Abstract
Aspirin possesses anti-bacterial activity that may prevent recurrence of Klebsiella pneumoniae pyogenic liver abscess (KP-PLA). In ex-vivo study, aspirin was administered before bactericidal assay against serotype K1 K. pneumoniae. We identified 5,912 patients with PLA who had no known pre-existing hepatobiliary diseases or malignancy in Taiwan from 1999 to 2013 from nationwide cohort study. Multivariate Cox proportional hazards regression models was used to estimate the hazard ratios [HR] for the association between aspirin use and recurrent PLA. The PLA recurrence rate in patients taking aspirin daily for 30 or more days, from 90 days before to 90 days after the first PLA episode (aspirin users), and aspirin non-users was 42.5 and 74.6 per 1,000 person-years of follow-up, respectively. The population-based study showed a HR for PLA recurrence in aspirin users of 0.50 (95% confidence interval, 0.35–0.69), relative to that in non-users, after adjustments for confounders. An ex-vivo study indicated that aspirin was able to significantly enhance bacterial killing by leukocytes, whether collected from diabetic patients with KP-PLA recurrence or from healthy volunteers. Our results suggest that aspirin is associated with reduced risk for PLA recurrence among Taiwanese with PLA who had no preexisting hepatobiliary diseases or malignancy.
Subject terms: Risk factors, Bacterial infection
Introduction
Pyogenic liver abscess (PLA) is a life-threatening infectious disease and most PLA cases used to be poly-microbial infections, with Escherichia coli the most common causative pathogen1. Since the 1980s, a highly virulent strain of Klebsiella pneumoniae (KP) has surpassed E. coli and emerged as the predominant etiology of PLA in Asia2, the USA3, Europe4, and globally5. Unlike PLAs caused by E. coli or other bacteria that are mostly associated with biliary tract disorders6, PLA caused by KP (KP-PLA) is often cryptogenic and most patients with KP-PLA have diabetes mellitus6,7. In addition, capsule serotypes K1 and K2 were the most common strains involved in KP-PLA8. The exact cause for the increasing incidence of KP-PLA is still not clear but increased intestinal colonization by these KP strains was postulated9. A recent study from Taiwan, where KP has been the leading etiology of cryptogenic PLA, revealed a fecal KP carriage rate of 75% in healthy adults and serotype K1/K2 isolates accounted for 23% of the typeable strains10, suggesting that colonization by KP strains may precede invasion of the intestinal mucosa and portal circulation, followed by the development of PLA. While the short-term morbidity and mortality of PLA are currently better understood, long-term surveillance is needed to determine the rate of PLA recurrence and factors that affect recurrence.
Aspirin has been used to prevent cardiovascular disease, and, by inhibiting cyclooxygenase-mediated arachidonic acid metabolism, can reduce the synthesis of prostaglandins, which may facilitate bactericidal activity by leukocytes11. In safe therapeutic concentrations, sodium salicylate (SAL), the major metabolite of aspirin, can reduce capsular polysaccharide (CPS) production by up to 70%12. We previously reported that SAL enhanced susceptibility of KP serotype K1 to leukocyte phagocytosis by reducing CPS production13; moreover, we found that patients with community-acquired KP bacteremia who had recent use of aspirin were at a lower risk of acquiring KP-associated invasive syndromes in multivariate analysis13, suggesting a potential role for aspirin uses in preventing recurrent KP-PLA. To date, no large-scale population-based study has investigated the relationship between KP-PLA recurrence and aspirin use. Using ex-vivo study model and nationwide population-based database in Taiwan, our primary aim was to determine whether aspirin use in patients with KP-PLA was associated with reduced risk for recurrence, relative to population-based controls without aspirin use.
Results
Patient characteristics
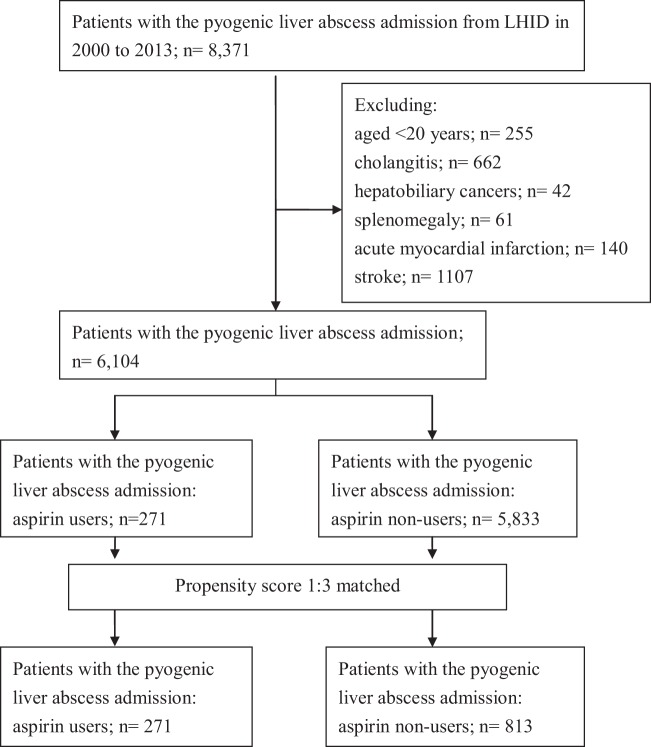
The medical records of 6,104 eligible patients with a first episode of PLA between 2000 and 2013 were reviewed; of these, 271 were in the aspirin-user cohort and 5,833 in the non-user cohort (Fig. 1). The demographic characteristics of both groups are summarized in Table 1. The aspirin-user group tended to be older than the non-user group. There were significant differences in sex and use of anti-diabetic medications (metformin, other OADs, and insulin), amoxicillin or ampicillin, and statins between the 2 groups. Except for cancer, with a higher rate among aspirin non-users, the aspirin-user group had higher rates of diabetic mellitus and hypertension. After 1:3 propensity-score matching, 271 aspirin users and 813 non-users were similar in terms of age, sex, baseline comorbidities, and medication use (Fig. 1).
