Abstract
Objective:
Although post-traumatic stress disorder (PTSD) is identified as a risk factor in the development of rheumatoid arthritis (RA), associations of PTSD with disease progression are less clear. To explore whether PTSD might influence disease-related measure of systemic inflammation in RA, we compared serum cytokine/chemokine (cytokine) concentrations in RA patients with and without PTSD.
Methods:
Participants were U.S. Veterans with RA and were categorized as having PTSD, other forms of depression/anxiety, or neither based on administrative diagnostic codes. Multiplex cytokines were measured using banked serum. Associations of PTSD with cytokine parameters (including a weighted cytokine score) were assessed using multivariable regression, stratified by anti-CCP status and adjusted for age, sex, race, and smoking status.
Results:
Among 1,460 RA subjects with mean (SD) age of 64 (11) years and disease duration of 11 (11) years, 91% were male, 77% anti-CCP positive, and 80% ever smokers. Of these, 11.6% had PTSD, 23.7% other depression/anxiety, and 64.7% had neither. PTSD, but not depression/anxiety, was associated with a higher cytokine score and number of high-concentration analytes in adjusted models, though this was limited to anti-CCP positive subjects. PTSD was associated with heightened expression of several individual cytokines including IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, IL-17, IFN-γ, GM-CSF, MCP-1, and TNF-α.
Conclusion:
Anti-CCP positive RA patients with PTSD have higher serum cytokine concentrations than those without PTSD, demonstrating that systemic inflammation characteristic of RA is heightened in the context of this relatively common psychiatric comorbidity.
Keywords: Rheumatoid arthritis, cytokines, post-traumatic stress disorder, anti-citrullinated protein antibody, depression, anxiety
There is a growing body of evidence suggesting that psychosocial trauma contributes to the risk of developing autoimmune diseases such as rheumatoid arthritis (RA) (1, 2). Whether stress-related disorders such as post-traumatic stress (PTSD), resulting from psychosocial trauma occurring before or after disease onset, also adversely affect the natural course of established autoimmune conditions has been subject to far less scrutiny. This is a clinically relevant question, as evidence linking the two would suggest that treatments aimed at reducing levels of psychosocial stress could yield meaningful benefits in the management of autoimmune disease. In an attempt to explore this issue, our group previously demonstrated that RA patients with comorbid PTSD reported more pain and physical impairment in addition to having higher tender joint counts and worse global activity scores over a mean follow-up period of three years compared to patients without PTSD (3). These differences were most striking for patient-reported outcomes such as pain, global well-being, and physical function, but were not seen for more objective physician- or laboratory-based measures including the swollen joint count, erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP).
Although mechanisms underpinning the relationship between PTSD and autoimmune disease are unknown, numerous reports have identified a chronic state of low-grade, systemic inflammation that accompanies psychosocial stress (4–6). Dysregulation of the hypothalamic-pituitary-adrenal axis that characterizes PTSD (7, 8) has been suggested to augment systemic inflammation, providing a potential mechanism for the increased incidence of RA and systemic lupus erythematosus observed among those with PTSD in epidemiologic studies (1, 2). The state of chronic inflammation accompanying PTSD also has the potential to augment intrinsic inflammatory processes in patients with established RA. Whether other more sensitive inflammatory mediators might intercede in the relationship between PTSD and heightened RA disease activity is unknown. This possibility has been raised by recent reports including a meta-analysis of reports from the general population demonstrating increased serum expression of interleukin (IL)-6, IL-1ß, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ in PTSD patients (9), some of the same inflammatory mediators implicated in the pathogenesis of RA (10).
Therefore, the objective of this study was to examine circulating cytokine and chemokine concentrations in RA patients with and without PTSD. Our goal in this cross-sectional study was to evaluate whether perturbations in inflammatory mediators previously identified in PTSD patients in the general population were also observed among those with RA. We hypothesized that in a cohort of U.S. Veterans with RA, PTSD would be associated with higher concentrations of select serum cytokines and chemokines.
Methods and Materials
Participants and procedures.
We studied participants from the Veterans Affairs Rheumatoid Arthritis (VARA) registry, a multicenter longitudinal observational cohort of U.S. Veterans with RA (11, 12). All study participants satisfied the 1987 American College of Rheumatology classification criteria for RA (13) and provided informed consent prior to enrollment. This analysis was limited to the first 1,460 participants enrolled in the registry for whom banked blood samples were available (3). The study was approved by the institutional review board at each participating center and was approved by the VARA Scientific and Ethics Advisory Committee.
Identification of PTSD and other psychiatric diagnoses.
