Abstract
Background
Somatic mosaicism is to date an uncommon finding in genetic autoinflammatory syndromes such as Cryopyrin‐associated periodic syndrome, Blau syndrome, and TNF receptor‐associated periodic syndrome (TRAPS). However, somatic mosaicism may be particularly important in adult‐onset or atypical phenotypes of these conditions. Herein, we report a unique adult‐onset TRAPS patient presenting with intermittent daily fever for 3 weeks, rash, peritonitis, and lymphadenopathy, who was found with hematopoietic mosaicism involving different white blood cell populations.
Methods
Patient's lymphocyte genomic DNA was initially analyzed by periodic fever seven‐gene next‐generation sequencing panel. Genomic DNAs extracted from patient's hair roots, buccal swab, and subpopulations of white blood cells were subsequently examined on the identified TNFRSF1A variant using Sanger sequencing.
Results
A de novo mosaic missense variant, c.265 T>C(p.Phe89Leu), in the TNFRSF1A gene was found in the patient's buccal swab, B cells, neutrophils, and NK cells but not detected in monocytes, T cells, and hair roots.
Conclusion
These data provide additional information about somatic mosaicism in autoinflammatory conditions and provide new insights regarding cellular players that are indispensable for the phenotypic expression of TRAPS.
Keywords: autoinflammatory, mosaicism, TRAPS
1. INTRODUCTION
Somatic mosaicism, the occurrence of two genetically distinct populations of cells within an individual, is an uncommon finding in the known genetic systemic autoinflammatory disorders NLRP3‐ and NLRC4‐mediated autoinflammatory syndromes (Kawasaki et al., 2017; Rowczenio et al., 2017), Blau syndrome, and a single early‐onset patient with TNF receptor‐associated periodic syndrome (TRAPS) (Mensa‐Vilaro et al., 2016; Rowczenio et al., 2016). Mosaicism may explain late‐onset and atypical presentations of monogenic autoinflammatory syndromes.
TNF receptor‐associated periodic fever syndrome is an autosomal dominant systemic autoinflammatory disorder. It typically manifests in early childhood with intermittent fever of prolonged duration (usually 10–14 days), rash, myalgia, oligoarthralgia, periorbital swelling and serositis, as well as amyloidosis. Adult‐onset TRAPS is reported in about 22% of the well characterized 158‐case cohort from the Eurofever/EUROTRAPS international registry (Lachmann et al., 2014). Similar symptoms are reported in all age groups except for cervical lymphadenopathy and periorbital edema that are more prevalent in children and chest pain that is more often seen in adults (Jacobelli, Andre, Alexandra, Dode, & Papo, 2007).
TRAPS is caused by missense mutations in the TNF receptor superfamily 1A (TNFRSF1A) gene (OMIM 191190) which encodes the 55 kDa receptor protein for TNF receptor 1 (TNFR1). Previously, gain of function mutations on the TNFRSF1A gene were thought to amplify TNF signaling, however, TNF blockade yielded partial or no benefit in TRAPS patients (jacobelli et al., 2007). Subsequent investigations indicated that an unfolded protein response due to the accumulation of misfolded receptors in the endoplasmic reticulum and consequent production of downstream pro‐inflammatory cytokines are key aspects of the pathobiology of TRAPS. The latter, in turn, provided insight for the successful clinical use of IL‐1 blockade (Lobito et al., 2006). Herein, we report the first case of adult‐onset TRAPS associated with a mosaic variant that, in turn, affects different white blood cell lineages.
