Abstract
Passive exercise/movement has a long history in both medicine and physiology. Early clinical applications of passive exercise/movement utilized pneumatic and direct limb compression to stimulate the vasculature and evoke changes in blood flow to avoid complications brought about by stasis and vascular disease. Over the last 50 years, passive exercise/movement has continued to progress and has provided physiologists with a reductionist approach to mechanistically examine the cardiorespiratory, hyperemic, and afferent responses to movement without the confounding influence of metabolism that accompanies active exercise. This review, in addition to providing an historical perspective, will focus on the recent advancements utilizing passive leg movement (PLM), and how the hyperemic response at the onset of this passive movement has evolved from a method to evaluate the central and peripheral regulation of blood flow during exercise to an innovative and promising tool to assess vascular function. As an assessment of vascular function, PLM is relatively simple to perform and provides a nitric oxide (NO)-dependent evaluation of endothelial function across the lifespan that is sensitive to changes in activity/fitness and disease state (heart failure, peripheral artery disease, sepsis). The continual refinement and characterization of PLM is aimed at improving our understanding of blood flow regulation and the development of a clinically-ready approach to predict and monitor the progression of cardiovascular disease.
1. Introduction
Passive exercise/movement, simply defined as the manipulation of the body or a limb (e.g. leg) without voluntary effort or muscle contraction, has a long and rich history in medicine and physiology. This intentionally broad definition, encompasses insight gained from over 200 years of investigations utilizing exercise achieved without voluntary activation and contraction, pneumatic or direct compression of a limb, and passive movement of a limb over a given range of motion. Each of these unique uses of involuntary exercise/movement provide differing physiological insight, highlighting the clinical and investigative utility of this reductionist approach. This review will first provide a brief historical overview of how passive exercise/movement developed and was first used to treat abnormalities of the circulatory system. Next, the focus will shift to the utilization of passive exercise as a mechanistic approach to identify and understand reflex pathways involved in the cardiorespiratory response to active exercise. Finally, the majority of this review will concentrate on the recently developed body of literature examining the hyperemic and vasodilatory response to passive leg movement (PLM), and how this novel approach may provide a valid and reliable assessment of vascular function. The research presented will be predominantly focused on passive exercise/movement, however, several investigations providing valuable insight from pharmacological and mechanistic studies have been included to better elucidate the pathways regulating blood flow and rapid vasodilation.
2. Historical Perspective
The use of passive exercise is deeply rooted in vascular medicine. Indeed, the earliest documentation of such an approach being applied in practice dates back to 1812 when Sir James Murray applied the principles of altering atmospheric pressure through an “air bath” (pneumatic compression) to the whole body to elicit changes in the circulation [1]. Shortly thereafter, in the 1830’s, Junod and Clanney independently reported the use of negative pressure isolated to a limb to treat various diseases [2, 3]. In 1887, Bluck pioneered the use of rhythmically alternating atmospheric pressure “to bring about an influx and efflux of the blood in the part or parts effected” [4] and the first published investigation to use this approach to treat peripheral artery disease (technically thrombo-angiitis obliterans) was published in 1917 by Sinkowitz and Gottlieb [5]. Their conservative approach to improve hyperemia through suction resulted in alleviation of pain and more rapid healing of ulcerations in these patients. Reid and Herrman, in 1934, developed an apparatus termed the PAVAEX, standing for passive vascular exercise, to non-surgically treat PAD and reported improvements in circulation in patients with arteriosclerosis and foot ulcers [6-8].
More recently, in the early 1970’s, intermittent compressions and passive exercise began to be routinely used post-surgery to prevent early post-operative deep venous thrombosis (DVT) [9, 10]. In modern medicine, intermittent pneumatic compression (IPC) is now commonly used to prevent DVT and thromboembolism during periods of reduced activity such as recovery from surgery or acute hospitalization [11]. The application of mechanical forces to the limb alters pressure within arteries and veins resulting in antegrade acceleration of blood. Depending on the magnitude and pattern/rhythmicity of compression, peak velocity of blood in the veins may increase by up to 200% while increases in popliteal blood flow have been increased by nearly 100% [12-14]. Clearly, in terms of the cardiovascular system, the use of passive exercise has evolved significantly from its earliest form as an alternative to bloodletting to a standard treatment to prevent DVT. Through mechanistic pharmacological studies passive movement continues to be better understood and potential applications of this approach in clinical practice are still being developed.
3. Passive exercise: gaining insight into the reflex nature of the cardiorespiratory response to exercise
The notion that passive movement of the limbs evokes an increase in ventilation has been studied for well over 100 years with the first report on this topic dating back to 1888 by Geppert and Zuntz [15]. Utilizing a comprehensive amalgamation of various body movements including passive arm movement and passive leg pedaling Dixon et al. documented that ventilation increased in response to passive movement, however, the mechanism responsible for this increase remained unidentified [16]. Not long after, Flandrois et al., examined the ventilatory response to passive limb movement in lightly anesthetized dogs and reported a significant contribution of limb mechanoreceptors to movement-induced hyperpnea [17]. Collectively, these early studies emphasized that the ventilatory response to passive exercise is in excess of the increase in metabolic demand, independent of central command and, predominantly, involves a peripherally activated afferent-derived neural mechanism. The central and peripheral cardiovascular responses to passive exercise were largely ignored in these early respiratory-centric investigations.
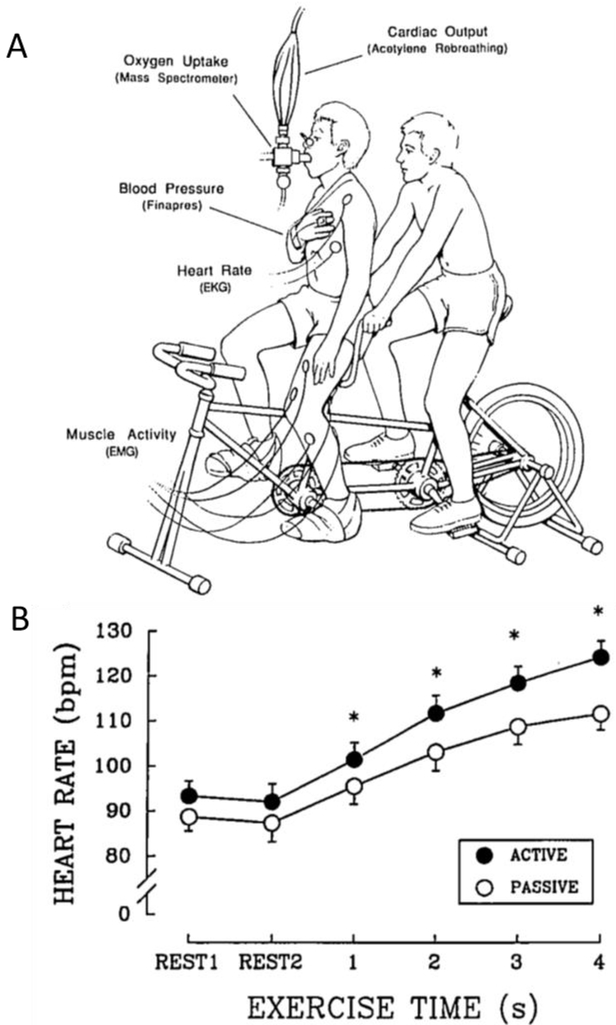
Utilizing a tandem bicycle ergometer, Nobrega et al. compared the cardiovascular response to active and passive cycling [18] (Figure 1). This creative model, in which two subjects sit on a tandem bicycle ergometer, allowed one subject, although undergoing the cycling motion, to remain completely passive while the other dictated their motion by performing dynamic cycling exercise. An advantage of this model is that it avoids the potentially confounding influence of pharmacological interventions and electrical stimulation which had been used previously to investigate the reflex nature of the cardiovascular response to exercise [19-27]. During passive exercise cardiac output (CO) increases as result of a modest, yet significant, increase in stroke volume (SV), while mean arterial pressure also increases [18]. Interestingly, heart rate (HR) did not change in this original passive cycling study [18]. This lack of cardioacceleration (i.e. an increase in HR) may have been the result of the timing of the HR measurements which were not performed until minutes 3 and 5 of the passive exercise. Previous work by Waisbren et al. reported significant cardioacceleration during the first minute of passive exercise which returned back to pre-exercise levels by the 5th minute of exercise [28]. Similarly, Nobrega and Araujo reported rapid cardioacceleration during 4 sec of fast passive cycling [29] (Figure 1). In combination, these findings suggest a transient cardiovascular response to passive exercise that appears to be evoked by stimulation of group III mechanoreceptors.
Figure 1: Reflex-driven cardiovascular responses to passive exercise.
A) Experimental setup used by Nobrega and Araujo 1993 and Nobrega et al. 1994 illustrating adapted tandem bicycling for the assessment of reflex-driven cardiovascular responses to passive exercise. During the protocol, the subject being studied, on the front of the bicycle, remained relaxed while breathing through a mouthpiece. The rider on the back of the bicycle actively performed the exercise task. During passive exercise, oxygen uptake (mass spectrometer), cardiac output (acetylene rebreathing), blood pressure (finometer), heart rate (EKG), and muscle activity (EKG) were assessed. B) The heart rate response during 4 sec of active (filled circles) and passive (open circles) exercise was recorded, with a clear passive exercise-induced increase in HR being documented. Modified from Nobrega et al. [29].
