Abstract
Pyrethroids are broad-spectrum insecticides and presence of chiral carbon differentiates among various forms of pyrethroids. Microbial approaches have emerged as a popular solution to counter pyrethroid toxicity to marine life and mammals. Bacterial and fungal strains can effectively degrade pyrethroids into non-toxic compounds. Different strains of bacteria and fungi such as Bacillus spp., Raoultella ornithinolytica, Psudomonas flourescens, Brevibacterium sp., Acinetobactor sp., Aspergillus sp., Candida sp., Trichoderma sp., and Candia spp., are used for the biodegradation of pyrethroids. Hydrolysis of ester bond by enzyme esterase/carboxyl esterase is the initial step in pyrethroid biodegradation. Esterase is found in bacteria, fungi, insect and mammalian liver microsome cells that indicates its hydrolysis ability in living cells. Biodegradation pattern and detected metabolites reveal microbial consumption of pyrethroids as carbon and nitrogen source. In this review, we aim to explore pyrethroid degrading strains, enzymes and metabolites produced by microbial strains. This review paper covers in-depth knowledge of pyrethroids and recommends possible solutions to minimize their environmental toxicity.
Keywords: biodegradation, pyrethroids, metabolic pathway, esterase enzyme, hydrolysis
Introduction
Pyrethroids are the most commonly used global pesticides. Chrysanthemum cinerariaefolium flowers are the natural source of pyrethroids and allethrin was developed as the first synthetic pyrethroid insecticide in 1949 (Ensley, 2018; Gammon et al., 2019; Xu et al., 2019). Pyrethroids can be divided into two groups, type I pyrethroids containing basic cyclopropane carboxylic (e.g., allethrin) and type II pyrethroids containing cyano group (Proudfoot, 2005; Wolansky and Harrill, 2008; Chang et al., 2016; Figure 1). Presence of cyano group in type II pyrethroids enhances their insecticidal properties as compared to type I pyrethroids. All pyrethroids contain at least four stereoisomers, which exhibit different biological activities (Table 1). Pyrethroids are either marketed as racemic mixture of stereoisomers or single chemical isomer. Piperonyl butoxide acts as synergist in commercial formulation of pyrethroids and inhibits the metabolic degradation of active compounds (Bradberry et al., 2005; Fai et al., 2017). Deltamethrin is used in different countries to control malaria-spreading mosquitoes. Pyrethroids are reported to be 2250 times more toxic to insect than mammals and disrupt sodium, chloride channels (Chrustek et al., 2018). At high concentrations pyrethroids inhibit the functioning of gamma amino butyric acid (GABA) gated chloride ion channel (Bradberry et al., 2005; Gammon et al., 2019). Pyrethroids are mainly used to control insect pests of agriculture, horticulture, forestry and household. Pyrethroids are considered comparatively safe but their extensive use makes them harmful for humans and animals (Kuivila et al., 2012; Burns and Pastoor, 2018; Bordoni et al., 2019). Previous reports have concluded their detrimental effects on non-target species including marine fish and aquatic insects (Burns and Pastoor, 2018; Lu et al., 2019). Pyrethroid toxicity biomarkers have been well documented in fish (Ullah et al., 2019). Frequent pyrethroids applications in agriculture and households can cause inappropriate effects on human growth. In humans, pyrethroids exposure leads to contaminated urine, low serum quality, and antiandrogenic activity. Bio-absorption of pyrethroids was detected in the urine samples of outdoor workers in California (Sullivan et al., 2019), which indicates the importance of this topic. In rats, the developmental of bifenthrin neurotoxicity was reported as mixed type (typeI/II) (Gammon et al., 2019) whereas non-target neurotoxicity of pyrethroids has also been investigated in zebrafish (Paravani et al., 2017; Awoyemi et al., 2019; Strungaru et al., 2019).
Figure 1.
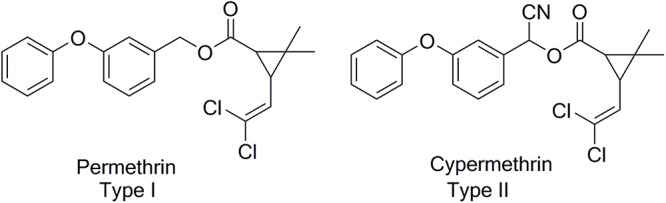
Structure of type I and type II pyrethroids.
Table 1.
Brief classification of indoor and agricultural pyrethroids.
| Type I | Type II | Racemic pyrethroids | |
|---|---|---|---|
| S.No. | pyrethroids | pyrethroids | (formulations with isomer) |
| 1 | Allethrin | Cyhalothrin | Resmethrin (bioresmethrin and cis-resmethrin |
| 2 | Bioallethrin | Cyfluthrin | Allethrin (d-allethrin, bioallethrin, esbiothrin, and s-bioallethrin) |
| 3 | Bifenthrin | γ-Cyhalothrin | Fenvalerate (esfenvalerate) |
| 4 | Permethrin | Cypermethrin | Cyhalothrin (γ-cyhalothrin) |
| 5 | D-Phenothrin | α-Cypermethrin | Phenothrin (d-phenothrin) |
| 6 | Prallethrin | Deltamethrin | Cypermethrin (d-cypermethrin) |
| 7 | Resmethrin | Fenpropathrin | |
| 8 | Bioresmethrin | Fenvalerate | |
| 9 | Tefluthrin | Esfenvalerate | |
| 10 | Tetramethrin | Flucythrinate | |
| 11 | Flumethrin | ||
| 12 | Tau-fluvalinate |
Microbial system is considered suitable for the biodegradation of synthetic pyrethroids (Bhatt et al., 2019a). Most of the previous work about pyrethroids is based on bacterial degradation. Bacterial strains from the genera Bacillus, Pseudomonas, Raoultella, Achromobacter, Acidomonas, Brevibacterium, Pseudomonas, Streptomyces, Serratia, Sphingobium, Clostridium, Klebsiella, and Lysinibacillus have been characterized for pyrethroid degradation (Cycoń and Piotrowska-Seget, 2016; Birolli et al., 2019; Hu et al., 2019; Zhao et al., 2019). Fungi also have the potential to degrade wide variety of pesticides (Maqbool et al., 2016). Only a few groups of fungi including Aspergillus niger, Aspergillus terricola, Trichoderma viridae, Phaenerochaete chrysosporium (Saikia and Gopal, 2004; Deng et al., 2015), Candia pelliculosa (Chen et al., 2012c), and Cladosporium sp. (Chen et al., 2011b) have been reported for pyrethroid biodegradation (Birolli et al., 2018). Fungi have been found to possess comparatively better pesticide degradation potential than bacteria (Bhatt, 2019; Gangola et al., 2019). Many researchers have predicted pyrethroid degradation metabolites and pathways. A few metabolites are common among all pyrethroids, which are used as metabolic markers (such as 3-phenoxybenzoic acid) during microbial degradation. Esterase enzymes are often studied for pyrethroid degradation, due to their presence in bacteria, fungi, insect, and human tissues (Liu et al., 2017; Wang et al., 2018; Bai et al., 2019). Different genes with complete open reading frames coding pyrethroid hydrolase/esterase enzymes have been reported in bacterial strains (Hu et al., 2019; Yang et al., 2019).
Previous studies have concluded that microbial cultures can efficiently remove pyrethroids from the environment. In this review, we have attempted to compile the related information about the toxicity and microbial degradation of pyrethroid insecticides.
Hazardous Effects of Pyrethroids
Toxicity studies have revealed several effects of pyrethroids on human and marine life (Table 2). Large-scale application of pyrethroids affects humans and animals. Indoor pyrethroids exposure studies revealed low levels of pyrethroids absorption in biological and environmental samples (Ghazouani et al., 2019). Measurement of absorbed daily dose (ADD) from biological samples is more reliable than environmental samples (Williams et al., 2003). Cyfluthrin studies on a medium pile of nylon carpet suggested that pyrethroids were absorbed in the surrounding surfaces and were also found in human urine samples (Williams et al., 2003; Sullivan et al., 2019). Presence of 4-fluro-3 phenoxybenzoic acid in urine samples indicated human exposure to pyrethroids and environmental measurements further confirmed the results (Williams et al., 2003). Studies on pyrethroid residues in children diaper revealed that pyrethroid metabolites were stable on the diaper up to 72 h (Hu et al., 2004). Pyrethroid residues have been reported in dust, cloth, union suit samples, diaper, military uniform, and urine samples (Bradman et al., 2007; Proctor et al., 2019). Pyrethroid residues in the urine samples of pregnant women have been reported from Jiangsu China and France (Qi et al., 2012; Dereumeaux et al., 2018; Kamai et al., 2019). Children in United States are more exposed to pyrethroids as compared to organic food taking children of other areas. Pyrethroid metabolite 3-phenoxybenzaldehyde was commonly found in the urine samples of exposed children (Lu et al., 2009; Dalsager et al., 2019). Presence of 3-phenoxybenzaldehyde metabolite in the semen of Japanese males suggested that their semen quality was decreased by pyrethroids (Toshima et al., 2012). Residues of organophosphorous and pyrethroids were also reported in Australian preschool children (Babina et al., 2012) and urinary concentration of pyrethroids from Queensland (Australia) pre-schoolers correlated with the age and sex (Li et al., 2019). Pyrethroids are also used to disinfect the aircrafts and presences of 3-phenoxybenzaldehyde in the urine samples of flight attendants (18–65 years old) clearly indicated pyrethroid exposure in different age groups of humans (Wei et al., 2012). Higher pyrethroids exposure was reported in farmers and consumers of northern Thailand (Hongsibsong et al., 2019) and extensive studies revealed their carcinogenic potential (Navarrete-Meneses and Pérez-Vera, 2019).
Table 2.
