Abstract
Objective:
To define caudate nucleus locations responsive to intraoperative direct electrical stimulation for tinnitus loudness modulation and relate those locations to functional connectivity maps between caudate nucleus subdivisions and auditory cortex.
Methods:
Six awake study participants who underwent bilateral deep brain stimulation (DBS) electrode placement in the caudate nucleus as part of a Phase I clinical trial (https://clinicaltrials.gov/ct2/show/NCT01988688) were analyzed for tinnitus modulation to acute stimulation at 20 locations. Resting-state 3T fMRI was used to compare connectivity strength between centroids of tinnitus loudness reducing and non-reducing caudate locations with auditory cortex in the 6 DBS Phase I trial participants and 14 other neuroimaging participants with Tinnitus Functional Index > 50.
Results:
Acute tinnitus loudness reduction was observed at 5 caudate locations, 4 were positioned at the body and 1 at the head of the caudate nucleus in normalized Montreal Neurological Institute space. The remaining 15 electrical stimulation interrogations of the caudate head failed to reduce tinnitus loudness. Compared to the caudate head, the body subdivision had stronger functional connectivity to the auditory cortex on fMRI (p<0.05).
Conclusion:
Acute tinnitus loudness reduction was more readily achieved by electrical stimulation of the caudate nucleus body. Compared to the caudate head, the caudate body has stronger functional connectivity to auditory cortex. These first-in-human findings provide insight into the functional anatomy of caudate nucleus subdivisions and may inform future target selection in our basal ganglia-centric neuromodulation approach to treat medically refractory tinnitus.
Keywords: Caudate Nucleus, Deep Brain Stimulation, fMRI, Tinnitus, Resting-State Functional Connectivity
Tinnitus is a disorder of elemental auditory percepts in the absence of physical sound stimuli. Its prevalence is 10–15%5,7,20 and incidence is 5.4%12 of the general population. The growing affinity for portable music appliances and widespread access to consumer electronics is worrisome for damaging hearing and triggering tinnitus, particularly in adolescents and young adults.4,24 Although the majority of people who experience subjective tinnitus are not distressed by their auditory phantoms, 1% of U.S. adults are severely bothered and significantly disabled.23 For those tinnitus sufferers who are unresponsive to conventional acoustical and behavioral treatments, novel treatment options remain limited.
An innovative, basal ganglia-centric treatment to reduce auditory phantom awareness by neuromodulation of caudate nucleus function has been proposed.2 This idea is principally based on perceptual evidence of tinnitus loudness and phantom sound quality modulation mediated by electrical stimulation of the caudate nucleus in awake Parkinson’s disease patients with chronic tinnitus who were undergoing deep brain stimulation (DBS) surgery,2,9 and long-term tinnitus reduction10 following focal vascular injury to area LC, an area at the junction of the head and body of the caudate nucleus. Neuroimaging evidence to support targeting area LC of the caudate nucleus specifically for neuromodulation therapy is increased auditory corticostriatal functional connectivity in a cohort with chronic tinnitus compared to a cohort without tinnitus, adjusted for hearing loss.6 It is hypothesized that electrical stimulation of the caudate nucleus may correct dysfunctional gating of auditory phantom percepts, thereby reducing tinnitus severity. This approach is conceptually distinct from auditory-centric treatments that are based on increased neuronal spontaneous firing rates following deafferentation of tonotopic central auditory structures,18 oscillatory brain activity in the primary auditory cortex,25 and downregulation of inhibitory functions in the central auditory system.21
We report on the relationship between short-term tinnitus loudness reduction and location of caudate nucleus stimulation on a cohort with medically refractory tinnitus who underwent awake deep brain stimulation (DBS) surgery as part of a Phase I clinical trial. The objectives of this study are to evaluate for functional distinctions of the caudate head and body subdivisions by querying acute tinnitus loudness modulation as a consequence of direct electrical stimulation and measuring strength of auditory corticostriatal functional connectivity of those psychoacoustically defined caudate subdivisions using resting-state 3T fMRI. Information derived from this study would contribute to a better understanding of human caudate nucleus functional organization and inform methodology to target the caudate nucleus more precisely for neuromodulation in a Phase II clinical trial of deep brain stimulation for medically refractory tinnitus.