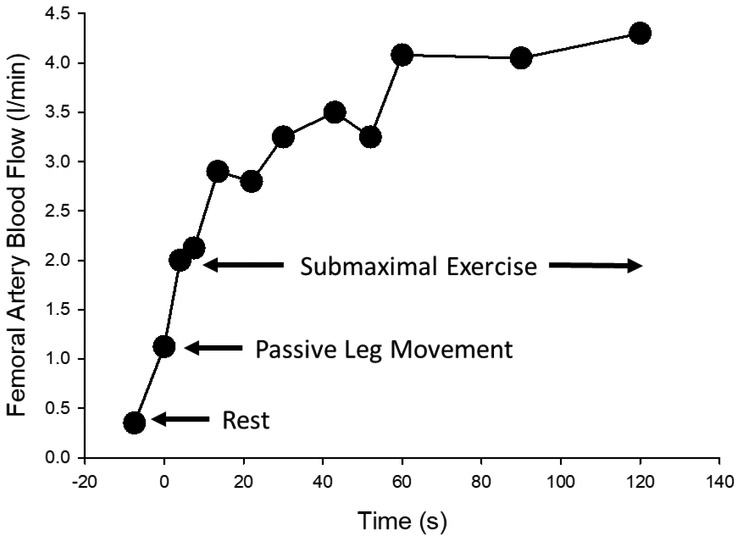
Despite promising clinical investigations that utilized passive exercise to augment blood flow and mitigate the development of DVT after surgery [9, 10], the peripheral hemodynamic response to passive exercise remained largely unexplored until the late 1990’s when Radegran and Saltin sought to determine the temporal relationship between blood flow, blood pressure, and muscle contractions during exercise [30]. Although not the primary focus of these investigations, passive exercise was performed prior to dynamic single leg knee extension and increases in blood flow were observed upon the first passive movement (Figure 2). Blood flow increased 3.3 fold above baseline values and peaked 4–5 duty cycles after the onset of movement. In retrospect, given the long history of investigation with respect to the vascular effect of mechanical compression (reviewed above) it is not surprising that passive exercise, which results in mechanical deformation of the vasculature and skeletal muscle, resulted in a significant increase in blood flow. Nevertheless, this was an interesting observation that inadvertently ignited a plethora of research aimed at understanding both the interplay between central and peripheral hemodynamics during passive exercise and the mechanisms contributing to the regulation of blood flow during exercise when metabolism is removed from the equation.
Figure 2: First evidence of peripheral hemodynamic response to passive exercise/movement.
Femoral artery blood flow (ml/min) at rest, during passive leg exercise/movement, and during submaximal single leg knee-extension exercise. Blood flow was measured by Doppler ultrasound during the transition from baseline (no movement) to 60 rpm, which was accomplished within 5 to 7 passive leg movements. Although not the focus of this investigation, this is the first evidence of increased leg blood flow during passive leg exercise/movement. Modified from Radegran and Saltin 1999 [46].
4. Passive leg movement (PLM) as a reductionist approach to study exercise hemodynamics
During the transition from rest to active exercise several mechanisms act in concert to regulate the onset of exercise-induced hyperemia, including vasodilation, the exercise pressor response, and the skeletal muscle pump. As a result of active exercise, metabolism and O2 demand increase and are matched by a concomitant increase in blood flow and O2 delivery. This increase in metabolism dominates the regulation of blood flow making it difficult to ascertain which other vasodilatory pathways may be contributing to the exercise-induced hyperemia. In order to better understand the vasodilatory, central hemodynamic, and mechanical mechanisms contributing to this response, Wray et al. compared passive and active knee-extension exercise in seated and supine positions [31]. Passive exercise, by definition, is void of voluntary contraction and evokes only a minor increase in O2 consumption. This allows for the evaluation of exercise-induced hyperemia without an increase in metabolism. Additionally, facilitated by the supine position, in one trial, the leg was positioned above heart level to facilitate venous emptying and minimize the contribution of the skeletal muscle pump. During the transition from rest to passive exercise a transient cardioacceleration and hyperemic response were observed. It was concluded that passive movement resulted in a reduction in vascular resistance as a result of mechanical compression. Although cardioacceleration occurred it was assumed that CO was not changed in response to passive movement, however, it should be noted that SV and CO were not directly assessed. Gonzalez-Alonso et al., in an effort to further identify the role of mechanical factors in exercising hyperemia, performed passive exercise (knee extension) with and without external thigh compressions, and reported increases in LBF, HR, and SV, but not CO [32]. When external thigh compressions were superimposed on passive exercise the increase LBF was doubled without any impact on central hemodynamics. Collectively, these findings suggested that the peripheral hyperemic response to PLM was disassociated from the central hemodynamic response.
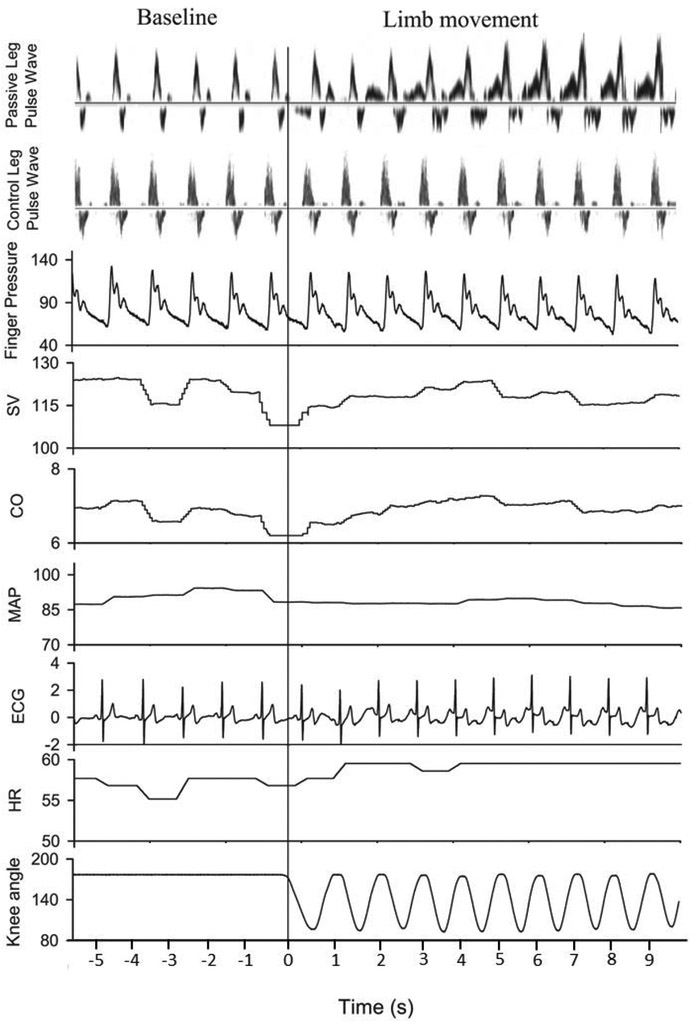
Building upon these studies, McDaniel et al. sought to determine the central hemodynamic response to PLM and paid particular attention to the transient nature of the movement-induced responses [33]. Notably, in an earlier study from our laboratory, it was observed that after 1 minute of continuous PLM, blood flow returned to baseline and it was postulated that a change in CO may follow a similar time course [31]. Using second by second assessment of peripheral and central hemodynamics (HR, SV, CO, and MAP), McDaniel et al. reported clear increases in LBF and CO during passive movement [33] (Figure 3). Interestingly, when blood flow to the passively moved limb was inhibited by suprasystolic compression around the thigh, the changes in HR, SV, and CO persisted, indicating a reflex-mediated central hemodynamic response to PLM. The transient nature of both the peripheral and central hemodynamic responses to PLM likely explains why previous investigations did not find an increase in CO and, in some instances, an increase in LBF [34], during passive exercise.
Figure 3: Temporal association between central and peripheral hemodynamics during passive leg movement (PLM).
Time-aligned sample tracing for central and peripheral variables during the transition from baseline (no movement) to PLM at a rate of 60 Hz. The vertical line occurs at time 0 signifying the onset of PLM, also confirmed by the change in knee angle (bottom plot). The top two plots are the Doppler intensity-weighted spectra representing blood velocity measured at the level of the common femoral artery in both the passively moved leg (top plot) and non-moved contralateral control leg (second plot). SV, stroke volume; CO, cardiac output; MAP, mean arterial pressure; HR, heart rate. Modified from McDaniel et al. 2010 [33].
Given the similar time course of central and peripheral hemodynamic responses to PLM, our group next sought to further examine the link between these responses by altering the communication between peripheral and central factors and by pharmacologically altering the magnitude of the peripheral feedback about the movement. Specifically, building upon earlier work utilizing anesthesia that identified a peripheral reflex driven central hemodynamic response to active exercise [35, 21, 27] we sought to inhibit this afferent feedback, from thinly myelinated group III/IV skeletal muscle fibers, and examine the impact on the central and peripheral response to PLM. In a group of healthy young men, fentanyl, a μ-opioid receptor antagonist, was administered via a lumbar intrathecal injection to inhibit the central projection of group III/IV afferent fibers [36]. Following fentanyl administration, both the central and peripheral hemodynamic responses to PLM were attenuated. Specifically, in healthy young individuals the hyperemic response to PLM was reduced by ~ 30% while the magnitude and duration of the CO response was also attenuated by ~50%. Additional work by our group in patients with a spinal cord injury, where afferent feedback is absent, did not reveal an increase in HR and CO during PLM, further supporting the afferent link between movement and the central hemodynamic response [37].
Building upon the finding that the mechanoreflex contributes to the magnitude of the hemodynamic response to PLM, Ives et al. examined the role of sex, as a biological variable, on this response [38]. Normalizing for the smaller body surface area and leg volume of the women revealed a similar peripheral hemodynamic responses to PLM between men and women. However, the central hemodynamic response in women remained attenuated suggesting a sex-specific attenuation of the mechanoreflex. Interestingly, and in contrast to our findings in healthy young individuals, afferent blockade in individuals with mechanoreflex hypersensitivity, such as heart failure, evokes an augmented, rather than attenuated, hyperemic response to PLM [39]. Together, these studies reveal a significant role of the mechanoreflex in the central and peripheral hemodynamic responses to PLM.