Hazardous effects of different pyrethroids.
| S. No. | Pyrethroids | Sample source/Study sample | Specific statement | References |
|---|---|---|---|---|
| 1 | Pyrofenofos, cypermethrin, permethrin, tefluthrin, trans-fenfluthrin, bifenthrin, indoxacarb, acephate, and spinosyn A | Larvae of tobacco budworm, Heliothis virescens (F.) | Detection of esterase resistance/susceptibility in insect larvae | Huang and Ottea, 2004 |
| 2 | Racemic (cis-bifenthrinm, fonofos, and profenofos), racemic trans-permethrin, cis-permethrin | Freshwater, invertebrates | Determination of enantioselectivity based toxicity of chiral pyrethroids | Liu et al., 2005 |
| 3 | Cyfluthrin | Human fetal astrocyte cells | Affecting growth, survival and functioning of human astrocyte cells | Mense et al., 2006 |
| 4 | Cis and trans permethrin | Human urine | Detected the presence of pyrethroid in urine | Bradman et al., 2007 |
| 5 | Cyfluthrin | Human peripheral lymphocytes | Genotoxic effect seen in the human peripheral lymphocytes due to mutation | Ila et al., 2008 |
| 6 | Type I and Type II pyrethroids | Mild poisoning sign | Hyper activity and hyper-excitability in mice and rat | Wolansky and Harrill, 2008; Wang et al., 2018 |
| 7 | Type I and Type II pyrethroids | Moderate to severe poisoning sign | Prostration, sinuous writhing, uncoordinated twitches, normothermia | Wolansky and Harrill, 2008; Wang et al., 2018 |
| 8 | Type I and Type II pyetrhoids | Nearly lethal syndrome | Clonic seizures, tonic seizure, and rigors occasionally just before death | Wolansky and Harrill, 2008 |
| 9 | Cyfluthrin and beta cyfluthrin | Androgen responsive cell line, MDA-kb2 | Antiangrogenic activity was reported in presence of pyrethroids | Zhang et al., 2008 |
| 10 | λ-Cyhalothrin and γ-cylaothrin | Aquatic invertebrate and Fish | Single enantiomer is less toxic than racemate of pyrethroid | Giddings et al., 2009 |
| 11 | Racemic pyrethroid | Urine sample | Detection of pyrethroid intermediate 3-phenoxybenzoic acid | Lu et al., 2009 |
| 12 | α-Cypermethrin | Human peripheral blood lymphocytes | High cytotoxic effect at >20 μg/ml | Kocaman and Topaktaş, 2009 |
| 13 | Bifenthrin, permethrin, fenvalerate | Yeast strains | Enantioselctivity in esterogenic activity | Wang et al., 2010 |
| 14 | Deltamethrin, β-cyfluthrin, cypermethrin, permethrin, bifenthrin, esfenvalerate, λ-cyhalothrin, tefluthrin, fenpropathrin, resmethrin, and S-bioallethrin | Swiss-webster mice | Pyrethroid actions affect the sodium influx in cerebrocortical neurons | Cao et al., 2011 |
| 15 | Beta-cypermethrin | Soil samples | Microbial community in soil affected by the action of cypermethrin | Zhuang et al., 2011 |
| 16 | Cypermethrin and decamthrein | Brinjal fruits | It was noticed that trace quantity persist in Brinjal upto a long time | Kaur et al., 2011 |
| 17 | Lambda-cyhalothrin | Developing rats | Cholinergic dysfunctions and oxidative stress is responsible for neurotoxicity in rats | Ansari et al., 2012 |
| 18 | Cypermethrin | Myrica opima | Hepatopancreas and gill have increased glycogen | Tendulkar and Kulkarni, 2012 |
| 19 | Deltamethrin | Human dopaminergic neuroblastoma SH-SY5Y cells | Oxidative stress mediated neurotoxicity | Romero et al., 2012 |
| 20 | Deltamethrin | Male BALB/c Mice | Deltamethrin inhibit the osteoclast development | Sakamoto et al., 2012 |
| 21 | Deltamethrin | Rat bone marrow cells | Testicular injury and genotoxicity due to pyrethroids when compare with biopesticide (Bacillus thuringiensis) | Ismail and Mohamed, 2012 |
| 22 | Cypermethrin | CV-1 cells (Cercopithecus aethiops monkey kidney Cells) | Cypermethrin inhibited the interaction of androgen receptor and steroid receptor coactivator-1 | Pan et al., 2012 |
| 23 | Deltamethrin | Trichogramma evanescencs, T. semblidis | Discrimination of sex pheromones affected by deltamethrin | Delpuech et al., 2012 |
| 24 | Bifenthrin | Rat adrenal pheochromocytoma cells (PC-12) | Bifenthrin affects the antioxidant enzyme due to enantioselectivity | Lu, 2013 |
| 25 | 3-Phneoxybenzoic acid | Urine and semen samples | Pyrethroid exposure reduced semen quality | Toshima et al., 2012 |
| 26 | Pyrethroids (3-phenoxybenzoic acid, 2-methyl 3-phenoxybenzoic acid) | Urine samples | Chronic exposure of pyrethroids on Australian preschool childrens | Babina et al., 2012 |
| 27 | Pyrethroids mixed | Urine samples of flight attendant | Detection of pyrethroid metabolites in urine analysis | Wei et al., 2012 |
| 28 | Lambda-cyhalothrin | Liver of Oreochromis niloticus | Apoptotic and oxidative effect due to piperonyl butoxide treatment with lambda- cypermethrin | Piner and Üner, 2012 |
| 29 | Permethrin | Mice | Reproductive toxicity due to enatioslectivity of permethrin | Jin et al., 2012 |
| 30 | Bifenthrin | Sandy loam soil | Difference of half life in sterile and non sterile soil indicated that bifenthrin persistence change microbial community | Sharma and Singh, 2012 |
| 31 | Twelve different pyrethroids | Marine animals (Dolphins) | Mother to calf transfer of pyrethroids by lactation and gestation in Dolphin | Alonso et al., 2012 |
| 32 | Thirteen different pyrethroids | Human breast milk | Analysis of pyrethroid in Brazil, Columbia and Spain by food samples to humans than transfer rate in infants | Corcellas et al., 2012 |
| 33 | Permethrin | Consumed human food, residential exposure | Mathematical modeling of EPA that is SHEDS-multimedia model | Zartarian et al., 2012 |
| 34 | Lambda-cyhalothrin | Male mice | Reproductive and Hepatotoxicity observed | Al-Sarar et al., 2014 |
| 35 | Pyrethroids and metabolites | Urinary sample of pregnant women | Data indicated effect of pyrethroid on pregnant women that will also affect infants | Qi et al., 2012 |
| 36 | Permethrin, cfluthrin, esfenvalerate, cypermethrin | Mammalian cells, fishes | Pyrethroids act as endocrine disruptor | Brander et al., 2016 |
| 37 | λ-Cyhalothrin, fenvalerate and permethrin | Embryo of Zebra fish (Danio rerio) | Triiodothyronine (T3) level decreased due to exposure of lambda cyhalothrin and Fenvalerate | Zhang et al., 2017 |
| 38 | Cypermethrin, deltamethrin and cyhalothrin | Cucumis sativus | Chlorophyll and caretonoids showed sensitive effect | Braganca et al., 2018 |
| 39 | Cypermethrin | Bacillus sp. | In vitro toxicity detected in human cell line | Sundaram et al., 2013 |
| 40 | Bifenthrin, λ-cyhalothrin, cyfluthrin, cypermethrin, cis-deltamethrin, esfenvalerate, and cis/trans permethrin | Solid food sample | Pyrethroid degradates not present in sufficient level in diet to substantially impact the adults | Birolli et al., 2016b; Morgan et al., 2018 |
| 41 | Cypermethrin | Salvator merianae (Argentine tegu) | Cypermethrin with other pesticides affect immune and endocrine system | Mestre et al., 2019 |
Macrophages are immune cells that play important role in pathogen removal from the cells. It was observed that β-cypermethrin and cyhalothrin treatment decreased phagocytic activity and nitric oxide production in macrophage cells (He et al., 2019). No activity was detected at low concentration of cyhalothrin whereas macrophage activity was blocked at higher concentration. Direct effect of cyhalothrin on macrophage cells is due to the activity of sodium ion membrane channels whereas activity of hypothalamus pituitary adrenal axis caused indirect effects in rats (Righi and Palermo-Neto, 2005; He et al., 2019). Different levels of pyrethroid toxicity in freshwater invertebrates Ceriodephnia dubia and Daphnia magna is due to a selective enantiomer in racemate (Liu et al., 2005). Comparative study of cyfluthrin and chlorpyrifos toxicity in human fetal astrocytes (star shaped glial cells in the brain and spinal cord) revealed that cyfluthrin exerts more toxic effects on survival, growth and proper functioning of human peripheral lymphocytes, and induces apoptosis (Mense et al., 2006; Segura et al., 2018). Cyfluthrin and chlorpyrifos over express pro-inflammatory mediators, and cyfluthrin can cause mutation to change chromosome number (Mense et al., 2006; Muzinic et al., 2018). Genotoxic and cytotoxic effects of cyfluthrin were detected by Salmonella/mammalian microsome mutagenicity test, chromosomal aberration, chromatid exchange, and micronucleus formation in cultured human peripheral blood lymphocytes in vitro (Ila et al., 2008; Chalap et al., 2018). Pyrethroid genotoxicity demands for their restricted use around children, elderly people, and pregnant women (Kocaman and Topaktaş, 2009).
Pyrethroids are neurotoxic pesticides and affect neurotransmitters (Gammon et al., 2019). Effect of low acute oral dose of pyrethroids has been investigated in small rodents. Neurobehavioral study suggested that pyrethroids block sodium chloride and GABA channels, which inhibit transfer of neurotransmitters between cells (Cao et al., 2011; Richardson et al., 2019). Permethrin was reported against Laccophilus minutus (Touylia et al., 2019) and in humans it is absorbed through dermal and non-dietary entry points (Nakagawa et al., 2019). Cypermethrin, allethrin, cis/trans permethrin and deltamethrin modified the strength and behavior of tested organisms, whereas decreased grip strength was noted after pyrethrum, cypermethrin, bifenthrin, β-cyfluthrin, deltamethrin, S-bioallethrin, and permethrin treatments. A coordination study of deltamethrin and α-cypermethrin with rotarod revealed that the compound with α-cyano group enhanced acoustic evoked startle response amplitude whereas opposite effect was observed without α-cyano group. Intensity of tremor and sensory response is rarely explored against pyrethroids (Wolansky and Harrill, 2008). Ansari et al. (2012) reported that long term exposure of λ-cypermethrin produces harmful neurochemical endpoints that cause behavioral variations in rats.
Antiandrogenic activity of cyfluthrin and β-cyfluthrin in a carcinogenic cell line MDA-kb2 has also been reported (Zhang et al., 2008). Bifenthrin evokes various toxicological effects in different human cells by modifying homeostasis and cell viability in human prostate cancer cells (Chien et al., 2019). Bifenthrin acts as endocrine disrupting chemical by inhibiting the expression of glucocorticoid and estrogen receptor (Ligocki et al., 2019). 5-Dihydrotestosterone induced androgen receptor activity was blocked by pyrethroids in MDA-kb2 cells and considered as moderate antiandrogenic (Zhang et al., 2008). Giddings et al. (2009) compared the effect of γ-cyhalothrin and λ-cyhalothrin, and suggested that the single active enantiomer (isomer) causes more toxicity than racemic mixture of both pyrethroids in marine fish and invertebrates (Giddings et al., 2009). Lambda-cyhalothrin and fenvalerate decreased triiodothyronine (T3) in the embryo of Zebra fish (Danio rerio) (Awoyemi et al., 2019). Due to specific binding between ERα receptor and pyrethroid isomer, synthetic pyrethroids act as estrogenic endocrine disrupting compounds (Wang et al., 2010; Lauretta et al., 2019).
