Abstract
Salt-sensing mechanisms in hypertension involving the kidney, vasculature and central nervous system have been well studied; however, recent studies suggest that immune cells can sense sodium (Na+). Antigen presenting cells (APCs) including dendritic cells (DCs) critically modulate inflammation by activating T cells and producing cytokines. We recently found that Na+ enters DCs through amiloride-sensitive channels including the alpha and gamma subunits of the epithelial sodium channel (ENaC) and mediates NADPH oxidase-dependent formation of immunogenic isolevuglandin (IsoLG)-protein adducts leading to inflammation and hypertension. Here, we describe a novel pathway in which the salt-sensing kinase serum/glucocorticoid kinase 1 (SGK1) in APCs mediates salt-induced expression and assembly of ENaCα and ENaCγ and promotes salt-sensitive hypertension by activation of the NADPH oxidase and formation of IsoLG-protein adducts. Mice lacking SGK1 in CD11c+ cells were protected from renal inflammation, endothelial dysfunction and developed blunted hypertension during the high salt feeding phase of the N-Nitro-L-arginine methyl ester hydrochloride (L-NAME)/high salt model of salt-sensitive hypertension. CD11c+ APCs treated with high salt exhibited increased expression of ENaCγ which co-immunoprecipitated with ENaCα. This was associated with increased activation and expression of various NADPH oxidase subunits. Genetic deletion or pharmacological inhibition of SGK1 in CD11c+ cells prevented the high salt induced expression of ENaC and NADPH oxidase. These studies indicate that expression of SGK1 in CD11c+ APCs contributes to the pathogenesis of salt-sensitive hypertension.
Keywords: Inflammation, Dendritic Cell, Hypertension, Isolevuglandins, SGK1
Graphical Abstract
INTRODUCTION
Hypertension is a leading cause of mortality and disability due to stroke, heart failure, myocardial infarction and kidney damage. Recently, the American Heart Association (AHA) and the American College of Cardiology (ACC) developed new guidelines that classified hypertension as blood pressure starting at 130/80 mmHg because cardiovascular disease is common in individuals with blood pressure at this level.1, 2 This new classification places nearly half of the American population in the hypertensive category. Despite its importance and extensive research, the etiology of hypertension is not well understood and blood pressure remains poorly controlled in a substantial portion of hypertensive individuals despite treatment with several drugs.3
Salt-sensitive hypertension affects nearly 50% of the population and reducing salt intake decreases blood pressure and cardiovascular events in the general population.4-7 The precise mechanism of how dietary salt contributes to blood pressure elevation, renal injury, and cardiovascular disease remains unclear. Recent evidence suggests that Na+ can accumulate in tissues and promote inflammation.8,9 Our laboratory and others have shown that cells of both the innate and adaptive immune system are involved in the genesis of hypertension.10-13 Various hypertensive stimuli, including angiotensin II, norepinephrine, and salt cause macrophages, monocytes and T lymphocytes to infiltrate the kidney and vasculature and promotes Na+ retention, vasoconstriction, blood pressure elevation and end-organ damage.10, 14-18
Prior studies have focused on the roles of kidney, vasculature and sympathetic activity in salt-sensitivity, but the contribution of immune cells is poorly understood. Antigen-presenting dendritic cells (DCs) present antigenic peptides and produce cytokines that lead to T cell activation, proliferation and differentiation. We recently found that NADPH oxidase-dependent formation of highly reactive γ-ketoaldehydes or isolevuglandin (IsoLG)-protein adducts in DCs contributes to inflammation and hypertension.12 In addition, we found that murine DCs sense Na+ through amiloride sensitive channels and trigger NADPH oxidase-dependent formation of IsoLG-protein adducts.10 The intracellular enzymes that are sensitive to Na+ in DCs are not known. An important intracellular sensor of salt is the serum/glucocorticoid-regulated kinase 1 (SGK1). This has been predominantly studied in cells of the collecting duct where it regulates expression of ENaC. Recently, SGK1 has been found to mediate salt-induced T cell activation and production of IL-17A.19-21 Given the importance of APCs in both hypertension and immune cell activation, we sought to determine if SGK1 in these cells play a role in their response to salt leading to salt-induced hypertension.
METHODS
The authors declare that all supporting data are available within the article.
Animals and Blood Pressure Measurements:
C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Male mice approximately 10-12 weeks of age were used for the study. TgCD11ccre (CD11cCre+/+) mice were purchased from Jackson Laboratories. SGK1fl/fl mice on the C57BL/6 background were obtained from Aniko Naray-Fejes-Toth (Dartmouth College, Hanover, NH, USA). SGK1 was deleted in DCs by crossing tgCre/CD11c with SGK1fl/fl mice (SGK1CD11cKO). The N(G)-Nitro-L-arginine methyl ester (L-NAME) high-salt protocol was performed as previously described.22 Briefly, male mice were randomly selected to initially receive L-NAME (0.5 mg/mL) in the drinking water for 2 weeks. Following this, mice were given regular water and chow ad libitum for a 2-week washout period. The mice were then fed a 4% high-salt diet for 3 weeks. Blood pressure was monitored invasively using radio-telemetry as previously described.12, 23 After radiotelemetry implantation, mice were allowed to recover for 10 days before administration of the L-NAME/high salt feeding protocol. In some studies, CD11c+ cells were isolated from the spleens of either SGK1CD11cKO or SGK1fl/fl mice and were exposed to 190 mmol/L NaCl in culture for 48 hours. These cells were then adoptively transferred to wild-type recipient mice that then received a 4-week infusion of angiotensin II. Blood pressure was measured in these mice using radiotelemetry. Mice were sacrificed at the end of all experiments by CO2 inhalation. All experimental procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in an AAALAC-accredited facility in accordance with the Guide for the Care and Use of Laboratory Animals and the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Cell isolation and culture:
We obtained splenic CD11c+ cells from C57BL/6J wild type, SGK1fl/fl and SGK1CD11cKO mice using a positive selection kit (Miltenyi Biotec). CD11c+ cells were cultured in 6-well plates at a density of 4 × 106 cells/well for 24 hours in either control RPMI media (150 mmol/L Na) or media containing 190 mmol/L Na+. To control for hyper-osmolality, other cells were exposed to mannitol (190 mmol/L).