PTSD, depression, and anxiety were ascertained using diagnostic codes within the Veterans Affairs (VA) Decision Support System as previously described (3). Briefly, we identified individuals with PTSD by using at least one International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code of 309.81. Participants with depression/anxiety included those with codes of 300.00, 300.01, 300.02, 309*, 296.2 to 296.36, 296.90, 311*, excluding 309.81. Positive predictive values of this approach to PTSD identification using administrative data have been shown to exceed 75% in the VA system (14). All psychiatric diagnoses were considered prevalent at the time of study enrollment to mitigate potential detection bias by later systematic VA screening efforts (15). Patients were categorized using a hierarchical classification into three mutually exclusive groups, those with: 1) PTSD (with or without comorbid depression or other form of anxiety); 2) depression or other form of anxiety in the absence of PTSD; or 3) none of the aforementioned diagnostic codes.
Cytokine and Chemokine Measurements.
Cytokine concentrations were measured in banked serum obtained at enrollment, using a Bio-Plex Pro Human Cytokine 17-plex Assay (Bio-Rad, Hercules, CA) run on a Luminex 200 system (Luminex, Austin, TX) (16, 17). These assays incorporated a proprietary blocking buffer to abrogate interference from the presence of heterophilic antibody (e.g. rheumatoid factor). Data processing was performed with Bio-Plex Manager software (Bio-Rad), and analyte concentrations were interpolated from standard curves. Seventeen serum analytes were measured: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-17, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1β (MIP-1β), and TNF-α. These analytes were selected based on their biologic relevance to synovial inflammation in RA and represent a subset of measures from 48-analyte panel shown to predict disease onset during the pre-clinical phase of RA (17). Specific cytokine concentrations were deemed to be “positive” in an individual if the value was more than two standard deviations (SD) above the mean for that analyte in the entire study population. A cytokine score was developed as a measure of overall inflammation and is calculated from the log transformed, normalized, and weighted summation of individual cytokines with the following formula: where Bi are regression coefficients (16–18).
Additional variables collected through the VARA registry at enrollment included age, sex, self-reported race, medication use and smoking status (never, former, and current). A count of eight select chronic conditions including diabetes mellitus, coronary artery disease, cerebrovascular disease, hypertension, hyperlipidemia, chronic obstructive and interstitial lung disease, and chronic kidney disease was utilized as an overall comorbidity score. Anti-cyclic citrullinated peptide (anti-CCP) antibody and rheumatoid factor (RF) were measured as previously described and deemed to be positive if values exceeded thresholds defined by the manufacturers (3).
Statistical analysis.
Baseline patient characteristics, cytokine score, and number of positive cytokines were compared between groups using ANOVA and chi-square tests. Associations between the cytokine score and measures of acute phase response (CRP and ESR) were examined using Spearman’s correlation coefficients. Cytokine score was considered as the primary outcome of interest while others including the number of positive cytokines and individual analyte concentrations were considered in secondary analyses. Multivariable least squares regression models (cytokine score and individual cytokine concentrations) and negative binomial regression models (number of positive cytokines) were adjusted for covariates identified a priori, including age, sex, race, and smoking status. Models were constructed with cytokine parameters as the dependent variable and PTSD status (PTSD vs. depression/anxiety vs. neither condition [referent group]) as the independent variable. Prior to inclusion in regression models, cytokine score and individual analyte concentrations were log-transformed for normality. Recognizing the relationship of PTSD with a higher frequency of cigarette smoking as well as the relationship of smoking with circulating cytokines (3), we also examined for evidence of a statistical interaction between PTSD and current smoking (vs. former/never) as well as anti-CCP antibody status in multivariable models examining cytokine score. Based on these results, multivariable regression models were further stratified by anti-CCP antibody status. Sensitivity analyses were performed requiring at least two separate ICD-9-CM codes for PTSD classification in addition to examining “fully-adjusted” models that included other factors identified as possible covariates based on univariate p-values < 0.1 (the aforementioned covariates plus disease duration, comorbidity count, body mass index, methotrexate, biologic, and prednisone use). P values < 0.05 were considered statistically significant. Analyses were completed using Stata v15 (StataCorp, College Station, TX) within the VA Informatics and Computing Infrastructure.