Damage of β-cypermethrin to soil microbial communities is less as compared to marine life (Zhuang et al., 2011). Bifenthrin affects microbial community in sandy loam soil and pyrethroids are generally considered as a threat to marine life (Sharma and Singh, 2012). Cypermethrin and deltamethrin residues were reported in Brinjal fruits which can be reduced by washing and boiling before cooking (Kaur et al., 2011). Deltamethrin induced shift of soil microorganisms was reported with cabbage plants after 30 days of treatment (Braganaca et al., 2019). Pyrethroids are highly toxic to aquatic organisms such as fish, shrimp, crab and shellfish. Effects of γ-cyhalothrin and modulator piperonyl butoxide were observed in fish Oreochromis niloticus. Study revealed that λ-cyhalothrin causes oxidative stress in the liver of O. niloticus and stress was further increased in the presence of piperonyl butoxide (Piner and Üner, 2012; Giddings et al., 2019). Pyrethroids transform into solid, liquid and gas phase and enter in food chains to pose high health risk. Pyrethroids accumulated in sediment are major source of aquatic toxicity (Tang et al., 2018). Toxicity of type I and type II pyrethroids was assessed in embryo of Zebrafish (D. rerio) that depicted different mechanistic effects of pyrethroids and their instability in marine environment (Awoyemi et al., 2019). Pyrethroids toxicity to red blood cells and brain cells is associated with physiological changes and DNA damage in fish (Paravani et al., 2019; Ullah et al., 2019).
Cypermethrin stress decreased total glycogen content in different organs/tissues of Marica opima and affected its metabolic activity (Tendulkar and Kulkarni, 2012). Oxidative stress produced by deltamethrin is one of the major mechanism of neurotoxicity (Romero et al., 2012). Deltamethrin inhibits the differentiation of osteoclast by regulating nuclear factor of activated T-cells cytoplasmic-1 (NFATc-1) and oxygenase-1 which is an important regulatory protein (Sakamoto et al., 2012). Deltamethrin is more hazardous than biopesticide (Bacillus thuringiensis) and has been reported to cause testicular injury in rats and affect sex pheromones (Delpuech et al., 2012; Ismail and Mohamed, 2012). Cypermethrin inhibits the androgen receptor (AR) activity by disrupting AR-SRC1 (steroid receptor coactivator-1) interaction (Pan et al., 2012). Toxicity of pyrethroids (cis-bifenthrin) is enantioselective in nature and particular degrading enzymes are more expressive. These previous studies provide detailed knowledge of chiral chemical toxicity at molecular level (Lu, 2013). High pesticide exposure leads to acute pesticide poisoning and damages central nervous system (CNS) (Starks et al., 2012).
Permethrin and its four chiral isomers caused severe histopathological testicular damage in mice at 100 mg/kg by decreasing testis weight and concentration of testosterone hormone (Jin et al., 2012). These pesticides have been noted to transfer from mother to calf in dolphins via gestation and lactation pathways (Alonso et al., 2012; Kondo et al., 2019). Studies conducted in Brazil, Columbia and Spain reported the presence of pyrethroids in human breast milk at concentrations of about 1.45–24.2 ng/gm lw (Corcellas et al., 2012).
Zartarian et al. (2012) studied the effects of permethrin in 3–5 years old children. Stochastic human exposure and dose stimulation model (SHEDS) for multimedia multi-pathway chemicals is commonly known as multimedia computer based method developed by environmental protection agency (EPA) for the study of toxic chemicals (Zartarian et al., 2012). Lambda-cypermethrin has been reported to cause reproductive toxicity, hepatotoxicity, splenotoxicity, and nephrotoxicity in male mice (Starks et al., 2012). Effect of pyrethroids on different fish suggested that highly lipophilic pyrethroids accumulate in sediments and organisms. These compounds also act as endocrine disruptor and block the hormonal signaling in aquatic animals and mammals (Brander et al., 2016).
A study of cypermethrin, deltamethrin, and cyhalothrin phytotoxicity on Cucumis sativus showed that these insecticides affected the production of cholorophyll and caretonoids in plants (Braganca et al., 2018). The study on cypermethrin biodegradation and metabolites detection in tomato, cabbage, rape, pepper, and cucumber revealed its rapid dissipation in plants. Enatioselective degradation was observed in pepper and cucumber (Yao et al., 2018).
Pyrethroid-Degrading Microorganisms and Their Degradation Characteristics
Many studies have confirmed that bacteria and fungi are capable of degrading pyrethroids in liquid cultures or soils (Table 3). Microorganisms can degrade pyrethroids by using either directly as a source of carbon or co-metabolically (Birolli et al., 2016b; Cycoń and Piotrowska-Seget, 2016; Chen and Zhan, 2019). Acidomonas sp. degraded more than 70% of allethrin in 72 h as carbon and nitrogen source (Paingankar et al., 2005). Micrococcus sp. strain CPN1 has been reported to biodegrade and completely mineralize cypermethrin through enzymatic cleavage of ester bond (Tallur et al., 2008; Zhao et al., 2015). Pyrethroid degrading bacterium Sphingobium sp. JZ-2 was isolated and characterized from activated sludge of pyrethroid manufacturing wastewater. Strain JZ-2 efficiently degraded cypermethrin, bifenthrin, and fenvalerate. Novel pyrethroid hydrolase purified from the cell extract was strongly inhibited by different ions (Ag+, Cu2+, Hg2+, and Zn2+) (Guo et al., 2009). Serratia spp. strain JC1 and JCN13 efficiently biodegraded beta-cypermethrin due to their higher hydrophobicity. Strain JC1 degraded 92% beta-cypermethrin within 10 days whereas strain JCN13 degraded 89% within 4 days. Growth conditions for better biodegradation were also optimized through response surface methodology (RSM) and Box-Behnken design (Zhang et al., 2010). Pyrethroid degrading bacterium Raoultella ornithinolytica ZK4 was isolated from the soil samples of a pesticide plant and it degraded lambda-cyhalothrin and deltamethrin (Zhang et al., 2019). Recently 3-phenoxybenzoic acid and other pyrethroids were degraded (96.37%) within 72 h of treatment by using Klebsiella pneumoniae strain BPBA052 (Tang et al., 2019).
Table 3.
Pyrethroid degrading microorganisms and their optimized conditions in lab/field.
| S. No | Bacteria/Fungi/ Insect/Other | Pyrethroid used | Standard condition for growth | Specific statement | References |
|---|---|---|---|---|---|
| 1 | Bacillus cereus, Pseudomonas fluorescence, and Achromobacter sp. | Permethrin, deltamethrin, fastac, fenvalerate, and fluvalinate | pH-7.0 Temp-30°C Tween,80 to maintain relatively insoluble compound in solution | 3-Phenoxybenzoic acid was the major product Permethrin transformed rapidly as compared to others | Maloney et al., 1988 |
| 2 | Pseudomonas sp. ET1 | 3-Phenoxybenzoate | pH-7.2 Temp-30°C | Phenoxy substituted benzyl aldehyde was metabolized whereas benzyl alcohol, benzene, phenol, and aniline were not | Toppw and Akhtar, 1991 |
| 3 | Trichoderma viridae, Trichoderma terricola, Aspergillus niger, and Phanerochate chrysosporium | Beta-cyfluthrin | pH-6.5°C czapek dox medium used | Cleavage of ether linkage result in metabolites formation. That is confirmed by NMR analysis | Saikia and Gopal, 2004; Deng et al., 2015 |
| 4 | Acidomonas sp. | Allethrin | pH-7.0 Temp-37°C with minimal salt medium | Allethrin is metabolized by hydrolytic pathway followed by dehydrogenation and oxidation | Paingankar et al., 2005 |
| 5 | Pseudomonas stutzeri S1 | Beta-cyfluthrin | pH-7.0 Temp-28°C Minimal salt media | Strain able to degrade the beta-cyfluthrin | Saikia et al., 2005 |
| 6 | Aspergillus niger ZD11 | Trans-permethrin, cis-permethrin, cypermethrin, fenvalerate, and deltamethrin | pH-6.8 Temp-30°C Minimal salt media | Novel pyrethroid hydrolase having the potential of wide range of pyrethroid degradation | Liang et al., 2005; Deng et al., 2015 |
| 7 | Micrococcus sp. CPN1 | Cypermethrin | Seuberts mineral salt medium at 150 rpm | Presence of 3-phenoxybenzoate, protochatachauate, and phenol were investigated | Tallur et al., 2008 |
| 8 | Bacillus sp. | Cypermethrin | pH-7.0 Temp.30°C Rpm-110 Minimal salt medium | 3-Phenoxybenzaldegyde and other metabolites of the pathway | Bhatt et al., 2016b, 2019b |
| 9 | Sphingobium sp. JZ-2 | Fenpropathrin, cypermethrin, permethrin, cyhalothrin, deltamethrin, fenvalerate, and bifenthrin | pH-7.0 Temp-30°C Luria Bertani medium | 3-Phenoxybenzadihyde, 2,2,3,3-tetramethylcyclopropanecarboxylic acid, 3-phenoxybenzaldehyde, 3-phenoxybenzoate, protocatechuate, and catechol | Guo et al., 2009 |
| 10 | Serratia spp. | Beta-cypermethrin | pH-6-9 Temp-20–38°C | 3-Phenoxybenzoic acid, phenol (92% degradation occurs within 10 days by Serratia strains) | Zhang et al., 2010 |
| 11. | Ochrobactrum tritici pyd-1 | Cis and trans permethrin, fenpropathrin | Luria Bertani medium Temp-30°C | 2,2,3,3-Tetramethylcyclopropane carboxylic, 3-phenoxybenzaldehyde, 3-phenoxybenzoic acid, 4-hydroxy-3-phenoxybenzoic acid, protocatechuate, and p-hydroquinone | Wang et al., 2011 |
| 12 | Clostridium sp. ZP3 | Fenpropathrin | pH-7.5 Temp-35°C | Benzyl alcohol, benzenemathanol, and 3,5-dimethylamphetamine | Zhang S. et al., 2011 |
| 13 | Pseudomonas aeruginosa CH7 | Beta-cypermethrin | pH-6-9 Temp-25–35°C | Biosurfactant production increased beta-cypermethrin degradation | Zhang C. et al., 2011 |
| 14 | Neustonic and epiphytic bacteria | Deltamethrin | pH-7.0 Temp-20°C Minimal salt medium | Bacteria reduced the initial concentration of cypermethrin | Kalwasinska et al., 2011 |