2. CASE PRESENTATION
The patient was enrolled in the autoinflammatory disease registry at Cleveland Clinic Foundation. Clinical data were obtained with the patient's written informed consent and procedures were approved with the ethical standards of the institutional review board on human research and in accordance with national standards. A 60‐year‐old Caucasian male presented with a 6‐year history of intermittent daily fever as high as 103.5°F, occurring every 7–8 weeks lasting 3–4 weeks with spontaneous resolution. His febrile episodes were associated with headaches, peritonitis, arthralgias, myalgias, painless cervical lymphadenopathy, bilateral episcleritis, and erythematous rash on his torso. He denied periorbital swelling or pleurisy. There was no family history of periodic fever syndromes. Extensive workup for infectious, malignant, and autoimmune etiology of fever of unknown origin was negative. Prednisone up to 60 mg daily, starting at the onset of each flare, only partially alleviated his symptoms, with both colchicine and nonsteroid antiinflammatory drugs (NSAIDs) being ineffective. On physical exam, he was febrile 102.4°F and presented with cervical lymphadenopathy, episcleritis, rebound abdominal tenderness, and an erythematous nonpruritic rash on his torso. His laboratory testing showed high inflammatory markers with C‐reactive protein (CRP) 21 mg/dl (normal range <0.9 mg/dl), erythrocyte sedimentation rate (ESR) 53 mm/h (normal range <20 mm/h), leukocytosis of 18.5 K (normal range 3.7–11 k/UL) with neutrophilia 17.5K (normal range 1.45–7.5 k/UL), ferritin 456ng/ml (normal range 18–300 ng/ml), normal liver function tests, and normal creatinine with no proteinuria. Serologic markers such as antinuclear antibodies, extractable nuclear antigen antibodies including SSA, SSB, double stranded DNA, Scl‐70, ANCA, antiribonucleoprotein were negative. The patient was administered subcutaneous injections of canakinumab 150 mg every 4 weeks with resolution of symptoms within 2 weeks after the first injection. CRP and ESR were normalized within the same time. The patient continued canakinumab without noticeable side effects. Given his clinical picture, TRAPS was highly suspected and genetic testing was performed.
3. GENETIC ANALYSIS
The patient's peripheral blood DNA was initially tested (GeneDx, Gaithersburg, MD) for a seven‐gene periodic fever syndrome next‐generation sequencing panel (ELANE, LPIN2, MEFV, MVK, NLRP3, PSTPIP1, and TNFRSF1A). Testing revealed a somatic missense mutation, c.265 T>C(p.Phe89Leu) (also referred to as F60L) (rs104895245), on TNFRSF1A exon 3 (NM_001065.3). No other pathogenic variants were found among the seven‐gene panel. The zygosity of this variant was stated as mosaic with allele fraction around 30%. To confirm the variant mosaic status, we performed PCR and Sanger sequencing on the TNFRSF1A exon 3 using genomic DNAs extracted from this patient's blood, hair root, and buccal swab. As shown in Figure 1a, the variant allele C as well as the wild‐type allele T, were identified in the patient's blood and buccal swab. No variant was seen in healthy control blood nor the patient's hair root. Our data confirmed the presence of the mosaic variant in this patient involving hematopoietic and nonhematopoietic lineages.
Figure 1.
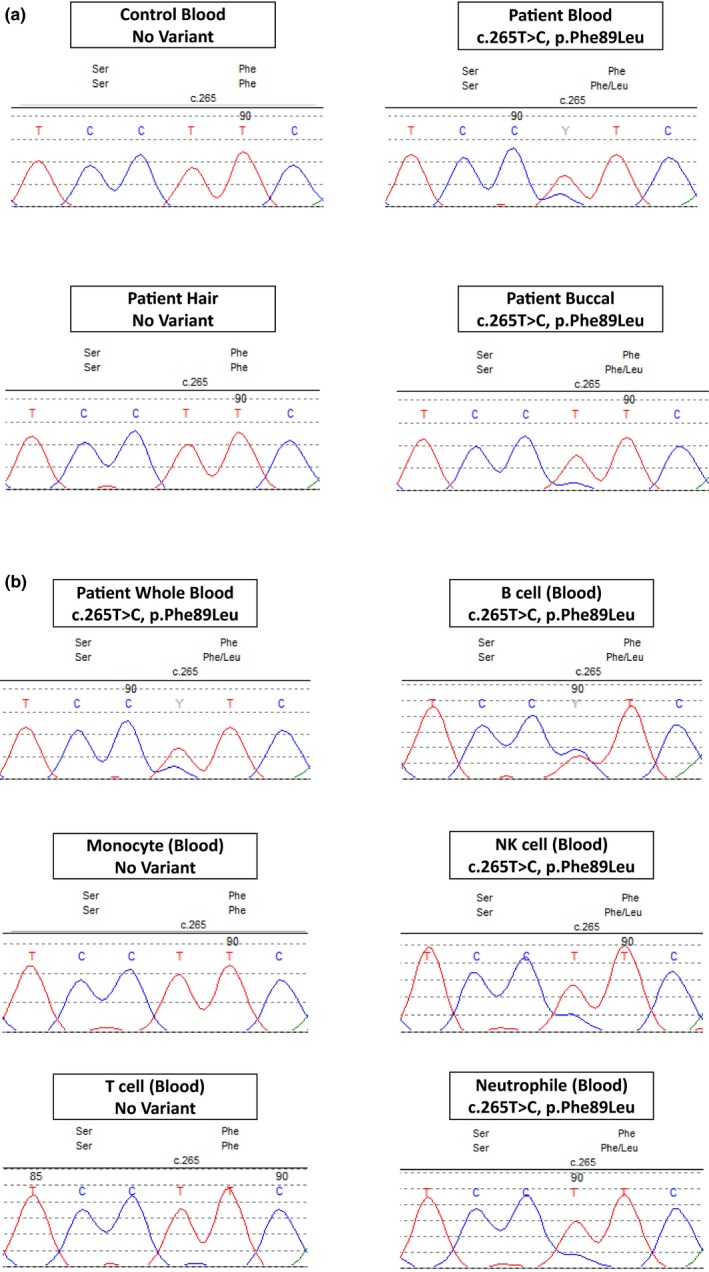