Results
Characteristics of the 1,460 U.S. Veterans with RA included in the analysis are summarized in Table 1. At the time of study enrollment, patients had a mean age of 64 years and were predominantly white (78%) and male (91%), reflecting the underlying demographics of the VA. Of these, 170 (11.6%) had at least one ICD-9-CM code for PTSD (309.81), a majority of whom had a concomitant diagnosis of depression (n = 145) or other forms of anxiety (n = 65). Requiring an ICD-9-CM code 309.81 on at least two separate dates yielded a lower frequency of 127 PTSD cases (9%). There were 346 (23.7%) patients diagnosed with other forms of anxiety or depression, in the absence of a PTSD diagnosis. The remaining 944 (64.7%) patients were not diagnosed with any of the aforementioned psychiatric disorders. Most patient characteristics were similar by diagnostic group with the exception that those with PTSD were younger (both at RA onset and study enrollment), trended towards a lower disease duration, were less likely to self-report white race, and were more likely to be ever smokers (current or former). Compared to those lacking either psychiatric classification, those with PTSD or with alternative forms of anxiety or depression had more comorbidities, higher disease activity, and worse functional status scores. There was no significant difference by diagnostic group in the frequency of RA treatments being used at enrollment, including the use of prednisone (P = 0.60), methotrexate (P = 0.18), or biologic agent (P = 0.59).
Table 1.
Characteristics of RA patients at the time of study enrollmenta
| Total (n = 1,460) |
No psychiatric diagnosis (n = 944) |
Depression/Anxiety Without PTSD (n = 346) |
PTSD (n = 170) |
P valueb | |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age at enrollment, years | 63.7 ± 11.3 | 64.9 ± 11.5 | 62.4 ± 11.4 | 59.3 ± 8.0 | <0.001 |
| Men, % | 90.8 | 91.6 | 88.7 | 90.6 | 0.28 |
| White, % | 77.9 | 79.1 | 78.3 | 70.6 | 0.05 |
| ≥HS education, % | 84.4 | 84.5 | 82.5 | 87.6 | 0.35 |
| Other factors | |||||
| Comorbidity count (0 to 8) | 1.8 ± 1.6 | 1.6 ± 1.5 | 2.3 ± 1.7 | 2.3 ± 1.6 | <0.001 |
| Smoking status, % | <0.001 | ||||
| Never | 20.4 | 21.7 | 19.7 | 15.3 | |
| Former | 52.3 | 54.5 | 52.6 | 40.0 | |
| Current | 27.2 | 23.9 | 27.8 | 44.7 | |
| BMI (kg/m2) | 28.3 ± 5.7 | 28.1 ± 5.7 | 28.1 ± 5.7 | 29.4 ± 5.9 | 0.05 |
| RA prognostic factors | |||||
| Disease duration, years | 11.2 ± 11.5 | 11.7 ± 11.7 | 10.6 ± 11.3 | 9.7 ± 10.2 | 0.07 |
| Radiographic damage | 52.4 | 52.5 | 51.6 | 53.4 | 0.93 |
| DAS28 | 4.0 ± 1.6 | 3.9 ± 1.6 | 4.3 ± 1.6 | 4.3 ± 1.6 | <0.001 |
| MD-HAQ (0 to 3) | 0.9 ± 0.6 | 0.9 ± 0.6 | 1.1 ± 0.6 | 1.1 ± 0.6 | <0.001 |
| RF positive, % | 80.9 | 80.6 | 81.1 | 82.2 | 0.90 |
| Anti-CCP positive, % | 77.1 | 77.8 | 75.6 | 76.9 | 0.71 |
| CRP (mg/L) | 1.3 ± 2.0 | 1.4 ± 2.2 | 1.2 ± 1.7 | 1.0 ± 1.5 | 0.08 |
| Prednisone, % | 43.1 | 42.4 | 43.4 | 46.8 | 0.60 |
| Methotrexate, % | 51.2 | 53.0 | 47.0 | 50.0 | 0.18 |
| Biologic agent, % | 22.2 | 22.6 | 20.3 | 24.0 | 0.59 |
| Age at diagnosis, years | 52.1 ± 14.1 | 52.9 ± 14.6 | 51.3 ± 13.7 | 49.3 ± 11.4 | 0.005 |
Values shown are mean ± standard deviation (SD) of percentage; RA = rheumatoid arthritis; PTSD = post-traumatic stress disorder; HS = high school (12 years); BMI = body mass index; DAS28 = 28-joint Disease Activity Score; MD-HAQ = multidimensional Health Assessment Questionnaire; CRP = C-reactive protein; RF = rheumatoid factor; anti-CCP = anti–cyclic citrullinated peptide.