Methods
Study Participants
One hundred and ninety-five prospective study participants were pre-screened for this Phase I Clinical Trial (https://clinicaltrials.gov/ct2/show/NCT01988688). A large number were eliminated from further consideration due to factors that included anxiety, depression and expressed suicidality, yielding 14 prospective participants who advanced to comprehensive audiological and neuropsychological screening, and resting-state 3T fMRI. Nine study participants met eligibility criteria and 6 elected to proceed with DBS implantation between August 2014 and February 2017, providing tinnitus perceptual data in response to acute DBS electrode macrostimulation during surgery. Inclusion criteria included men and women between the ages of 22 and 75, subjective unilateral or bilateral non-pulsatile tinnitus of one year’s duration or greater, Tinnitus Functional Index (TFI) > 50 (moderate problem or more severe),13 tinnitus unsatisfactorily responsive to acoustical or behavioral therapy, and Montreal Cognitive Assessment ≥ 26.14 Exclusion criteria included hyperacusis and profound hearing loss in both ears.
We report on two sets of experiments. For the intraoperative dataset to evaluate acute tinnitus loudness differences between the head and body subdivisions of the caudate nucleus to acute electrical stimulation, there were 6 participants (2 females; age mean (SD) = 51.5 (11) years, range = 37–62 years; TFI mean (SD) = 74.2 (9.8), range 62–89). For the resting-state 3T fMRI dataset to evaluate auditory corticostriatal connectivity differences between caudate nucleus subdivisions, all 14 prospective participants were included and another 6 tinnitus patients with TFI > 50 who participated in a neuroimaging imaging using the same scanner were added to the cohort, for a total of 20 (7 females; age mean (SD) = 53.5 (8.9) years, range = 37–66 years; TFI mean (SD) = 71.9 (10.8), range 50–89; ‘big problem’ or relatively severe). Demographic information for these 20 participants is summarized in Table 1. All participants gave written informed consent following explanation of study procedures that were approved by the UCSF Committee on Human Research (IRB# 13–11641). All experiments were conducted in accordance with the Declaration of Helsinki.
TABLE 1.
Study Cohort Demographic Information
| Study Participant | DBS Implant Status | Gender | Age | Tinnitus Functional Index |
|---|---|---|---|---|
| U01–01 | No | Male | 42 | 71 |
| U01–02 | Yes | Female | 38 | 77 |
| U01–03 | Yes | Male | 58 | 66 |
| U01–04 | Yes | Male | 58 | 62 |
| U01–05 | No | Female | 58 | 50 |
| U01–06 | Yes | Male | 56 | 74 |
| U01–07 | No | Female | 54 | 71 |
| U01–08 | No | Male | 66 | 54 |
| U01–09 | No | Female | 60 | 84 |
| U01–10 | Yes | Female | 37 | 76 |
| U01–11 | No | Male | 66 | 85 |
| U01–12 | Yes | Male | 62 | 89 |
| U01–13 | No | Male | 62 | 72 |
| U01–14 | No | Female | 53 | 69 |
| R1877 | No | Male | 54 | 67 |
| R2318 | No | Male | 61 | 85 |
| R2327 | No | Male | 44 | 56 |
| R2345 | No | Female | 44 | 73 |
| R2379 | No | Male | 46 | 73 |
| R2593 | No | Male | 51 | 83 |
U01 – Participants screened for Phase I DBS trial, R – Participants of tinnitus fMRI study
Caudate Nucleus Mapping with Electrical Stimulation
Awake stereotactic functional neurosurgery was performed using a Leksell Frame (Elekta, Stockholm, Sweden) and Framelink stereotactic software (Medtronic StealthStation, Minneapolis, MN). The caudate nucleus was targeted using an entry point at or just anterior to the coronal suture. A trajectory was planned to the subthalamic region, avoiding sulci, visible blood vessels and the ventricles. The trajectory was then shortened to the caudate nucleus and medialized in the coronal plane to place the bottom of the trajectory at the base of the caudate. The depth of trajectory was adjusted to center the 10.5 mm length electrode array of a model 3387 DBS electrode (Medtronic, Minneapolis, MN) within the caudate nucleus in the coronal plane. Targeting was modified in the anterior-posterior direction in the caudate nucleus to interrogate different loci as intraoperative mapping progressed.
