI groups (p>.05). Given the effects of topiramate and zonisamide on language performance17, we compared the proportion of patients that were on each medication across the two groups. Patients in the TLE-LI group were not more likely to be on zonisamide (p>.05); interestingly, there were more TLE-NLI patients on topiramate, though it was not significant and the overall number of patients on either drug was low (p>.05). Finally, a Fisher’s Exact demonstrated no differences between patient groups in language laterality (i.e., Laterality index = ([L-R]/[L+R])) based on fMRI activation patterns within any language ROIs (all p>.05).
There were differences in age of seizure onset (p<.001) and duration of epilepsy (p<.05) between the TLE-LI and TLE-NLI groups, with TLE-LI demonstrating an earlier age of seizure onset and longer disease duration than the TLE-NLI.
fMRI activation profiles
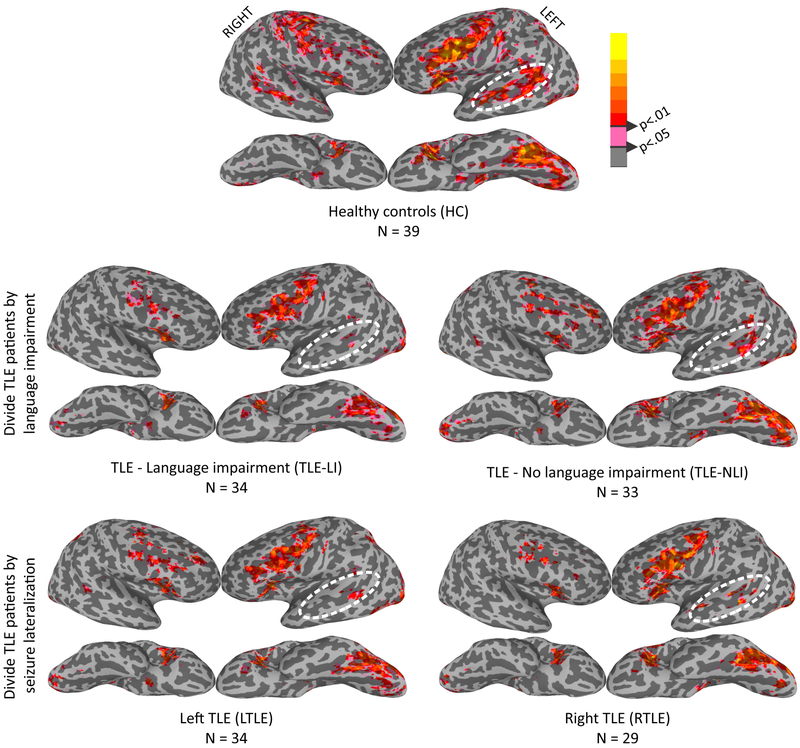
Figure 1 displays cluster-thresholded fMRI activation maps for HC (top row), TLE-LI vs TLE-NLI (middle row), and LTLE vs RLTE (bottom row). The HC pattern reveals a distributed, lexical-semantic network activation pattern, observed predominantly in the left hemisphere for ventral temporal, superior lateral temporal, and inferior frontal activation, with more modest activations observed in homologous right hemisphere regions. As can be seen, TLE-LI demonstrated a similar pattern of activation in left ventral temporal and frontal regions relative to HC and TLE-NLI, but with much less activation in the left superior temporal lobe relative to the other groups. In contrast, splitting patients into LTLE and RTLE (excluding the 5 bilateral TLE patients) revealed similar patterns of activation for both groups in the three main regions, with reduced activations in the left temporal region in both LTLE and RTLE groups.
Figure 1. fMRI cortical surface maps during a lexical-semantic task demonstrate activation differences in superior temporal regions.
Surface fMRI activation for the new words - false-font contrast for HC (top row), TLE patients split by language impairment (middle row), and TLE patients split by side of seizure onset (bottom row).