P values were generated as a global test for differences across groups using the chi-square test for categorical variables and one-way analysis of variance for continuous variables
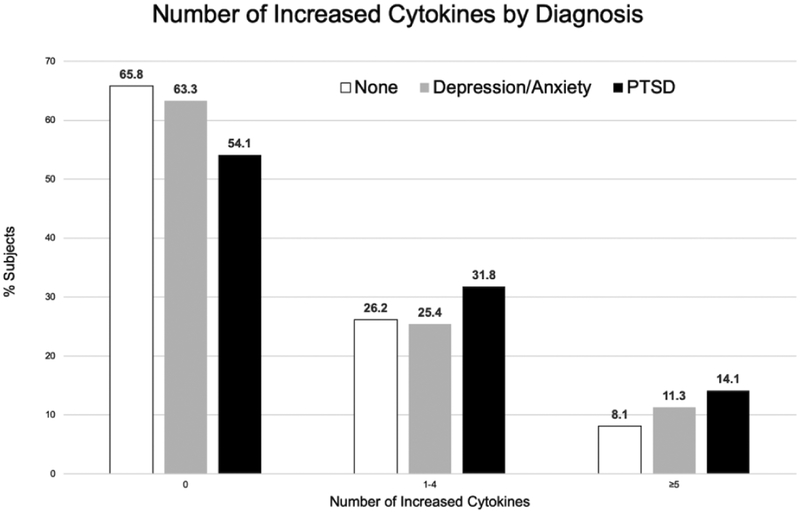
Cytokine scores demonstrated weak, but highly significant, associations with both CRP (r = 0.17; P < 0.001) and ESR (r = 0.13; P < 0.001). Results of multivariable analyses examining the associations of diagnostic groups with serum cytokine concentrations are shown in Table 2. After accounting the effects of age, sex, race, and smoking status, those with PTSD had a higher cytokine score (β = 0.179, P = 0.018) in the primary analysis as well as number of positive cytokines (β = 0.339, P < 0.001) compared to those without PTSD or other forms of anxiety or depression. Similar associations were not observed for other forms of anxiety and depression. These results were not changed after excluding individuals with other forms of anxiety in the absence of depression (data not shown). We found no evidence of interaction between PTSD and current smoking in models examining cytokine score (P value of interaction term = 0.73; data not shown). Associations of PTSD with serum cytokine concentrations were limited to anti-CCP positive individuals (P value of interaction term = 0.04). Among anti-CCP antibody positive RA patients, PTSD was associated with a significantly higher cytokine score (β = 0.273, P = 0.002) as well as a significantly greater number of positive analytes (β = 0.409, P < 0.001). No interaction was present between cytokine concentrations and anxiety/depression (P value of interaction term = 0.37). A significantly greater proportion of those with PTSD (45.9%; p = 0.017 for difference) demonstrated at least one positive cytokine, defined as a concentration more than two SD above the mean, compared to those with other forms of anxiety or depression (36.7%) or those without either psychiatric classification (34.2%) (Figure 1). Results in sensitivity analyses requiring two or more ICD-9-CM codes for PTSD or using fully adjusted models (adjusting for age, sex, race, smoking status, disease duration, comorbidity count, body mass index, methotrexate, biologic, and prednisone use) provided similar results (Supplemental Table 1).
Table 2.
Associations of serum cytokines with PTSD in RA (n = 1,460)
| No Psychiatric Diagnosis | Depression/Anxiety Without PTSD | PTSD | |
|---|---|---|---|
| Values, mean (SD)a | |||
| Cytokine score | 2.33 (0.89) | 2.39 (0.91) | 2.55 (0.94) |
| Cytokine number positive | 1.08(2.23) | 1.23 (2.42) | 1.61 (2.66) |
| All subjectsb | |||
| Cytokine score | Reference | 0.06 (−0.06, 0.17) | 0.18 (0.03, 0.33)c |
| Cytokine number positive | Reference | 0.14 (−0.05, 0.34) | 0.34 (0.19, 0.49)d |
| Anti-CCP positiveb | |||
| Cytokine score | Reference | 0.10 (−0.03, 0.23) | 0.27 (0.10, 0.45)d |
| Cytokine number positive | Reference | 0.16 (−0.07, 0.39) | 0.41 (0.21, 0.61)d |
| Anti-CCP negativeb | |||
| Cytokine score | Reference | −0.02 (−0.22, 0.18) | −0.08 (−0.36, 0.19) |
| Cytokine number positive | Reference | 0.22 (−0.42, 0.86) | −0.24 (−0.70, 0.22) |
Values were log transformed to achieve Gaussian distribution; Models adjusted for age, sex, race, and smoking status.
Values represent β coefficients (95% confidence interval)
P < 0.05
P <0.01
Figure 1. Number of elevated cytokines by PTSD or Depression/Anxiety diagnosis.
The proportion of subjects with 0, 1–4, or ≥5 elevated cytokines (defined as >2 SD above the mean) are shown for those with post-traumatic stress disorder (PTSD), other forms of anxiety or depression, or neither of these classifications. Rheumatoid arthritis patients with PTSD were more likely to have 1–4 or ≥5 elevating cytokines compared to those without either diagnosis (p = 0.017 for difference across groups).