Flow Cytometry of Renal and Aortic Leukocytes:
Single-cell suspensions of one kidney or the thoracic aorta with surrounding perivascular fat were prepared as previously described.21 Tissue homogenates were filtered through a 70 μm cell strainer after digestion. Single-cell suspensions were stained for flow cytometry using the following antibodies and fluorophores: Pacific LIVE/DEAD Fixable Violet Dead Cell Stain, allophycocyanin (APC) anti-CD45 antibody, phycoerythrin-conjugated (PE) anti-CD3 antibody (BD Bioscience, Franklin Lakes, NJ, USA), peridinin chlorophyll protein (PerCP) anti-CD4 antibody, phycoerythrin-cyanin-7-conjugated (PE-Cy7) anti-CD8 antibody, Amcyan anti-F4/80 antibody, and allophycocyanin-cyanin-7 (APC-Cy7) anti-CD19 antibody. We used intracellular staining with the single chain antibody D-11 to detect IsoLG protein adducts. The D11 ScFv antibody was labeled with a fluorochrome using the APEXTM Alexa Fluor 488 Antibody Labeling kit. Cells were then fixed and permeabilized for intracellular detection of IsoLG-adducts using a cell permeabilization kit. A known quantity of calibration (counting) beads were added to each sample prior to analysis. Samples were run on a BD FACSCanto II system and analyzed using FloJo software. Gates were set using fluorescence minus one (FMO) controls. Results were normalized using the bead count and expressed as number of cells per kidney or per aorta.
Immunohistochemistry and Masson’s trichrome staining:
Both anti-CD3 immunolabeling and Masson’s trichrome staining were performed and analyzed by a blinded observer. Kidneys were fixed in 10% neutral buffered formalin, routinely processed and paraffin embedded, then cut to 5 μm sections. Briefly, renal T cell infiltration was analyzed in kidney sections obtained from SGK1fl/fl controls and SGK1CD11c KO mice after L-NAME/high salt feeding. All steps besides dehydration, clearing and coverslipping were performed on the Leica Bond-Max IHC autostainer. Slides were deparaffinized. Heat-induced antigen retrieval was performed using the Epitope Retrieval 2 solution for 10 minutes. Slides were incubated with a monoclonal rabbit anti-mouse CD3 antibody at a 1:250 dilution for 60 minutes. The Bond Polymer Refine Detection system was used for visualization. Slides were then dehydrated, cleared and coverslipped. Quantification of CD3 positive cells in the renal cortex was conducted by manually counting the number of immunolabeled cells in 10 consecutive high-power (400X) fields. Mason’s trichrome staining was performed on the Gemini autostainer. Perivascular fibrosis index was assessed at the level of the arcuate arteries and their arteriolar branches throughout the renal cortices on a semiquantitative scale of 0-3.
Immunoblotting:
CD11c+ cells were cultured in normal media, mannitol, high salt, high salt plus GSK650394 100 nmol/L. We performed Western blots using protein extracts from DCs antibodies against NADPH oxidase subunits (p47phox, p22phox, gp91phox and p67phox). To investigate association of p47phox with gp91phox, protein lysates from CD11c+ APCs were immunoprecipitated using the gp91phox antibody followed by western-blotting using p47phox antibody as previously described.10 To determine association of ENaCγ with ENaCα, protein lysates from CD11c+ cells were immunoprecipitated using the ENaCα antibody followed by western-blotting using ENaCγ.
Statistics:
Data are expressed as mean ± SEM. P values <0.05 are considered significant. One tail-unpaired Student’s t-test were used to compare between two groups. In case of skewed distribution, the nonparametric test one-tailed Mann-Whitney U test was used. Blood pressure and vascular relaxation were analyzed using two-way ANOVA.
RESULTS
Loss of CD11c+ SGK1 attenuates L-NAME/high salt-induced hypertension:
To determine the role of CD11c+ APC-specific SGK1 expression in mediating salt induced inflammation and hypertension, we created mice with deletion of SGK1 in CD11c+ cells using the Cre-loxP system (Figure 1A). Western blot analysis confirmed a marked reduction in expression of SGK1 in the CD11c+ APCs of SGK1CD11cKO mice when compared to the Cre negative littermate controls (SGK1fl/fl) (Figure 1B). To induce salt-sensitive hypertension, we employed the L-NAME/HS protocol as previously described.22 As shown in Figure 1C, we found that DC specific deletion of SGK1 had no effect on baseline blood pressure or the hypertensive response to administration of L-NAME. The systolic blood pressure in both SGK1fl/fl controls and SGK1CD11cKO mice increased to equal extents and returned to baseline when L-NAME was discontinued. In contrast, the SGK1CD11cKO mice were protected from the salt-induced increase in systolic pressure (Figure 1C top panel). Diastolic blood pressure was similar between both groups throughout the L-NAME/high salt protocol (Figure 1C middle panel). Interestingly, during high salt feeding, there was a significant increase in heart rate in SGK1fl/fl when compared to SGK1CD11cKO mice suggesting increased sympathetic outflow in the former (Figure 1C bottom panel). As an estimate of sympathetic outflow, we assessed the low frequency to high frequency variability of heart rate and found that this was reduced in SGK1CD11cKO compared to the SGK1fl/fl controls in response to the L-NAME/HS protocol (Figure S1).