| 15 | Bacillus cereus MTCC1305 | Fenvalerate | pH-6-7.4 | HPLC analysis showed 500 ppm fenvalerate degradation by the bacterium | Selvam et al., 2013 |
| 16 | Pseudomonas viridoflava | Fenvalerate | pH-6.2-7.0 | HPLC analysis showed the pyrethroid is degraded with different peak areas | Selvam et al., 2013 |
| 17 | Serratia marcescens | Deltamethrin | No data | 3-Phenoxybenzaldehyde and peaks of other metabolites | Cycoń et al., 2014 |
| 18 | Acinetobactor calcoaceticus Mcm5 | Cypermethrin, bifenthrin, cyhalothrin, and deltamethrin | pH-7.0 Temp-30°C | All the pyrethroid degraded by the bacterial strain Mcm5 | Akbar et al., 2015b |
| 19 | Azorcus indigens HZ5 | Cypermethrin | pH-7.0 Temp-30°C | 70% cypermethrin degradation after 144 h | Burns and Pastoor, 2018 |
| 20 | Bacillus sp. SG2 | Cypermethrin | pH-7.0 Temp-32°C | 82% cypermethrin degraded after 15 days of experiment | Bhatt et al., 2016b, 2019a |
| 21 | Bacillus sp. DG-02 | Fenpropathrin, cypermethrin, cyfluthrin, lambda-cyhalothrin, deltamethrin, permethrin, and bifenthrin | pH-7.5 Temp-30°C | Different biodegradation patterns followed with distinct concentration | Chen et al., 2012b, 2014 |
| 22 | Bacillus amyloliquifaciens AP01 | Cypermethrin | pH-7.0 Temp-30°C | Approximately 45% cypermethrin degradation observed in 5 days | Lee et al., 2016 |
| 23 | Bacillus megaterium Jcm2 Brevibacillus parabrevis Jcm4 | Cypermethrin, bifenthrin, cyhalothrin, and deltamethrin | pH-7.0 Temp-30°C | Maximum 89% degradation obtained in cypermethrin | Akbar et al., 2015a |
| 24 | Brevibacterium aureum DG-12 | Cyfluthrin, cyhalothrin, fenpropathrin, deltamethrin, bifenthrin, and cypermethrin | pH-7.0 Temp-27°C | Maximum 84.7% biodegradation observed with cyfluthrin | Chen et al., 2013a |
| 25 | Catellibacterium sp. CC-5 | Cypermethrin, fenvlerate, fenpropathrin, deltamethrin, permethrin, and cyhalothrin | pH-7.0 Temp-30°C | 90% biodegradation achieved after 7 days with cypermethrin and deltamethrin | Zhao et al., 2013 |
| 26 | Lysinbacillus sphaericus FLQ-11-1 | Cyfluthrin | pH-7.0 Temp-35°C | Approximately 80% cyfluthrin removal after 5 days | Hu et al., 2014 |
| 27 | Ochrobactrum lupini DG-S-01 | Cypermethrin, cyfluthrin, fenpropathrin, cyhalothrin, and deltamethrin | pH-7.0 Temp-30°C | Maximum 90% biodegradation obtained with cypermethrin within 5 days | Chen et al., 2011a |
| 28 | Pseudomonas aeruginosa JQ-41 | Fenpropathrin, cypermethrin, deltamethrin, bifenthrin, and cyhalothrin | pH-7.0 Temp-30°C | Maximum 91.7% biodegradation obtained with fenpropathrin after 7 days of experiment | Song et al., 2015 |
| 29 | Pseudomonas flourescens | Cypermethrin | pH-7.0 Temp-25°C | 37.2% cypermethrin degraded in absence of sucrose after 96 h | Grant et al., 2002 |
| 30 | Rhodococcus sp. Jcm5 | Cypermethrin, bifenthrin, cyhalothrin, and deltamethrin | pH-7.0 Temp-30°C | 100% cypermethrin catabolism occures in 10 days | Akbar et al., 2015b |
| 31 | Stenotrophomonas sp. ZS-S-01 | Fenvalerate, deltamethrin, cypermethrin, cyfluthrin, and cyhalothrin | pH-7.0 Temp-30°C | Catabolic degradation in case of fenvalerate complete degradation occurs in 6 days | Chen et al., 2011d |
| 32 | Streptomyces sp. HU-S-01 | Cypermethrin | pH-7.5 Temp-26–28°C | 90% cypermethrin degradation in 24 h | Lin et al., 2011 |
| 33 | Streptomyces aureus HP-S-01 | Cypermethrin, deltamethrin, cyfluthrin, bifenthrin, fenvalerate, fenpropathrin, and permethrin | pH-7.5-7.8 Temp-27–28°C | Cyfluthrin, bifenthrin and fenvalerate degraded completely within 5 days | Chen et al., 2011c, 2012d |
| 34 | Candia pelliculosa ZS-02 | Bifenthrin, cyfluthrin, deltamethrin, fenvalerate, cypermethrin, and fenpropathrin | pH-7.2 Temp-32°C | Only bifenthrin degraded completely within 5 days | Chen et al., 2012c |
| 35. clc | Cladosporium sp. HU | Fenvalerate, fenpropathrin, cypermethrin, deltamethrin, bifenthrin, and permethrin | pH-7.2 Temp-26°C | Fenvalerate, fenpropathrin, cypermethrin degraded completely within 5 days | Chen et al., 2011b |
| 36 | Phaenerochate chrysosporium | Cyfluthrin | pH-6.5 Temp-28°C | Co-metabolic degradation (60%) after 30 days of experiment | Saikia and Gopal, 2004 |
| 37 | Bacillus cereus BCC01 | Beta-cypermethrin, deltamethrin, cypermethrin, permethrin, fenvalerate, and cyhalothrin | pH-7.0 Temp-30°C | Six metabolites were detected after biodegradation: α-hydroxy-3-phenoxy-benzeneacetonitrile, 3-phenoxybenzaldehyde, methyl-3-phenoxybenzoate, 3,5-dihydroxybenzoic acid, 3,4-dihydroxybenzoic acid, and 3,5-dimethoxyphenol | Hu et al., 2019 |
| 38 | Bacillus subtilis BSF01 | Cypermethrin, deltamethrin, cyhalothrin, and β-cyfluthrin | pH-6.7 Temp-34.5°C | Cis/trans β-cypermethrin, 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate, α-hydroxy-3-phenoxybenzeneacetonitrile, 3-phenoxybenzaldehyde, 3-pheoxybenzoic acid, and 3,5-dimethoxyphenol | Xiao et al., 2015 |
| 39 | Pseudomonas fulva P31 | D-phenothrin | pH-7.3 Temp-29.5°C | 3-Phenoxybenzaldegyde and 1,2- benzene dicarboxylic butyl dacyl ester identified as major intermediates | Yang et al., 2018 |
| 40 | Sepedonium maheswarium | Beta-cyfluthrin | PDA media Temp-25 ± 2°C | Dissipation study | Mukherjee and Mittal, 2007 |
| 41 | Penicillum raistrickii CBMAI 93, Aspergillus sydowii CBMAI935, Cladosporium sp. CBMAI 1237, Microsphaeropsis sp. CBMAI1675, Acremonium sp. CBMAI 1676, Westerdykella sp. CBMAI 1679, and Cladosporium sp. CBMAI1678 | Esfenvalerate | pH-7 Temp-32°C | All fungal strains degraded esfenvalerate with different efficiencies | Birolli et al., 2016a; Zhao et al., 2016 |
| 42 | Aspergillus sp. CBMAI 1829, Acremonium sp. CBMAI 1676, Microsphaeropsis sp. CBMAI 1675, and Westerdykella sp. CBMAI 1679 | Lambda-cyhalothrin | pH-7 Temp-32°C | Enantioselctive degradation of cyhalothrin by the fungal strains | Birolli et al., 2016a; Zhao et al., 2018 |
| 43 | Cunninghamella elegans DSM1908 | Cyhalothrin | pH-5.6 Temp-28°C | Intermediate metabolites and proposed pathways identified | Birolli et al., 2018 |
| 44 | Phtobacterium genghwense PGS6046 | Cyfluthrin | pH-8 Temp-30°C | Characterized metabolites in different culture conditions | Wang et al., 2018 |
| 45 | Acinetobacter baumanii ZH-14 | Permethrin | pH-7.0 Temp-30°C | Strain degraded permethrin as well as wide variety of pyrethroids | Zhan et al., 2018 |
| 46 | Raoultella ornithinolytica ZK4 | Pyrethroids | pH-6.5, Temp-37°C | Bacteria was isolated from the soil sediment that degraded different pyrethroids | Zhang et al., 2019 |
| 47 | Klebsiella pneumoniae BPBA052 | 3-Phenoxybenzoic acid | pH-7.7 Temp-35.01°C | Bacterium uses 3-phenoxybenzoic acid as carbon and energy source | Tang et al., 2019 |
| 48 | Rhodopseudomonas sp. PSB07-21 | Fenpropathrin | pH-7.0 Temp-35°C | Photoheterotrophic mode of growth was better as compared to photoautotrophic growth mode | Luo et al., 2019 |
Aerobic and anaerobic soil biodegradation of pyrethroid etofenprox was investigated in the rice fields of California. 3-Phenoxybenzoic acid, a hydrolytic product of ester bond cleavage was not detected in any sample. Microbial population in a flooded soil (anaerobic) played role in conversion and dissipation of etofenprox (Vasquez et al., 2011; Furihata et al., 2019). β-Cyfluthrin is commonly used by Indian farmers at Indian Agriculture Research Institute Delhi for controlling Lepidepteran pests of Solanaceous crops. To degrade its soil residues, Pseudomonas stutzeri was isolated, and identified using enrichment culture technique and intermediate metabolites were confirmed according to the previous reported pathway (Saikia et al., 2005; Birolli et al., 2019). Cyfluthrin degradation by bacterium Photobacterium ganghwense was confirmed by comparative metabolomics (Wang et al., 2019). Pyrethroid degradation capability of Ochrobacterium tritici strain pyd1 is dependent upon the molecular structure of synthetic pyrethroids (Wang et al., 2011). Strain pyd-1 effectively degraded both, cis and trans isomers at the same rate. Detailed metabolic pathway of fenpropathrin biodegradation through strain pyd-1 was also identified. Specific enzyme activities of pyrethroid hydrolase, 3-phenoxybenzaldehyde (PBD) dehydrogenase, 3-phenoxybenzoic acid (PBA) hydroxylase, 4-hydroxy-PBA dioxygenase, and p-hydroquinone hydroxylase have been studied in relation to pyrethroid fenpropethrin (Dehmel et al., 1995; Wang et al., 2011; Luo et al., 2019). Pseudomonas pseudoalcaligenes strain POB310 has been reported for the degradation of 3- and 4-carboxydiphenyl ethers (Dehmel et al., 1995). A genetically engineered strain of Pseudomonas putida also degraded other pesticides similar to pyrethroids (Gong et al., 2018). Anaerobic bacterium Clostridium strain ZP3 isolated from the mixed wastewater and sludge samples degraded higher concentrations of fenpropathrin by co-metabolic activity and was used to analyze complex redox reaction in fenpropathrin biodegradation (Zhang S. et al., 2011; Zhao et al., 2016). Co-metabolic biodegradation of β-cypermethrin was explored with Bacillus licheniformis B-1 (Zhao et al., 2019). Pseudomonas aeruginosa CH7 degraded 90% of beta-cypermethrin by isomerization within 12 days. Bio-surfactant (rhamnolipid) promotes the adsorption and hydrophobicity of chemical compounds (Zhang C. et al., 2011). Neustonic and epiphytic bacteria and their mixed cultures were noted to similarly, degrade deltamethrin (Kalwasinska et al., 2011). Chen et al. (2012d) also validated the cypermethrin biodegradation through Bacillus cereus ZH-3 and S. aureus HP-S-01 cells.