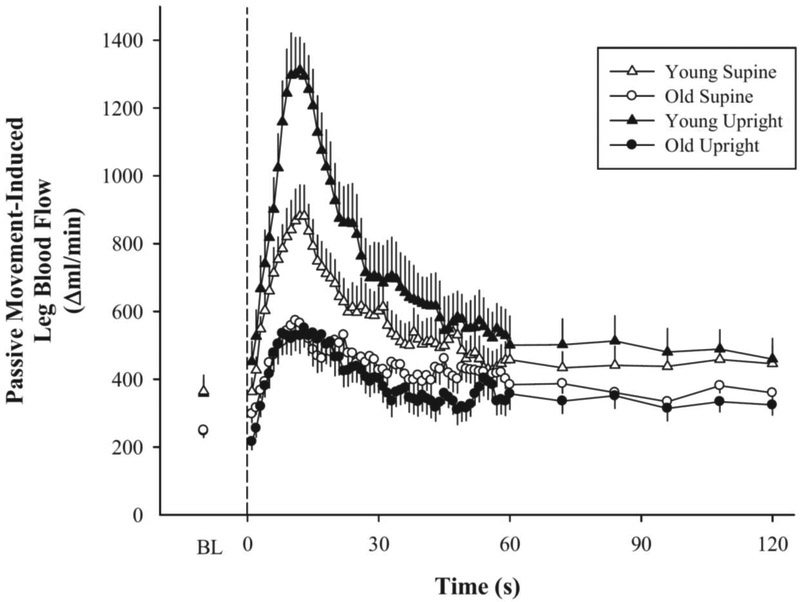
With the recognition that pressure (specifically, the arterial to venous pressure difference) is a critical component of blood flow, according to Ohm’s law, we next compared PLM in the upright seated and supine positions, as a means to manipulate perfusion pressure [40]. In the upright seated position the increase in blood flow during PLM was markedly higher than the supine position, interpreted to be consequence of the elevated perfusion pressure, due to the increased hydrostatic pressure afforded by the upright position (Figure 4). Although the mechanical stimulus (i.e. movement of the leg) was identical between body positions, the upright position magnified the hyperemic response by setting in motion a series of cascading events leading to greater vasodilation, including increased shear stress and subsequent increase in nitric oxide (NO) bioavailability. Overall, these findings provide a rather compelling evidence of a strong link between the peripheral and central hemodynamic responses to PLM.
Figure 4: Comparison of the hyperemic response to upright and supine passive leg movement (PLM).
Leg blood flow (ml/min) was measured in the common femoral artery by Doppler Ultrasound with participants in the supine or upright seated positions. In young individuals the upright seated position resulted in an augmented leg blood flow response to PLM. Interestingly, and in contrast to the young, healthy old individuals did not exhibit a difference in the leg blood flow to PLM between the supine and seated positions. Modified from Groot et al. 2015 [52].
5. Mechanistic studies to better understand and further identify central and peripheral components
The vasodilatory and subsequent hyperemic response to PLM is robust and provides a promising model to assess how factors such as aging and disease impact the regulation of blood flow. During active exercise aging is associated with a reduction in leg blood flow even after correcting for age-related differences in skeletal muscle oxidative capacity and differences in absolute work rates [41, 42] . By comparing young and old subjects during PLM we were able to remove the confounding influence of exercise metabolism and observed that, with advancing age, the PLM-induced hyperemic and vasodilatory responses were reduced by ~ 30 to 50% in the supine position [43]. Older subjects also exhibited attenuated central hemodynamic responses, further linking the peripheral and central responses during PLM.
The link between central and peripheral factors becomes more apparent when considered in the context of the innovative study of Bada et al. that utilized atrial pacing to alter HR during active exercise and further characterized the cause and effect relationship between central and peripheral hemodynamics [44]. Specifically, by comparing the peripheral vasodilatory and CO responses during rest, exercise, and ATP infusion with and without atrial pacing, it became apparent that the magnitude of the CO response was dictated by the peripheral vasodilatory response and not vice versa. Thus, in the context of PLM, the LBF and CO responses are linked but it appears that the CO response does not dictate the peripheral response [45, 40]. Rather, the CO response serves to prevent a precipitous drop in BP which would be expected in response to peripheral vasodilation.
6. Nitric oxide studies of PLM
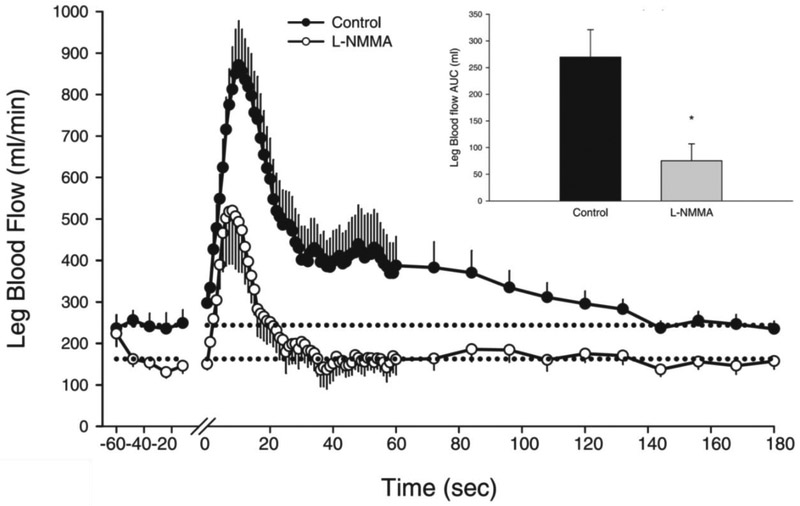
Although it was evident that the magnitude of the central hemodynamic response is associated with, but does not dictate, the peripheral response to PLM, the vasodilatory factors contributing to the robust increase in LBF remained largely unexplained. As previously described, Radegran and Saltin, in their attempt to determine the temporal relationship between blood flow, blood pressure, and muscle contraction during exercise, reported an increase in LBF during passive exercise [30]. Shortly thereafter, these investigators examined the role of NO as a regulator of vasomotor tone in skeletal muscle during the transition from rest to exercise [46]. Interestingly, and in contrast to more recent work, the inhibition of nitric oxide synthase (NOS) with NG-Methyl-L-arginine (L-NMMA) was reported to have no effect of LBF during passive exercise. While, as published, the exact timing of the LBF measurements were not entirely clear in this experiment, it is now apparent that the assessment of LBF was likely made after the initial peak response, thereby missing any impact of NOS inhibition. Indeed, with greater temporal resolution employed to assess LBF, combined with direct intra-arterial infusion of L-NMMA to inhibit NOS, Trinity et al. [40] and Mortensen et al. [45] independently, but in a similar time frame, reported that up to 80% of the overall increase in LBF during PLM was NO-dependent in healthy young individuals (Figure 5). Interestingly, the central hemodynamic response remained unchanged during NOS inhibition, again, indicating that peripheral factors, predominantly NO in healthy young people, dictate the hyperemic response to PLM.
Figure 5: Contribution of nitric oxide to the hyperemic response to passive leg movement (PLM).
Inhibition of nitric oxide synthase was accomplished by the local intra-arterial infusion of L-NMMA (NG-monomethyl-L-arginine) into to common femoral artery. L-NMMA diminished the absolute leg blood flow (LBF, ml/min) and the overall hyperemic response measured by the LBF area under the curve (ml) during the first 60 sec of PLM (inset). One minute of baseline (resting) data was collected before PLM and the transition from rest to PLM occurred at time 0 on the x-axis. Modified from Trinity et al. 2012 [40].
As aging is associated with vascular dysfunction [47-50], our group performed an initial study which included older subjects and documented an age-related reduction in hyperemia during PLM [43]. To confirm the role of NO in these age-related findings and advance our understanding of the potential clinical role of PLM, we performed a series of studies investigating the role of NO in the PLM-induced hyperemic response in young and old individuals. Specifically, Trinity et al. [51] reported that NOS inhibition in healthy older individuals had an insignificant impact on the already diminished hyperemic response to PLM. Groot et al. [52] expanded upon this finding and reported that older subjects, unlike the young, failed to augment their LBF response to PLM when in the upright seated position compared to being supine (Figure 4). By comparing supine and upright LBF responses, it was determined that an NO-dependent vasodilatory reserve is present in the young, but not the old. Temporal analyses, focused on the immediate vasodilatory response to PLM with and without NOS blockade, revealed that the accelerated rise in LBF in the young compared to old in the upright position is predominantly a consequence of greater NO bioavailability in the young. Although the invasive mechanistic PLM studies have been limited to men, women exhibit the same age-related attenuation in PLM-induced hyperemia [53], thus bolstering the utility of PLM as a novel assessment of vascular function across the life span in humans.
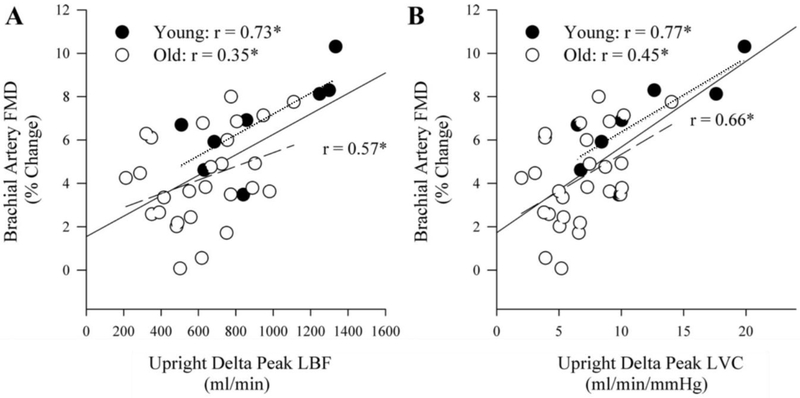
Investigations comparing PLM to more established methods of assessing vascular function, including the invasive infusion of ACh [45] and the technically challenging flow mediated dilation technique (FMD) [54], support the premise that PLM is a valid approach to determine vascular function and NO bioavailability. Specifically, Mortensen et al. [52] reported a significant positive relationship between PLM-induced hyperemia and the LBF response to the intra-arterial infusion of acetylcholine (ACh) (r = 0.84, p < 0.05), an established and direct approach to assess endothelial dependent vascular function. While Rossman et al. [54] directly compared the hyperemic and vasodilatory response to PLM with brachial artery FMD and also reported significant positive relationships between these two measures (Figure 6). Additionally, in another investigation by Walker et al. [55], the reactive hyperemic response following occlusion of the popliteal artery was positively correlated with the PLM-induced blood flow response. These relationships, particularly between BA FMD and PLM, are important as numerous large scale trials have identified BA FMD as a predictive tool to assess cardiovascular disease (CVD) risk [56-59]. Overall, these findings support the use of PLM as a novel approach to assess NO-mediated vascular function.
Figure 6: Relationship between passive leg movement (PLM) and flow-mediated dilation (FMD).
Correlations between the A) hyperemic response to PLM (Δ ml/min) and B) vasodilatory response to PLM, measured by change in leg vascular conductance during PLM (Δ ml/min/mmHg), to FMD assessed in the brachial artery (% change in dilation). For these comparisons PLM was performed in the upright seated position. The solid lines represent the correlation for the whole group, while the dotted and dashed lines represent the correlations for just the young and old groups, respectively. All correlations were significant at p < 0.05. Modified from Rossman et al. 2016 [54].