Figure 1.
Flow chart of study cohort. Note: LHID, Longitudinal Health Insurance Database.
Table 1.
Study cohort characteristics in patients with pyogenic liver abscess.
| Before propensity-score matched | After propensity-score matched | ||||||
|---|---|---|---|---|---|---|---|
| Aspirin user | Aspirin non-user | p value | Standardized difference | Aspirin non-user | p value | Standardized difference | |
| Case number | 271 | 5,833 | 813 | ||||
| Age group (years-old) | |||||||
| 20–44 | 6 (2.2) | 1,046 (17.9) | <0.01 | 0.541 | 23 (2.8) | 0.59 | 0.008 |
| 45–64 | 62 (22.9) | 2,425 (41.6) | <0.01 | 0.408 | 166 (20.4) | 0.39 | 0.045 |
| 65–74 | 81 (29.9) | 1,113 (19.1) | <0.01 | 0.253 | 229 (28.2) | 0.59 | 0.096 |
| ≥75 | 122 (45.0) | 1,249 (21.4) | <0.01 | 0.517 | 395 (48.6) | 0.31 | 0.121 |
| Mean (sd) | 71.3 (11.8) | 60.1 (16.0) | <0.01 | 0.799 | 72.2 (12.4) | 0.29 | 0.113 |
| Gender | 0.01 | 0.44 | |||||
| Male | 155 (57.2) | 3,778 (64.8) | 0.156 | 443 (54.5) | 0.062 | ||
| Female | 116 (42.8) | 2,055 (35.2) | 0.156 | 370 (45.5) | 0.062 | ||
| Co-morbidity | |||||||
| Diabetic mellitus | 156 (57.6) | 1,619 (27.8) | <0.01 | 0.631 | 427 (52.5) | 0.15 | 0.059 |
| Hypertension | 218 (80.4) | 2,030 (34.8) | <0.01 | 1.040 | 684 (84.1) | 0.16 | 0.054 |
| Cancer | 41 (15.1) | 1,449 (24.8) | <0.01 | 0.244 | 119 (14.6) | 0.84 | 0.044 |
| Medication use | |||||||
| A moxicillin or ampicillin | 26 (9.6) | 348 (6.0) | 0.02 | 0.136 | 72 (8.9) | 0.71 | 0.043 |
| Metformin | 84 (31.0) | 616 (10.6) | <0.01 | 0.520 | 221 (27.2) | 0.23 | 0.056 |
| Other oral anti-diabetic drugs | 105 (38.7) | 857 (14.7) | <0.01 | 0.564 | 291 (35.8) | 0.38 | 0.069 |
| Insulin | 41 (15.1) | 477 (8.2) | <0.01 | 0.218 | 94 (11.6) | 0.12 | 0.042 |
| Statins | 53 (19.6) | 236 (4.0) | <0.01 | 0.495 | 117 (14.4) | 0.04 | 0.097 |
| Follow-up time, year Mean (sd) | 3.4 (3.5) | 3.9 (4.1) | 0.04 | 0.135 | 3.6 (3.7) | 0.35 | 0.041 |
| Propensity score, Mean (sd) | 0.1237 (0.0985) | 0.0407 (0.0578) | <0.01 | 1.021 | 0.1190 (0.0901) | 0.46 | 0.046 |
Note: Sd, standard deviation.
Effects of aspirin in patients with PLA recurrence
Follow-up summation was performed for 23,780 person-years of follow-up [PYFU] during the study period. The mean follow-up time was 3.4 years in aspirin users and 3.9 years in non-users. A total of 1,449 (23.7%) patients had PLA recurrence after a first episode. The PLA recurrence rate was 42.5 per 1,000 PYFU in the aspirin-user group and 74.6 per 1,000 PYFU in the non-user group. Compared with patients in the non-user group, those in the aspirin-user group had a significantly lower risk of PLA recurrence after adjustments for age, gender, diabetic, hypertension, cancer, amoxicillin or ampicillin, metformin, other OADs, insulin, statins, aspirin used (adjusted HR: 0.62; 95% CI: 0.44–0.87, p < 0.01); however, the differences in all-cause mortality (adjusted HR: 0.92; 95% CI: 0.75–1.13, p = 0.45) and risk of cancer (adjusted HR: 0.76; 95% CI: 0.54–1.08, p = 0.12; Table 2) were not statistically significant between the two groups.