Ochrobactrum anthropi strain YZ-1 is quite potent to degrade pyrethroids. Role of bacterial esterase PytZ (606 bp) in biodegradation without any cofactor has been confirmed (Zhai et al., 2012). Pyrethroid hydrolase of molecular weight 53 KDa was purified and characterized from A. niger strain ZD11. Pyrethroid activity was not detected in the presence of glucose and it indicates that pyrethroid hydrolase only expresses in fungus after pyrethroid stress. Optimum pH for A. niger was found to be lower than B. cereus (7.3) whereas the optimum temperature was comparatively higher (45°C) as compared to B. cereus strain SM3 (37°C). Enzyme activity inhibition by thiol modifying enzyme (PCMB) p-chloromercuribenzoate suggested that sufhydryl group was involved in the catalytic center of enzyme (Liang et al., 2005). Cypermethrin reportedly caused toxicity to human hepatocarcinoma cell line H4H7 (Sundaram et al., 2013). Bacillus sp. helps to biodegrade cypermethrin in soil microcosm and B. cereus MTCC 1305 has been reported to biodegrade fenvalerate (Selvam et al., 2013). Pyrethroid toxicity and biodegradation efficiency of Pseudomonas viridoflava has also been thoroughly investigated (Selvam et al., 2013; Thatheyus and Selvam, 2013). Two strains of S. marcescens DeI-1 and DeI-2, enhanced the disappearance of cypermethrin (Cycoń et al., 2014). Application of ammonium nitrate as external nitrogen at the rate of 122.1 kg/ha-1 increased cypermethrin degradation by 80% (Xie et al., 2008). External nitrogen might accelerate microbial metabolism in lag phase.
Metabolic and ecological potential of fungi makes them suitable for bioremediation and waste treatment (Harms et al., 2011). Cell free extracts of fungi are known to effectively degrade chlorpyrifos and pyrethroids (Yu et al., 2006). β-Cyhalothrin degradation by different fungi has been reported including Trichoderma viridae strain 5-2, Trichoderma viridae strain 2211, Phanerochaete chryosogenum, Aspergillus terricola, and A. niger. Study was followed by the extraction and identification of major degradation metabolites (Saikia et al., 2005; Birolli et al., 2019).
Radiolabeled (14C) permethrin was used to understand the mechanism of pyrethroid degradation in soil and sediment. It was observed that R-enantiomer of both trans and cis permethrin mineralized rapidly as compared to S-enantiomer and degradation product of cis permethrin was more persistent in the soil environment (Qin and Gan, 2006). Enantioselective degradation of pyrethroids was also performed at southern California under field condition (soil and sediment) and enantioselective degradation of cis-bifenthrin, cypermethrin and permethrin occurred at half-life of 270–277 days, 52–135 days, and 99–141 days, respectively. Absence of enantioselectivity in biodegradation represents preferential condition for transformation (Qin et al., 2006).
Axenic culture of Pseudomonas fluorescens, B. cereus, and Achromobacter sp. degraded different pyrethroids such as permethrin, fenvalerate, fastac, deltamethrin, and fluvalinate in the presence of Tween-80 and 3-phenoxybenzoic acid was the major metabolite. Permethrin rapidly transformed into 3-phenoxybenzoic acid as compared to other pyrethroids (less than 5 days). In soils, pyrethroids were degraded into a diaryl ether metabolite 3-phenoxybenzoate. Efficiency of Pseudomonas strain ET1 in 3-phenoxybenzoate metabolism per cell was calculated as 2.6 ± 0.9 × 10-13 gm/cell/hour. Strain Pseudomonas ET1 morphologically resembles with Pseudomonas delafieldii but differs in 3-phenoxybenzoate degradation (Toppw and Akhtar, 1991). One strain cannot degrade all aromatic compounds due to the structural differences except genetically modified strains, which can be modified to simultaneously degrade different compounds (Gong et al., 2018).
Gene Cloning and Enzymatic Characterization of Pyrethroid Carboxylesterases
Esterease (carboxyl ester hydrolase) play an important role in initial transformation of parent pyrethroid by attacking ester bond or cytochrome P-450 dependent monooxygenase on acid or alcohol moieties (Kamita et al., 2016). Many researchers have studied carboxylesterase isolation and purification from B. cereus SM3, Klebsiella sp. ZD112, Sphingobium sp. JZ2, Pseudomonas flourescens SM-3, A. niger ZD11, Ochrobactrum lupini DG-S-01, Streptomyces aureus HP-S-01, Streptomyces sp. HU-S-01, Pseudomonas stutzeri, Micrococcus sp. CPN 1, Serratia sp. JC1 and Serratia sp. JCN13, Pichia pastoris (Cycoń and Piotrowska-Seget, 2016; Liu et al., 2017; Tang et al., 2017). Limitations in culture dependent approaches are popularizing the metagenomics tools. Thermostable pyrethroid esterase Sys410 was investigated by metagenomic approach and enzyme contained 280 amino acids having a molecular mass of 30.8 KDa (Fan et al., 2012; Popovic et al., 2017). Cloning was carried out from metagenomic library of soil samples and sequence analysis revealed that 819 bp pye3 gene codes for 273 amino acid protein. Enzyme was further characterized on the basis of enzyme kinetics (Km and Kcat activity) (Li et al., 2008; Luo et al., 2018).
Reported pyrethroid hydrolases have different pH and molecular weight. Carboxyl esterase enzymes can catabolize wide array of similar ester containing compounds. Because of enantioselectivity, a few essterases exhibit specific or moderate kinetic abilities, which differ from pyrethroid degrading enzymes. Enzyme expression and metabolites production during pyrethroid degradation is differential and can be sequentially up-regulated or down regulated (Bhatt et al., 2019b). Metagenomic based library was useful for the identification and mining of pyrethroid degrading genes, such as pytY and pytZ (O. anthropi strain YZ1), estP (Klebsiella sp. JD112), pytH (Sphingobium sp. JZ-1), and pye (soil). These genes can be used for isolation and comparison of novel pyrethroid degrading microbial strains.
Bacterial cells produce CO2 from 3-phenoxybenzoate at Km (Michaelis constant) value of 1.4 ± 0.8 μM that reveals high affinity of bacterial cells to 3-phenoxybenzoate. Metabolism of this pyrethroid intermediate is constitutive rather than catabolite repression. Maloney et al. (1993) were the first to report enzymatic catalysis of pyrethroids in B. cereus strain SM3. Enzyme initially named as permethrinase (61 ± 3 KDa) was finally termed as carboxylesterase after successive studies. Pure culture and cell free extract of B. cereus SM3 successfully hydrolyzed 2nd and 3rd generation pyrethroids. Permethrin was hydrolyzed more rapidly as compared to flumethrin.
Esterase is ranked under subcategory of hydrolases and International Union of Biochemistry classified carboxylesterase as subgroup 3.1.1. Active site of this enzyme contains serine residue that plays role in acylation during pyrethroid biodegradation through nucleophilic attack by hydroxyl group (OH). Transformed pyrethroid metabolites are easily excreted in urine because of their better water solubility than original pyrethroids. It justifies high concentrations of carboxylesterase enzyme in mammalian serum and liver (Sogorb and Vilanova, 2002). There are two major categories of carboxylestearases in human body (carbocylesterase-1 and carboxylesterase-2), which can degrade pyrethroids (Wang et al., 2018). Pyrethroid trans forms are more easily degraded by carboxylesterases as compared to cis-isomer. Due to high affinity for Na+ channels, trans isomers are more toxic to mammalian tissues. Rabbit serum contains higher cypermethrin degradation activity (WHO Task Group on Environmental Health Criteria for Permethrin et al., 1990).
Novel pyrethroid hydrolyzing esterase was reported from Klebsiella sp. strain ZD112. Gene estP contains an open reading frame of 1914 bp, encoding a protein of 637 amino acids and molecular mass of 73 KDa. Purified enzyme can effectively degrade wide variety of ester bond containing pesticides. Km value for trans and cis permethrin indicated that EstP has higher catalytic power than carboxylesterase enzyme (Wu et al., 2006). A novel pytH esterase gene, coding pyrethroid hydrolyzing carboxylesterase was also reported in Sphingobium sp. strain JZ1 having an open reading frame of 840 bp. Further cloning and purification of this enzyme revealed its molecular weight of about 31 KDa, isoelectric point (pI) of 4.85, and it does not require any cofactor for degrading different pyrethroids (Wang et al., 2009). Degradation of fenpropathrin and fenvalerate in alkaline and acidic soil was observed as enantioselective under aerobic conditions (Li et al., 2009).
Co-expression of two target genes [organophosphate hydrolase (opd) and carboxylesterase B1 (b1)] from Falovobacterium sp. and Culex pipens is used for degradation of organophosphorous, carbamate, and pyrethroid pesticides. Carboxylesterase 001D that was isolated from Helicoverpa armigera and heterologously expressed in bacteria (E. coli) potentially hydrolysed cypermethrin and fenvalerate (Li et al., 2016). Advanced genetic engineering techniques can enable a single microorganism to degrade multiple pesticides (Lan et al., 2006).
Fungal enzymes have also been reported for pesticide biodegradation. Some fungal enzymes catalyze esterification, hydroxylation, dehydrogenation, and deoxygenation during the degradation process. A. niger YAT carries out etherification reaction during cypermethrin biodegradation. Similar to metabolites of pyrethroid bacterial biodegradation, degrading enzymes of fungal strains have been confirmed as well (Maqbool et al., 2016).