Microelectrode recording (MER) was performed using an Alpha Omega recording system (Alpha Omega, Alpharetta, GA). A single MER pass was performed at the originally planned target in all cases. This was followed by placement of the DBS lead along the same tract, with the contacts spanning the caudate top to bottom in the coronal oblique trajectory plane based on the depth of the superior and inferior borders as determined by MER. If stimulation induced tinnitus loudness modulation (defined below) was observed at the original target, no further MER passes were made. If no significant tinnitus loudness modulation was observed, the DBS lead was removed and a second MER pass was performed along a parallel tract 5mm anterior or posterior to the original target within the caudate. The DBS lead was placed in the second tract, and macrostimulation was again performed. This process was repeated until a location in the caudate that produced tinnitus modulation with macrostimulation was identified or a maximum of three passes were made per hemisphere.
Bipolar macrostimulation was performed initially with the most distal contact (contact 0) set as the cathode and the most proximal (contact 3) set as the anode. On a tinnitus loudness numeric rating scale (NRS) that ranged from 0–10 (0 - no tinnitus, 5 – conversation level, 10 - jet engine), participants provided baseline values for both ears. Intraoperative use of the TFI, a 25 item validated instrument, to assess tinnitus was not feasible. The stimulation parameters of frequency, amplitude and pulse width were varied only one at a time in step-wise fashion and study participant were queried to assess for change in tinnitus loudness rating. A total two point change from baseline summed across both ears was used as the threshold to determine stimulation induced tinnitus loudness modulation. The receiver operating characteristics of this change in tinnitus loudness to change in tinnitus severity are: sensitivity = 0.84 and specificity = 0.38.3 Tinnitus loudness modulation effects typically lasted no more than 1 minute following return of stimulation amplitude to the lowest setting (≤ 2 Volts), which was performed without subject knowledge. DBS electrode locations within the caudate nuclei were transformed from anatomical coordinates to normalized Montreal Neurological Institute (MNI) brain template coordinates for subsequent analysis.
Resting-State fMRI Data Acquisition
Data were collected using a GE Discovery 3.0T magnetic resonance imaging scanner (General Electric Healthcare MR, Waukesha, WI). Participants underwent both a high-resolution FSPGR BRAVO anatomical T1 (0.5mm × 0.5mm, TR=7ms, TE=3ms) and a resting-state EPI scan (1.88mm × 1.88mm, 3.0mm slice thickness, TR=2000ms, TE=28ms, 100 repetitions). Data were preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/spm12) and functional connectivity metrics were estimated using the CONN toolbox (http://www.nitrc.org/projects/conn).
Data Preprocessing
Resting-state fMRI data was spatially pre-processed and EPI images were spatially realigned to a mean image and coregistered with the T1 image for each individual subject using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Preprocessing with the default pipeline in the CONN functional connectivity toolbox included functional realignment and unwarp, slice-timing correction, structural segmentation and normalization, functional normalization, ART-based functional outlier detection and scrubbing, and functional smoothing with an 8-mm Gaussian kernel in MNI space.