Table 2.
Incidence of recurrent pyogenic liver abscess events and relative incidence in patients with pyogenic liver abscess.
| Aspirin user | Aspirin non-user | Crude HR (95% confidence interval) | p value | Adjusted HR (95% confidence interval) | p value | |||
|---|---|---|---|---|---|---|---|---|
| Incidence case | Incidence rate | Incidence case | Incidence rate | |||||
| Recurrence of pyogenic liver abscess | 35 | 42.5 | 1,414 | 74.6 | 0.50 (0.35–0.69) | <0.01 | 0.62 (0.44–0.87) | <0.01 |
| All-cause mortality | 99 | 107.4 | 2,554 | 111.7 | 0.85 (0.70–1.04) | 0.13 | 0.92 (0.75–1.13) | 0.45 |
| Acute myocardial infraction | 6 | 6.8 | 80 | 3.5 | 1.96 (0.85–4.50) | 0.11 | 1.45 (0.61–3.46) | 0.40 |
| Cancer | 34 | 39.6 | 1,083 | 52.1 | 0.67 (0.48–0.94) | 0.02 | 0.76 (0.54–1.08) | 0.12 |
Incidence rate, 1000 person-years.
Note: HR, hazard ratios.
Adjusted HR: adjusted age, gender, diabetic, hypertension, cancer, amoxicillin or ampicillin, metformin, other oral anti-diabetic drugs, insulin, statins, aspirin used.
For development of the propensity score, a logistic regression model was applied (Table S1). In the propensity-score matched and competing adjusted-risk model, aspirin users still had a lower risk of PLA recurrence, with an adjusted HR of 0.65 (95% CI: 0.45–0.95; p = 0.02; Table 3). Meanwhile, comorbidity with diabetes or hypertension and use of metformin or insulin were also risk factors for PLA recurrence (Table 3). The Kaplan-Meier analysis for cumulative incidence of PLA recurrence showed a significantly lower risk of recurrent PLA in the aspirin-user group compared with that in the non-user group both before propensity-score matching (p < 0.001; Fig. 2A) and after propensity-score matching (p = 0.014; Fig. 2B).
Table 3.
Factors associated with recurrent pyogenic liver abscess events from multivariate analysis.
| Crude model | Adjusted model | Adjusted model after PS matched | Competing risk Adjusted model after PS matched | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p value | HR (95%CI) | p value | HR (95%CI) | p value | HR (95%CI) | p value | |
| Aspirin | 0.50 (0.35–0.69) | <0.01 | 0.62 (0.44–0.87) | <0.01 | 0.65 (0.45–0.95) | 0.02 | 0.62 (0.44–0.88) | <0.01 |
| Age group (years-old) | ||||||||
| 20–44 | 0.91 (0.79–1.04) | 0.17 | 0.87 (0.69–1.08) | 0.20 | 0.31 (0.09–1.06) | 0.06 | 0.86 (0.69–1.07) | 0.18 |
| 45–64 | 0.92 (0.80–1.06) | 0.27 | 1.07 (0.88–1.32) | 0.49 | 1.48 (0.82–2.66) | 0.19 | 1.07 (0.87–1.31) | 0.51 |
| 65–74 | ||||||||
| 75+ | 0.65 (0.56–0.76) | <0.01 | 0.83 (0.61–1.13) | 0.23 | 1.38 (0.56–3.35) | 0.48 | 0.79 (0.58–1.08) | 0.14 |
| Male vs. Female | 1.38 (1.23–1.54) | <0.01 | 1.32 (1.18–1.48) | <0.01 | 1.46 (1.07–1.99) | 0.02 | 1.24 (1.11–1.39) | <0.01 |
| Co-morbidity | ||||||||
| Diabetes | 1.08 (0.97–1.21) | 0.16 | 1.35 (1.16–1.57) | <0.01 | 1.53 (1.01–2.30) | 0.04 | 1.32 (1.14–1.54) | <0.01 |
| Hypertension | 0.86 (0.77–0.96) | <0.01 | 0.98 (0.87–1.11) | 0.80 | 0.83 (0.58–1.18) | 0.30 | 0.99 (0.87–1.13) | 0.91 |
| Cancer | 1.77 (1.57–1.99) | <0.01 | 1.78 (1.57–2.01) | <0.01 | 1.79 (1.22–2.64) | <0.01 | 1.10 (0.98–1.25) | 0.12 |
| Prescribed | ||||||||
| Amoxicillin or ampicillin | 0.54 (0.42–0.71) | <0.01 | 0.56 (0.43–0.73) | <0.01 | 0.78 (0.45–1.35) | 0.37 | 0.61 (0.47–0.80) | <0.01 |
| Metformin | 0.76 (0.64–0.90) | <0.01 | 0.65 (0.53–0.80) | <0.01 | 0.59 (0.40–0.88) | <0.01 | 0.67 (0.55–0.83) | <0.01 |
| Other oral anti- diabetic drugs | 0.99 (0.87–1.14) | 0.92 | 1.15 (0.95–1.39) | 0.15 | 1.09 (0.74–1.62) | 0.65 | 1.23 (1.02–1.48) | 0.03 |
| Insulin | 1.00 (0.83–1.20) | 0.99 | 0.92 (0.76–1.13) | 0.44 | 0.98 (0.63–1.50) | 0.91 | 0.94 (0.77–1.15) | 0.54 |
| Statins | 0.54 (0.40–0.74) | <0.01 | 0.63 (0.46–0.86) | <0.01 | 0.56 (0.35–0.90) | 0.02 | 0.67 (0.49–0.92) | 0.01 |
Note: HR, hazard ratios.