Figure 1:
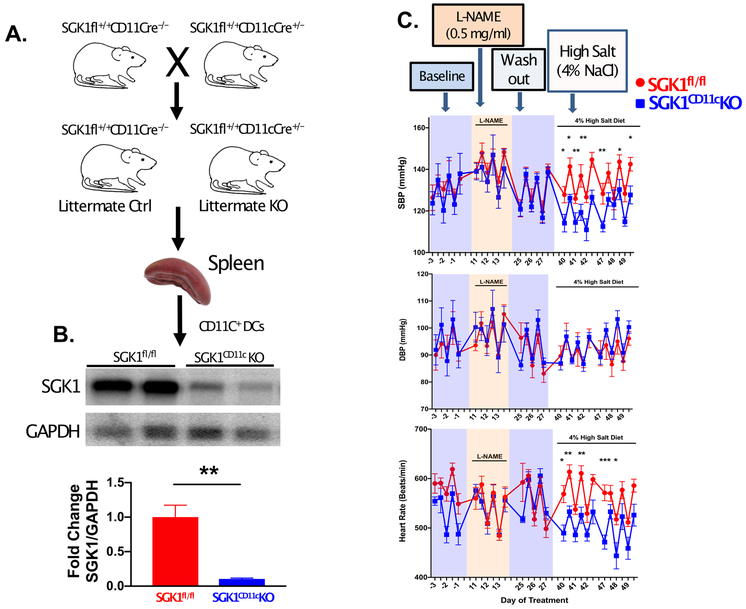
Loss of SGK1 in CD11c+ cells attenuates L-NAME/high salt-induced hypertension. (A) SGK1CD11cKO mice were generated by crossing SGK1fl/fl mice with mice transgenic for Cre recombinase driven by a CD11c promoter. (B) Western blot analysis of SGK1 expression in CD11c+ cells isolated from spleens of SGK1fl/fl controls and SGK1CD11cKO mice. SGK1fl/fl controls and SGK1CD11cKO mice were placed on a L-NAME/high salt diet and (top of panel C) systolic blood pressure (SBP, top panel C) diastolic blood pressure (DBP, middle panel C), and heart rate (lower panel C) were measured invasively using carotid radiotelemetry in freely moving conscious mice. *P<0.05; n = 4-6 per group. All data are expressed as a mean ± SEM.
As an additional approach to understanding the role of SGK1 in APCs, we isolated CD11c+ splenocytes from SGK1fl/fl and SGK1CD11cKO mice and exposed these to 190 mmol/L of NaCl for 48 hours. These cells were then adoptively transferred to WT mice and 10 days later a 4-week infusion of low dose (140 ng/kg/min) ang II was begun. As shown in figure S2, while the initial response to ang II in these animals was similar, those that received the SGK1CD11cKO APCs demonstrated a blunted hypertensive response to ang II at 4 weeks compared to those that received SGK1fl/fl APCs.
In additional experiments, we found that SGK1 in CD11c+ APCs mediates vascular dysfunction during salt-sensitive hypertension. As shown in Figure 2A and B, at baseline there was no significant difference in mesenteric arteriole endothelium-dependent relaxation to acetylcholine (Ach) or endothelium-independent responses evoked by sodium nitroprusside (SNP) between the SGK1fl/fl controls and the SGK1CD11cKO mice. However, we found that mesenteric arterioles from SGK1fl/fl controls exposed to L-NAME/high salt feeding displayed impaired relaxation in response to Ach, but not SNP, indicating an impairment of endothelium-dependent relaxation. Importantly, mesenteric arterioles form SGK1CD11cKO mice displayed no impairment in endothelium-dependent or -independent vasodilatation following L-NAME/high salt feeding (Figure 2C and D).
Figure 2:
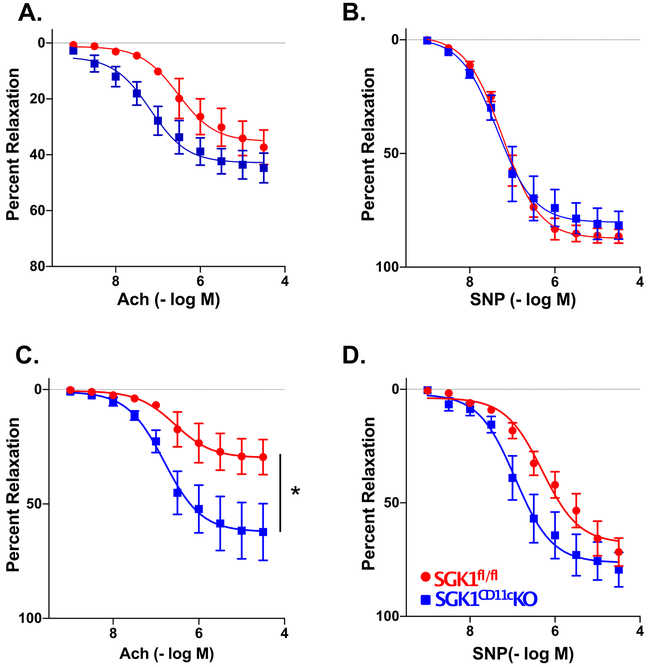
SGK1 in CD11c+ cells modulates vascular function in salt-sensitive hypertension. Endothelium-dependent and endothelium-independent relaxations to increasing doses of acetylcholine (Ach) and sodium nitroprusside (SNP) were measured before (panels A and B) and following (panels C and D) administration of L-NAME/high salt. (C.) *P<0.05; two-way ANOVA post-hoc Holm-Sidak. n=4-6 per group. All data are expressed as mean ± SEM.