Seed Definition
Seed regions were generated using the MarsBar Matlab toolbox (http://marsbar.sourceforget.net). A 5mm radius sphere was centered on a region of interest (ROI) defined by the average X,Y,Z coordinates of 1) the two left posterior DBS electrode locations that resulted in decreased tinnitus loudness, 2) the two right DBS electrode locations that resulted in decreased tinnitus loudness, 3) the nine left DBS electrode locations that did not result in decreased tinnitus loudness, and 4) the six right DBS electrode locations that did not result in decreased tinnitus loudness, for a total of four seed ROIs. The one left anterior DBS electrode location that resulted in decreased tinnitus loudness was treated as an outlier and was not included in the generation of seed regions.
Functional Connectivity Analysis
The CONN toolbox was used for functional connectivity analysis. Seed-to-voxel analysis was carried out to compare the positive contrast of functional networks connected to the more posterior caudate seed generated from DBS locations that resulted in decreased tinnitus loudness and the more anterior caudate seed generated from DBS locations that did not result in decreased tinnitus loudness. The analyses were performed separately for the right and left hemispheres. Thresholds for differences were set at p<0.05 with an additional cluster correction threshold set at p<0.05 using a false discovery rate correction.
Results
Tinnitus Loudness Modulation with Caudate Stimulation
In the 6 participants who underwent DBS device implantation, acute tinnitus modulation by DBS electrode macrostimulation was assessed in 12 locations of the left caudate and 8 locations of the right caudate nuclei. All intraoperative induced changes to tinnitus loudness perception returned to baseline values within 1 minute of returning stimulation amplitude to the lowest level. The number of DBS electrode passes in the left and right hemispheres ranged between 1 and 3 and the stimulation parameters of frequency, pulse width, and amplitude varied widely (Table 2). Hearing loss profile was asymmetrical in 4 participants (U01–02, −03, −04, and −06) with tinnitus loudness rated higher in the poorer ear in 3 of 4 and symmetrical in 2 participants (U01–10, −12) with tinnitus loudness rated at the same level (Table 3), an expected finding.22 Reports of tinnitus loudness modulation, defined as a total 2 point change from baseline summed across both ears, or change in tinnitus sound quality from awake participants during caudate nucleus mapping procedures guided final DBS electrode placement for long-term, chronic stimulation.9 During acute macrostimulation, 4 participants (U01–02, −04, −10, and −12) reported decreased tinnitus loudness at specific stimulation parameters (Table 3). One of these 4 participants, U01–12 also reported increased tinnitus loudness. Of the remaining 2 participants, U01–06 reported no change in tinnitus loudness and U01–03 reported only increased tinnitus loudness.
TABLE 2.
Intraoperative Caudate Nucleus Stimulation Parameters
| Study Participant | Number of Electrode Passes on Left | Number of Electrode Passes on Right | Frequency (Hertz) |
Pulse Width (microseconds) | Amplitude (Volts) |
|---|---|---|---|---|---|
| U01–02 | 1 | 1 | 10–180 | 50–200 | 1–10 |
| U01–03 | 3 | 1 | 10–150 | 60–450 | 2–10 |
| U01–04 | 2 | 2 | 10–150 | 60–100 | 2–10 |
| U01–06 | 3 | 1 | 10–250 | 90–210 | 2–10 |
| U01–10 | 1 | 1 | 10–150 | 90–200 | 2–10 |
| U01–12 | 2 | 2 | 10–250 | 90–150 | 2–10 |
TABLE 3.
Acute Tinnitus Loudness Modulation by Caudate Nucleus Stimulation
| Study Participant | Hearing Loss Profile by Ear | Baseline Loudness | Lowest Rating | Highest Rating |
|---|---|---|---|---|
| U01–02 | Mild L | 8 L 0 R |
5 L 0 R |
8 L 0 R |
| Normal R | ||||
| U01–03 | Severe L | 6 L 6 R |
6 L 6 R |
8.5 L 8 R |
| Moderate R | ||||
| U01–04 | Mild L | 7 L 6 R |
7 L 3 R |
7 L 6 R |
| Normal R | ||||
| U01–06 | Severe L | 7 L 6 R |
7 L 6 R |
7 L 6 R |
| Moderate R | ||||
| U01–10 | Moderate L | 8 L 8 R |
5 L 2 R |
8 L 8 R |
| Moderate R | ||||
| U01–12 | Severe L | 7 L 7 R |
6 L 6 R |
8 L 8 R |
| Severe R |
Tinnitus Loudness Numeric Rating Scale (0 - no tinnitus, 5 – conversation level, 10 - jet engine). L – Left ear. R – Right ear.