Figure 2.
(A) Cumulative proportion of patients developing recurrent pyogenic liver abscess events during follow-up according to aspirin uses and non-uses, before propensity-score matching. Kaplan-Meier analysis for cumulative incidence of pyogenic liver abscess recurrence showed significantly lower risk in the aspirin users group than in the non-users group before propensity-score matching (p < 0.001). (B) Cumulative proportion of patients developing recurrent pyogenic liver abscess events during follow-up according to aspirin users and non-users, after propensity-score matching. Kaplan-Meier analysis for cumulative incidence of pyogenic liver abscess recurrence showed significantly lower risk in the aspirin users group than in the non-users group after propensity-score matching (p = 0.014).
The aspirin users in subgroup analysis had lower competing adjusted risk for PLA recurrence after a first episode of PLA; this was especially significant for females, those with diabetes or a non-cancer comorbidity, amoxicillin and ampicillin non-users, other anti-diabetic drug non-users, and insulin non-users (Fig. S1). Cause-specific hazard analysis also yielded similar results (data not shown). Aspirin use in the 6–12 months and 3–6 months prior to a first episode of PLA did not show a lower risk for PLA recurrence. The HR for PLA recurrence among patients with aspirin use at the time of the first episode of PLA was 0.62 (95% CI: 0.41–0.93; p = 0.02), and the HR for PLA recurrence among patients with aspirin use after the first episode was 0.98 (95% CI: 0.97–0.98, p < 0.01), with both groups having a lower risk of PLA recurrence (Table 4).
Table 4.
Estimated hazard ratio for pyogenic liver abscess recurrence with use of aspirin for various durations.
| Aspirin used time | aspirin users | aspirin non-users | Adjusted HR | p value |
|---|---|---|---|---|
| Prior 6–12 months before first episode of PLA | 558 | 5,546 | 1.28 (0.98–1.68) | 0.07 |
| Prior 3–6 months before first episode of PLA | 452 | 5,652 | 0.89 (0.64–1.25) | 0.52 |
| During first episode of PLA | 271 | 5,833 | 0.62 (0.41–0.93) | 0.02 |
| After first episode of PLA | 1,251 | 4,853 | 0.98 (0.97–0.98) | <0.01 |
Note: HR, hazard ratios; PLA, pyogenic liver abscess.
Adjusted HR: adjusted age, gender, diabetic, hypertension, cancer, amoxicillin or ampicillin, metformin, other oral anti-diabetic drugs, insulin, statins, aspirin used and aspirin used after pyogenic liver abscess first even as a time-dependent covariate.
The restricted cubic spline Cox model indicated that aspirin use was associated with a lower recurrent risk for PLA, especially in diabetic patients who did not receive an OAD or those who received a high-dose OAD for glucose control (Fig. 3). Aspirin use did not show a significant difference in risk for PLA recurrence in diabetic patients with and without insulin therapy.
Figure 3.
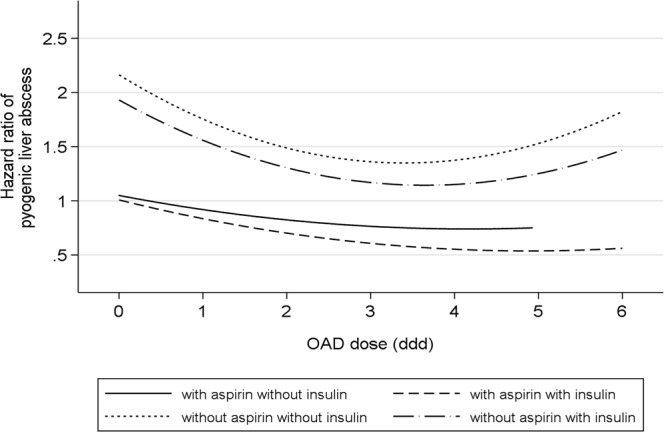
Dose-response association between oral anti-diabetic drug use (daily defined dose) and hazard ratio for recurrent pyogenic liver abscess in diabetic patients. Aspirin use was associated with lower risk for recurrent pyogenic liver abscess, especially in diabetic patients who did not receive an oral anti-diabetic drug (OAD) or those who received a high OAD dose for sugar control. Aspirin use did not show a significant difference in risk for recurrent pyogenic liver abscess in a comparison between diabetic patients with and without insulin therapy. Note: ddd, daily defined dose.