Carboxylesterases of Lucila cuprina and Drosophila melanogaster with mutagenesis in active site were used to study pyrethroid degradation. Carboxylesterase was cloned and expressed by genetic engineering to observe their pyrethroid degradation efficiency (Heidari et al., 2005). Human liver carboxylesterase hcE1 and hcE2 degraded both type I and type II pyrethroids with stereoselcetivity. trans-isomers were degraded more rapidly by these enzymes as compared to cis-isomer (Table 4). Km values of enzyme catalysis were lower as compared to pyrethroid compounds (Nishi et al., 2006). Human, rat and rabbit hepatocarboxylesterases also depicted capability to degrade pyrethroids (Ross et al., 2006). Esterases in Heliothis virescens larvae were found to be associated with their resistance to pyrethroids (Huang and Ottea, 2004). Development of next generation sequencing (NGS) methods has enabled us to use genetically engineered microorganism for large-scale pyrethroid hydrolases. Heterologous expression of human and insect pyrethroid hydrolases can be more beneficial for pyrethroid removal from contaminated sites.
Table 4.
Pyrethroid degrading enzymes from different sources.
| S. No. | Pyrethroid isomer used for study | Microbes/other source | Enzymes | Metabolites | References |
|---|---|---|---|---|---|
| 1 | Trans-permethrin, cis-permethrin, and racemic permethrin | Bacillus cereus | Permethrinase with molecular weight 61KDa | B-naphthylacetate was used as substrate, no specific data of pathway reported | Maloney et al., 1993 |
| 2 | Trans-permethrin and cis-permethrin | Lucila cuprina and Drosophila melanogaster Helicoverpa armigera | Carboxylestease enzyme plays role in pyrethroid hydrolysis | No data | Heidari et al., 2005; Li et al., 2019 |
| 3 | Trans-permethrin, cis-permethrin, cypermethrin, fenvalerate, and deltamethrin | Aspergillus niger ZD11 | Novel pyrethroid hydrolase degrades permethrin and similar compounds | No data | Liang et al., 2005 |
| 4 | Deltamethrin, bifenthrin, cyfluthrin, and λ-cyhalothrin | Human liver | hCE-1 and hCE-2 carboxylesterases hydrolyze the pyrethroids and pyrethroid like fluorescent surrogates | No data | Nishi et al., 2006; Wang et al., 2018 |
| 5 | Pyrethroids and organophosphate | Falobacterium sp., Culex pipiens | Co-expression of organophosphate hydrolase and carboxylesterase B1 gene that can degrade many pesticides together | No data | Lan et al., 2006 |
| 6 | Trans and cis-permethrin | Klebsiella sp. ZD112 | Esterase enzyme with molecular weight 73KDa has high efficiency than insect and mammals | p-Nitrophenyl ester was used for enzyme catalysis | Wu et al., 2006 |
| 7 | Permethrin, deltamethrin, cypermethrin, and esfenvalerate | Intestinal, liver and serum carboxylesterse | Hydrolysis of pyrethroids by humans and rat tissues | No data of metabolite | Crow et al., 2007 |
| 8 | Bioresmethrin α-cypermethrin deltamethrin | Hepatic cells | Hepatic carboxylesterase | Hepatic carboxylesterase metaboloize ester comtaining xenobiotics | Ross et al., 2006 |
| 9 | Cypermethrin | Soil samples | Soil dehydrogenase | Increased dehydrogenase activity when nitrogen was added into cypermethrin | Xie et al., 2008 |
| 10 | Prethroids in soil | Soil samples | Pyrethroid hydrolyzing esterase | The genes coding esterase cloned and expressed from metagenomic library | Li et al., 2008 |
| 11 | Cypermethrin | Bacillus spp. | Esterase and aldehyde dehydrogenase | Upregulation of the enzymes in response to pesticide stress | Bhatt et al., 2019a |
| 12 | Cypermethrin | Bacillus sp. | Esterase, dehydrogenease, and many other proteins and enzymes | Differential expression was observed with cypermethrin in Bacillus sp. | Bhatt et al., 2016a |
| 13 | Permethrin, fenpropathrin, cypermethrin, deltamethrin, cyhalothrin, fenvalerate, and bifenthrin | Sphingobium sp. JZ-1 | Pyrethroid hydrolyzing carboxylesterase | 840bp of gene coding for the enzyme carboxylesterase (molecular mass-31 KDa and PI-4.85) | Wang et al., 2009 |
| 14 | Fenpropathrin, cypermethrin, permethrin, cyhalothrin, deltamethrin, fenvalerate, and bifenthrin | Sphingobium sp. JZ-2 | Pyrethroid hydrolase | This enzyme was a monomer of a 31KDa with pI-4.85. | Guo et al., 2009 |
| 15 | Cyhalothrin, cypermethrin, and deltamethrin | Soil samples | Thermostable pyrethroid esterase | Isolated and identified from metagenomic approach. Molecular mass of the enzyme was 30.8 KDa | Fan et al., 2012 |
| 16 | Lambda-cyhalothrin, beta-cypermethrin, beta cyfluthrin, deltamethrin, and permethrin | Ochrobactrum anthropi YZ-1 | Novel pyrethroid hydrolyzing carboxylesterase | Hingh enzyme specificity, broad substrate activity makes this enzyme as a potential candidate for pyrethroid degradation | Zhai et al., 2012 |
| 17 | Beta-cypermethrin, deltamethrin, cypermethrin, permethrin, fenvalerate, and Cyhalothrin | Bacillus cereus BCC01 | Carboxylesterase EstA | Enzyme showed excellent adaptability under various circumstances | Hu et al., 2019 |
| 18 | Fenpropathrin | Rhodopseudomonas palustris PSB-S | Esterase (Est3385) | The optimal temperature (35°C) and pH (6.0) for esterase | Luo et al., 2018, 2019 |
| 19 | Cypermethrin | Bacillus subtilis | Esterase and laccase | pH-7.0 Temp-32°C | Gangola et al., 2018 |
Metabolic Pathways of Pyrethroid Biodegradation
Every living cell that can survive in different environmental conditions must has metabolic pathways, which help to fetch required food (nutrition) from the surroundings (soil, water). Oxygenases (monooxygenases and dioxygensases) play important role in biodegradation of pesticides by common pathways (Fuchs et al., 2011; Birolli et al., 2016b; Bhatt et al., 2019b). Pyrethroid degrading cells (bacteria, fungi and some animal cells) produce metabolites and make them accessory for downstream pathways (Figure 2). Casida identified the pyrethroid breakdown pathway in 1960. Pyrethroids are metabolized in human body via catabolic pathway. Distribution of carboxylesterases in different tissues has been reported and major esterase of intestine is called carboxylesterase 2 (hCE2) that has higher catalytic activity as compared to liver and other tissue cells (Crow et al., 2007).
Figure 2.
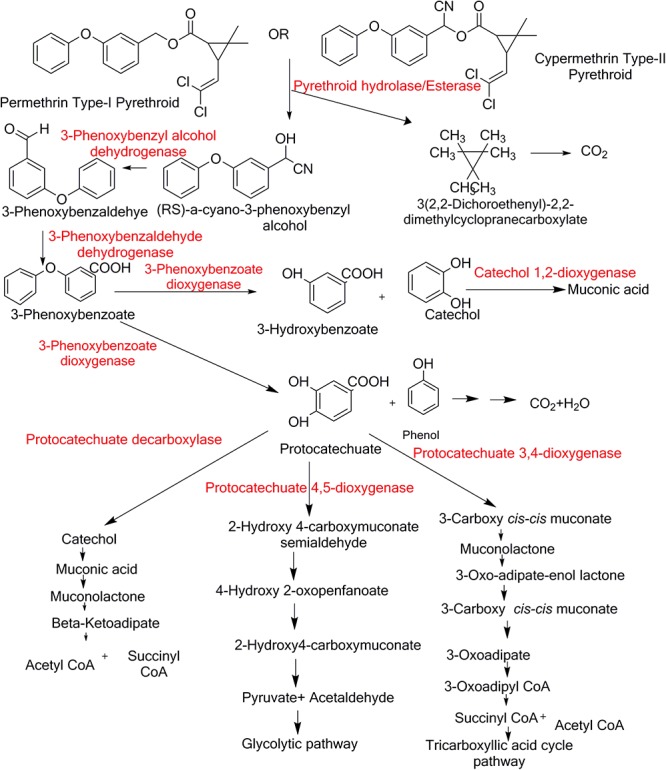
Detailed metabolic pathways of pyrethoids in microorganisms.
Hydroxyester metabolites are produced during oxidative pathways whereas oxidative ester cleavage is the minor pathway of some pyrethroids. Isomers of various pyrethroids are affected differently by initial hydrolytic attack. Pyrethroid degradation by hydroxyl group (OH-) nucleophilic attack under alkaline conditions is similar to simple aliphatic ester. Chemically, ester or nitrile hydrolysis occurs under alkaline conditions. Ester (bond) hydrolysis produces acid (RCOO-) and 3-phenoxybenzaldehyde, via fast decomposition of intermediary compound cyanohydrins. Another parallel hydrolysis pathway produces primary amide that again hydrolyze into RCOO- acid and 3-phenoxybenzaldehyde (Wang et al., 2018). Microbial degradation follows the same pattern and most of the metabolites are common in all microbial pathways with only a few exceptions. Strains belonging to genera Bacillus, Micrococcus, Staphylococcus aureus, R. ornithinolytica, and Catellibacterium are used for pyrethroid detection (Tallur et al., 2008; Chen et al., 2013b; Zhao et al., 2013; Zhang et al., 2019). Consortium biodegradation pathways of B.cereus ZH3 and S. aureus, and Bacillus licheniformis B1 and Sphingomonas sp. SC-1 have also been reported (Chen et al., 2012b; Liu et al., 2013; Wang et al., 2019). 3-Phenoxybenzaldehyde and 2,2,3,3 tetramethylcyclopropanecarboxylic acid were detected during cypermethrin degradation by Bacillus sp. SG2 and Bacillus subtilis BSF01 (Tallur et al., 2008; Bhatt et al., 2016b). Cyclopropanecarboxylic acid, 2,2-dimethyl-3 (2-methyl-1- propenyl), 2-ethyl, 1,3 dimethyl cyclopent 2-ene carboxylic acid, chrysanthemic acid and allethrolone (2-cyclopenten-1-one-4 hydroxy-3 methyl 2 (2 propenyl) were found as major metabolites of allethrin during degradation by Acidomonas sp. Hydrolysis, oxidation and dehydrogenation reactions mediated allethrin biodegradation (Paingankar et al., 2005; Bhatt et al., 2016b; Birolli et al., 2016b).