Caudate Subdivisions and Tinnitus Loudness Modulation
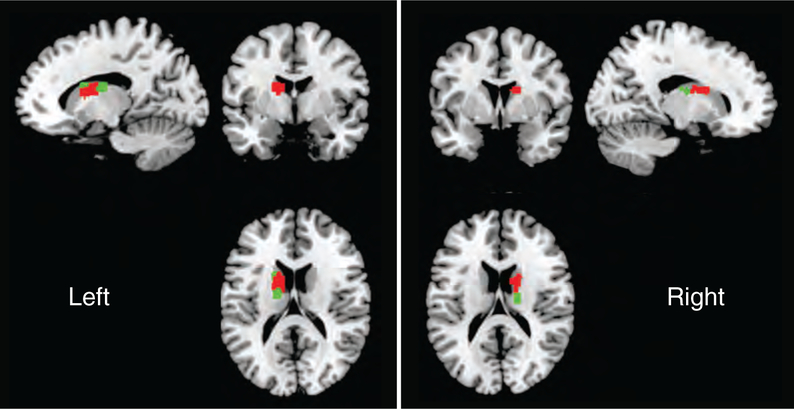
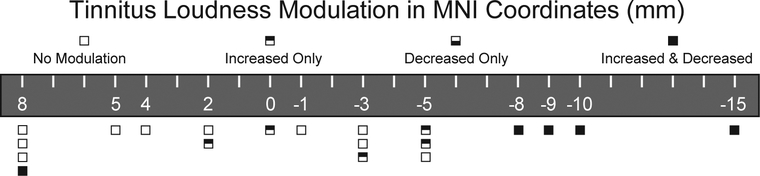
Macrostimulation at 5 DBS electrode locations resulted in decreased tinnitus loudness. The remaining 15 electrode locations resulted in either no change or increased tinnitus loudness. Four of the 5 electrode locations that resulted in decreased tinnitus loudness were positioned more posteriorly in the caudate body, whereas all 15 locations that did not result in decreased tinnitus loudness were located anteriorly, toward the caudate head. Electrode positions in the left and right caudate nuclei in MNI space with color coding of stimulation locations that resulted in tinnitus loudness reduction (green) and without tinnitus reduction (red) are displayed in Figure 1. By collapsing right and left hemispheric caudate nucleus interrogation data, an anteroposterior map of the caudate nucleus for tinnitus modulation can be constructed. The caudate nucleus head is anterior (positive) and body is posterior (negative). Combined decreases and increases in tinnitus loudness modulation are strongly clustered for MNI coordinates in the caudate body subdivision, between −8mm and −15mm (Figure 2).
FIG. 1.
All twenty locations of deep brain stimulation electrode placement with macrostimulation are displayed in MNI space. Caudate nucleus locations with tinnitus loudness reduction (green) and without tinnitus reduction (red) are displayed. Within the caudate head, there is 1 location with tinnitus loudness reduction and 15 locations without.
FIG. 2.
Anteroposterior map of the caudate nucleus for tinnitus modulation. Caudate head is anterior (positive, left) and body is posterior (negative, right). Data are aggregated from both hemispheres. Outcome of tinnitus loudness interrogation at each AP coordinate is coded by a box. Increase and decrease in tinnitus loudness modulation is more strongly expressed for MNI coordinates between −8 and −15 (caudate body). MNI – Montreal Neurological Institute.