Effects of aspirin on ex vivo leukocyte bactericidal activity
Of 5 diabetic patients admitted to KCGMH between 2013 and 2016 with a diagnosis of recurrent KP-PLA, 3 were males and 2 females, with a mean age of 59.8 years (range: 54–68 years). These 5 patients did not take aspirin before or after a first episode of KP-PLA. The average time between first occurrence and KP-PLA recurrence was 1.7 years (range: 0.8–2.4 years). Poor glycemic control with hemoglobin A1c above 9.0% (average: 10.7%; range: 9.4–12.5%) was observed in these 5 patients. The demographic information and data gathered from these patients are shown in Table S2.
The difference between the rate of KP killing by leukocytes from these 5 diabetic patients and 5 healthy volunteers before oral administration of 100 mg of aspirin was significant (p < 0.01; Fig. 4). KP was significantly more susceptible to killing after incubation with leukocytes from healthy volunteers. The ex vivo bactericidal activity of leukocytes collected 1 h following oral administration of 100 mg of aspirin from 5 diabetic patients with KP-PLA recurrence and 5 healthy male volunteers was significantly greater than that of leukocytes collected from the same patients or from volunteers before aspirin treatment (p < 0.01; Fig. 4). The results indicated that aspirin was able to significantly enhance bacterial killing by leukocytes, whether collected from diabetic patients with KP-PLA recurrence or from healthy volunteers.
Figure 4.

Ex-vivo human leukocyte bactericidal activity assay. Peripheral WBCs from 5 diabetic patients with recurrent pyogenic liver abscess caused by KP and 5 healthy volunteers (controls) were collected before and 1 h after oral administration of 100 mg of ASA, and were incubated with KP that had previously been opsonized with normal human serum. ASA treatment significantly enhanced leukocyte killing of KP (p < 0.01), regardless of whether leukocytes were collected from patients or healthy volunteers. Note: ASA, aspirin; KP, Klebsiella pneumoniae; WBC, white blood cells.
Discussion
We provided evidence that exposure to aspirin for more than 30 days, from 90 days before to 90 days after the first episode, was associated with decreased risk for recurrence of PLA in a nationwide population-based study in Taiwan. The duration relationship for aspirin use (both at the time of the first episode and at the time after the first episode of PLA; Table 4) was evident in the current study. Furthermore, the effect of aspirin use on a lower risk for PLA recurrence was found especially in diabetic patients who did not receive an OAD or in those on a higher OAD dose for glucose control (Fig. 4). The risk of recurrence may vary with glycemic status in diabetic patients; however, no laboratory tests that could serve as a proxy for glycemic status (HbA1c) were available in this dataset. Our findings imply that, with aspirin use, PLA may be preventable, especially in patients with a new diagnosis of diabetes and in diabetic patients with poor glucose control. We further demonstrated that aspirin enhanced leukocyte bactericidal activity against KP in diabetic patients with KP-PLA recurrence and in healthy volunteers in an ex-vivo study. To our knowledge, this is the first longitudinal study that specifically examined the role of aspirin in the prevention of PLA recurrence, using both population-based and ex-vivo findings.
Recurrent KP-PLA has seldom been reported14,15. Recently, a tertiary teaching hospital in China reported that 4 (4.1%) of 98 cases of KP-PLA had a prior history of PLA; therefore, these 4 cases were thought to represent recurrences, with onset between 7 and 96 months after the first episode16. In another study conducted in Taiwan that included 601 patients with PLA with a mean follow-up of 6 years, the cumulative recurrence rate of PLA was lower in both the patients with cryptogenic PLA (2.0%) and diabetic patients with PLA (4.4%) than those pre-existing hepatobiliary diseases with PLA (23.8%)1; moreover, 80% of diabetic patients initially infected with KP in this study were again infected with KP when the PLA recurred, which suggests that the recurrence rate of PLA in diabetic patients was low and most PLA recurrence was due to KP1. However, these single-center retrospective studies were limited by selection and recall bias1,16, and our large-scale population-based study might overcome these limitations. Furthermore, our cohort excluded patients with cholangitis, hepatobiliary cancer, or splenomegaly to reduce the complexity of analysis. Meanwhile, we also excluded patients with prior myocardial infarction or stroke to ensure comparability between aspirin users and non-users. The results showed that aspirin therapy decreased the risk of PLA recurrence. This finding was also confirmed in multivariate analysis after adjusting for a number of potential confounders, which was further reinforced by the duration relationship for aspirin use.
Adding to the long list of benefits, researchers have found that aspirin can reduce virulent bacteria associated with serious infections. One study found that SAL, a byproduct of aspirin, disrupted bacterial ability to adhere to host tissue, reducing propagation and spread of infection. Animals treated with aspirin have fewer bacteria at sites of infection, and develop smaller abscesses17. Additionally, our previous study has demonstrated that SAL could affect CPS production in KP, therefore attenuating the pathogenicity of KP strains. Aspirin also promotes human leukocyte phagocytosis and bactericidal activity against KP13. The majority of KP-PLA cases are preceded by colonization of the gastrointestinal tract, as suggested in epidemiological studies9. We found that patients who had used aspirin during the month prior to the diagnosis of community-acquired KP bacteremia appeared to have a lower risk for invasive syndromes including PLA13. As a result, aspirin may not be curative, but it could reduce the ability of bacteria to cause infection. We speculate that, with time, the immune system can adapt to compensate for or overcome the deficits, thereby decreasing the risk of PLA associated with use of aspirin. More studies are needed to confirm this hypothesis. The ex-vivo study still suggests that aspirin renders the host susceptible to KP. However, aspirin may simply arrest invasion by KP through the loss of resistance to colonization by KP. The consequences of an altered intestinal microbiota due to aspirin therapy are not fully understood. We did not explore how aspirin administration disturbs host-microbiota homeostasis that leads to KP-PLA. This also presents an opportunity for intervention, if established intestinal colonization can be eliminated. Use of aspirin to enhance innate immune defenses and inhibit pathogen colonization of the intestinal lumen and translocation across mucosal barriers may provide new strategies for management of this emerging infectious disease.