Subsequent analyses examined the associations of PTSD and other forms of anxiety or depression with individual serum cytokine concentrations (Table 3). After accounting for the effects of age, sex, race, and smoking status, PTSD was associated with significantly higher serum concentrations of IL-1β (β = 0.252, P = 0.010), GM-CSF (β = 0.239, P = 0.039), and MCP-1 (β = 0.156, P = 0.024) than was observed in individuals lacking PTSD or other form of anxiety or depression. Although not achieving statistical significance, there were trends towards higher serum concentrations of IL-2 (β = 0.284, p = 0.073), IL-6 (β = 0.198, p = 0.089), IL-10 (β = 0.235, p = 0.058) and IFN-γ (β = 0.301, p = 0.098) in PTSD. In contrast, there were no associations of other forms of anxiety or depression in the absence of PTSD with any individual circulating cytokine with the exception that these patients demonstrated higher concentrations of GM-CSF (β = 0.224, P = 0.009) compared to those lacking either psychiatric classification.
Table 3.
Associations of PTSD, anxiety and depression with serum cytokine and chemokine concentrations in RA patients
| Analyte | Neither (n = 944) |
Depression/Anxiety without PTSDa (n = 346) |
PTSDa (n = 170) |
|---|---|---|---|
| IL-1β | Reference | 0.109 (−0.033, 0.252) | 0.252 (0.061, 0.443)b |
| IL-2 | Reference | 0.109 (−0.122, 0.341) | 0.284 (−0.026, 0.595) |
| IL-4 | Reference | 0.139 (−0.021, 0.300) | 0.182 (−0.033, 0.398) |
| IL-5 | Reference | 0.054 (−0.090, 0.198) | 0.122 (−0.071, 0.315) |
| IL-6 | Reference | 0.048 (−0.122, 0.218) | 0.198 (−0.030, 0.426] |
| IL-7 | Reference | 0.121 (−0.025,0.267) | 0.144 (−0.053, 0.340) |
| IL-8 | Reference | 0.069 (−0.153, 0.290) | 0.205 (−0.093, 0.503) |
| IL-10 | Reference | 0.127 (−0.054, 0.309) | 0.235 (−0.008, 0.479) |
| IL-12 | Reference | 0.083 (−0.109, 0.275) | 0.157 (−0.101, 0.415) |
| IL-13 | Reference | −0.020 (−0.162, 0.123) | 0.110 (−0.081, 0.302) |
| IL-17 | Reference | 0.110 (−0.135, 0.354) | 0.194 (−0.133, 0.523) |
| G-CSF | Reference | 0.129 (−0.069, 0.328) | 0.129 (−0.137, 0.396) |
| GM-CSF | Reference | 0.224 (0.055, 0.393)b | 0.239 (0.012, 0.466)c |
| IFN-γ | Reference | 0.168 (−0.097, 0.433) | 0.301 (−0.056, 0.657) |
| MCP-1 | Reference | 0.031 (−0.070,0.131) | 0.156 (0.021,0.291)c |
| MIP-1β | Reference | 0.030 (−0.053, 0.113) | 0.074 (−0.037, 0.185) |
| TNF-α | Reference | 0.129 (−0.092, 0.349) | 0.202 (−0.094, 0.499) |
Values represent β coefficients (95% confidence interval) predicting log-transformed cytokine concentration after adjusting for age, sex, race, and smoking status.
P < 0.01
P < 0.05
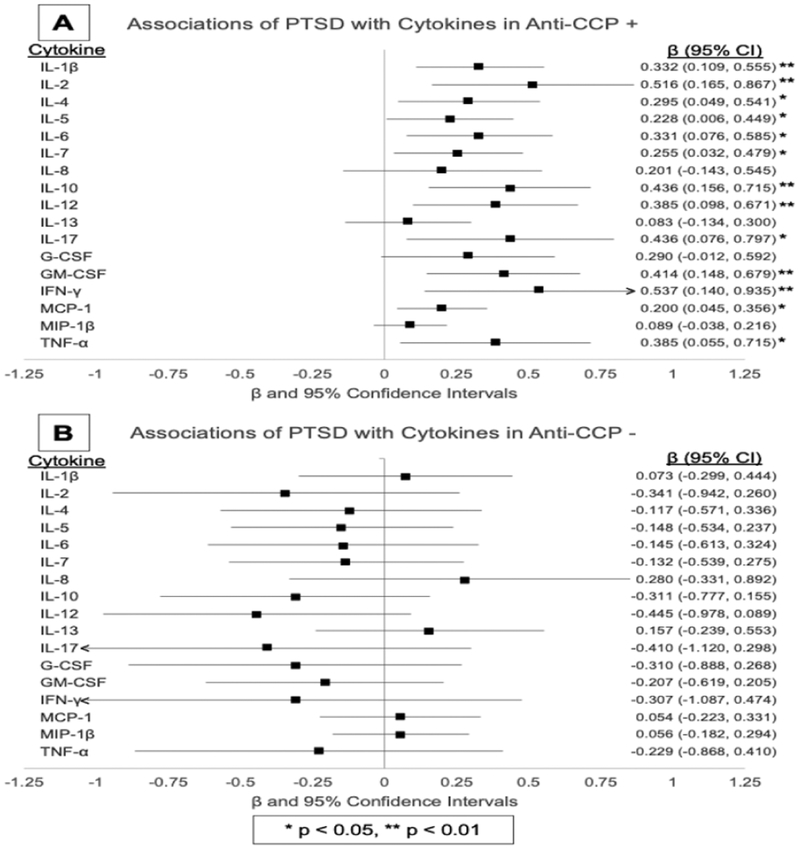
Associations of PTSD with individual analytes, following stratification by anti-CCP antibody status, are shown in Figure 2. Among anti-CCP antibody positive patients (Figure 2A), those with PTSD demonstrated higher serum concentrations for 13 of the 17 analytes examined. This included all three of the aforementioned analytes identified in overall analysis (IL-1β, GM-CSF, and MCP-1; all with higher effect sizes) in addition to IL-2, IL-4, IL-5, IL-6,IL-7, IL-10, IL-12, IL-17, IFN-γ, and TNF-α. As in overall analyses, other forms of anxiety and depression were associated with higher circulating levels of GM-CSF (β = 0.390, P < 0.001) in addition to IL-10 (β = 0.233, P = 0.029) in analyses limited to anti-CCP antibody positive individuals. There were no associations of PTSD (Figure 2B) or other forms of anxiety and depression with any individual analyte concentration in those seronegative for anti-CCP antibody.