It should be noted that, with the exception of a single study [60], the evaluation of hyperemia in response to PLM has been predominantly limited to the leg, however, limb differences in vascular function have been recognized with other approaches and may be impacted by both aging and disease [61, 62]. Importantly, age-associated vascular dysfunction, assessed by FMD, persists when assessed in the leg compared to the arm [61], suggesting that the vasculature of the leg is at least as indicative of overall vascular health as measurements made in the arm. Passive limb movement in the arm, with movement occurring at the elbow, induces a robust and transient hyperemic response similar to that observed in the leg. Compared to the leg, the absolute blood flow response during arm PLM is lower, however, following normalization for the smaller volume of the arm, the relative change in blood flow between the limbs is actually quite similar [60]. However, it is currently unknown if the hyperemic response to arm PLM is affected by age, disease, or NO-bioavailability. Future research addressing these knowledge gaps is warranted to both assess potential limb differences and determine the utility of arm PLM.
7. PLM-induced vasodilation: going beyond NO
Although the overall PLM-induced hyperemic response is largely NO-mediated (~80%) in healthy young individuals, a significant blood flow response persists following NOS inhibition in this population [40]. Furthermore, older individuals exhibit a significant movement-induced hyperemia, albeit attenuated compared to the young, which is minimally impacted by NOS blockade [52, 51]. Clearly, other vasodilatory pathways, in addition to NO, must be contributing to these responses. A number of studies have found important vasodilatory roles for several shear stimulated endothelial-dependent vasodilators including prostaglandins (PG) and endothelium derived hyperpolarizing factor (EDHF) resulting from the metabolism of arachidonic acid by cyclooxygenase and cytochrome P450, respectively [63-68]. Interestingly, PG appears to contribute to the heterogeneity of vasodilation in men and women [68] while EDHF-mediated control of vascular tone is prevalent in conditions of attenuated NO bioavailability that typically accompanies aging and cardiovascular disease such as hypertension [63, 64]. Although investigations into the mechanisms responsible for these compensatory or redundant vasodilatory pathways have yet to be performed with PLM, several studies employing single contraction, occlusion, or compression have been performed with the inhibition of multiple vasodilatory pathways. The hyperemic response to PLM and a single contraction are remarkably similar in regards to the transient nature of the response and the role of NO [69] (Figure 7), suggesting that findings obtained during a single contraction may help elucidate other, yet unidentified, mechanisms contributing the PLM-induced hyperemia.
Figure 7: Comparison of vasodilatory response to passive leg movement (PLM) and single forearm contraction.
A) Vasodilatory response (measured as leg vascular conductance) to PLM in young and old subjects with and without L-NMMA to inhibit nitric oxide synthase. B) Vasodilatory response (measured as change in forearm vascular conductance) over 30 cardiac cycles following a single forearm contraction at 40% of maximal voluntary contraction. Despite differing modes of movement (i.e. single contraction versus passive movement) and limb differences (arm versus leg), the transient nature of the vasodilatory response, age-related reduction, and impact of L-NMMA is remarkably similar between studies. Modified from Groot et al. 2015 [52] and Casey et al. 2013 [69].
During the transition from rest to movement, or exercise, the increase in blood flow is rapid, occurring after the first cardiac cycle. Identifying factors governing this response has proven to be a difficult task, however, neural mechanisms and the muscle pump do not appear to be contributing to this rapid vasodilation [70-74]. Moreover, metabolically derived vasodilators are unlikely to account for this response given the rapid onset [75], therefore, muscle and/or endothelial-derived agents are likely playing a primary role. Hamann et al. [76] documented that, somewhat intuitively, without vasodilation the blood flow response to muscle contraction is nearly abolished. At the onset of exercise, the appearance of potassium (K+) in venous blood and the blood flow response follow a nearly identical time course, suggesting that K+ derived from the muscle may be involved in rapid vasodilation [77, 78]. In support of this link, animal models utilizing isolated muscle preparations have identified a critical role of K+ in rapid contraction induced vasodilation [79]. Indeed, the infusion of K+ to elevate the K+ concentration results in a depolarized membrane potential which renders any vasodilatory agent acting through hyperpolarization incapable of evoking a vasodilatory response [80]. Through the inhibition of multiple K+ channels, including the K+ voltage dependent, Na+ K+ ATPase, and inwardly rectifying K+ channels (KIR) Armstrong et al. revealed that K+ is a significant contributor to the rapid single contraction induced vasodilation [81].
Building upon these animal studies, Dinenno and colleagues [82-84] performed several elegant human-based pharmacological studies examining mechanisms responsible for rapid vasodilation in the arm following compression, occlusion, and contraction. Combined blockade of NO, PG, and K+ stimulated vascular smooth muscle hyperpolarization with BaCl2 and oubain nearly eliminated the vasodilatory response to a single contraction, especially at the lower intensities of muscle contraction (10% and 20% MVC) [82]. These investigators also reported that following 5 minutes of ischemia, reactive hyperemia, a predominantly microvascular response, was completely abolished when K+ channels were blocked, whereas there was no effect of combined NO and PG inhibition [83]. Given the documented relationship between the microvascular response, vascular health, and cardiovascular disease risk, these findings indicate that factors beyond the endothelium, involving the regulation of vascular hyperpolarization, may be critical in understanding how aging and disease fundamentally impact vascular function. Future investigations examining the role of K+ channels in the PLM-induced hyperemic response are warranted to further identify the vasodilatory mechanisms contributing to PLM-induced vasodilation, potentially increasing the utility of PLM for clinical diagnosis and the assessment of vascular health.
Adenosine triphosphate (ATP) is a potent vasodilator released in response to compression of the vasculature and, to a lesser extent, flow-mediated shear stress. ATP-mediated vasodilation occurs independent of traditional endothelial cell signaling including NO and PG and appears to act predominantly through KIR channels [85]. During PLM arterial and venous [ATP], assessed by intravascular microdialysis, did not change suggesting that ATP may not contribute to PLM-induced vasodilation [86]. However, a recognized limitation of intravascular microdialysis is the slow sampling rate which may not have been capable of detecting the rapid transient changes that likely occur during PLM. Despite these previous measurements, the role of ATP in PLM-induced vasodilation is currently unknown.
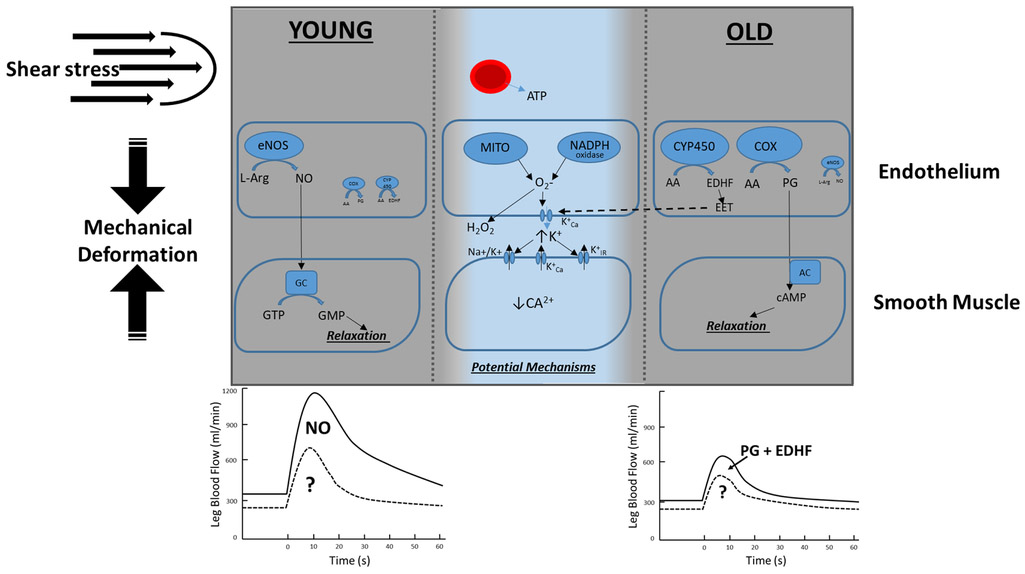
Taking into account the previous investigations focused on non NO pathways responsible for rapid vasodilation, it is apparent that the vasodilatory response to PLM, although highly NO mediated, likely requires a combination of endothelial dependent and independent mechanisms [87] (summarized in Figure 8). Further investigations, focused on factors other than NO that contribute to the PLM response, in both aged and diseased individuals, are currently underway.
Figure 8: Vasodilatory factors contributing to passive leg movement (PLM)-induced hyperemia.
PLM concomitantly increases shear stress and results in a mechanical deformation that serve as stimuli to trigger a cascade of vasodilatory events in the vasculature. In young healthy individuals a robust and transient increase in leg blood flow occurs immediately after the onset of PLM that is primarily NO-mediated (80%), as confirmed by several studies utilizing L-NMMA to inhibit nitric oxide synthase (NOS). Based on this high NO-dependency it is hypothesized that there will be a minimal contribution of other endothelial-derived vasodilators, including PG and EDHF, in young healthy individuals. In older individuals the magnitude of the hyperemic response is attenuated compared to the young and is not NO-mediated. Therefore, it is hypothesized that PG and EDHF will contribute substantially to the hyperemic response in older individuals. In both young and old, a variety of potential mechanisms maybe contributing to the remaining hyperemic response including but not limited to vasodilatory roles of adenosine triphosphate (ATP), potassium (K+), or hydrogen peroxide (H2O2) as previously reported for single contraction and in isolated vessels.
8. Impact of physical activity on PLM
PLM has been used by our group and others to assess vascular function in a multitude of individuals differing in levels of physical activity/fitness and in disease state [88, 53, 52, 89, 39, 33, 43, 90, 36, 51, 40, 37, 91-93]. As discussed previously, aging is associated with an attenuated hyperemic and vasodilatory response to PLM [88, 53, 43, 51]. Much of this age-related reduction appears to be related to diminished NO-bioavailability [88, 51] and physical activity restores the age-related reduction in vascular function assessed by FMD and intra-arterial ACh infusions [48]. With this as a backdrop, Groot et al. [88] compared the vasodilatory response to PLM in young sedentary, old sedentary, old active, and old exercise trained subjects. The reduction in PLM-induced vasodilation was graded with respect to activity/fitness level in the old individuals, such that, the least fit (i.e. old sedentary) exhibited the lowest PLM response. Additionally, the old sedentary group was the only group that failed to exhibit an increase in vasodilation, or vasodilatory reserve, when comparing the upright seated to supine PLM response, the magnitude of which is NO-dependent. Interestingly, despite having a higher absolute and relative maximal aerobic capacity (VO2max) than the young sedentary subjects, the PLM response in the old trained was lower than these young subjects, suggesting a persistent age-related attenuation in vascular function that is not completely reversed by physical activity/fitness.