This study had some limitations. First, this was an observational study and could not prove a causal relationship. However, the ex-vivo leukocyte bactericidal experiment showed that aspirin was able to significantly enhance bacterial killing by leukocytes, subsequently resulting in lower risk for PLA recurrence. Second, smoking, drinking, exercise habits, diet, and other behaviors were not included in our model, but these may influence the relationship between aspirin and PLA recurrence. To minimize bias, we used propensity-score matching and subgroup analyses, which showed a strong relationship with health-seeking behaviors, but still indicated that aspirin was associated with reduced risk for PLA recurrence. Third, the disease and drug records collected from the databases may have been incorrect. We considered PLA as a critical event. Although, and used ICD-9-CM code 041.3 to identify sources of infection other than the lung. This code may have been omitted in the dataset. Our PLA cohort excluded patients with a history of hepatobiliary malignancy, cholangitis, common bile duct stones, and portal hypertension with splenomegaly to limit PLA to cryptogenic cases18.
In conclusion, our ex-vivo study suggested that aspirin enhanced the bactericidal activity of leukocytes against KP. The translation of these insights into the clinical arena demonstrated that exposure to aspirin was associated with reduced risk for PLA recurrence; moreover, a duration relationship was evident in the population-based study. Future studies are warranted to clarify the mechanism by which aspirin alters the pathogenesis of PLA by KP and how aspirin influences the risk of KP-PLA recurrence.
Methods
Data sources
The present study used data from the Taiwan National Health Insurance Research Database (NHIRD). The NHIRD, initiated in 1995, has been managed and released to the public by the National Health Research Institute (NHRI) of Taiwan, and presently covers more than 99% (approximately 23 million) of the residents in Taiwan. For use in research and health policy development, the Taiwan NHRI created a longitudinal health insurance database (LHID) that included 1 million randomly sampled residents in 2005. There were no significant differences in characteristics, including age and sex, between enrolled residents in the LHID and those in the NHIRD19,20. Any information in the LHID that could reveal the identities of individual patients was de-identified. All procedures including experimental protocol in this study followed the directives of the Declaration of Helsinki and informed consent were approved by the Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital (KCGMH; 201601500B0).
We identified patients hospitalized with the diagnosis of PLA (ICD-9 code 572.0) from the LHID between January 1, 2000 and December 31, 2013. Aspirin use (Anatomical Therapeutic Chemical code B01AC06) was defined as dosing of aspirin for more than 30 days, from 90 days before to 90 days after the first PLA episode during the study period. To confirm duration relationship, we also analyzed aspirin use for 6–12 months and 3–6 months prior to the first PLA episode, as well as use after the PLA episode.
Baseline characteristics
Baseline characteristics recorded within 3 years before the first episode of PLA included age, sex, comorbidities, and medication usage. Comorbidities of diabetes mellitus (ICD-9 codes 250, 357.2, 366.41, 362.0A181), hypertension (ICD-9 codes 401, 402, 403, 404, 405, A260, A269), and cancer (ICD-9 codes 140–208) were defined as diagnoses made at 2 outpatient visits or during 1 hospitalization within 3 years before the first episode of PLA. Prescriptions for amoxicillin or ampicillin, metformin, other oral anti-diabetes drugs (OADs) (sulfonylureas, alpha-glucosidase inhibitors, thiazolidinediones, dipeptidyl peptidase-4 inhibitors), insulin, and statins were also defined as dosing for more than 30 days, from 90 days before to 90 days after the first episode; these were treated as covariates in this study.
Those who did not receive aspirin during the study period were considered controls and were assigned an index date corresponding to the same year of the case. Patients younger than 20 years and those with prior stroke or acute myocardial infarction were excluded. The etiology of cryptogenic PLA was nearly always KP in Taiwan18. Therefore, we further excluded patients with a history of hepatobiliary malignancy, cholangitis, common bile duct stones, or portal hypertension with splenomegaly in order to limit our cohort to PLA caused by KP. Based on intention-to-treat principle, analyses were conducted according to initial assignment of aspirin modality, regardless of any subsequent changes.
Outcome variables: PLA recurrence, complications, and mortality
The observation period started 30 days after discharge for the first episode of PLA and ended when the patient died or developed recurrence, or December 31, 2013, which ever occurred earlier. The incidence of cancer or myocardial infarction was also followed up until death or December 31, 2013. Mortality records were retrieved from patients who died in the hospital or withdrawn from the NHIRD.