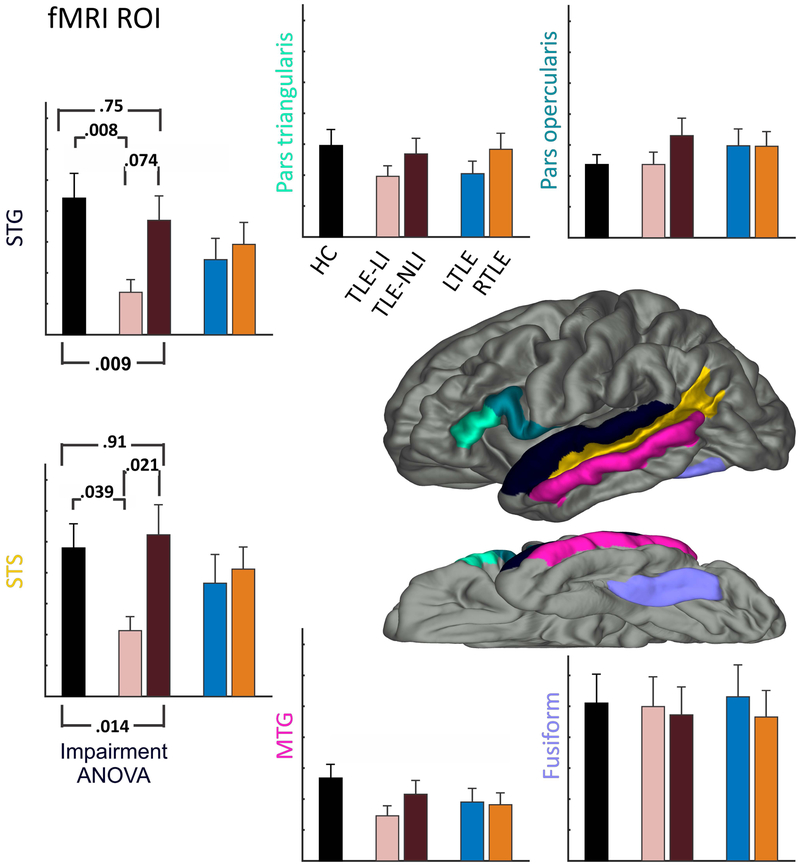
ROI-level analysis confirmed the differences between groups in the left lateral temporal lobe observed in the fMRI surface maps (see Figure 2). ANOVA revealed differences in the STG [F(2,98)=4.89, p<.01] and STS [F(2,98)=4.42, p<.05] among the HC, TLE-LI and TLE-NLI. Follow-up tests identified the same pattern in both ROIs, with the TLE-LI group having decreased activity compared to both the HC [STG: p<.01; STS: p<.05] and the TLE-NLI group [STG: p=.074; STS: p<.05], but no differences between the HC and TLE-NLI groups [STG: p=.75; STS: p=.91]. No differences were found for the other regions (all p>.05). In addition, post-hoc analyses of the same six ROIs in the right hemisphere did not reveal any differences between the TLE-LI and TLE-NLI groups (all p>.05).
Figure 2. ROIs confirm that decreases in fMRI activation for language impairment restricted to superior temporal regions.
Six ROIs associated with lexical-semantic processing are displayed on the brain. The surrounding bar graphs represent the number of active voxels in each ROI for the HC (black), followed by TLE split by language impairment into TLE-LI (light green) and TLE-NLI (dark green), then TLE split by side of seizure onset into LTLE (blue) and RTLE (red). Error bars are standard error of the mean. Significant p-values for the Impairment ANOVA are noted, and p-values are reported for follow-up pairwise tests between HC vs TLE-LI and TLE-NLI vs TLE-LI.
Cortical Thickness ROIs
There were no significant differences in cortical thickness within any of the ROIs (all p>.05).
DTI tractography
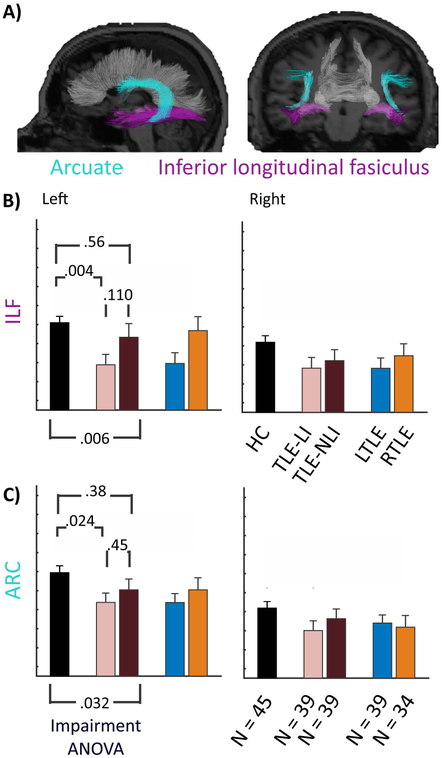
An ANOVA revealed group differences in FA of the left ILF [F(2,117)=5.27, p<.01] and left ARC [F(2,117)=3.53, p<.05] (see Figure 3). Follow-up tests revealed differences between TLE-LI and HC [left ILF: p<.01 ; left ARC: p<.05]. There were no differences between TLE-NLI and HC [left ILF: p=.56; left ARC: p=.38] or TLE-LI and TLE-NLI [left ILF: p=.11; left ARC: p=.45].