(a) Sanger sequencing of the TNFRSF1A exon 3 shows the variant allele C, instead of the wild‐type allele T, in the patient's blood and buccal swab. No variant was identified in the wild‐type control blood and the patient hair root. (b) Sanger sequencing of the TNFRSF1A exon 3 in the white blood cells displays the variant allele in the B cells, NK cells, and neutrophils. With an estimated variant allele fraction at 25%–45% by measuring the fluorescence peak heights between the wild‐type and the variant alleles. There was no variant identified in the monocyte and T cell subpopulations
We further conducted genetic analysis of blood cell subpopulations. The TNFRSF1A exon 3 genomic region was amplified from isolated leukocyte subpopulations, and Sanger sequenced. As shown in Figure 1b, the variant allele was identified in the B cells, NK cells, and neutrophils. We estimated the variant allele fraction at 25%–45% by measuring the fluorescence peak heights between the wild‐type and the variant alleles. There was no variant identified in the monocyte and T cell subpopulations.
4. DISCUSSION
Herein we report the first case of somatic mosaicism in adult‐onset TRAPS caused by a missense mutation, c.265 T>C(p.Phe89Leu), at the exon 3 of TNFRSF1A gene. The presence of somatic mosaicism suggests that this mutation is a de novo event. According to EUROFEVER registry, 158 variants have been described in TRAPS as pathogenic or variants of unclear significance and the majority of them are missense. The high penetrance TNFRSF1A variants, usually involving the cysteine residues, are less frequently reported than those with low penetrance (Hull et al., 2002). The high penetrance variants affecting the highly conserved cysteine residues of the extracellular cysteine‐rich domains are associated with an early‐onset and more severe phenotype. The low penetrance variants affecting nonconserved regions are associated with a milder phenotype, a later disease onset even in adulthood, and a lower risk of amyloidosis (aganna et al., 2003; Hull et al., 2002).
Of note, functional studies have shown that p.Phe89Leu change elevates NF‐kB activity, resulting in substantial increase in the secretion of IL‐1β, IL‐6, and IL‐12, which triggers proinflammatory response (Nedjai et al., 2011). Additionally, the variant c.265T>C(p.Phe89Leu) (aka F60L) is not present in the public databases such as ExAC and gnomAD, corroborates the prediction that the variant is pathogenic as it is not found in healthy individuals.
Furthermore, the patient's severe phenotype with prolonged systemic symptoms and short symptom‐free intervals as well as dramatic improvement upon IL‐1 blockade corroborates pathogenicity and suggests that variants in modestly conserved regions may be associated with aggressive phenotypes as well. The p.Phe89Leu variant has been published previously in association with two TRAPS cases (Aganna et al., 2001; Papa et al., 2017), in which, symptoms presented in childhood consisting of febrile episodes lasting more than 20 days, urticarial rash, arthralgia, and abdominal pain. One patient responded to NSAIDs and steroids and the other one responded to anakinra as maintenance therapy (papa et al., 2017). Functional studies have shown that p.Phe89Leu exerts a pro‐inflammatory phenotype by increasing TNF‐stimulated c‐Rel activity in TRAPS patients (Nedjai et al., 2011).