Figure 2. Forest plot of the associations of PTSD with individual cytokine concentrations stratified by anti-CCP antibody status.
β coefficients demonstrating the association of post-traumatic stress disorder (PTSD) with individual cytokine concentrations are illustrated in the forest plot for rheumatoid arthritis patients who are anti-CCP antibody positive (A) and anti-CCP antibody negative (B).
Discussion
The purpose of this cross-sectional study was to assess associations of PTSD with circulating inflammatory mediators implicated in RA, as reflected in serum cytokine and chemokine (cytokine) profiles. Affecting approximately 12% of this Veteran population with RA, PTSD was associated with higher circulating cytokine concentrations compared to those without comorbid PTSD. Although we have previously demonstrated similar associations of PTSD and other forms of anxiety and depression with select measures of RA disease activity (pain, physical impairment, tender joint count, and global well-being scores) (3), associations observed in this study with circulating cytokine concentrations were specific to PTSD. In contrast to associations observed with cytokine concentrations, we previously found no associations between PTSD and measures of acute phase response (ESR or CRP), likely owing to the weak correlations between these measures and cytokine score. These findings suggest that along the spectrum of different anxiety and mood disorders, PTSD appears to be unique in its capacity of promoting heightened inflammatory responses and thus may be more likely to adversely influence the natural course of disorders characterized by systemic inflammation.
Of note, the associations with cytokine score and the number of analytes positive appear to be driven by higher circulating concentrations of several cytokines implicated in RA pathogenesis (10), including IL-1β and IL-6, and other pro-inflammatory cytokines that have also been shown to be influenced by PTSD in non-RA populations (9). Whether available therapies specifically targeting these inflammatory mediators might yield particular benefit to RA patients suffering from comorbid PTSD, above and beyond that observed with other approved treatments, is unknown but merits further consideration. In addition to being driven by a number of inflammatory mediators, our results suggest that the observed associations between PTSD and serum cytokine concentrations are limited to anti-CCP antibody positive patients. Given the marked difference in effect sizes observed by autoantibody status as well as the significant interaction observed between PTSD and anti-CCP antibody status, these results do not appear to result from a lack of power in the smaller seronegative patient subgroup. These results contrast from a large study demonstrating a “dose-dependent” relationship between the number of different PTSD symptoms present in women with future RA risk, a risk that was similar between seropositive and seronegative disease (1). These differences suggest the possibility that PTSD and its incumbent symptoms could influence disease susceptibility and progression through different biologic pathways and these effects could differ by gender. It is also possible that PTSD influences inflammatory pathways in a manner that is dependent on the presence of anti-CCP antibody. It is also possible that PTSD is more relevant with more severe disease that accompanies anti-CCP antibody positivity or that RA misclassification (more likely among anti-CCP negative individuals) could affect these results.