Figure 3. White matter integrity is compromised in TLE-LI compared to HC in the ILF and ARC.
A) Sagittal and coronal rendering of the arcuate fasciculus (ARC) and inferior longitudinal fasciculus (ILF). B) ILF and C) ARC bar graphs representing the FA of the HC, followed by TLE split by language impairment into TLE-LI (light green) and TLE-NLI (dark green), then TLE split by side of seizure onset into LTLE (blue) and RTLE (red). Error bars are standard error of the mean. Significant p-values for the Impairment ANOVA are noted, and p-values are reported for follow-up pairwise tests between HC vs TLE-LI and TLE-NLI vs TLE-LI.
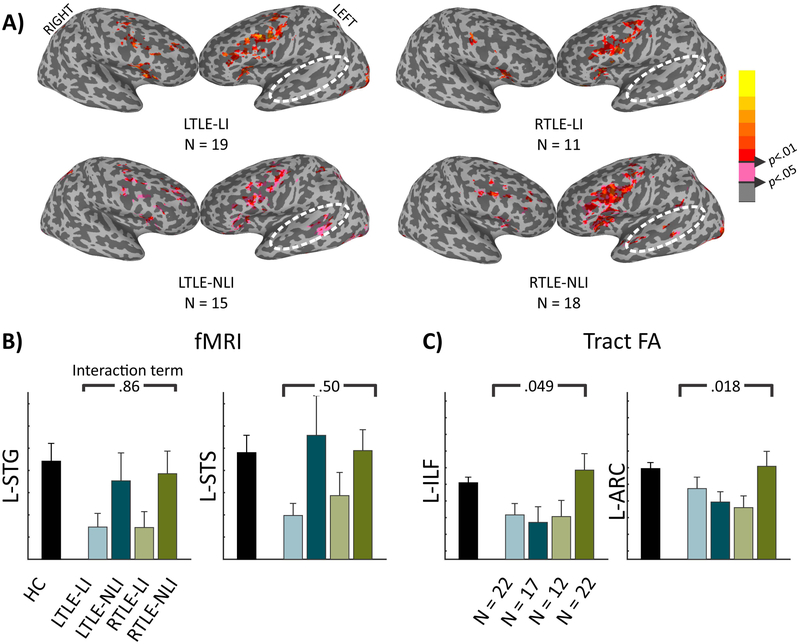
Impairment-by-Side of Seizure Onset
Figure 4a displays surface maps of the TLE-LI and TLE-NLI groups further subdivided into LTLE and RTLE. As can be seen, there is minimal lateral temporal activation in TLE-LI patients for either the RTLE or LTLE patients. Conversely, lateral temporal activations are observed in both LTLE and RTLE in the TLE-NLI subjects. A 2×2 ANOVA follow-up was run on the STG and STS (Figure 4b). The main effect for language impairment was still present [STG: F(1,54)=5.6, p<.05; STS: F(1,54)=5.24, p<.05]. However, no significant main effects of RTLE vs LTLE or interaction effects were found (all p>.05).
Figure 4. Splitting by side of seizure onset reveals preserved fMRI language-impairment effects but an interaction for tract FA.
A) Surface fMRI activation for the new words - false-font contrast for the 4 TLE groups. B) Number of active voxels in each ROI for HC (black) followed by the TLE split into LTLE-LI (light teal), LTLE-NLI (dark teal), RTLE-LI (light green), and RTLE-NLI (dark green). The p-value is from the interaction term of a 2 (TLE-LI, TLE-NLI) by 2 (LTLE, RTLE) ANOVA. C) Tract FA in each tract, the same as in B).
When investigating the two left hemisphere tracts in the 2×2 ANOVA, neither the language impairment nor the side of seizure onset main effects were significant (all p>.05). However, in both tracts the interaction term was significant [L-ILF: F(1,65)=3.99, p<.05, L-ARC: F(1,65)=5.8, p<.05] (Figure 4c). This was driven by RTLE TLE-NLI having a higher FA relative to the lower FA in the other three patient groups.