9. Assessment of PLM in clinical populations: impact of disease
Vascular dysfunction is a hallmark characteristic of cardiovascular disease. Heart failure and heart transplant patients fall on the extreme end of the cardiovascular disease spectrum, exhibiting both central and peripheral hemodynamic dysfunction. Utilizing PLM, Witman et al. [92] compared heart failure (reduced ejection fraction, HFrEF) patients and healthy age-matched controls. The vasodilatory response to continuous PLM was reduced by nearly 90% in HFrEF patients compared to healthy age-matched controls. In an effort to partition the central and peripheral factors that contribute to PLM hemodynamics, a novel single PLM approach consisting of one cycle of movement through a 90⁰ range of motion and back which minimizes the central hemodynamic response, was also performed. As the reduction in PLM in the patients with HFrEF remained with single PLM, it was concluded that this difference was primarily driven by peripheral dysfunction and diminished NO bioavailability. It is interesting to note that, as a whole, HFrEF patients who fall on the extreme end of the cardiovascular disease spectrum, exhibit the most attenuated PLM response measured to date.
Peripheral artery disease (PAD) is a debilitating atherosclerotic condition resulting in diminished blood flow, muscle pain (intermittent claudication), and limb ischemia in severe cases. Furthermore, PAD is characterized by diminished NO bioavailability and vascular function [94]. Several investigations have reported a diminished PLM response in PAD patients [95, 45, 55]. Interestingly, elevated levels of NADPH oxidase, a key enzyme involved in the generation of superoxide in the vasculature, were reported in this population suggesting that oxidative stress may be contributing to the lower PLM response in PAD [55]. These clearly attenuated PLM-induced responses in patients with PAD, further support the clinical utility of this approach to assess NO-mediated vascular function [45, 40].
Sepsis, a systemic life threatening condition resulting from a complex interaction between the infecting organism and the host’s inflammatory, immune, and coagulation system, has major implications in terms of mortality rate. The pathophysiology of sepsis has a critical vascular component as toxic mediators evoke increased inducible NOS activity leading to a loss of vascular tone, vasodilatory capacity, and ultimate organ failure. Our group recently documented that upon initial diagnosis patients with sepsis exhibit an attenuated PLM response compared to well matched controls, indicative of vascular dysfunction [90]. By day 3, patient baseline femoral artery blood flow returned to levels observed in controls, likely a reflection of normalized CO and a resolution of the initial inflammatory response, however, the hyperemic response to PLM remained attenuated, indicative of vascular dysfunction [90]. Of note, FMD was also significantly attenuated in these patients, further corroborating the vascular dysfunction assessed by PLM.
Thus, the assessment of PLM-induced hyperemia and vasodilation in relation to physical activity and in clinical populations lends credence to the use of PLM as a novel approach to assess NO-mediated vascular function (Figure 9). It should be noted that direct inhibition of NOS has not been performed in patient groups, therefore, the substantial reduction in PLM-induced hyperemia and the role of NO in these clinical populations is inferred from studies in healthy young and older adults. Clearly, the hyperemic response to PLM is altered by both physical activity and disease state, whether chronic (heart failure) or acute (sepsis).
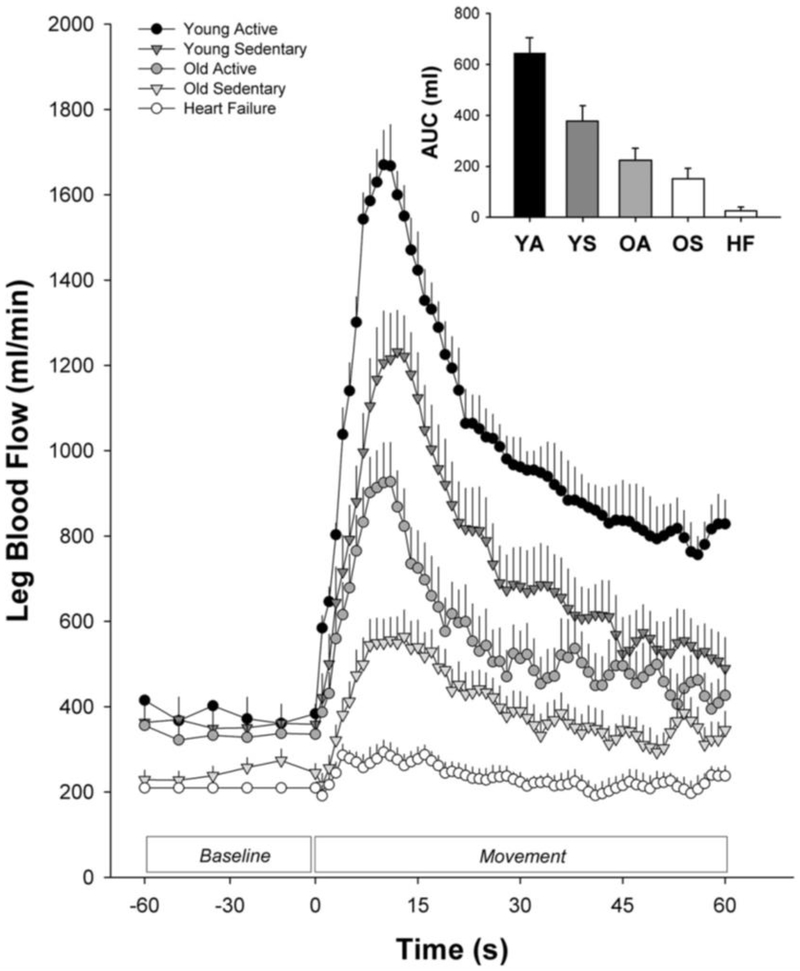
Figure 9: Passive leg movement (PLM)-induced hyperemia in groups typified by differing levels of cardiovascular health.
As the level of physical fitness/activity declines and the prevalence of cardiovascular disease risk factors increases the hyperemic response to PLM is impaired. YA, young physically active (n=5; JR Gifford, R. Broxterman, and RS Richardson, unpublished observations). YS, young sedentary (n=12, Groot et al. 2016 [88]). OA, old physically active (n=10, Groot et al. 2016 [88]). HF, heart failure (n=14, Witman et al. 2015 [92]). AUC, area under the curve.
10. Passive movement: beyond the transient hyperemic response
Up to this point, the discussion of PLM has focused primarily on the acute peripheral hemodynamic response, the factors regulating this response, and the alterations in this response that occur with aging, physical activity, and disease. Given the robust blood flow response, PLM has been used as a reductionist approach to better understand the vascular adaptations to active exercise, including increased capillarity and elevations in endothelial NOS (eNOS). Importantly, active exercise induces an increase in metabolic demand, an increase in blood flow, and passive stretching of the vasculature, all of which are associated with capillary growth and vascular adaptation. Therefore, during active exercise it is not possible to isolate the impact of increased blood flow (as well as the subsequent increase in shear stress) and the increase in mechanical stress on the vasculature from the increase in metabolism. Therefore, Hellsten et al. [96] performed 90 minutes of continuous PLM in healthy young participants resulting in increased blood flow, shear stress, and mechanical stress without an increase in VO2 and reported consequential increases in VEGF protein concentration, a 3-fold increase in endothelial cell proliferation rate, and a 4-fold increase in eNOS mRNA levels. In healthy older subjects and patients with PAD a similar, albeit slightly shorter, period of passive movement (60 versus 90 min), evoked a modest increase in VEGF and angiogenic factors [97]. This same group performed follow-up work utilizing a PLM training paradigm consisting of 4 weeks of PLM performed for 90 minutes, 4 times per week, to examine the angiogenic potential of chronically increasing shear and mechanical stresses [95]. After 2 weeks of PLM training, healthy young subjects displayed an increase in the number of capillaries around a fiber and an increase in endothelial cell proliferation. After 4 weeks, capillary density was increased and was associated with an increase in eNOS mRNA. Taken together, these findings reveal a potent angiogenic stimulus evoked by PLM. Additional research is warranted to determine if chronic PLM is effective at stimulating angiogenesis in aged and/or clinical populations characterized by vascular dysfunction. Somewhat surprisingly, the hyperemic response to PLM was not assessed following the 4 weeks of PLM training, therefore it is unknown if vascular function, per se, was improved in conjunction the improved angiogenic profile.
The impact of exercise training, or detraining, on the hyperemic response to PLM is currently under investigation. However, based upon previous findings, the hyperemic response to PLM is largely NO-dependent and appears to be altered by training status and level of physical activity [88, 45, 40]. Therefore, it would seem reasonable to expect that exercise training (or detraining) would result in improvements (or decrements) in the hyperemic response, reflecting changes in vascular function. Lepsen et al. [98] performed 8 weeks of either endurance or resistance exercise in patients with COPD and despite improvements in exercise capacity, vascular function, assessed by PLM and FMD was not improved. This 8 week training protocol did not evoke increased eNOS protein expression in the quadriceps indicating that the adaption to training may have been blunted in these patients. Additional investigations examining the ability of exercise training to remediate age and disease related reductions in PLM are required.
11. Comparison of PLM to other assessments of vascular function
Vascular dysfunction, defined as an inadequate vascular tone response to a given stimulus, often precedes CVD. Moreover, changes in vascular function predict CVD [56, 99, 58, 59]. Therefore, being able to simply and reliably assess vascular function is of critical importance in assessing overall health and avoiding cardiovascular events. Current strategies to assess vascular function include both invasive and non-invasive approaches such as: intra-coronary and intra-arterial infusion of ACh [100, 101], flow mediated dilation [50], and finger plethysmography [102, 103]. Excellent, in-depth reviews presenting the pros and cons of these approaches are available [104-106]. Here, these approaches will be briefly highlighted as well as the currently recognized advantages and limitations associated with PLM as an assessment of vascular function (Table 1).