An additional noteworthy finding in our case was the differential variant presence within white blood cell lineages. The c.265T>C(p.Phe89Leu) change was specifically present in NK cells, neutrophils, and B cells but not detected in monocytes and T cells. These data support the important role of neutrophils in the TRAPS pathogenesis and that macrophages may be dispensable. The Sanger sequencing method used in this study, although is semiquantitative, has the ability to identify a specific, known variant near 15%–20% allele fraction. Upon further study, the patient's hair, monocyte, and T‐cell DNAs were analyzed by next‐generation sequencing (NGS) targeting the low level c.265T>C variant in each sample with >1000x read‐depth. Consistent with Sanger sequencing data, NGS results showed no detectable variant in hair and monocyte samples, while the variant in T cells was observed near the detection limit (~2%) (data not shown). Our sequencing results demonstrated that some samples lack the TNFRSF1A c.265 T>C variant, which warrant the somatic, instead of germline variant status. Patients with TRAPS have reduced TNFα‐induced apoptosis in neutrophils (D'Osualdo et al., 2006), and canakinumab treatment results in reduction of neutrophil count and decreased pro‐inflammatory signaling corresponding to clinical response (Torene et al., 2017). In our patient, neutrophil count decreased from 17.5K to 3.61K post first canakinumab injection. Rapid resolution of symptoms with IL‐1 blockade in our patient with a high clinical suspicion of TRAPS attests to the critical importance of IL‐1 pathway in disease pathogenesis. Mosaicism in the first reported TRAPS patient was present in all cell types, with variant allele fractions ranging 4%–30%, reflecting an early event in the pluripotent stage of embryogenesis (Rowczenio et al., 2016). It was assumed that the variant was present in the white blood cells leading to patient's symptoms. In our case we were able to further delineate which cellular players, namely monocytes, may be dispensable for TRAPS pathogenesis.
Mosaicism in systemic autoinflammatory diseases is increasingly recognized but its prevalence across the autoinflammatory spectrum has not been systematically investigated especially among patients with milder, atypical or late onset phenotypes. A molecular diagnosis affirms the clinical suspicion in these cases and allows for early institution of appropriate treatment such as IL‐1 blockers. In conclusion, this is the first report of somatic mosaicism in TRAPS and the second ever reported in this autoinflammatory disease. Our findings corroborate the role of neutrophils in TRAPS pathogenesis. Mosaicism can explain adult onset and mutation negative autoinflammatory phenotypes. High clinical suspicion and targeted sequencing may be warranted to determine a cause and tailor individualized treatment.
CONFLICT OF INTEREST
AK has received consultant fees and honorariums from Novartis and Horizon
Kontzias A, Zarabi SK, Calabrese C, et al. Somatic mosaicism in adult‐onset TNF receptor‐associated periodic syndrome (TRAPS). Mol Genet Genomic Med. 2019;7:e791 10.1002/mgg3.791
Funding information
Financial support for the research was provided by the Department of Laboratory Medicine, Cleveland Clinic, The R.J. Fasenmyer Center for Clinical Immunology and the Department of Rheumatology and Immunology, Cleveland Clinic.
REFERENCES
- Aganna, E. , Aksentijevich, I. , Hitman, G. A. , Kastner, D. L. , Hoepelman, A. I. M. , Posma, F. D. , … McDermott, M. F. (2001). Tumor necrosis factor receptor‐associated periodic syndrome (TRAPS) in a Dutch family: Evidence for a TNFRSF1A mutation with reduced penetrance. European Journal of Human Genetics, 9, 63–66. 10.1038/sj.ejhg.5200573 [DOI] [PubMed] [Google Scholar]
- Aganna, E. , Hammond, L. , Hawkins, P. N. , Aldea, A. , McKee, S. A. , van Amstel, H. K. P. , … McDermott, M. F. ( 2003). Heterogeneity among patients with tumor necrosis factor receptor‐associated periodic syndrome phenotypes. Arthritis and Rheumatism, 48, 2632 – 2644. [DOI] [PubMed] [Google Scholar]