Loss of SGK1 in CD11c+ APCs prevents renal inflammation:
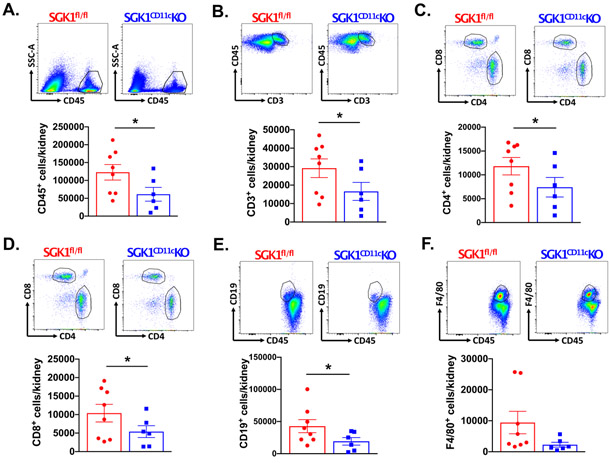
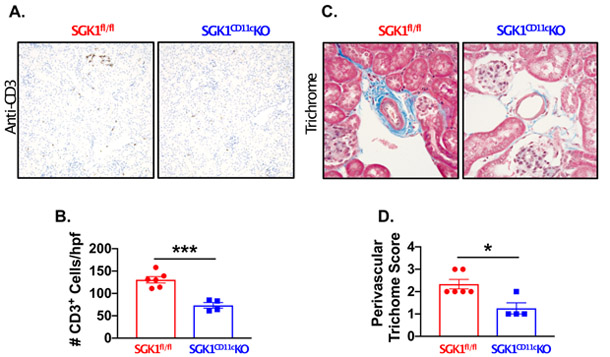
To determine the role of CD11c+ APC SGK1 on renal and vascular inflammation, we performed flow cytometry on single-cell suspensions of kidneys and thoracic aortas following L-NAME/high salt feeding in SGK1CD11cKO mice and SGK1fl/fl controls. Gating strategies to detect total leukocytes (CD45+ cells), total T lymphocytes (CD45+ CD3+ cells), CD4+ T lymphocytes, CD8+ T lymphocytes, B Cells (CD19+ cells), and monocyte/macrophages (F4/80+ cells) in kidney and thoracic aorta are shown in Figures S3 and S4. Following L-NAME/high salt feeding, total leukocytes, T lymphocytes, CD4+ T lymphocytes, CD8+ T lymphocytes, CD19+ B cells were reduced in the SGK1CD11cKO mice when compared to the SGK1fl/fl controls (Figure 3A-E). The F4/80+ and CD11c+ cells were not different in the SGK1CD11cKO mice compared to SGK1fl/fl controls (Figure 3F and S5). In keeping with prior findings in this model of salt-sensitive hypertension,22 aortic immune cell infiltration including total leukocytes, T cells, CD4+ T cells, CD8+ T cells, B cells and monocyte/macrophages was minimal and not different between SGK1CD11cKO mice and SGK1fl/fl controls (Figure S6). As shown in Figure 4A and B, immunostaining with anti-CD3 revealed a striking reduction in renal infiltration of T cells in response to salt-sensitive hypertension in SGK1CD11cKO mice when compared to the SGK1fl/fl littermate controls. In addition, the SGK1CD11cKO mice exhibited less renal perivascular fibrosis when compared to the SGK1fl/fl controls following the L-NAME/high salt protocol as detected by Masson’s Trichrome staining (Figure 4C and D). These results suggest that CD11c+ APC specific expression of SGK1 contributes to the genesis of salt-sensitive hypertension and its associated renal inflammation and damage.
Figure 3:
SGK1 in CD11c+ APCs mediates renal inflammation in salt sensitive hypertension. Representative images and summary data for (A) total leukocytes (CD45+ cells), (B) total T lymphocytes (CD45+ CD3+ cells), (C) CD4 T lymphocytes (CD45+ CD4+ cells), CD8 T lymphocytes (CD45+ CD8+ cells), (D) B cells (CD45+ CD19+) and (E) single-cell suspensions from the kidney of SGK1fl/fl controls and SGK1CD11c mice after L-NAME/high-salt feeding. *P<0.05; student’s t-test; n=6-8 per group. All data are expressed as a mean ± SEM. SSC-A indicates side scatter area.
Figure 4:
Loss of CD11c+ APC SGK1 prevents salt-sensitive hypertension induced renal immune cell infiltration and fibrosis. Representative images of (A) renal CD3+ T lymphocytes (original magnification 200X) and (B) summary data from SGK1fl/fl controls and SGK1CD11cKO mice after L-NAME/high-salt feeding. Masson’s Trichrome staining of (A) renal perivascular fibrosis (original magnification 600X) and (B) semiquantitative scoring in SGK1fl/fl and SGK1CD11cKO mice after L-NAME/high-salt feeding. *P<0.05; ***P<0.001; student’s t-test; n=4-6 per group. All data are expressed as a mean ± SEM.