Logistic Model
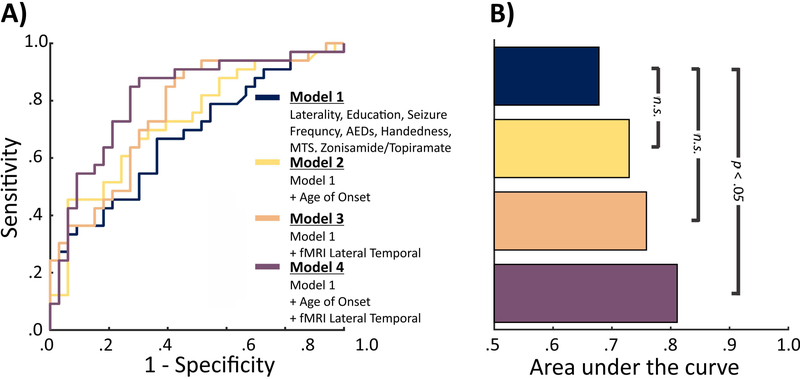
Given the significant differences observed in left lateral temporal fMRI activation and age of onset between the TLE-LI and TLE-NLI patient groups, we compared four models to determine if fMRI activation and age of seizure onset offer independent discriminant information for classification of language impairment (see Figure 5). Our baseline model included demographic/clinical variables known to influence cognition and the language network: Education, Handedness, MTS, side of seizure onset, seizure frequency, number of AEDs, and treatment with zonisamide or topiramate39,43. All clinical variables were available for 66 of the 67 patients with fMRI (1 patient was missing seizure frequency). This model had an AUC significantly above chance (AUC=.67, p<.05). Two additional models, which included the demographic/clinical variables plus the combined fMRI activations from the STS and STG (AUC=.76) or age of onset (AUC=.73) numerically increased the AUC, but neither model performed significantly better than the baseline model (both p>.05). However, including both lateral temporal lobe fMRI activation and age of onset in the same model produced a larger AUC increase than either model independently (AUC=.81), which was significantly better performance relative to the baseline model (p<.05).
Figure 5. ROC curves and Area Under the Curve comparing model performance when discriminating TLE-LI from TLE-NLI.
A) The ROC curves associated with 4 logistic regression models. Models 2-4 include Model 1 plus additional variable(s). B) The area under the curve associated with each ROC curve. Significance values show whether models 2-4 significantly outperformed model 1 as determined by confidence intervals from 10,000 bootstrapped samples.
Discussion
In this study, we identify a neurobiological substrate of language impairment in patients with TLE based on structural, functional and diffusion imaging. Specifically, a decrease in functional activations within the left superior temporal region during a lexical-semantic task differentiated patients with and without language impairment, independent of side of seizure onset. This decrease in functional activity was not related to differences in cortical atrophy, as there were no differences between patients in cortical thickness in perisylvian regions. However, TLE-LI patients showed microstructural compromise to two key language WM tracts relative to HC—the left ARC and ILF—which was not observed in the TLE-NLI patients. Thus, decreases in functional activations within the left superior temporal region coupled with disconnections within the broader language network may lead to the language impairments observed in the TLE-LI phenotype. Finally, we demonstrate that in addition to demographic and clinical information, fMRI adds to the discrimination of TLE patients with and without language impairment, indicating that task-based fMRI provides unique information regarding the neurobiology of language impairment in TLE that is not entirely explained by clinical factors.
In our study, the strongest indicator of language impairment was a decrease in functional activation within the left superior temporal lobe, which was not observed in other key language-related regions (i.e., left inferior frontal gyrus and fusiform). Although this localized decrease was observed during a lexical-semantic task designed to activate temporal lobe language cortex, it is of interest that it appeared to differentiate patients with broad language impairment (i.e., those with impairments in visual and auditory naming, and semantic fluency) from those with intact language performances. In addition, there were no differences between patients with LTLE and RTLE and no interaction with side of seizure onset, indicating that decreased left temporal activation during a lexical-semantic task is a robust signature of language network dysfunction that is not specific to patients with LTLE. Although this finding may seem surprising given that many studies have found language impairment to predominantly affect patients with LTLE7-9, it is of note that many other studies have not revealed LTLE versus RTLE differences13-16 or have only included patients with LTLE (Chang et al.29). Interestingly, our lexical-semantic task was associated with preserved left frontal activation in TLE-LI. This is important to note because many fMRI tasks currently implemented for language mapping (e.g., covert word generation6) primarily demonstrate robust and reliable frontal lobe activations but less reliable temporal lobe activations. Our findings suggest that lexical-semantic tasks designed to elicit temporal lobe activation may be a promising way to assess language network dysfunction in TLE.