Table 1:
Comparison of methods for assessment of vascular function
| Methods for the Assessment of Vascular Function | |||||||
|---|---|---|---|---|---|---|---|
| Technique | Noninvasive | Repeatable | Reproducible | Reflects Physiology | Remediable | Predicts Outcomes | Ease of Use |
| Direct Catheterization with Vasoactive Drug Infusion | − | + / − | − | + | + | + | Low |
| Flow-Mediated Dilation | + | + | + | Controversial | + | + | Moderate |
| Finger Plethysmography | + | + | + | − | − | + / − | Very High |
| Passive Leg Movement | + | + | + | + | + | Under Investigation | High |
Not long after the discovery that ACh evoked endothelial-dependent vasodilation, predominantly through NO in isolated arteries by Furchgott and Zawadski [101], investigators began administering ACh intra-arterially to directly assess endothelial function in-vivo. This clinical assessment was first performed in the coronary vasculature involving quantitative coronary angiography [107] and was later adapted to the peripheral circulation in combination with venous occlusion plethysmography to assess the blood flow response [108, 100]. Although limited in terms of routine clinical utility, due to the invasiveness and complexity (combined drug infusion and blood flow assessment) involved with this approach, a major advantage of direct drug infusion studies is that dose-dependent assessments of endothelial-dependent and endothelial-independent (sodium nitroprusside) vascular function can be assessed.
In an effort to circumvent the limitations associated with the assessment of vascular function utilizing drug infusions, Celermajer and colleagues [50] developed the FMD technique which employs reactive hyperemia, following peripheral circulatory occlusion with the direct assessment of conduit artery diameter by Doppler ultrasound, to non-invasively assess vascular function. Several reviews and tutorials provide an excellent overview of this approach [109, 110]. Briefly, the FMD technique results in a transient increase in shear stress and a subsequent vasodilation of the interrogated artery. FMD provides a useful and accessible method with which to study vascular function and has greatly advanced our understanding of vascular health and physiology. Most noteworthy, is the ability of FMD to predict the incidence of cardiovascular disease [105, 111]. Additionally, FMD assessed in the periphery, reflects coronary vessel function and, although highly variable, appears to at least partially, reflect NO bioavailability [112, 113]. The major limitation of FMD is in the technical application, as this approach requires advanced sonography skills and is highly dependent on the precise measurement of, often very minimal, changes in artery diameter.
Finger plethysmography (EndoPAT, Itmar Medical) is a relatively recently developed approach to assess endothelial function [102, 103]. Similar to FMD, an occlusion cuff is placed on the arm and the reactive hyperemia in the microvessels of the finger is measured following cuff release. A major advantage of this approach is that the technical requirements of FMD are removed, however, this technique is minimally reflective of NO bioavailability [114]. Additionally, the ability of finger plethysmography to reflect disease state is not clear as reports indicate that this technique failed to detect differences between healthy individuals and patients with renal impairment and type 2 diabetes [115] while others have reported that this approach predicts cardiovascular events [103, 116, 117] and is correlated with coronary microvascular function [118]. Interestingly, interventions with profound vascular consequences including smoking and glucose loading did not negatively impact finger plethysmography-assessed vascular function [115]. Unlike FMD, finger plethysmography reflects microvascular function, the plasticity of which may not be as malleable as conduit vessel function.
PLM is an easy to perform non-invasive assessment of vascular function that has been validated against intra-arterial infusion of ACh [45], FMD [54], and is up to 80% NO-dependent [45, 40]. A detailed description of the methodology and interpretation PLM was recently published by Gifford and Richardson [87]. Briefly, the participant is in an upright seated position and the leg is passively moved through a 90⁰ range of motion at 1 Hz by a member of the research team. During movement the change in blood flow through the common femoral artery is assessed by Doppler ultrasound and analyzed on sec by sec basis. Recent modifications to PLM have been examined in an effort to further simplify the procedure and improve the clinical utility of this technique. Most notably, a single movement of the leg, referred to as single PLM, has been reported to reflect NO-bioavailability while also diminishing the central hemodynamic response [91, 119]. Preliminary findings also indicate that single PLM is capable of clearly detecting age-related reductions in vascular function. During PLM, as the diameter of the common femoral artery remains constant, the hyperemic response is solely dependent upon the change in blood velocity and, therefore, the technical requirement for sonography is substantially less compared to FMD. Although the blood velocity is assessed at the level of the conduit artery, the hyperemic response during PLM is driven by downstream microvascular vasodilation. Importantly, and unlike finger plethysmography, this microvascular-centric response has been documented to be predominantly largely NO-mediated [45, 40]. As described earlier, the PLM-induced hyperemic (or vasodilatory) response is progressively attenuated as the number of cardiovascular risk factors is increased and, ultimately, reaches a nadir with the presence of CVD. Investigations examining the relationship between PLM and coronary vessel function and the ability of PLM to predict future CV events in clinical cohorts are underway.
12. Conclusion
In medicine, starting nearly 2 centuries ago, direct and pneumatic limb compression was applied to patients suffering from vascular abnormalities to aid in the healing process and similar practices continue today. In physiology, passive exercise/movement studies were instrumental in identifying the importance of feedback from peripherally located mechanosensitive and metabosensitive skeletal muscle afferent fibers in the cardiovascular response to exercise. However, the long and rich history of passive exercise/movement, originating in medicine and physiology, continues to evolve. Specifically, through a series of studies, performed by our group and others, the hyperemic response, induced by passive leg movement (PLM), is becoming recognized as a novel assessment of vascular function with real clinical relevance.
Key Points.
Passive exercise/movement provides a reductionist approach to mechanistically examine the cardiorespiratory, hyperemic, and afferent responses to movement without the confounding influence of factors associated with voluntary exercise.
Passive movement has evolved and continues to be refined as a clinically-ready assessment of vascular function.
Acknowledgements:
This work was supported by Veterans Affairs Rehabilitation Research and Development Career Development (IK2RX001215), Merit (E6910-R and E1697-R), Spire (E1433-P), Senior Research Career Scientist (E9275-L) awards, American Heart Association (14SDG18850039) and NIH Heart, Lung, and Blood Institute grant (HL-091830).
Footnotes
Conflicts of Interest: Joel D. Trinity and Russell S. Richardson declare they have not conflicts of interest.
References:
- 1.Murray J Nature and treatment of cholera–new method proposed. Lond Med Surg J. 1832;1:749–52. [Google Scholar]
- 2.Clanny WR. APPARATUS FOR REMOVING THE PRESSURE OF THE ATMOSPHERE FROM THE BODY OR LIMBS. Lancet. 1835;23(601):804–5. [Google Scholar]
- 3.Junod V-T. Recherches physiologiques et thérapeutiques sur les effets de la compression et de la raréfaction de l’air, tant sur le corps que sur les membres isolés. 1834. [Google Scholar]
- 4.Bluck E Improved means or appliances for promoting or modifying the circulation of the blood in a living body. London, Darling and Sons; 1888. [Google Scholar]
- 5.Sinkowitz S, Gottlieb I. THROMBO-ANGIITIS OBLITERANS: THE CONSERVATIVE TREATMENT BY BIER’S IIYPEREMIA SUCTION APPARATUS. JAMA. 1917;68(13):961–3. [Google Scholar]
- 6.Herrmann LG, Reid MR. The Conservative Treatment of Arteriosclerotic Peripheral Vascular Diseases: Passive Vascular Exercises (Pavaex Therapy). Ann Surg. 1934;100(4):750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrmann LG, Reid MR. Passive vascular exercises: treatment of peripheral obliterative arterial diseases by rhythmic alternation of environmental pressure. Arch Surg. 1934;29(5):697–704. [Google Scholar]
- 8.Reid MR, Herrmann LG. Treatment of obliterative vascular diseases by means of an intermittent negative pressure environment. J Med. 1933;14(June):200. [Google Scholar]
- 9.Roberts V, Sabri S, Pietroni M, Gurewich V, Cotton L. Passive flexion and femoral vein flow: a study using a motorized foot mover. Br Med J. 1971;3(5766):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabri S, Roberts V, Cotton L. Prevention of early postoperative deep vein thrombosis by intermittent compression of the leg during surgery. Br Med J. 1971;4(5784):394–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen A, Frangos S, Kilaru S, Sumpio B. Intermittent pneumatic compression devices–physiological mechanisms of action. Eur J Vasc Endovasc Surg. 2001;21(5):383–92. [DOI] [PubMed] [Google Scholar]
- 12.Morgan RH, Carolan G, Psaila JV, Gardner AMN, Fox RH, Woodcock JP. Arterial Flow Enhancement by Impulse Compression. Vascular Surg. 1991;25(1):8–16. doi:Doi 10.1177/153857449102500102. [DOI] [Google Scholar]
- 13.Nicolaides AN, Fernandes e Fernandes J, Pollock AV. Intermittent sequential pneumatic compression of the legs in the prevention of venous stasis and postoperative deep venous thrombosis. Surgery. 1980;87(1):69–76. doi: 10.5555/uri:pii:0039606080902731. [DOI] [PubMed] [Google Scholar]
- 14.van Bemmelen PS, Mattos MA, Faught WE, Mansour MA, Barkmeier LD, Hodgson KJ et al. Augmentation of blood flow in limbs with occlusive arterial disease by intermittent calf compression. J Vasc Surg. 1994;19(6):1052–8. doi: 10.1016/S0741-5214(94)70217-9. [DOI] [PubMed] [Google Scholar]
- 15.Geppert J, Zuntz N. Ueber die Regulation der Athmung. Archiv für die gesamte Physiologie des Menschen und der Tiere. 1888;42(1):189–245. doi: 10.1007/bf01669357. [DOI] [Google Scholar]
- 16.Dixon ME, Stewart PB, Mills FC, Varvis CJ, Bates DV. Respiratory consequences of passive body movement. J Appl Physiol. 1961;16(1):30–4. doi: 10.1152/jappl.1961.16.1.30. [DOI] [PubMed] [Google Scholar]