CD11c+ specific expression of SGK1 mediates expression and association of ENaCα and ENaCγ subunits:
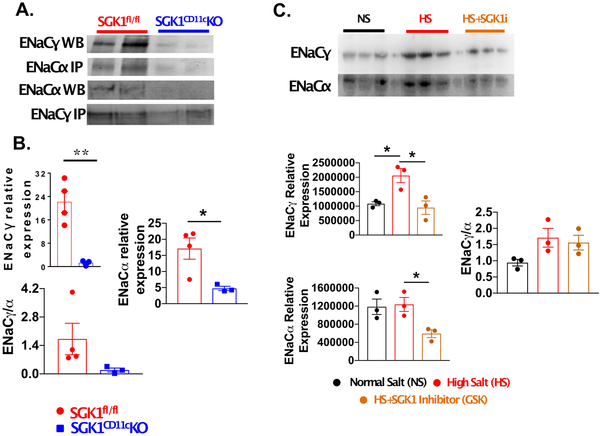
Recently, we found that sodium enters DCs through the α and γ subunits of ENaC and mediates NADPH oxidase-dependent formation of IsoLG-protein adducts leading to inflammation and hypertension.10 In the kidney, SGK1 functions to stabilize ENaC expression. To determine if SGK1 modulates these channels in APCs, we isolated splenic CD11c+ cells, immunoprecipitated ENaCα and performed Western blots for associated ENaCγ. As shown in Figure 5A and B, compared to SGK1fl/fl littermate controls, the SGK1CD11cKO mice exhibited less association of ENaC α and γ than observed in the SGK1fl/fl controls following the L-NAME/high salt protocol.
Figure 5:
CD11c+ APC SGK1 mediates expression of ENaCα and ENaCγ during salt-sensitive hypertension. Splenic CD11c+ APCs were isolated from SGK1fl/fl controls and SGK1CD11c mice after L-NAME/high-salt feeding. (A) Western blot showing the effect of loss of APC SGK1 on ENaCα and ENaCγ expression after salt-sensitive hypertension. (B) Summary data of ENaCα and ENaCγ in DCs after L-NAME/high-salt feeding. (C) Western blot analysis of ENaCα and ENaCγ expression from mouse splenic CD11c+ APCs cultured for 24 hours in either normal salt (NS; 150 mmol/L NaCl), high-salt (HS; 190 mmol/L), or high salt + SGK inhibitor (HS + SGK1i). (D) Quantification of the effect of SGK1 inhibition of ENaC channel expression in CD11c+ APCs. * P<0.05; **P<0.01; student’s t-test; n=3-4 per group. All data are expressed at mean ± SEM.
In additional experiments, we cultured isolated mouse splenic CD11c+ cells in normal salt (150 mmol/L NaCl) or high salt media (190 mmol/L NaCl) with or without pharmacological inhibition of SGK1 using GSK650394 (100 nmol/L) for 24 hours. As shown in Figure 5C, we found that SGK1 inhibition prevented the increase in expression of ENaCγ in response to high salt exposure (Figure 5C). There was no difference in expression of ENaCα between normal salt and high salt but inhibition of SGK1 significantly reduced its baseline expression (Figure 5C, lower panel).
CD11c+ APC expression of SGK1 modulates the APC NADPH oxidase and isoLG adduct formation:
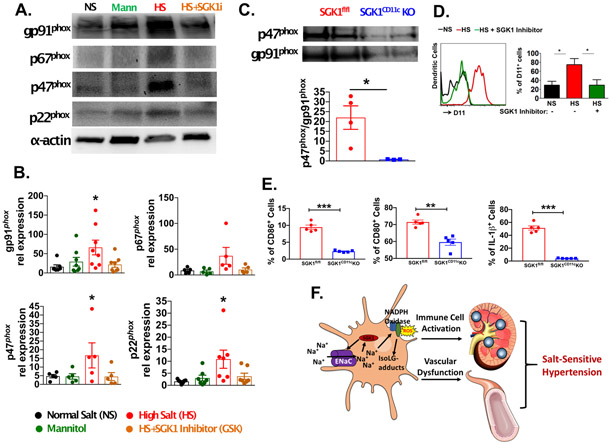
Because sodium entry through ENaC can induce the NADPH oxidase,10 we performed western blots for p22phox, p47phox, p67phox and gp91phox in CD11c+ APCs exposed to either normal or high salt conditions treated with or without SGK1 inhibition (Figure 6A). To control for osmolarity, mannitol (80mM) was added to normal salt media. We found that while equiosmolar mannitol was without effect, high salt exposure markedly induced expression of all NADPH oxidase subunits and co-incubation with the SGK1 inhibitor GSK650394 prevented this (Figure 6A and 6B).