In addition to decreased temporal lobe activations, we found the TLE-LI group showed damage to the left ILF and ARC relative to HC. These two pathways constitute the ventral and dorsal language pathways respectively41. Recent evidence from connectome analyses of white matter found that the STG has abnormal network properties in patients with the language impairment phenotype5. We found no regional cortical thickness abnormalities in the lateral temporal cortex of interest, which is suggestive that the decreased activation is not related to a lack of cortex in these areas (i.e. cortical thinning). Additionally, patients with visible cortical defects were not enrolled in the study. Therefore, we propose that the lateral temporal functional language decrease observed in our TLE-LI patients relative to controls may be related to disconnection within the wider language network rather than local lateral superior temporal cortical pathology. According to this hypothesis, damage to the ILF and ARC disrupts communication between preserved visual word form processing44 (i.e., fusiform) and superior temporal regions important for lexical-semantic processing45.
However, it is of note that microstructural integrity of the ILF and ARC did not differ between the TLE-LI and TLE-NLI patients at a group level; instead side of seizure onset interacted with language impairment. This interaction showed that within the TLE-NLI group, LTLE (but not RTLE) patients also had damage to these two tracts. Therefore, damage to the left ARC and ILF appears to be necessary (i.e. damage present in both RTLE-LI and LTLE-LI) but not sufficient (i.e. damage also present in LTLE-NLI) to produce a language impairment. A possibility is that there are unmeasured white matter differences distinguishing LTLE-LI and LTLE-NLI, either additional damage in LTLE-LI or an alternative route available to LTLE-NLI. Much recent work on connectomes in TLE46 and in other syndromes47 emphasize the importance of whole-brain connectomes to understanding cognitive co-morbidities in different clinical syndromes. A second possibility is the potential for language reorganization in LTLE-NLI, which may have preserved language ability in this group. Language reorganization is generally considered in the context of functional activation migration39. Here we found no differences between LTLE-LI and LTLE-NLI groups in fMRI language laterality, and TLE-NLI showed preserved left perisylvian activation patterns, which suggests that functional reorganization did not explain the intact language performance in the LTLE-NLI group.
We found that the TLE-LI and TLE-NLI groups did not differ significantly in most clinical or demographic variables. Interestingly, side of seizure onset did not differ significantly between TLE-LI (22 LTLE / 16 RTLE) and TLE-NLI (20 LTLE / 22 RTLE). The notable clinical feature which did differentiate TLE-LI and TLE-NLI was age of seizure onset. This is not surprising, as an extensive literature ties earlier age of seizure onset to poor WM development and cognitive outcomes18,48. Because age of seizure onset and left temporal lobe fMRI activation were the only two variables that consistently discriminated between TLE-LI and TLE-NLI patients at the group level, we tested the ability of these two variables to classify patients after taking other clinical variables (including side of seizure onset) into account. Although neither age of seizure onset nor fMRI alone outperformed the baseline model, these two variables combined added significantly to the prediction of language impairment. This suggests that fMRI measures of lexical-semantic processing contribute to an understanding of language impairment in combination with clinical features, particularly age of seizure onset. Our results point toward an underlying neurobiological feature of language impairment in TLE being dysfunction within left superior temporal areas, for both LTLE and RTLE that may be driven by poor development of (or damage to) left perisylvian WM tracts in patients with an early age of seizure onset48-50.
Implications for Cognitive Phenotypes
Our goal in the present work was to establish the underlying neuropathological substrates in a commonly occurring cognitive phenotype characterized by language impairment5. Epilepsy can result from diverse genetic and acquired causes, which are unified by the common clinical presentation of recurring seizures51. The argument for cognitive phenotypes is that similarly, diverse causes flow into shared neural dysfunctions unified by the presentation of cognitive dysfunction. Indeed, different cognitive phenotypes in TLE display unique patterns of microstructural WM compromise5.
Here, we identify a neural signature shared by patients with language impairment, i.e., decreased functional activation coupled with microstructural loss in the left temporal language network. These pathological changes in functional activation provide a more direct indicator of language dysfunction than side of seizure onset or many other clinical variables, excepting age of seizure onset. This may be particularly relevant to consider in patients with RTLE, who often present with language impairment that may be overlooked or dismissed as secondary to medication effects. In our study, TLE-LI were not more likely to be on topiramate or zonisamide relative to TLE-NLI, nor did this variable add to the prediction of language impairment in our ROC model. Therefore, the language deficits observed in these RTLE patients were better explained by the underlying functional and microstructural abnormalities found within the left temporal language network.