Study design and participants for ex-vivo human leukocyte bactericidal activity assay
Diabetic patients admitted to KCGMH between January 1, 2013 and December 31, 2016 with community-acquired mono-microbial bacteremia caused by KP were included to determine the patients with KP-PLA recurrence21. PLA recurrence was defined as having a typical clinical presentation, with new imaging findings of abscess recurrence after the first episode of abscess had fully resolved. All isolated KP strains were stored at −80 °C before use. Peripheral blood samples were collected from 5 healthy male volunteers aged 25–40 years and from participants with recurrent KP-PLA for an ex-vivo bactericidal assay as described below. All participants provided written informed consent.
Determination of K. pneumoniae serotype and rmpA genes
Genotyping of 7 clinically significant capsular types (K1, K2, K5, K20, K54, K57, and KN1) for all KP isolates was performed with a polymerase chain reaction (PCR) assay using primers designed for the cps variable region22. PCR was also used to determine the presence of rmpA. Previously published primers for rmpA were used, and the expected PCR products of rmpA were 535 bp in length23.
Ex-vivo human leukocyte bactericidal activity assay
Bactericidal activity was measured using a standard assay method24. The KP isolate from each enrolled patient during the first episode of KP-PLA (study for each patient, respectively) and serotypes K1 KP, KP-M1 (study for volunteers) were cultured on Tryptic Soy Agar medium overnight at 37 °C, and then opsonized with addition of 10% pooled serum collected from 5 healthy male volunteers who did not take any aspirin prior to donation. The suspension was mixed at a 10:1 ratio of infected to whole blood leukocytes, which had been collected from diabetic patients with recurrent KP PLA and 5 volunteers, either before or 1 h after a 100-mg oral dose of aspirin. Samples were collected 1 h later and diluted in H2O (pH 11.0, adjusted with NaOH) to lyse the leukocytes and disperse the bacteria for the power-plate colony assay25. All tests were performed in triplicate to ensure reproducibility.
Statistical analysis
Baseline characteristics were compared using either a 2-sided t-test or χ2 test as suitable. In multivariate Cox proportional hazards regression models, the effects of aspirin were adjusted for age, sex, comorbidities, and prescriptions used. Results were expressed as hazard ratios (HRs) and compared with those aspirin non-users. All time-independent covariates were compared using estimated log-log survival curves to ensure a constant HR over time. Adjusted HRs and 95% confidence intervals (CIs) for PLA recurrence with aspirin use were based on participant characteristics (see below). After adjusting for covariates, the cumulative hazards of PLA recurrence over time were compared between aspirin users and non-users with Cox proportional hazards regression models and a cause-specific, PLA recurrence and mortality, competing adjusted-risk model26. Propensity-score matched logistic regression analysis for the likelihood of aspirin use was performed using the baseline covariates. Adequacy of balance for the covariates in the matched sample was assessed using the standardized mean difference between the 2 groups, with differences less than 10% reflecting good balance.
In subgroup analysis for subjects with diabetes, restricted cubic spline curves were used to examine non-linear associations of aspirin users and non-users with recurrent PLA, with adjustments for confounding variables, to further characterize the nature of the relationships between OAD doses with the risk of PLA recurrence. Four knots were chosen to produce a curve that appeared adequately smooth27. To control for consistent medication effect across different durations, the aspirin used after the first episode of PLA was assessed as a duration relationship covariate in the Cox model. The counts of surviving bacteria in the bactericidal activity assay were analyzed using the log rank test. The 2-tailed test was used in statistical significance testing and a p value < 0.05 was considered significant. All statistical analyses were carried out using STATA 15.1 (STATA Corp., College Station, TX, USA) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Supplementary information
Aspirin use is associated with reduced risk for recurrence of pyogenic liver abscess: a propensity score analysis
Acknowledgements
The work was supported by a grant from the Ministry of Science and Technology of Taiwan (MOST 107-2314-B-182A-141 -MY3). We thank Dr. Chien-Ching Hung at the Department of Internal Medicine, National Taiwan University Hospital, for his critical review of this manuscript.
Author Contributions
J.S.L., C.H.L. and S.K.C. conceived and designed the study; J.S.L., C.H.L., S.K.C., W.C.T., C.C.C. and F.J.C. collected the data; J.S.L. and C.H.L. analyzed and interpreted the data; J.S.L. and C.H.L. conceptualized and drafted the manuscript. All authors have reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-48017-3.