Figure 6:
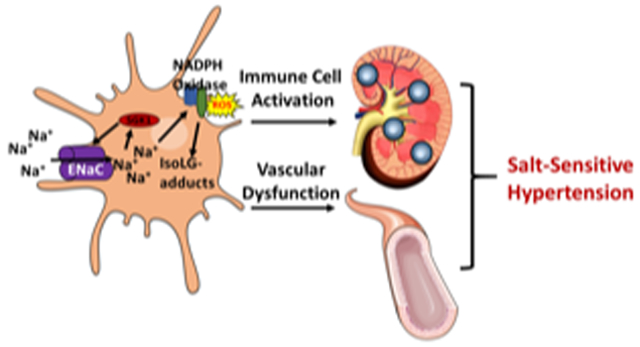
CD11c+ APC SGK1 mediates NADPH oxidase expression and activation in DCs leading to the formation of IsoLG-protein adducts. (A) Western blot showing expression of NADPH oxidase subunits in splenic CD11c+ APCs cultured for 24 hours in either normal salt (NS; 150 mMol/L NaCl), Manntiol (Mann), high salt (HS; 190 mMol/L), or HS + SGK1 inhibitor (HS + SGK1i). (B) Quantification of p22phox, p47phox, p67phox and gp91phox in CD11c+ APCs as determined by densitometry of Western blots. (C) Co-immunoprecipitation studies to define the association of p47phox and gp91phox in SGK1fl/fl controls and SGK1CD11cKO mice after L-NAME/high-salt feeding. (D) Flow cytometry of intracellular staining of IsoLG-protein adducts with D11 antibody in APCs after 24 hours of culture in NS, HS, or HS + SGK1i. (E) Effect of high sodium exposure on the percent of SGK1fl/fl and SGK1CD11cKO cells expressing CD80, CD86 and producing IL-1β (F) Pathway illustrating how high salt may lead to an inflammatory state. SGK1 increases expression of ENaC in antigen presenting DCs. This allows increased sodium entry into DCs. The elevated sodium increases activation and expression of NADPH oxidase which increases production of reactive oxygen species and accumulation of IsoLG-protein adducts. This leads to activation of immune cells which infiltrate the kidney and the vasculature, promoting sodium retention, vascular dysfunction and salt-sensitive hypertension. *p<0.05; **P<0.01; student’s t-test and one-way ANOVA; n=3-8 per group. All data are expressed at mean ± SEM.
A key step in activation of the NADPH oxidase is movement of p47phox to the membrane and its docking to gp91phox. To determine if SGK1 in CD11c+ APCs mediates activation of NADPH oxidase, we immunoprecipitated gp91phox and performed Western blots for associated p47phox. We found that compared to the SGK1fl/fl controls, there was less association of p47phox with gp91phox in the SGK1CD11cKO mice treated with L-NAME/high salt (Figure 6C). Given that SGK1 has such a profound effect on NADPH oxidase expression and activation, and that reactive oxygen species from this enzyme seem to promote formation of IsoLG-protein adducts, we determined if SGK1 modulates IsoLG-protein adduct formation in CD11c+ APCs. Using intracellular staining with the D11 antibody that recognizes IsoLG-protein adducts, we found that the salt-induced increase in IsoLG-protein adducts in CD11c+ APCs was prevented by SGK1 inhibition (Figure 6D).
Role of APC SGK1 in modulating co-stimulatory molecules and IL-1β:
We have previously shown that both ang II and salt-induced hypertension cause an increase in expression of co-stimulatory molecules CD80 and CD86 in CD11c+ APCs and that these are critical for development of hypertension.24 We and others have also shown that hypertension is associated with an increase in production of IL-1β by APCs.12, 25 To determine if these activation events are influenced by SGK1, we exposed splenic CD11c+ cells from SGK1fl/fl and SGK1CD11cKO mice to 190 mmol/L of NaCl for 48 hours and used flow cytometry to assess the ultimate phenotype of these cells. As shown in figure S7A, high sodium skewed the SGK1fl/fl containing cells to a CD11c high phenotype, while the SGK1CD11cKO remained CD11c intermediate following high salt exposure. The CD11c high population demonstrated higher expression of CD80, CD86 and IL-1β compared to the CD11c intermediate population (Figure S7B-D). In both populations, deletion of SGK1 markedly diminished the percent of cells positive for CD80, CD86 and IL-1β (Figure 6E). Thus, SGK1 in CD11c+ APCs markedly influences NADPH oxidase activation, surface expression of co-stimulatory molecules, and production of IL-1β.
DISCUSSION
In this study, we demonstrate that expression of SGK1 in CD11c+ cells promotes salt-induced hypertension, likely via a pathway illustrated in figure 6F. The absence of SKG1 in these cells markedly reduces renal inflammation and prevents the alterations of vascular reactivity that occur in hypertension. Likewise, the expression of ENaCα and ENaCγ subunits in CD11c+ cells was found to be dependent on SGK1 expression and activity. In CD11c+ cells lacking SGK1, the expression of these subunits was markedly reduced. In vitro we found that a modest increased in extracellular sodium stimulates ENaC α and γ expression and that this is prevented by a selective SGK1 inhibitor. In in vitro studies we show that extracellular sodium increases expression of the major NADPH oxidase subunits in CD11c+ cells and the formation of immunogenic IsoLG protein adducts in an SGK1 dependent manner. The association of p47phox and gp91phox in these cells was markedly reduced by the absence of SGK1 following the L-NAME/HS protocol.
To induce salt-sensitive hypertension, we employed a model of L-NAME followed by high salt feeding. Administration of L-NAME creates a condition of endothelial dysfunction, which has been associated with salt-sensitivity. Mice lacking the endothelial nitric oxide synthase exhibit salt sensitivity26 Laffer et al. found that salt sensitive people are unable to vasodilate in response to salt loading.27 Indeed, we found that L-NAME followed by high salt feeding increases renal inflammation, and that this is reduced by CD11c+ specific deletion of SGK1. Randolph et al. demonstrated that monocytes differentiate into DCs during trans-endothelial trafficking.28 Loss of NO increases endothelial expression adhesion molecules and chemokines that in turn would promote monocyte transmigration.29, 30 Upon entry into the interstitium of sites like the kidney and perhaps other high sodium reservoirs, APCs are activated in an SGK1 dependent fashion. In this fashion, sodium activation of APCs can mediate salt sensitivity.