Caudate Subdivisions and Resting-State fMRI Connectivity
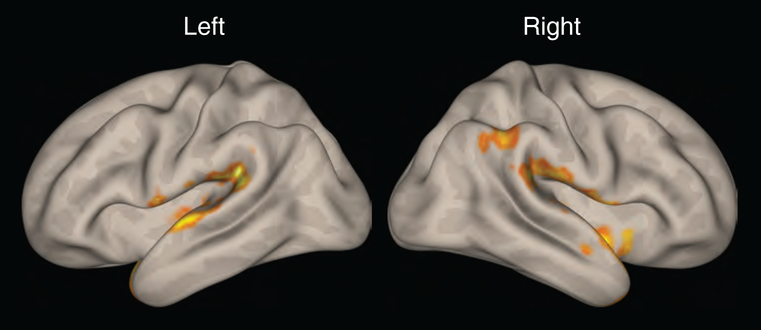
Short-term tinnitus loudness reduction derived from acute intraoperative stimulation experiments motivated a comparison of functional connectivity patterns between the caudate head and body subdivisions. For this analysis a total of 20 participants with chronic bothersome tinnitus defined by TFI > 50 were evaluated using the CONN toolbox. The seeded connectivity analysis was performed for both hemispheres. While the right hemisphere did not show a statistically significant difference, the left hemisphere revealed the more posteriorly positioned caudate body to have increased auditory corticostriatal functional connectivity to the left and right superior temporal gyri as shown in Figure 3.
FIG. 3.
Heat map display of functional connectivity of left posterior caudate body seed compared to left anterior caudate head seed. The left caudate body demonstrates increased auditory corticostriatal functional connectivity with both superior temporal gyri. Positive contrast performed using second level analysis in CONN toolbox, with height threshold of p<0.05 and cluster correction threshold of p<0.05, using a false discovery rate correction.
Discussion
By identifying a subdivision of the caudate nucleus in which acute electrical stimulation led to decreased tinnitus loudness and employing fMRI techniques to show that the caudate body has increased functional connectivity to auditory cortex, the results of this research relates tinnitus perceptual data to functional neuroanatomy. This first-in-human striatal mapping study provides further insight into the corticostriatal networks that are involved in chronic bothersome tinnitus and enables more precise targeting for clinical intervention.
Functional connectivity between the caudate nucleus and auditory cortex in chronic tinnitus is supported by related studies in animals. In monkeys, there are corticostriatal connections between the body of the caudate and the superior temporal cortex, secondary auditory cortex, and the superior temporal gyrus.19,26 In cats, similar anatomical connections have been identified.17 More recently, Ahsan et al. (2017) evaluated DBS of the caudate nucleus for tinnitus modulation in rats.1 In that study, the behavioral assay for tinnitus was a gap-detection acoustic startle reflex. Electrical stimulation of the rat caudate nucleus, whose subdivisions are substantially not as well defined compared to primates, modulated tinnitus for up to five days and induced temporary disruption of synchronous striatal and auditory cortical activity. Tinnitus animal model studies permit certain levels of analyses that cannot be practically performed in humans. However, there remains an essential need for human studies to evaluate tinnitus modulation beyond go no-go auditory phantom detection, such as modulation of tinnitus percept sound quality.9
Functional connectivity studies in humans using fMRI have shown unique patterns of connectivity that differentiate anterior and posterior aspects of the caudate nucleus. However, the functional consequences of these differential patterns of connectivity on tinnitus modulation have been unclear to date. In our study, we found that posterior region of the caudate, corresponding to the body subdivision, modulated tinnitus loudness more consistently to electrical stimulation. This finding is consistent with results from Jung et al. (2014)8 who found that posterior clusters of the caudate show functional connectivity with medial temporal cortices, suggesting that the posterior aspects of the caudate may potentially play a greater role in tinnitus modulation.