Our study joins the expanding field of studies that have attempted to understand the heterogeneity in cognitive ability by subdividing TLE into cognitive phenotypes and characterizing the neuroanatomical correlates of each subtype. This type of phenotyping could enable a personalized medicine approach to patients for predicting cognitive progression and potentially cognitive decline following surgery2-5. Understanding the shared neurobiology within cognitive phenotypes will be a key aspect in tying together the descriptive and predictive elements of this effort as part of ongoing epilepsy-wide efforts in sub-dividing patients to improve treatment decision making52,53.
Limitations and Future Directions
A key caveat of our approach is that the main neural biomarkers identified were based on functional and microstructural alterations detected with non-invasive imaging. It is possible that other pathological changes were present in our TLE patients that were not captured in our analysis, but that influenced our language findings. In addition, it is known that fMRI is subject to signal loss in anteromedial temporal lobe regions. Although we used a robust method to correct for geometric distortions and signal loss31, it is possible that susceptibility artifacts limited our ability to detect anterior temporal lobe activations that could have further differentiated our groups. In addition, there are many approaches for probing language processing. We used a visual semantic judgement task and found dysfunction in the superior temporal region; however, an auditory semantic judgement task might produce different, albeit complementary, language network patterns that could provide additional information regarding language impairment in TLE. Therefore, identifying replicable and stable functional biomarkers for clinical care necessitates the comparison of the efficacy of multiple tasks54.
An important consideration for our study is the certainty of the hemisphere of seizure onset through continuous video-EEG monitoring of spontaneous seizures. It is of course possible that some RTLE-LI patients may have experienced independent left temporal lobe seizure onset not detected during their pre-surgical work-up. While possible, we think this is not probable. In our sample, of the 16 RTLE-LI patients, 9 underwent surgery of which 7 patients achieved Engle 1 surgery outcomes and 2 are awaiting one-year Engle outcome scores. Of the 9 patients without Engle scores (7 who have not yet undergone surgery and 2 awaiting Engle outcomes) seizure onset laterality was determined by intracranial EEG (n=4) or continuous video scalp EEG monitoring with strong evidence of right temporal lobe seizure onset (n=5). Our contention is that language impairment in our RTLEs represents bilateral pathology48-50, not bilateral seizure onsets. As the field of epilepsy is moving toward establishing more meaningful taxonomies55 and identifying stable cognitive phenotypes, understanding the underlying neurobiology of these phenotypes could improve our ability to match patients to treatments and improve a range of epilepsy-related outcomes.
Supplementary Material
Key Point Box.
Both left and right TLE patients were identified with a systemic impairment in language across multiple language tests.
Patients with and without language impairment were not well discriminated by clinical features, except for language-impaired patients having an earlier age of onset.
Patients with language impairment displayed decreased left lateral temporal activation during a language task regardless of side of seizure onset.
Patients with language impairment also displayed decreased white matter tract integrity compared with controls as well as an earlier age of seizure onset.
The best performing model discriminating language-impaired and non-impaired patients included both age of seizure onset and lateral temporal functional activation.
Acknowledgements
We would like to acknowledge funding support from the National Institute of Health (R01 NS065838 to C.R.M.).
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011;7(3):154–164. doi: 10.1038/nrneurol.2011.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermann B, Seidenberg M, Lee E-J, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc 2007;13(01). doi: 10.1017/S135561770707004X [DOI] [PubMed] [Google Scholar]
- 3.Dabbs K, Jones J, Seidenberg M, Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav. 2009;15(4):445–451. doi: 10.1016/j.yebeh.2009.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez-Cruces R, Velázquez-Pérez L, Rodríguez-Leyva I, et al. Association of white matter diffusion characteristics and cognitive deficits in temporal lobe epilepsy. Epilepsy Behav 2018;79:138–145. doi: 10.1016/j.yebeh.2017.11.040 [DOI] [PubMed] [Google Scholar]