Findings from this study parallel those of a meta-analysis examining associations of PTSD with elevated inflammatory markers in non-RA populations (9). Pooling data from 20 studies, Passos and colleagues identified significant associations of PTSD with increased circulating concentrations of IL-1β, IL-6, TNF-α, and IFN-γ (9), cytokines that are also implicated in the pathogenesis of RA. Our study is among the first to corroborate these findings in a cohort of anti-CCP antibody positive RA patients, demonstrating similar elevations in the same four cytokines in relation to PTSD in addition to identifying nine additional analytes elevated that included IL-2, IL-4, IL-5, IL-7, IL-10, IL-12, IL-17, GM-CSF, and MCP-1. Of interest, increased serum concentrations of IL-1β, IL-6, GM-CSF, IFN-γ, and TNF-α are compatible with a profile associated with bone marrow derived mesenchymal cells, recently speculated to play a pathogenic role in RA as well as select neurologic disorders (19–21). Though beyond the scope of this study, future work examining the potential role of this cell population in patients with RA and comorbid PTSD could provide important mechanistic insight to our observations.
In their meta-analysis of non-RA patients, Passos and colleagues (9) reported that comorbid depression, the use of psychotropic medications and the duration of PTSD related symptoms served as potential sources of study heterogeneity, suggesting that these factors could influence the findings observed in the present study. Since the vast majority of RA patients with PTSD in this study also suffered from comorbid depression (85%), meaningful comparisons between those with PTSD in isolation and those concomitantly affected by depression were not possible. Likewise, data specific to the use of non-pharmacologic therapies (e.g. cognitive behavioral therapy), psychotropic medications (including measures of medication adherence for these treatments), and PTSD symptom duration and/or severity were not available for this study Future studies with alternative designs are needed to evaluate the effect of psychotropic medications on inflammatory measures, and the potential effect of psychotropic medications on our findings would only bias towards the null.
There are additional limitations to this study. The VARA registry is a sample comprised primarily of older, white men. Thus, these results may not be generalizable to other populations, particularly women who are two- to three-times more likely to have RA. Given our reliance on administrative data for PTSD case identification, misclassification is possible. Concerns over misclassification bias, however, are mitigated with prior reports demonstrating a positive predictive value of this approach exceeding 75% (14) in addition to the fact that resulting misclassification would only act to bias these results towards the null. The cross-sectional study design prohibits any causal inference or conclusions regarding the direction of the relationship between PTSD and the cytokines that were measured. It is also possible that unmeasured confounding could impact our study results. Although we observed no differences in the use of RA-related medications (prednisone, methotrexate, or biologic treatments) based on PTSD classification, it is possible that PTSD or other psychiatric diagnoses could impact adherence to these medicines that, in turn, could impact values of these inflammatory mediators.
However, this study also has several important strengths. The VARA registry provides a unique, large, and well-characterized cohort that is inherently at higher risk for PTSD. With systematic screening and treatment programs in place, the VA health care system provides an ideal setting for this study, as these findings are unlikely to reflect significant referral or detection bias. Moreover, the registry includes rich levels of annotation for a number of covariates that could directly affect disease-related outcomes. Such indices include the availability of standardized assessments of disease activity as well as autoantibody measures generated using banked samples. The latter may be particularly salient, as prior studies examining risk factors of disease susceptibility have consistently shown differing risk profiles for seropositive and seronegative disease. These prior observations parallel those from the current study in which PTSD was associated with serum cytokine expression only in anti-CCP antibody positive patients.
In summary, we found that RA patients with PTSD demonstrate higher serum cytokine concentrations in comparison to those without PTSD, findings that were limited to those with anti-CCP antibody positive disease. In addition to the need for large-scale longitudinal assessments examining the relationship of PTSD symptoms and symptom management with RA disease activity, additional insight into the complex mechanisms that underpin the relationship of PTSD with measures of systemic inflammation will be needed to optimize future interventions in this population.
Supplementary Material
Acknowledgements:
The authors wish to thank Ms. Debra Bergman for her contributions in preparing this manuscript.
Funding:
TRM is supported by grants from the National Institutes of Health (NIH) / National Institute of Arthritis and Musculoskeletal and Skin Diseases (P50AR060772), National Institute of Alcohol Abuse and Alcoholism (R25AA020818), National Institute of General Medical Sciences (U54GM115458), Veterans Affairs Office of Research & Development (Merit Award, CX000896), Bristol Myers Squibb, and Ironwood Pharmaceuticals. BRE is supported by a University of Nebraska Medical Center (UNMC) Internal Medicine Scientist Development Award, the UNMC Mentored Scholars Program, and the UNMC College of Medicine Physician-Scientist Training Program. LC is supported by Department of Veterans Affairs HSR&D Merit IIR 14-048-3. JS was supported Department of Veterans Affairs IK2 BX001301, NIH/NHLBI R56HL12277, and a Rheumatology Research Foundation Disease Targeted Research Award. JS is now a full time employee of AbbVie (all work reported was completed prior to current employment). JFB is supported by a Veterans Affairs Clinical Science Research and Development Career Development Award (IK2 CX000955). GWC is supported by by Specialty Care Center of Innovation, Veterans Health Administration and Department of Veterans Affairs, Health Services Research and Development.