The perceptual and connectional distinctions of caudate head and body subdivisions in tinnitus modulation have clinical implications. While the critical advantage of DBS is reversibility of stimulation-dependent outcomes and adjustability of therapy and target location to titrate benefits against side effects in an ongoing fashion, this neuromodulatory approach nonetheless requires implantation of hardware into the brain and skull, and obligates battery recharging or replacement surgeries to enable stimulation. A maintenance free alternative approach would be to lesion the caudate nucleus irreversibly, accepting unknown risks that may be small,16 motivated by case reports of vascular infarction involving the caudate nucleus that have conferred enduring tinnitus loudness reduction.10,11 Although a lesioning approach may be more attractive in terms of avoiding the risks associated with an implanted device, not enough known at the present time about the optimal target for intervention to pursue lesioning. It is also not clear yet if the target is actually the same from patient to patient. In both approaches, reversible and irreversible, treatment of medically refractory tinnitus by neuromodulation can perhaps be made more effective by targeting the caudate body between −5mm and −15mm in MNI space. This is a region where tinnitus loudness became softer or louder in response to electrical stimulation. With a chronically implanted electrode in place, optimization of DBS stimulation parameter selection would likely reduce or further reduce tinnitus loudness. Adoption of this method of caudate body targeting can be implemented by warping the structural MRI into MNI space, marking the target zone, and exporting the target zone back to the structural MRI. A further refinement in treatment target selection may be to reconstruct a personalized corticostriatal connectivity map to evaluate the most promising segment within the 10 mm target zone.
This study has several limitations. First, as a Phase I trial, acute intraoperative tinnitus modulation data were collected from 6 study participants. The physiological and connectional distinction between the head and body caudate subdivisions and possible left hemispheric lateralization in relatively severe chronic tinnitus will require replication data from Phase II and Phase III trials, where much larger cohorts will enable more detailed analyses. Still, confidence in the key finding is bolstered by the fMRI result of stronger caudate body auditory corticostriatal connectivity in a larger cohort of 20 participants with TFI > 50. Second, in addition to the anteroposterior analysis presented in this article, mediolateral and craniocaudal functional analyses may reveal further insights. The caudate tapers to a thin structure along its body and tail subdivisions. To address connectional distinctions in those other dimensions, smaller seeds in much larger cohorts will be required. Third, the relationship between acute tinnitus loudness modulation and long-term tinnitus severity outcome has not yet been determined. For those without acute tinnitus modulation, it may be possible that chronic stimulation of the caudate head could eventually reduce tinnitus severity through long-term circuit modification or act through indirect pathways.15 Treatment outcomes reporting of this Phase I trial at its conclusion will provide deeper insights into this and other findings.
Conclusions
Acute deep brain stimulation of the caudate nucleus in a small Phase I clinical trial cohort reveals auditory phantom neuromodulatory and functional connectivity distinctions between the head and body subdivisions. The posteriorly located caudate body more reliably results in short-term tinnitus loudness reduction. Compared to the caudate head, the caudate body has stronger functional connectivity to auditory cortex. These first-in-human findings provide important insight into the functional anatomy of caudate nucleus subdivisions and may inform future target selection in our basal ganglia-centric neuromodulation approach to treat medically refractory tinnitus.
Financial Support:
NIH-NIDCD 5 U01DC013029 (S.W.C.), Department of Defense W81XWH-13-1-0494 (S.W.C.), and Coleman Memorial Fund (S.W.C.).
Footnotes
Meeting presentation: Portions of this work were presented in abstract form and oral presentation at meetings of the American Society for Stereotactic and Functional Neurosurgery, Denver, Colorado, June 2–5, 2018 and The Triological Society, National Harbor, Maryland, April 18–22, 2018.
ClinicalTrials.gov Identifier:
Disclosures
Co-authors Paul S. Larson and Susan Heath have received honoraria from Medtronic, the company that manufactures the deep brain stimulation device used for this study.