Bacillus sp. DG-02 primarily degraded fenpropathrin through carboxylester linkage cleavage to yield 2,2,3,3-tetramethylcyclopropanecarboxylic acid phenyl ester and α-hyroxy-3-phenoxybenzeneacetonitrile which transformed into 3-phenoxybenzaldehyde spontaneously, followed by the oxidization of 3-phenoxybenzaldehyde via diaryl cleavage (Chen et al., 2014). B. thuringiensis ZS-19 transformed cyhalothrin by cleavage of both the ester linkage and diaryl bond to yield six intermediate products including α-hydroxy-3-phenoxy-benzeneacetonitrile, 3-phenoxyphenyl acetonitrile, N-(2-isoproxy-phenyl)-4-phenoxy-benzamide, 3-phenoxybenzaldehyde, 3-phenoxybenzoate, and phenol, respectively (Chen et al., 2015; Wang et al., 2018). Esterase is essential for ester bond cleavage during pyrethroid degradation. Initially carboxylesterase activity forms two metabolites (RS)-α-cyano-3-phenoxybenzyl alcohol and 2,2,3,3 tetramethylcyclopropanecarboxylic acid. Finally 2,2,3,3 tetramethylcyclopropanecarboxylic acid is converted into CO2 after few steps but (RS)-α-cyano-3-phenoxybenzyl alcohol is transformed into stable 3-phenoxybenzaldehyde (3-PBA). This step is catalyzed by 3-phenoxybenzaldehyde alcohol dehydrogenase that transroms 3-phenoxybenzaldehyde to 3-phenoxybenzoate. Another enzyme phenoxybenzoate 1,2-dioxygenase transforms 3-phenoxybenzoate into 3,4-dihydroxy- benzoate (protocatechuate) and phenol. Protocatechuate and phenols are further converted into primary and secondary metabolites by microorganisms (Wang et al., 2019). Tricarboxylic acid and glycolysis pathways are mainly used by microbes to produce energy from the pyrethroids (Wang et al., 2014; Gajendiran and Abraham, 2018).
Recent Tools for Pyrethroid Biodegradation
Traditional identification of microorganisms was based on biochemical tests. Inaccurate results of these tests usually resulted in wrong isolation and characterization. Development of molecular biology tools have facilitated the isolation and identification of pyrethroid degrading microbes (16S rRNA for bacteria and ITS sequencing for fungi) (Gangola et al., 2018; Gupta et al., 2018). Degradation of pyrethroids is commonly analyzed by chromatographic techniques such as high performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectroscopy (MS) (Castellarnau et al., 2016). Due to their low pyrethroid detection limit in soil samples, combination of solid phase extraction and gas chromatography mass spectrometry (GC-MS) was developed as a new method (Chen et al., 2012a; Braganca et al., 2018). After microbial degradation, these methods can efficiently detect pyrethroid metabolites up to ng/gm of soil (Braganca et al., 2018). RSM is generally used for the optimization of pyrethroids and different kinetics of pesticides have been reported (Chen et al., 2012e; Bhatt et al., 2016a,b; Morales et al., 2019). First order reaction is followed for pyrethroid degradation and for the impact analysis on humans whereas cell culture techniques are employed for pyrethroid toxicity detection. Development of rapid genomic tools could analyze the whole genome of pyrethroid catabolizing microorganisms (Bhatt, 2018; Bhatt and Barh, 2018).
Conclusion and Further Aspects
To feed the world’s rapidly growing population, large-scale use of pesticides in agricultural systems cannot be stopped. Pyrethroid insecticides are used in most of the countries and exhibit comparatively less toxicity than organophosphate and organochlorine pesticides. Recently, toxicity of pyrethroids on marine life (fish), humans and phytotoxicity has been reported. Esterase can degrade ester bond of pyrethroids to produce metabolite 3-penoxybenzaldehyde. Pyrethroid degrading esterase and 3-phenoxybenzaldehyde can be used as signature molecule for pyrethroid biodegradation. Based on this potential marker, pyrethroids degrading microorganism can be selected in a shorter period. Molecular chronometer based coverage of esterase enzyme is possible with existing data. Consortium based pesticide biodegradation approach is more suitable but it has not been significantly studied for pyrethroid degradation. Previous data favors the development of pyrethroid degradation mechanism through microbial system. In future, omics technologies could potentially be used for pyrethroid degradation and to understand molecular biology, enzyme kinetics, and metabolic pathways. System biology of pyrethroid degradation can be further useful for the investigation of multiple information at one platform.
Author Contributions
SC conceived the idea. PB wrote the manuscript and prepared the figures and tables. YH, HZ, and SC revised the manuscript. All authors approved the final manuscript for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful to the global researchers for their contribution.
Footnotes
Funding. We acknowledge the grants from National Natural Science Foundation of China (31401763), National Key Project for Basic Research (2015CB150600), Guangdong Province Science and Technology Innovation Strategy Special Fund (2018B020206001), Guangdong Natural Science Funds for Distinguished Young Scholar (2015A030306038), Guangdong Special Branch Plan for Young Talent with Scientific and Technological Innovation (2017TQ04N026), and Science and Technology Planning Project of Guangdong Province (2017A010105008).
References
- Akbar S., Sultan S., Kertesz M. (2015a). Bacterial community analysis of cypermethrin enrichment cultures and bioremediation of cypermethrin contaminated soils. J. Basic Microbiol. 55 819–829. 10.1002/jobm.201400805 [DOI] [PubMed] [Google Scholar]
- Akbar S., Sultan S., Kertesz M. (2015b). Determination of cypermethrin degradation potential of soil bacteria along with plant growth-promoting characteristics. Curr. Microbiol. 70 75–84. 10.1007/s00284-014-0684-7 [DOI] [PubMed] [Google Scholar]
- Alonso M. B., Feo M. L., Corcellas C., Vidal L. G., Bertozzi C. P., Marigo J., et al. (2012). Pyrethroids: a new threat to marine mammals? Environ. Int. 47 99–106. 10.1016/j.envint.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Al-Sarar A. S., Abobakr Y., Bayoumi A. E., Hussein H. I., Al-Ghothemi M. (2014). Reproductive toxicity and histopathological changes induced by lambda-cyhalothrin in male mice. Environ. Toxicol. 29 750–762. 10.1002/tox.21802 [DOI] [PubMed] [Google Scholar]
- Ansari R. W., Shukla R. K., Yadav R. S., Seth K., Pant A. B., Singh D., et al. (2012). Cholinergic dysfunctions and enhanced oxidative stress in the neurobehavioral toxicity of lambda-cyhalothrin in developing rats. Neurotox. Res. 22 292–309. 10.1007/s12640-012-9313-z [DOI] [PubMed] [Google Scholar]
- Awoyemi O. M., Kumar N., Schmitt C., Subbiah S., Crago J. (2019). Behavioral, molecular and physiological responses of embryo-larval zebrafish exposed to types I and II pyrethroids. Chemosphere 219 526–537. 10.1016/j.chemosphere.2018.12.026 [DOI] [PubMed] [Google Scholar]
- Babina K., Dollard M., Pilotto L., Edwards J. W. (2012). Environmental exposure to organophosphorus and pyrethroid pesticides in South Australian preschool children: a cross sectional study. Environ. Int. 48 109–120. 10.1016/j.envint.2012.07.007 [DOI] [PubMed] [Google Scholar]
- Bai L. S., Zhao C. X., Xu J. J., Feng C., Li Y. Q., Dong Y. L., et al. (2019). Identification and biochemical characterization of carboxylesterase 001G associated with insecticide detoxification in Helicoverpa armigera. Pest. Biochem. Physiol. 157 69–79. 10.1016/j.pestbp.2019.03.009 [DOI] [PubMed] [Google Scholar]
- Bhatt P. (2018). “Insilico tools to study the bioremediation in microorganisms,” in Handbook of Research on Microbial Tools for Environmental Waste Management, eds Pathak V., Navneet (Hershey, PA: IGI Global; ), 389–395. 10.4018/978-1-5225-3540-9.ch018 [DOI] [Google Scholar]
- Bhatt P. (2019). Smart Bioremediation Technologies: Microbial Enzymes. Amsterdam: Elsevier Science. [Google Scholar]
- Bhatt P., Barh A. (2018). “Bioinformatic tools to study the soil microorganisms: an in silico approach for sustainable agriculture,” in In Silico Approach for Sustainable Agriculture, eds Choudhary D., Kumar M., Prasad R., Kumar V. (Singapore: Springer; ). [Google Scholar]
- Bhatt P., Gangola S., Chaudhary P., Khati P., Kumar G., Sharma A., et al. (2019a). Pesticide induced up-regulation of esterase and aldehyde dehydrogenase in indigenous Bacillus spp. Bioremediat. J. 23 42–52. 10.1080/10889868.2019.1569586 [DOI] [Google Scholar]
- Bhatt P., Pathak V. M., Joshi S., Bisht T. S., Singh K., Chandra D. (2019b). “Major metabolites after degradation of xenobiotics and enzymes involved in these pathways,” in Smart Bioremediation Technologies, (Cambridge, MA: Academic Press), 205–215. 10.1016/B978-0-12-818307-6.00012-3 12093357 [DOI] [Google Scholar]
- Bhatt P., Negi G., Gangola S., Khati P., Kumar G., Srivastava A., et al. (2016a). Differential expression and characterization of cypermethrin-degrading potential proteins in Bacillus thuringiensis strain, SG4. 3 Biotech. 6:225. 10.1007/s13205-016-0541-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P., Sharma A., Gangola S., Khati P., Kumar G., Srivastava A. (2016b). Novel pathway of cypermethrin biodegradation in a Bacillus sp. strain SG2 isolated from cypermethrin-contaminated agriculture field. 3 Biotech. 6:65. 10.1007/s13205-016-0372-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birolli W. G., Alvarenga N., Seleghim M. H. R., Porto A. L. M. (2016a). Biodegradation of the pyrethroid pesticide esfenvalerate by marine-derived fungi. Mar. Biotechnol. 18 511–520. 10.1007/s10126-016-9710-z [DOI] [PubMed] [Google Scholar]
- Birolli W. G., Arai M. S., Nitschke M., Porto A. L. M. (2019). The pyrethroid (±)-lambda-cyhalothrin enantioselective biodegradation by a bacterial consortium. Pestic. Biochem. Physiol. 156 129–137. 10.1016/j.pestbp.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Birolli W. G., Borges E. M., Nitschke M., Romao L. P. C., Porto A. L. M. (2016b). Biodegradation pathway of the pyrethroid pesticide esfenvalerate by bacteria from different biomes. Water, Air Soil Pollut. 227:271 10.1007/s11270-016-2968-y [DOI] [Google Scholar]