- 17.Flandrois R, Lacour JR, Islas-Maroquin J, Charlot J. Limbs mechanoreceptors inducing the reflex hyperpnea of exercise. Respir Physiol. 1967;2(3):335–43. doi: 10.1016/0034-5687(67)90038-2. [DOI] [PubMed] [Google Scholar]
- 18.Nobrega AC, Williamson JW, Friedman DB, Araujo CG, Mitchell JH. Cardiovascular responses to active and passive cycling movements. Med Sci Sports Exerc. 1994;26(6):709–14. [DOI] [PubMed] [Google Scholar]
- 19.Asmussen E, Johansen SH, Jorgensen M, Nielsen M. On the Nervous Factors Controlling Respiration and Circulation during Exercise. Experiments with Curarization. Acta Physiol (Oxf). 1965;63(3):343–50. doi: 10.1111/j.1748-1716.1965.tb04073.x. [DOI] [PubMed] [Google Scholar]
- 20.Asmussen E, Nielsen M, Wieth-Federsen G. On the Regulation of Circulation during Muscular Work. Acta Physiol (Oxf). 1943;6(4):353–8. doi: 10.1111/j.1748-1716.1943.tb02861.x. [DOI] [Google Scholar]
- 21.Galbo H, Kjaer M, Secher NH. Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. J Physiol. 1987;389(1):557–68. doi: 10.1113/jphysiol.1987.sp016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollander AP, Bouman LN. Cardiac acceleration in man elicited by a muscle-heart reflex. J Appl Physiol. 1975;38(2):272–8. [DOI] [PubMed] [Google Scholar]
- 23.Hornbein TF, Sorensen SC, Parks CR. Role of muscle spindles in lower extremities in breathing during bicycle exercise. J Appl Physiol. 1969;27(4):476–9. doi: 10.1152/jappl.1969.27.4.476. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto GA, Mitchell JH, Mizuno M, Secher NH. Cardiovascular-Responses at the Onset of Exercise with Partial Neuromuscular Blockade in Cat and Man. J Physiol. 1987;384(1):39–47. doi:DOI 10.1113/jphysiol.1987.sp016442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjaer M, Hanel B, Worm L, Perko G, Lewis SF, Sahlin K et al. Cardiovascular and neuroendocrine responses to exercise in hypoxia during impaired neural feedback from muscle. Am J Physiol. 1999;277(1):R76–85. doi: 10.1152/ajpregu.1999.277.1.R76. [DOI] [PubMed] [Google Scholar]
- 26.KJÆR M, PERKO G, SECHER NH, BOUSHEL R, BEYER N, POLLACK S et al. Cardiovascular and ventilatory responses to electrically induced cycling with complete epidural anaesthesia in humans. Acta Physiol (Oxf). 1994;151(2):199–207. doi:doi: 10.1111/j.1748-1716.1994.tb09738.x. [DOI] [PubMed] [Google Scholar]
- 27.Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH et al. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol. 1993;470(1):693–704. doi:doi: 10.1113/jphysiol.1993.sp019883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waisbren SJ, Whiting CS, Nadel ER. Effects of passive limb movement on pulmonary ventilation. Yale J Biol Med. 1990;63(6):549–56. [PMC free article] [PubMed] [Google Scholar]
- 29.Nobrega AC, Araujo CG. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc. 1993;25(1):37–41. [PubMed] [Google Scholar]
- 30.Radegran G, Saltin B. Muscle blood flow at onset of dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 1998;274(1):H314–22. [DOI] [PubMed] [Google Scholar]
- 31.Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol. 2005;565(3):1053–60. doi: 10.1113/jphysiol.2005.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R et al. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol. 2008;586(9):2405–17. doi: 10.1113/jphysiol.2008.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol. 2010;108(1):76–84. doi: 10.1152/japplphysiol.00895.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ter Woerds W, De Groot PCE, van Kuppevelt DHJM, Hopman MTE. Passive Leg Movements and Passive Cycling Do Not Alter Arterial Leg Blood Flow in Subjects With Spinal Cord Injury. Phys Ther. 2006;86(5):636–45. [PubMed] [Google Scholar]
- 35.Fernandes A, Galbo H, Kjaer M, Mitchell JH, Secher NH, Thomas SN. Cardiovascular and ventilatory responses to dynamic exercise during epidural anaesthesia in man. J Physiol. 1990;420(1):281–93. doi: 10.1113/jphysiol.1990.sp017912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barret-O’Keefe Z, Runnels S et al. Limb movement-induced hyperemia has a central hemodynamic component; Evidence from a neural blockade study. Am J Physiol Heart Circ Physiol. 2010:ajpheart.00482.2010. doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venturelli M, Amann M, Layec G, McDaniel J, Trinity JD, Fjeldstad AS et al. Passive leg movement-induced hyperaemia with a spinal cord lesion: evidence of preserved vascular function. Acta Physiol (Oxf). 2014;210(2):429–39. doi: 10.1111/apha.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ives SJ, McDaniel J, Witman MA, Richardson RS. Passive limb movement: evidence of mechanoreflex sex specificity. Am J Physiol Heart Circ Physiol. 2013;304(1):H154–61. doi: 10.1152/ajpheart.00532.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ives SJ, Amann M, Venturelli M, Witman MA, Groot HJ, Wray DW et al. The Mechanoreflex and Hemodynamic Response to Passive Leg Movement in Heart Failure. Med Sci Sports Exerc. 2016;48(3):368–76. doi: 10.1249/MSS.0000000000000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S et al. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol. 2012;590(6):1413–25. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285(3):H1023–31. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- 42.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol. 2003;284(4):H1251–9. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- 43.McDaniel J, Hayman MA, Ives SJ, Fjeldstad AS, Trinity JD, Wray DW et al. Attenuated Exercise Induced Hyperemia with Age: Mechanistic Insight From Passive Limb Movement. J Physiol. 2010:jphysiol.2010.198770. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bada AA, Svendsen JH, Secher NH, Saltin B, Mortensen SP. Peripheral vasodilatation determines cardiac output in exercising humans: insight from atrial pacing. J Physiol. 2012;590(8):2051–60. doi: 10.1113/jphysiol.2011.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol. 2012;590(17):4391–400. doi: 10.1113/jphysiol.2012.235952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol. 1999;276(6):H1951–60. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- 47.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging Is Associated with Endothelial Dysfunction in Healthy-Men Years before the Age-Related Decline in Women. J Am Coll Cardiol. 1994;24(2):471–6. doi:Doi 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 48.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F et al. Physical Activity Prevents Age-Related Impairment in Nitric Oxide Availability in Elderly Athletes. Circulation. 2000;101(25):2896–901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 49.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–7. [DOI] [PubMed] [Google Scholar]
- 50.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–5. [DOI] [PubMed] [Google Scholar]
- 51.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Morgan DE et al. Passive leg movement and nitric oxide-mediated vascular function: the impact of age. Am J Physiol Heart Circ Physiol. 2015;308(6):H672–9. doi: 10.1152/ajpheart.00806.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE et al. The role of nitric oxide in passive leg movement-induced vasodilatation with age: insight from alterations in femoral perfusion pressure. J Physiol. 2015;593(17):3917–28. doi: 10.1113/jp270195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groot HJ, Rossman MJ, Trinity JD, Layec G, Ives SJ, Richardson RS. Passive leg movement-induced vasodilation in women: the impact of age. Am J Physiol Heart Circ Physiol. 2015;309(5):H995–H1002. doi: 10.1152/ajpheart.00422.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossman MJ, Groot HJ, Garten RS, Witman MA, Richardson RS. Vascular function assessed by passive leg movement and flow-mediated dilation: initial evidence of construct validity. Am J Physiol Heart Circ Physiol. 2016;311(5):H1277–H86. doi: 10.1152/ajpheart.00421.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker MA, Hoier B, Walker PJ, Schulze K, Bangsbo J, Hellsten Y et al. Vasoactive enzymes and blood flow responses to passive and active exercise in peripheral arterial disease. Atherosclerosis. 2016;246:98–105. doi: 10.1016/j.atherosclerosis.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 56.Gokce N, Keaney JF, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO et al. Predictive value of noninvasivelydetermined endothelial dysfunction for long-term cardiovascular events inpatients with peripheral vascular disease. J Am Coll Cardiol. 2003;41(10):1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 57.Hirsch L, Shechter A, Feinberg MS, Koren-Morag N, Shechter M. The impact of early compared to late morning hours on brachial endothelial function and long-term cardiovascular events in healthy subjects with no apparent coronary heart disease. International journal of cardiology. 2011;151(3):342–7. doi: 10.1016/j.ijcard.2010.08.069. [DOI] [PubMed] [Google Scholar]
- 58.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 59.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(6):502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burns KJ, Pollock BS, McDaniel J. The cardiovascular response to passive movement is joint dependent. Physiol Rep. 2016;4(5). doi: 10.14814/phy2.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol. 2008;105(5):1661–70. doi: 10.1152/japplphysiol.90612.2008. [DOI] [PubMed] [Google Scholar]
- 62.Richardson RS, Secher NH, Tschakovsky ME, Proctor DN, Wray DW. Metabolic and vascular limb differences affected by exercise, gender, age, and disease. Med Sci Sports Exerc. 2006;38(10):1792–6. doi: 10.1249/01.mss.0000229568.17284.ab. [DOI] [PubMed] [Google Scholar]
- 63.Bellien J, Iacob M, Gutierrez L, Isabelle M, Lahary A, Thuillez C et al. Crucial role of NO and endothelium-derived hyperpolarizing factor in human sustained conduit artery flow-mediated dilatation. Hypertension. 2006;48(6):1088–94. doi: 10.1161/01.HYP.0000246672.72188.bd. [DOI] [PubMed] [Google Scholar]
- 64.Bellien J, Iacob M, Remy-Jouet I, Lucas D, Monteil C, Gutierrez L et al. Epoxyeicosatrienoic acids contribute with altered nitric oxide and endothelin-1 pathways to conduit artery endothelial dysfunction in essential hypertension. Circulation. 2012;125(10):1266–75. doi: 10.1161/CIRCULATIONAHA.111.070680. [DOI] [PubMed] [Google Scholar]
- 65.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23(8):374–80. [DOI] [PubMed] [Google Scholar]