Abbreviations
- ANOVA
analysis of variance
- CCP
cyclic citrullinated peptide
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IFN
interferon
- IL
interleukin
- MCP
monocyte chemoattractant protein
- PTSD
post-traumatic stress disorder
- RA
rheumatoid arthritis
- RF
rheumatoid factor
- SD
standard deviation
- TNF
tumor necrosis factor
- VA
Veterans Affairs
- VARA
Veterans Affairs Rheumatoid Arthritis (registry)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
US Government Disclaimer: The contents of this report do not represent the views of the US Department of Veterans Affairs or the United States Government.
Conflict of Interest Statement
The authors declare no conflicts of interest.
References:
- 1.Lee YC, Agnew-Blais J, Malspeis S, Keyes K, Costenbader K, Kubzansky LD, et al. Post-Traumatic Stress Disorder and Risk for Incident Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2016;68(3):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts AL, Malspeis S, Kubzansky LD, Feldman CH, Chang SC, Koenen KC, et al. Association of Trauma and Posttraumatic Stress Disorder With Incident Systemic Lupus Erythematosus in a Longitudinal Cohort of Women. Arthritis Rheumatol. 2017;69(11):2162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikuls TR, Padala PR, Sayles HR, Yu F, Michaud K, Caplan L, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65(2):227–34. [DOI] [PubMed] [Google Scholar]
- 4.Reus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–54. [DOI] [PubMed] [Google Scholar]
- 5.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339(6116):166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15(1):43–53. [DOI] [PubMed] [Google Scholar]
- 7.Bremner JD, Vythilingam M, Anderson G, Vermetten E, McGlashan T, Heninger G, et al. Assessment of the hypothalamic-pituitary-adrenal axis over a 24-hour diurnal period and in response to neuroendocrine challenges in women with and without childhood sexual abuse and posttraumatic stress disorder. Biol Psychiatry. 2003;54(7):710–8. [DOI] [PubMed] [Google Scholar]
- 8.Santa Ana EJ, Saladin ME, Back SE, Waldrop AE, Spratt EG, McRae AL, et al. PTSD and the HPA axis: differences in response to the cold pressor task among individuals with child vs. adult trauma. Psychoneuroendocrinology. 2006;31(4):501–9. [DOI] [PubMed] [Google Scholar]
- 9.Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2(11):1002–12. [DOI] [PubMed] [Google Scholar]
- 10.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–42. [DOI] [PubMed] [Google Scholar]
- 11.Mikuls TR, Kazi S, Cipher D, Hooker R, Kerr GS, Richards JS, et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol. 2007;34(7):1480–4. [PubMed] [Google Scholar]
- 12.Miriovsky BJ, Michaud K, Thiele GM, O’Dell JR, Cannon GW, Kerr G, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. [DOI] [PubMed] [Google Scholar]
- 14.Gravely AA, Cutting A, Nugent S, Grill J, Carlson K, Spoont M. Validity of PTSD diagnoses in VA administrative data: comparison of VA administrative PTSD diagnoses to self-reported PTSD Checklist scores. J Rehabil Res Dev. 2011;48(1):21–30. [DOI] [PubMed] [Google Scholar]
- 15.Prins AKR, Leskin G. PTSD in Iraq War veterans: implications for primary care In: Iraq War clinician guide. 2nd ed. Washington, DC: US Department of Veterans Affairs; 2004. [Google Scholar]
- 16.Sokolove J, Johnson DS, Lahey LJ, Wagner CA, Cheng D, Thiele GM, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(4):813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7(5):e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.England BR, Sokolove J, Robinson WH, Thiele GM, Ganti AK, Sayles H, et al. Associations of Circulating Cytokines and Chemokines With Cancer Mortality in Men With Rheumatoid Arthritis. Arthritis Rheumatol. 2016;68(10):2394–402. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen C, Djouad F, Fritz V, Apparailly F, Plence P, Noel D. Mesenchymal stem cells and rheumatoid arthritis. Joint Bone Spine. 2003;70(6):483–5. [DOI] [PubMed] [Google Scholar]
- 20.Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6(5):552–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melero-Jerez C, Ortega MC, Moline-Velazquez V, Clemente D. Myeloid derived suppressor cells in inflammatory conditions of the central nervous system. Biochim Biophys Acta. 2016;1862(3):368–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.