It remains to be defined how and where immune cells encounter the elevated tissue Na+ in vivo. It is likely that a major site for such exposure is the renal interstitium. We have previously shown that a major site of DC activation in hypertension is the kidney, as renal denervation prevented formation of IsoLG adducts in DCs and reduced their production of cytokines12. Recently, Berry et al. found that in a model of urinary tract infection, renal regional hypersalinity leads to the production of CCL2 by tubular epithelial cells leading to the recruitment and localization of monocyte-derived mononuclear phagocytes to the medulla.31 Therefore, it is likely that monocytes are recruited to the renal interstitium in hypertension where they encounter elevated sodium concentrations. Indeed, in the present study, a variety of myeloid and non-myeloid immune cells were found in the kidney of the L-NAME/HS treated mice and these were uniformly reduced by the absence of SGK1. It is also possible that DCs and related cells sense increased levels of sodium in other tissues such as the skin.8
The role of SGK1 and its effects on blood pressure has been best described in the renal epithelial cells of the distal convoluted tubule where it increases expression of ENaC and NCC.32 Recent studies have also demonstrated a role of SGK1 in activation of immune cells. In 2013, Wu et al. and Kleinewietfeld et al. found that SGK1 mediates polarization of T cells to Th17 cells by phosphorylation and inactivation of FoxO1, a direct repressor of the IL-23 receptor, in response to elevated Na+ concentrations.19, 20 Subsequent studies by Safa et al. and Hernandez et al. demonstrated that SGK1 signaling inhibits T regulatory cells by phosphorylating and inhibiting FoxO1 and FoxO3, which regulate Foxp3 expression.33, 34 Both Th17 and T regulatory cells have been shown to play a role in the pathogenesis of hypertension and end organ damage.21, 35 Thus our finding that loss of SGK1 in CD11c+ cells also protects against hypertension and renal inflammation adds to the expanding roles of this kinase in immune activation by sodium.
An interesting finding in our study is that SGK1 regulates expression of ENaC in CD11c+ cells. While ENaC is best recognized for modulating renal sodium reabsorption,36-38 recent studies suggest that ENaC subunits are also expressed in non-renal cells, including the lung and gut. Recently we found that DCs respond to elevated extracellular Na+ in an ENaC-dependent manner and this leads to production of ROS and formation of IsoLG-protein adducts.10 These DCs activate T cells and prime development of hypertension when adoptively transferred into recipient naïve mice.10, 12 In the kidney, the expression of ENaC is primarily regulated by SGK1. Thus, in addition to regulating renal ENaC, our current study shows that SGK1 mediates CD11c+ cell expression of ENaC.
In conclusion, we have defined a novel pathway whereby the salt-sensing kinase SGK1 in APCs contributes to salt-sensitive hypertension through increased expression of ENaC α and γ, NADPH oxidase subunit expression and activation, isoLG-adduct formation, surface expression of co-stimulatory molecules and IL-1β production. Thus, in a model of salt sensitive hypertension, SGK1 has profound effects of the phenotype of CD11c+ antigen presenting cells, ultimately leading to T cell activation, renal inflammation and vascular dysfunction.
Supplementary Material
Perspectives: This is the first study to demonstrate that SGK1 in response to excessive salt activates CD11c+ cells by increasing expression and activation of NADPH oxidase, IsoLG-adduct formation, and regulation of co-stimulatory molecules leading to salt-sensitive hypertension. SGK1 may provide an important therapeutic target for treatment of salt-sensitive hypertension, that affects nearly 50% of all hypertensive patients.
NOVELTY AND SIGNIFICANCE.
- What Is New?
- Deletion of SGK1 in CD11c+ cells prevents salt-sensitive hypertension, vascular dysfunction and renal inflammation in mice.
- Deletion of SGK1 in CD11c+ cells prevents salt-induced surface expression of co-stimulatory molecules CD86 and CD80 and production of IL-1β.
- Inhibition of SGK1 in CD11c+ antigen presenting pharmacologically or genetically cells prevents the increase in NADPH oxidase subunits and ENaC subunit association in CD11c+ cells.
- Pharmacological inhibition of SGK1 prevents formation of IsoLG-adducts in CD11c+ antigen presenting cells.
- What Is Relevant?
- Salt-sensitive hypertension affects nearly 50% of all hypertensive patients.
- Renal inflammation and vascular dysfunction are key components in experimental models of salt-sensitive hypertension, and the current study demonstrates that SGK1 plays a critical role in the activation of antigen presenting cells via NADPH oxidase, ENaC association, IsoLG-adduct formation, surface expression of co-stimulatory molecules, and the production of IL-1β.
- Summary
- Activation of antigen presenting cells have been shown to play a critical role in the promotion of hypertension in experimental animals. We provide the first evidence that SGK1 in antigen presenting cells are activated by sodium leading to the development of salt-sensitive hypertension.