- 5.Reyes A, Kaestner E, Bahrami N, et al. Cognitive phenotypes in temporal lobe epilepsy are associated with distinct patterns of white matter network abnormalities. Neurology. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balter S, Lin G, Leyden KM, Paul BM, McDonald CR. Neuroimaging correlates of language network impairment and reorganization in temporal lobe epilepsy. Brain Lang. July 2016. doi: 10.1016/j.bandl.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busch RM, Frazier TW, Haggerty KA, Kubu CS. Utility of the Boston Naming Test in Predicting Ultimate Side of Surgery in Patients with Medically Intractable Temporal Lobe Epilepsy. Epilepsia. 2005;46(11):1773–1779. doi: 10.1111/j.1528-1167.2005.00300.x [DOI] [PubMed] [Google Scholar]
- 8.Raspall T, Doñate M, Boget T, et al. Neuropsychological tests with lateralizing value in patients with temporal lobe epilepsy: Reconsidering material-specific theory. Seizure. 2005;14(8):569–576. doi: 10.1016/j.seizure.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 9.Keary TA, Frazier TW, Busch RM, Kubu CS, Iampietro M. Multivariate Neuropsychological Prediction of Seizure Lateralization in Temporal Epilepsy Surgical Cases: NP PREDICTION OF SEIZURE LATERALIZATION. Epilepsia. 2007;48(8):1438–1446. doi: 10.1111/j.1528-1167.2007.01098.x [DOI] [PubMed] [Google Scholar]
- 10.Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological Characteristics of the Syndrome of Mesial Temporal Lobe Epilepsy. Arch Neurol. 1997;54(4):369–376. doi: 10.1001/archneur.1997.00550160019010 [DOI] [PubMed] [Google Scholar]
- 11.Alessio A, Bonilha L, Rorden C, et al. Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav. 2006;8(3):593–600. doi: 10.1016/j.yebeh.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 12.Bell BD, Hermann BP, Woodard AR, et al. Object naming and semantic knowledge in temporal lobe epilepsy. Neuropsychology. 2001;15(4):434. [DOI] [PubMed] [Google Scholar]
- 13.Ogden-Epker M, Cullum CM. Quantitative and qualitative interpretation of neuropsychological data in the assessment of temporal lobectomy candidates. Clin Neuropsychol. 2001;15(2):183–195. [DOI] [PubMed] [Google Scholar]
- 14.Langfitt JT, Rausch R. Word-finding deficits persist after left anterotemporal lobectomy. Arch Neurol. 1996;53(1):72–76. [DOI] [PubMed] [Google Scholar]
- 15.Cherlow DG, Serafetinides EA. Speech and memory assessment in psychomotor epileptics. Cortex. 1976;12(1):21–26. [DOI] [PubMed] [Google Scholar]
- 16.Stafiniak P, Saykin AJ, Sperling MR, et al. Acute naming deficits following dominant temporal lobectomy: prediction by age at 1st risk for seizures. Neurology. 1990;40(10):1509–1509. [DOI] [PubMed] [Google Scholar]
- 17.Ojemann LM, Ojemann GA, Dodrill CB, Crawford CA, Holmes MD, Dudley DL. Language Disturbances as Side Effects of Topiramate and Zonisamide Therapy. Epilepsy Behav. 2001;2(6):579–584. doi: 10.1006/ebeh.2001.0285 [DOI] [PubMed] [Google Scholar]
- 18.Lee C-Y, Tabesh A, Benitez A, Helpern JA, Jensen JH, Bonilha L. Microstructural integrity of early- versus late-myelinating white matter tracts in medial temporal lobe epilepsy. Epilepsia. 2013;54(10):1801–1809. doi: 10.1111/epi.12353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oyegbile TO, Dow C, Jones J, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62(10):1736–1742. [DOI] [PubMed] [Google Scholar]
- 20.McDonald CR, Hagler DJ Jr, Ahmadi ME, et al. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia. 2008;49(5):794–803. [DOI] [PubMed] [Google Scholar]
- 21.Slinger G, Sinke MR, Braun KP, Otte WM. White matter abnormalities at a regional and voxel level in focal and generalized epilepsy: A systematic review and meta-analysis. NeuroImage Clin. 2016;12:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Pro-ed; 2001. [Google Scholar]
- 23.Hamberger MJ, Seidel WT. Auditory and visual naming tests: Normative and patient data for accuracy, response time, and tip-of-the-tongue. J Int Neuropsychol Soc. 2003;9(03). doi: 10.1017/S135561770393013X [DOI] [PubMed] [Google Scholar]
- 24.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System: Examiners Manual. Psychological Corporation; 2001. [Google Scholar]
- 25.Drane DL, Pedersen NP. Knowledge of language function and underlying neural networks gained from focal seizures and epilepsy surgery. Brain Lang. 2019;189:20–33. doi: 10.1016/j.bandl.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edmonds EC, Eppig J, Bondi MW, et al. Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Neurology. 2016;87(20):2108–2116. doi: 10.1212/WNL.0000000000003326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wechsler D WASI (Wechsler adult scale–Reduced). N Y Psychol Corp. 1999. [Google Scholar]