References
- 1.Cheng HC, et al. Long-term outcome of pyogenic liver abscess. Factors related with abscess recurrence. J. Clin. Gastroenterol. 2008;42:1110–1115. doi: 10.1097/MCG.0b013e318157e4c1. [DOI] [PubMed] [Google Scholar]
- 2.Siu LK, et al. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from non-infectious subjects in Hong Kong, Singapore, and Taiwan. J. Clin. Microbiol. 2011;49:3761–3765. doi: 10.1128/JCM.00977-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pope JV, Teich DL, Clardy P, McGillicuddy DC. Klebsiella pneumoniae liver abscess: an emerging problem in North America. J. Emerg. Med. 2011;41:e103–e105. doi: 10.1016/j.jemermed.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 4.Moore R, O’Shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection. 2013;41:681–686. doi: 10.1007/s15010-013-0408-0. [DOI] [PubMed] [Google Scholar]
- 5.Turton JF, et al. Genetically similar isolates of Klebsiella pneumoniae serotype K1 causing liver abscesses in three continents. J. Med. Microbiol. 2007;56:593–597. doi: 10.1099/jmm.0.46964-0. [DOI] [PubMed] [Google Scholar]
- 6.Chen SC, et al. Comparison of Escherichia coli and Klebsiella pneumoniae liver abscesses. Am. J. Med. Sci. 2007;334:97–105. doi: 10.1097/MAJ.0b013e31812f59c7. [DOI] [PubMed] [Google Scholar]
- 7.Tsai FC, Huang YT, Chang LY, Wang JT. Pyogenic liver abscess as endemic disease, Taiwan. Emerg. Infect. Dis. 2008;14:1592–1600. doi: 10.3201/eid1410.071254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung CP, et al. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut. 2002;50:420–424. doi: 10.1136/gut.50.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fung CP, et al. Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerg. Infect. Dis. 2012;18:1322–1325. doi: 10.3201/eid1808.111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin YT, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol. 2012;12:13. doi: 10.1186/1471-2180-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stables MJ, et al. Priming innate immune responses to infection by cyclooxygenase inhibition kills antibiotic-susceptible and –resistant bacteria. Blood. 2010;116:2950–2959. doi: 10.1182/blood-2010-05-284844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domenico P, Schwartz S, Cunha BA. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect. Immun. 1989;57:3778–3782. doi: 10.1128/iai.57.12.3778-3782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CH, et al. Aspirin enhances opsonophagocytosis and is associated to a lower risk for Klebsiella pneumoniae invasive syndrome. BMC Infect. Dis. 2014;14:47. doi: 10.1186/1471-2334-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang YS, et al. Recurrent Klebsiella pneumoniae liver abscess: Clinical and microbiological characteristics. J. Clin. Microbiol. 2009;47:3336–3339. doi: 10.1128/JCM.00918-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weng SW, Liu JW, Chen JW, Wang PW. Recurrent Klebsiella pneumoniae liver abscess in a diabetic patient followed by Streptococcus bovis endocarditis–occult colon tumor plays an import role. Jpn. J. Infect. Dis. 2005;58:70–72. [PubMed] [Google Scholar]
- 16.Qian Y, et al. A retrospective study of pyogenic liver abscess focusing on Klebsiella pneumoniae as a primary pathogen in China from 1994 to 2015. Sci. Rep. 2016;6:38587. doi: 10.1038/srep38587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kupferwasser LI, et al. Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J. Clin. Invest. 2003;112:222–233. doi: 10.1172/JCI200316876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YT, Liu CJ, Yeh YC, Chen TJ, Fung CP. Ampicillin and amoxicillin use and the risk of Klebsiella pneumoniae liver abscess in Taiwan. J. Iinfect. Dis. 2013;208:211–217. doi: 10.1093/infdis/jit157. [DOI] [PubMed] [Google Scholar]
- 19.Longitudinal Health Insurance Database 2000, http://nhird.nhri.org.tw/en/Data_Subsets.html#S3 (cited 2019 Mar).
- 20.Lai SW, Lin CL, Liao KF. Association between oral corticosteroid use and pyogenic liver abscesses in a case-control study. Biomedicine-Taiwan. 2018;8:33–39. doi: 10.1051/bmdcn/2018080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CH, Chuah SK, Tai WC, Chen IL. Platelet reactivity in diabetic patients with invasive Klebsiella pneumoniae liver abscess syndrome. Infect. Drug Resist. 2018;11:1669–1676. doi: 10.2147/IDR.S174913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenover FC, et al. Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. J. Clin. Microbiol. 2008;46:2231–2240. doi: 10.1128/JCM.00480-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu WL, et al. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin. Infect. Dis. 2006;42:1351–1358. doi: 10.1086/503420. [DOI] [PubMed] [Google Scholar]
- 24.Hampton MB, Vissers MC, Winterbourn CC. A singleassay for measuring the rates of phagocytosis and bacterial killing by neutrophils. J. Leukoc. Biol. 1994;55:147–152. doi: 10.1002/jlb.55.2.147. [DOI] [PubMed] [Google Scholar]
- 25.Green JN, Winterbourn CC, Hampton MB. Analysis of neutrophil bactericidal activity. Methods Mol. Biol. 2007;412:319–332. doi: 10.1007/978-1-59745-467-4_21. [DOI] [PubMed] [Google Scholar]
- 26.Mohammad KA, Fatima-Tuz-Zahura M, Bari W. Fine and Gray competing risk regression model to study the cause-specific under-five child mortality in Bangladesh. BMC Int. Health Hum. Rights. 2017;17:3. doi: 10.1186/s12914-017-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrell, F. E. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. (Springer-Verlag, 2001).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Aspirin use is associated with reduced risk for recurrence of pyogenic liver abscess: a propensity score analysis