Acknowledgments
Sources of Funding: This work was supported by the American Heart Association grant 19POST3480779 and SFRN204200, and National Institutes of Health grant F32HL142937 and K01HL130497. The Translational Pathology Shared Resource is supported by NCI/NIH Cancer Center Support Grant 5P30 CA68485-19 and the Vanderbilt Mouse Metabolic Phenotyping Center Grant 2 U24 DK059637-16. CTSA award No. UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Conflicts of Interest/Disclosures: None
REFERENCES
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr. 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension. 2017 [Google Scholar]
- 2.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913 [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV. Shattuck lecture. The hypertension paradox--more uncontrolled disease despite improved therapy. N Engl J Med. 2009;361:878–887 [DOI] [PubMed] [Google Scholar]
- 4.He FJ, MacGregor GA. Reducing population salt intake worldwide: From evidence to implementation. Progress in cardiovascular diseases. 2010;52:363–382 [DOI] [PubMed] [Google Scholar]
- 5.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 6.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: Systematic review and meta-analyses. Bmj. 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: Meta-analysis of outcome trials. Lancet. 2011;378:380–382 [DOI] [PubMed] [Google Scholar]
- 8.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-c-dependent buffering mechanism. Nat Med. 2009;15:545–552 [DOI] [PubMed] [Google Scholar]
- 9.Kopp C, Linz P, Dahlmann A, Hammon M, Jantsch J, Muller DN, Schmieder RE, Cavallaro A, Eckardt KU, Uder M, Luft FC, Titze J. 23na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–640 [DOI] [PubMed] [Google Scholar]
- 10.Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, Dikalov S, Titze JM, Knollmann BC, Harrison DG, Kirabo A. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 2017;21:1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon KB, Davies SS, Kirabo A. Dendritic cells and isolevuglandins in immunity, inflammation, and hypertension. Am J Physiol Heart Circ Physiol. 2017;312:H368–H374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J 2nd, Harrison DG. Dc isoketal-modified proteins activate t cells and promote hypertension. J Clin Invest. 2014;124:4642–4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol. 2010;10:203–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin ii-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin ii promotes kidney injury in hypertension. Am J Physiol Renal Physiol. 2008;295:F515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JD, Patel MB, Song YS, Griffiths R, Burchette J, Ruiz P, Sparks MA, Yan M, Howell DN, Gomez JA, Spurney RF, Coffman TM, Crowley SD. A novel role for type 1 angiotensin receptors on t lymphocytes to limit target organ damage in hypertension. Circ Res. 2012;110:1604–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic th17 cells by inducible salt-sensing kinase sgk1. Nature. 2013;496:513–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic th17 cells. Nature. 2013;496:518–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norlander AE, Saleh MA, Pandey AK, Itani HA, Wu J, Xiao L, Kang J, Dale BL, Goleva SB, Laroumanie F, Du L, Harrison DG, Madhur MS. A salt-sensing kinase in t lymphocytes, sgk1, drives hypertension and hypertensive end-organ damage. JCI Insight. 2017;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE, Harrison DG. Cd70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ Res. 2016;118:1233–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ. Inhibition and genetic ablation of the b7/cd28 t-cell costimulation axis prevents experimental hypertension. Circulation. 2010;122:2529–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J 2nd Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin ii-induced hypertension. Circ Res. 2015;117:547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leonard AM, Chafe LL, Montani JP, Van Vliet BN. Increased salt-sensitivity in endothelial nitric oxide synthase-knockout mice. Am J Hypertens. 2006;19:1264–1269 [DOI] [PubMed] [Google Scholar]
- 27.Laffer CL, Scott RC 3rd, Titze JM, Luft FC, Elijovich F Hemodynamics and salt-and-water balance link sodium storage and vascular dysfunction in salt-sensitive subjects. Hypertension. 2016;68:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483 [DOI] [PubMed] [Google Scholar]
- 29.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA Jr., Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan BV, Harrison DG, Olbrych MT, Alexander RW, Medford RM. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc Natl Acad Sci U S A. 1996;93:9114–9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berry MR, Mathews RJ, Ferdinand JR, Jing C, Loudon KW, Wlodek E, Dennison TW, Kuper C, Neuhofer W, Clatworthy MR. Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell. 2017;170:860–874 e819 [DOI] [PubMed] [Google Scholar]
- 32.Ellison DH. Ubiquitylation and the pathogenesis of hypertension. The Journal of clinical investigation. 2013;123:546–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Safa K, Ohori S, Borges TJ, Uehara M, Batal I, Shimizu T, Magee CN, Belizaire R, Abdi R, Wu C, Chandraker A, Riella LV. Salt accelerates allograft rejection through serum- and glucocorticoid-regulated kinase-1-dependent inhibition of regulatory t cells. Journal of the American Society of Nephrology : JASN. 2015;26:2341–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, Deng S, Herold KC, Kuchroo VK, Kleinewietfeld M, Hafler DA. Sodium chloride inhibits the suppressive function of foxp3+ regulatory t cells. The Journal of clinical investigation. 2015;125:4212–4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin ii-induced hypertension and vascular injury. Hypertension. 2011;57:469–476 [DOI] [PubMed] [Google Scholar]
- 36.Kleyman TR, Satlin LM, Hallows KR. Opening lines of communication in the distal nephron. The Journal of clinical investigation. 2013;123:4139–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleyman TR, Kashlan OB, Hughey RP. Epithelial na(+) channel regulation by extracellular and intracellular factors. Annu Rev Physiol. 2018;80:263–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The na+-dependent chloride-bicarbonate exchanger slc4a8 mediates an electroneutral na+ reabsorption process in the renal cortical collecting ducts of mice. The Journal of clinical investigation. 2010;120:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.