References
- 1.Ahsan SF, Luo H, Zhang J, Kim E, Xu Y: An animal model of deep brain stimulation for treating tinnitus: A proof of concept study. Laryngoscope, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Cheung SW, Larson PS: Tinnitus modulation by deep brain stimulation in locus of caudate neurons (area LC). Neuroscience 169:1768–1778, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Dewyer NA, Kiringoda R, Kram YA, Chang JL, Chang CY, Cheung SW: Stapedectomy Effects on Tinnitus: Relationship of Change in Loudness to Change in Severity. Otolaryngol Head Neck Surg 153:1019–1023, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Gilles A, Van Hal G, De Ridder D, Wouters K, Van de Heyning P: Epidemiology of noise-induced tinnitus and the attitudes and beliefs towards noise and hearing protection in adolescents. PLoS One 8:e70297, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry JA, Dennis KC, Schechter MA: General review of tinnitus: prevalence, mechanisms, effects, and management. J Speech Lang Hear Res 48:1204–1235, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Hinkley LB, Mizuiri D, Hong O, Nagarajan SS, Cheung SW: Increased striatal functional connectivity with auditory cortex in tinnitus. Front Hum Neurosci 9:568, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman HJ, Reed GW: Epidemiology of tinnitus, in Snow JB (ed): Tinnitus: theory and management. Hamilton, ON: BC Decker, 2004, pp 16–41 [Google Scholar]
- 8.Jung WH, Jang JH, Park JW, Kim E, Goo EH, Im OS, et al. : Unravelling the intrinsic functional organization of the human striatum: a parcellation and connectivity study based on resting-state FMRI. PLoS One 9:e106768, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larson PS, Cheung SW: Deep brain stimulation in area LC controllably triggers auditory phantom percepts. Neurosurgery 70:398–405; discussion 405–396, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Larson PS, Cheung SW: A stroke of silence: tinnitus suppression following placement of a deep brain stimulation electrode with infarction in area LC. J Neurosurg 118:192–194, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Lowry LD, Eisenman LM, Saunders JC: An absence of tinnitus. Otol Neurotol 25:474–478, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Martinez C, Wallenhorst C, McFerran D, Hall DA: Incidence rates of clinically significant tinnitus: 10-year trend from a cohort study in England. Ear Hear 36:e69–75, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meikle MB, Henry JA, Griest SE, Stewart BJ, Abrams HB, McArdle R, et al. : The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear 33:153–176, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. : The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Pennartz CM, Berke JD, Graybiel AM, Ito R, Lansink CS, van der Meer M, et al. : Corticostriatal Interactions during Learning, Memory Processing, and Decision Making. J Neurosci 29:12831–12838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pross SE, Allen CA, Hong OS, Cheung SW: Willingness-to-accept Gamma knife radiosurgery for tinnitus among career San Francisco firefighters. Otol Neurotol 35:1026–1032, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Reale RA, Imig TJ: Auditory cortical field projections to the basal ganglia of the cat. Neuroscience 8:67–86, 1983 [DOI] [PubMed] [Google Scholar]
- 18.Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA: Ringing ears: the neuroscience of tinnitus. J Neurosci 30:14972–14979, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selemon LD, Goldman-Rakic PS: Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci 5:776–794, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shargorodsky J, Curhan GC, Farwell WR: Prevalence and characteristics of tinnitus among US adults. Am J Med 123:711–718, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Syka J: Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev 82:601–636, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Tsai BS, Sweetow RW, Cheung SW: Audiometric asymmetry and tinnitus laterality. Laryngoscope 122:1148–1153, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Vio MM, Holme RH: Hearing loss and tinnitus: 250 million people and a US$10 billion potential market. Drug Discov Today 10:1263–1265, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Vogel I, Brug J, Hosli EJ, van der Ploeg CP, Raat H: MP3 players and hearing loss: adolescents’ perceptions of loud music and hearing conservation. J Pediatr 152:400–404, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Weisz N, Muller S, Schlee W, Dohrmann K, Hartmann T, Elbert T: The neural code of auditory phantom perception. J Neurosci 27:1479–1484, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeterian EH, Pandya DN: Corticostriatal connections of the superior temporal region in rhesus monkeys. J Comp Neurol 399:384–402, 1998 [PubMed] [Google Scholar]