- 28.Thesen T, McDonald CR, Carlson C, et al. Sequential then interactive processing of letters and words in the left fusiform gyrus. Nat Commun. 2012;3:1284. doi: 10.1038/ncomms2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang Y-HA, Javadi SS, Bahrami N, Uttarwar VS, Reyes A, McDonald CR. Mapping lexical-semantic networks and determining hemispheric language dominance: Do task design, sex, age, and language performance make a difference? Brain Lang. 2018;179:42–50. doi: 10.1016/j.bandl.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald CR, Thesen T, Carlson C, et al. Multimodal imaging of repetition priming: using fMRI, MEG, and intracranial EEG to reveal spatiotemporal profiles of word processing. Neuroimage. 2010;53(2):707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland D, Kuperman JM, Dale AM. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. NeuroImage. 2010;50(1):175–183. doi: 10.1016/j.neuroimage.2009.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- 33.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 34.Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- 36.Saad ZS, Reynolds RC. SUMA. NeuroImage. 2012;62(2):768–773. doi: 10.1016/j.neuroimage.2011.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor JSH, Rastle K, Davis MH. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol Bull. 2013;139(4):766–791. doi: 10.1037/a0030266 [DOI] [PubMed] [Google Scholar]
- 38.Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart CC, Swanson SJ, Sabsevitz DS, Rozman ME, Janecek JK, Binder JR. Predictors of language lateralization in temporal lobe epilepsy. Neuropsychologia. 2014;60:93–102. doi: 10.1016/j.neuropsychologia.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonald CR, Leyden KM, Hagler DJ, et al. White matter microstructure complements morphometry for predicting verbal memory in epilepsy. Cortex. 2014;58:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319 [DOI] [PubMed] [Google Scholar]
- 42.Hagler DJ, Ahmadi ME, Kuperman J, et al. Automated white-matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Hum Brain Mapp. 2009;30(5):1535–1547. doi: 10.1002/hbm.20619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sass KJ, Sass A, Westerveld M, et al. Specificity in the correlation of verbal memory and hippocampal neuron loss: Dissociation of memory, language, and verbal intellectual ability. J Clin Exp Neuropsychol. 1992;14(5):662–672. doi: 10.1080/01688639208402854 [DOI] [PubMed] [Google Scholar]
- 44.McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7(7):293–299. doi: 10.1016/S1364-6613(03)00134-7 [DOI] [PubMed] [Google Scholar]
- 45.Friederici AD. The Role of Left Inferior Frontal and Superior Temporal Cortex in Sentence Comprehension: Localizing Syntactic and Semantic Processes. Cereb Cortex. 2003;13(2):170–177. doi: 10.1093/cercor/13.2.170 [DOI] [PubMed] [Google Scholar]
- 46.Bernhardt BC, Bonilha L, Gross DW. Network analysis for a network disorder: The emerging role of graph theory in the study of epilepsy. Epilepsy Behav. 2015;50:162–170. doi: 10.1016/j.yebeh.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 47.Shen X, Finn ES, Scheinost D, et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc. 2017;12(3):506–518. doi: 10.1038/nprot.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hermann B, Seidenberg M, Bell B, et al. The neurodevelopmental impact of childhood-onset temporal lobe epilepsy on brain structure and function. Epilepsia. 2002;43(9):1062–1071. [DOI] [PubMed] [Google Scholar]
- 49.Seidenberg M, Kelly KG, Parrish J, et al. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia. 2005;46(3):420–430. [DOI] [PubMed] [Google Scholar]
- 50.Kaaden S, Quesada CM, Urbach H, et al. Neurodevelopmental disruption in early-onset temporal lobe epilepsy: evidence from a voxel-based morphometry study. Epilepsy Behav. 2011;20(4):694–699. [DOI] [PubMed] [Google Scholar]
- 51.Lytton WW. Computer modelling of epilepsy. Nat Rev Neurosci. 2008;9(8):626–637. doi: 10.1038/nrn2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Commission on Classification and Terminology of the ILAE. Proposal for a revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–99. [DOI] [PubMed] [Google Scholar]
- 53.Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–530. doi: 10.1111/epi.13670 [DOI] [PubMed] [Google Scholar]
- 54.Gaillard WD, Balsamo L, Xu B, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63(8):1403–1408. [DOI] [PubMed] [Google Scholar]
- 55.Hermann B, Loring DW, Wilson S. Paradigm Shifts in the Neuropsychology of Epilepsy. J Int Neuropsychol Soc 2017;23(9–10):791–805. doi: 10.1017/S1355617717000650 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.