- D'Osualdo, A. , Ferlito, F. , Prigione, I. , Obici, L. , Meini, A. , Zulian, F. , … Gattorno, M. (2006). Neutrophils from patients with TNFRSF1A mutations display resistance to tumor necrosis factor‐induced apoptosis: Pathogenetic and clinical implications. Arthritis and Rheumatism, 54, 998–1008. 10.1002/art.21657 [DOI] [PubMed] [Google Scholar]
- Hull, K. M. , Drewe, E. , Aksentijevich, I. , Singh, H. K. , Wong, K. , Mcdermott, E. M. , … Kastner, D. L. (2002). The TNF receptor‐associated periodic syndrome (TRAPS): Emerging concepts of an autoinflammatory disorder. Medicine (Baltimore), 81, 349–368. 10.1097/00005792-200209000-00002 [DOI] [PubMed] [Google Scholar]
- Jacobelli, S. , Andre, M. , Alexandra, J. F. , Dode, C. , & Papo, T. (2007). Failure of anti‐TNF therapy in TNF receptor 1‐associated periodic syndrome (TRAPS). Rheumatology (Oxford), 46, 1211–1212. 10.1093/rheumatology/kel298 [DOI] [PubMed] [Google Scholar]
- Kawasaki, Y. , Oda, H. , Ito, J. , Niwa, A. , Tanaka, T. , Hijikata, A. , … Saito, M. K. (2017). Identification of a high‐frequency somatic NLRC4 mutation as a cause of autoinflammation by pluripotent cell‐based phenotype dissection. Arthritis & Rheumatology, 69, 447–459. 10.1002/art.39960 [DOI] [PubMed] [Google Scholar]
- Lachmann, H. J. , Papa, R. , Gerhold, K. , Obici, L. , Touitou, I. , Cantarini, L. , … Gattorno, M. (2014). The phenotype of TNF receptor‐associated autoinflammatory syndrome (TRAPS) at presentation: A series of 158 cases from the Eurofever/EUROTRAPS international registry. Annals of the Rheumatic Diseases, 73, 2160–2167. 10.1136/annrheumdis-2013-204184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobito, A. A. , Kimberley, F. C. , Muppidi, J. R. , Komarow, H. , Jackson, A. J. , Hull, K. M. , … Siegel, R. M. (2006). Abnormal disulfide‐linked oligomerization results in ER retention and altered signaling by TNFR1 mutants in TNFR1‐associated periodic fever syndrome (TRAPS). Blood, 108, 1320–1327. 10.1182/blood-2005-11-006783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensa‐Vilaro, A. , Cham, W. T. , Tang, S. P. , Lim, S. C. , Gonzalez‐Roca, E. , Ruiz‐Ortiz, E. , … Aróstegui, J. I. (2016). Brief report: First identification of intrafamilial recurrence of blau syndrome due to gonosomal NOD2 mosaicism. Arthritis & Rheumatology, 68, 1039–1044. 10.1002/art.39519 [DOI] [PubMed] [Google Scholar]
- Nedjai, B. , Hitman, G. A. , Church, L. D. , Minden, K. , Whiteford, M. L. , McKee, S. , … Turner, M. D. (2011). Differential cytokine secretion results from p65 and c‐Rel NF‐κB subunit signaling in peripheral blood mononuclear cells of TNF receptor‐associated periodic syndrome patients. Cellular Immunology, 268, 55–59. 10.1016/j.cellimm.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Papa, R. , Doglio, M. , Lachmann, H. J. , Ozen, S. , Frenkel, J. , Simon, A. , … Gattorno, M. (2017). A web‐based collection of genotype‐phenotype associations in hereditary recurrent fevers from the Eurofever registry. Orphanet Journal of Rare Diseases, 12, 167 10.1186/s13023-017-0720-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowczenio, D. M. , Gomes, S. M. , Aróstegui, J. I. , Mensa‐Vilaro, A. , Omoyinmi, E. , Trojer, H. , … Hawkins, P. N. (2017). Late‐onset cryopyrin‐associated periodic syndromes caused by somatic NLRP3 mosaicism‐UK single center experience. Frontiers in Immunology, 8, 1410 10.3389/fimmu.2017.01410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowczenio, D. M. , Trojer, H. , Omoyinmi, E. , Arostegui, J. I. , Arakelov, G. , Mensa‐Vilaro, A. , … Lachmann, H. J. (2016). Brief report: Association of tumor necrosis factor receptor‐associated periodic syndrome with gonosomal mosaicism of a novel 24‐nucleotide TNFRSF1A deletion. Arthritis & Rheumatology, 68, 2044–2049. 10.1002/art.39683 [DOI] [PubMed] [Google Scholar]
- Torene, R. , Nirmala, N. , Obici, L. , Cattalini, M. , Tormey, V. , Caorsi, R. , … Gattorno, M. (2017). Canakinumab reverses overexpression of inflammatory response genes in tumour necrosis factor receptor‐associated periodic syndrome. Annals of the Rheumatic Diseases, 76, 303–309. 10.1136/annrheumdis-2016-209335 [DOI] [PMC free article] [PubMed] [Google Scholar]