- 66.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C et al. Nitric-Oxide Is Responsible for Flow-Dependent Dilatation of Human Peripheral Conduit Arteries in-Vivo. Circulation. 1995;91(5):1314–9. doi:Doi 10.1161/01.Cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 67.Okahara K, Sun B, Kambayashi J-i. Upregulation of prostacyclin synthesis–related gene expression by shear stress in vascular endothelial Cells. ATVB. 1998;18(12):1922–6. [DOI] [PubMed] [Google Scholar]
- 68.Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol. 2011;301(3):H1118–26. doi: 10.1152/ajpheart.00400.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Casey DP, Walker BG, Ranadive SM, Taylor JL, Joyner MJ. Contribution of nitric oxide in the contraction-induced rapid vasodilation in young and older adults. J Appl Physiol. 2013;115(4):446–55. doi: 10.1152/japplphysiol.00446.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol. 1998;85(6):2249–54. doi: 10.1152/jappl.1998.85.6.2249. [DOI] [PubMed] [Google Scholar]
- 71.Buckwalter JB, Clifford PS. Autonomic control of skeletal muscle blood flow at the onset of exercise. Am J Physiol Heart Circ Physiol. 1999;277(5):H1872–H7. doi: 10.1152/ajpheart.1999.277.5.H1872. [DOI] [PubMed] [Google Scholar]
- 72.Corcondilas A, Koroxenidis GT, Shepherd JT. Effect of a Brief Contraction of Forearm Muscles on Forearm Blood Flow. J Appl Physiol. 1964;19(1):142–6. doi: 10.1152/jappl.1964.19.1.142. [DOI] [PubMed] [Google Scholar]
- 73.Dyke CK, Dietz NM, Lennon RL, Warner DO, Joyner MJ. Forearm blood flow responses to handgripping after local neuromuscular blockade. J Appl Physiol. 1998;84(2):754–8. doi: 10.1152/jappl.1998.84.2.754. [DOI] [PubMed] [Google Scholar]
- 74.Naik JS, Valic Z, Buckwalter JB, Clifford PS. Rapid vasodilation in response to a brief tetanic muscle contraction. J Appl Physiol. 1999;87(5):1741–6. doi: 10.1152/jappl.1999.87.5.1741. [DOI] [PubMed] [Google Scholar]
- 75.Wunsch SA, Muller-Delp J, Delp MD. Time course of vasodilatory responses in skeletal muscle arterioles: role in hyperemia at onset of exercise. Am J Physiol Heart Circ Physiol. 2000;279(4):H1715–23. doi: 10.1152/ajpheart.2000.279.4.H1715. [DOI] [PubMed] [Google Scholar]
- 76.Hamann JJ, Buckwalter JB, Clifford PS. Vasodilatation is obligatory for contraction-induced hyperaemia in canine skeletal muscle. J Physiol. 2004;557(Pt 3):1013–20. doi: 10.1113/jphysiol.2004.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiens B, Saltin B, WallØSe L, Wesche J. Temporal relationship between blood flow changes and release of ions and metabolites from muscles upon single weak contractions. Acta Physiol (Oxf). 1989;136(4):551–9. doi: 10.1111/j.1748-1716.1989.tb08701.x. [DOI] [PubMed] [Google Scholar]
- 78.Mohrman DE, Sparks HV. Myogenic hyperemia following brief tetanus of canine skeletal muscle. Am J Physiol. 1974;227(3):531–5. doi: 10.1152/ajplegacy.1974.227.3.531. [DOI] [PubMed] [Google Scholar]
- 79.Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol. 2006;572(Pt 2):561–7. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Juel C, Olsen S, Rentsch RL, González-Alonso J, Rosenmeier JB. K+ as a vasodilator in resting human muscle: implications for exercise hyperaemia. Acta Physiol (Oxf). 2007;190(4):311–8. doi: 10.1111/j.1748-1716.2007.01678.x. [DOI] [PubMed] [Google Scholar]
- 81.Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol. 2007;581(Pt 2):841–52. doi: 10.1113/jphysiol.2007.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2013;305(1):H29–H40. doi: 10.1152/ajpheart.00298.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circ Res. 2013;113(8):1023–32. doi: 10.1161/CIRCRESAHA.113.301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction‐induced rapid vasodilatation. J Physiol. 2007;583(3):861–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crecelius AR, Kirby BS, Richards JC, Dinenno FA. Mechanical effects of muscle contraction increase intravascular ATP draining quiescent and active skeletal muscle in humans. J Appl Physiol. 2013;114(8):1085–93. doi: 10.1152/japplphysiol.01465.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mortensen SP, Thaning P, Nyberg M, Saltin B, Hellsten Y. Local release of ATP into the arterial inflow and venous drainage of human skeletal muscle: insight from ATP determination with the intravascular microdialysis technique. J Physiol. 2011;589(Pt 7):1847–57. doi: 10.1113/jphysiol.2010.203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gifford JR, Richardson RS. CORP: Ultrasound assessment of vascular function with the passive leg movement technique. J Appl Physiol. 2017;123(6):1708–20. doi: 10.1152/japplphysiol.00557.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Groot HJ, Rossman MJ, Garten RS, Wang E, Hoff J, Helgerud J et al. The Effect of Physical Activity on Passive Leg Movement-Induced Vasodilation with Age. Med Sci Sports Exerc. 2016;48(8):1548–57. doi: 10.1249/MSS.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayman MA, Nativi JN, Stehlik J, McDaniel J, Fjeldstad AS, Ives SJ et al. Understanding exercise-induced hyperemia: central and peripheral hemodynamic responses to passive limb movement in heart transplant recipients. Am J Physiol Heart Circ Physiol. 2010;299(5):H1653–H9. doi: 10.1152/ajpheart.00580.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nelson AD, Rossman MJ, Witman MA, Barrett-O’Keefe Z, Groot HJ, Garten RS et al. Nitric oxide-mediated vascular function in sepsis using passive leg movement as a novel assessment: a cross-sectional study. J Appl Physiol. 2016;120(9):991–9. doi: 10.1152/japplphysiol.00961.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Venturelli M, Layec G, Trinity J, Hart CR, Broxterman RM, Richardson RS. Single passive leg movement-induced hyperemia: a simple vascular function assessment without a chronotropic response. J Appl Physiol. 2017;122(1):28–37. doi: 10.1152/japplphysiol.00806.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Witman MA, Ives SJ, Trinity JD, Groot HJ, Stehlik J, Richardson RS. Heart failure and movement-induced hemodynamics: partitioning the impact of central and peripheral dysfunction. International journal of cardiology. 2015;178:232–8. doi: 10.1016/j.ijcard.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burns KJ, Pollock BS, Stavres J, Kilbane M, Brochetti A, McDaniel J. Passive limb movement intervals results in repeated hyperemic responses in those with paraplegia. Spinal Cord. 2018;56(10):940–8. doi: 10.1038/s41393-018-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Böger RH, Bode-Böger SM, Thiele W, Junker W, Alexander K, Frölich JC. Biochemical Evidence for Impaired Nitric Oxide Synthesis in Patients With Peripheral Arterial Occlusive Disease. Circulation. 1997;95(8):2068–74. doi: 10.1161/01.cir.95.8.2068. [DOI] [PubMed] [Google Scholar]
- 95.Hoier B, Rufener N, Bojsen-Moller J, Bangsbo J, Hellsten Y. The effect of passive movement training on angiogenic factors and capillary growth in human skeletal muscle. J Physiol. 2010;588(Pt 19):3833–45. doi: 10.1113/jphysiol.2010.190439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R975–82. doi: 10.1152/ajpregu.00677.2007. [DOI] [PubMed] [Google Scholar]
- 97.Hoier B, Walker M, Passos M, Walker PJ, Green A, Bangsbo J et al. Angiogenic response to passive movement and active exercise in individuals with peripheral arterial disease. J Appl Physiol. 2013;115(12):1777–87. doi: 10.1152/japplphysiol.00979.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Iepsen UW, Munch GD, Rugbjerg M, Rinnov AR, Zacho M, Mortensen SP et al. Effect of endurance versus resistance training on quadriceps muscle dysfunction in COPD: a pilot study. Int J Chron Obstruct Pulmon Dis. 2016;11:2659–69. doi: 10.2147/COPD.S114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26(6):631–40. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 100.Taddei S, Virdis A, Mattei P, Salvetti A. Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypertension. 1993;21(6):929–33. doi: 10.1161/01.hyp.21.6.929. [DOI] [PubMed] [Google Scholar]
- 101.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 102.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J.146(1):168–74. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 103.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31(9):1142–8. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 104.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753–67. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–95. [DOI] [PubMed] [Google Scholar]
- 106.Joannides R, Bellien J, Thuillez C. Clinical methods for the evaluation of endothelial function-- a focus on resistance arteries. Fundam Clin Pharmacol. 2006;20(3):311–20. doi: 10.1111/j.1472-8206.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 107.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315(17):1046–51. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 108.Linder L, Kiowski W, Bühler FR, Lüscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation. 1990;81(6):1762–7. doi: 10.1161/01.cir.81.6.1762. [DOI] [PubMed] [Google Scholar]
- 109.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound Assessment of Flow-Mediated Dilation. Hypertension. 2010;55(5):1075–85. doi: 10.1161/hypertensionaha.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Green DJ, Jones H, Thijssen D, Cable N, Atkinson GJH. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57(3):363–9. [DOI] [PubMed] [Google Scholar]
- 112.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235–41. [DOI] [PubMed] [Google Scholar]
- 113.Broxterman RM, Witman MA, Trinity JD, Groot HJ, Rossman MJ, Park S-Y et al. Strong Relationship Between Vascular Function in the Coronary and Brachial Arteries. Hypertension. 2019:HYPERTENSIONAHA.119.12881. doi:doi: 10.1161/HYPERTENSIONAHA.119.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101(2):545–8. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 115.Moerland M, Kales AJ, Schrier L, van Dongen MG, Bradnock D, Burggraaf J. Evaluation of the EndoPAT as a Tool to Assess Endothelial Function. Int J Vasc Med. 2012;2012:904141. doi: 10.1155/2012/904141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM et al. Cross-Sectional Relations of Digital Vascular Function to Cardiovascular Risk Factors in the Framingham Heart Study. Circulation. 2008;117(19):2467–74. doi: 10.1161/circulationaha.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS et al. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57(3):390–6. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr., Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44(11):2137–41. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 119.Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Kithas AC, Hydren JR et al. Single passive leg movement assessment of vascular function: contribution of nitric oxide. J Appl Physiol. 2017;123(6):1468–76. doi: 10.1152/japplphysiol.00533.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]