- Birolli W. G., Vacondio B., Alvarenga N., Seleghim M. H. R., Porto A. L. M. (2018). Enantioselective biodegradation of the pyrethroid (±)-lambda-cyhalothrin by marine-derived fungi. Chemosphere 197 651–660. 10.1016/j.chemosphere.2018.01.054 [DOI] [PubMed] [Google Scholar]
- Bordoni L., Nasuti C., Fedeli D., Galeazzi R., Laudadio E., Massaccesi L., et al. (2019). Early impairment of epigenetic pattern in neurodegeneration: additional mechanisms behind pyrethroid toxicity. Exp. Gerontol. 124:110629. 10.1016/j.exger.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Bradberry S. M., Cage S. A., Proudfoot A. T., Vale J. A. (2005). Poisoning due to pyrethroids. Toxicol. Rev. 24 93–106. 10.2165/00139709-200524020-00003 [DOI] [PubMed] [Google Scholar]
- Bradman A., Whitaker D., Quirós L., Castorina R., Henn B. C., Nishioka M., et al. (2007). Pesticides and their metabolites in the homes and urine of farmworker children living in the Salinas Valley, CA. J. Expo. Sci. Environ. Epidemiol. 17 331–349. 10.1038/sj.jes.7500507 [DOI] [PubMed] [Google Scholar]
- Braganaca I., Mucha A. P., Tomasino M. P., Santos F., Lemos P. C., Matos C. D., et al. (2019). Deltamethrin impact in a cabbage planted soil: degradation and effect on microbial community structure. Chemosphere 220 1179–1186. 10.1016/j.chemosphere.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Braganca I., Lemos P. C., Barros P., Delerue-Matos C., Domingues V. F. (2018). Phytotoxicity of pyrethroid pesticides and its metabolite towards Cucumis sativus. Sci. Total Environ. 619-620 685–691. 10.1016/j.scitotenv.2017.11.164 [DOI] [PubMed] [Google Scholar]
- Brander S. M., Gabler M. K., Fowler N. L., Connon R. E., Schlenk D. (2016). Pyrethroid pesticides as endocrine disruptors: molecular mechanisms in vertebrates with a focus on fishes. Environ. Sci. Technol. 50 8977–8992. 10.1021/acs.est.6b02253 [DOI] [PubMed] [Google Scholar]
- Burns C. J., Pastoor T. P. (2018). Pyrethroid epidemiology: a quality-based review. Crit. Rev. Toxicol. 48 297–311. 10.1080/10408444.2017.1423463 [DOI] [PubMed] [Google Scholar]
- Cao Z., Shafer T. J., Crofton K. M., Gennings C., Murray T. F. (2011). Additivity of Pyrethroid actions on sodium influx in cerebrocortical neurons in primary culture. Environ. Health Perspect. 119 1239–1246. 10.1289/ehp.1003394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellarnau M., Azcon J. R., Lopez J. F., Grimalt J. O., Marco M. P., Nieuwenhuijsen M., et al. (2016). Assessment of analytical methods to determine pyrethroids content of bednets. Tropic. Med. Int. Health 22 41–51. 10.1111/tmi.12794 [DOI] [PubMed] [Google Scholar]
- Chalap E. D., Abdulhussein F. S., Aljuboory D. S. A. (2018). The cellular genetic effect of pyrethroid on lab rabbit. Int. J. Res. Pharma Sci. 10 415–418. [Google Scholar]
- Chang J., Wang Y., Wang H., Li J., Xu P. (2016). Bioaccumulation and enantioselectivity of type I and type II pyrethroid pesticides in earthworm. Chemosphere 144 1351–1357. 10.1016/j.chemosphere.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Chen L., Guan L. M., Wu Y. N., Xu L. J., Fu F. F. (2012a). Study on the residue and degradation of fluorine-containing pesticides in Oolong tea by using gas chromatography-mass spectrometry. Food Control 25 433–440. 10.1016/j.foodcont.2011.11.027 [DOI] [Google Scholar]
- Chen S., Geng P., Xiao Y., Hu M. (2012b). Bioremediation of β-cypermethrin and 3-phenoxybenzaldehyde contaminated soils using Streptomyces aureus HP-S-01. Appl. Microbiol. Biotechnol. 94 505–515. 10.1007/s00253-011-3640-5 [DOI] [PubMed] [Google Scholar]
- Chen S., Hu W., Xiao Y., Deng Y., Jia J., Hu M. (2012c). Degradation of 3-phenoxybenzoic acid by a Bacillus sp. PLoS One 7:e50456. 10.1371/journal.pone.0050456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Luo J., Hu M., Geng P., Zhang Y. (2012d). Microbial detoxification of bifenthrin by a novel yeast and its potential for contaminated soils treatment. PLoS One 7:e30862. 10.1371/journal.pone.0030862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.,, Luo J., Hu M., Lai K., Geng P., Huang H. (2012e). Enhancement of cypermethrin degradation by a coculture of Bacillus cereus ZH-3 and Streptomyces aureus HP-S-01. Bioresour. Technol. 110 97–104. 10.1016/j.biortech.2012.01.106 [DOI] [PubMed] [Google Scholar]
- Chen S., Chang C., Deng Y., An S., Dong Y. H., Zhou J., et al. (2014). Fenpropathrin biodegradation pathway in Bacillus sp. DG-02 and its potential for bioremediation of pyrethroid-contaminated soils. J. Agric. Food Chem. 62 2147–2157. 10.1021/jf404908j [DOI] [PubMed] [Google Scholar]
- Chen S., Deng Y., Chang C., Lee J., Cheng Y., Cui Z., et al. (2015). Pathway and kinetics of cyhalothrin biodegradation by Bacillus thuringiensis strain ZS-19. Sci. Rep. 5:8784. 10.1038/srep08784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Dong Y. H., Chang C., Deng Y., Zhang X. F., Zhong G., et al. (2013a). Characterization of a novel cyfluthrin-degrading bacterial strain Brevibacterium aureum and its biochemical degradation pathway. Bioresour. Technol. 132 16–23. 10.1016/j.biortech.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Chen S., Lin Q., Xiao Y., Deng Y., Chang C., Zhong G., et al. (2013b). Monooxygenase, a novel beta-cypermethrin degrading enzyme from Streptomyces sp. PLoS One 8:e75450. 10.1371/journal.pone.0075450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Hu M., Liu J., Zhong G., Yang L., Rizwan-ul-Haq M., et al. (2011a). Biodegradation of beta-cypermethrin and 3-phenoxybenzoic acid by a novel Ochrobactrum lupini DG-S-01. J. Hazard. Mater. 187 433–440. 10.1016/j.jhazmat.2011.01.049 [DOI] [PubMed] [Google Scholar]
- Chen S., Hu Q., Hu M., Luo J., Weng Q., Lai K. (2011b). Isolation and characterization of a fungus able to degrade pyrethroids and 3-phenoxybenzaldehyde. Bioresour. Technol. 102 8110–8116. 10.1016/j.biortech.2011.06.055 [DOI] [PubMed] [Google Scholar]
- Chen S., Lai K., Li Y., Hu M., Zhang Y., Zeng Y. (2011c). Biodegradation of deltamethrin and its hydrolysis product 3-phenoxybenzaldehyde by a newly isolated Streptomyces aureus strain HP-S-01. Appl. Microbiol. Biotechnol. 90 1471–1483. 10.1007/s00253-011-3136-3 [DOI] [PubMed] [Google Scholar]
- Chen S., Yang L., Hu M., Liu J. (2011d). Biodegradation of fenvalerate and 3-phenoxybenzoic acid by a novel Stenotrophomonas sp. strain ZS-S-01 and its use in bioremediation of contaminated soils. Appl. Microbiol. Biotechnol. 90 755–767. 10.1007/s00253-010-3035-z [DOI] [PubMed] [Google Scholar]
- Chen S., Zhan H. (2019). “Biodegradation of synthetic pyrethroid insecticides,” in Microbial Metabolism of Xenobiotic Compounds, ed. Arora P. K. (Singapore: Springer Nature; ), 10.1007/978-981-13-7462-3_11 [DOI] [Google Scholar]
- Chien J. M., Liang W. Z., Liao W. C., Kuo C. C., Chou C. T., Hao L. J., et al. (2019). Ca2+ movement and cytotoxicity induced by the pyrethroid pesticide bifenthrin in human prostate cancer cells. Hum. Exp. Toxicol. 10.1177/0960327119855129 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Chrustek A., Hołyńska-Iwan I., Dziembowska I., Bogusiewicz J., Wróblewski M., Cwynar A., et al. (2018). Current research on the safety of pyrethroids used as insecticides. Medicina 54:61. 10.3390/medicina54040061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcellas C., Feo M. L., Torres J. P., Malm O., Ocampo-Duque W., Eljarrat E., et al. (2012). Pyrethroids in human breast milk: occurrence and nursing daily intake estimation. Environ. Int. 47 17–22. 10.1016/j.envint.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Crow J. A., Borazjani A., Potter P. M., Ross M. K. (2007). Hydrolysis of pyrethroids by human and rat tissues: examination of intestinal, liver and serum carboxylesterases. Toxicol. Appl. Pharmacol. 221 1–12. 10.1016/j.taap.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycoń M., Piotrowska-Seget Z. (2016). Pyrethroid-degrading microorganisms and their potential for the bioremediation of contaminated soils: a review. Front. Microbiol. 7:1463. 10.3389/fmicb.2016.01463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycoń M., Żmijowska A., Piotrowska-Seget Z. (2014). Enhancement of deltamethrin degradation by soil bioaugmentation with two different strains of Serratia marcescens. Int. J. Environ. Sci. Technol. 11 1305–1316. 10.1007/s13762-013-0322-0 [DOI] [Google Scholar]
- Dalsager L., Larsen B. F., Bilenberg N., Jensen T. K., Nielson F., Kyhl H. B., et al. (2019). Maternal urinary concentrations of pyrethroid and chlorpyrifos metabolites and attention deficit hyperactivity disorder (ADHD) symptoms in 2-4-year-old children from the odense child cohort. Environ. Res. 176:108533. 10.1016/j.envres.2019.108533 [DOI] [PubMed] [Google Scholar]
- Dehmel U., Engesser K. H., Timmis K. N., Dwyer D. F. (1995). Cloning, nucleotide sequence, and expression of the gene encoding a novel dioxygenase involved in metabolism of carboxydiphenyl ethers in Pseudomonas pseudoalcaligenes strain POB310. Arch. Microbiol. 163 35–41. 10.1007/s002030050168 [DOI] [PubMed] [Google Scholar]
- Delpuech J. M., Dupont C., Allemand R. (2012). Effects of deltamethrin on the specific discrimination of sex pheromones in two sympatric Trichogramma species. Ecotoxicol. Environ. Saf. 84 32–38. 10.1016/j.ecoenv.2012.06.007 [DOI] [PubMed] [Google Scholar]
- Deng W., Lin D., Yao K., Yuan H., Wang Z., Li J., et al. (2015). Characterization of a novel β-cypermethrin-degrading Aspergillus niger YAT strain and the biochemical degradation pathway of β-cypermethrin. Appl. Microbiol. Biotechnol. 99 8187–8198. 10.1007/s00253-015-6690-2 [DOI] [PubMed] [Google Scholar]
- Dereumeaux C., Saoudi A., Goria S., Wagner V., Chanel P. D. C., Pecheux M., et al. (2018). Urinary levels of pyrethroid pesticides and determinants in pregnant French women from the Elfe cohort. Environ. Int. 119 89–99. 10.1016/j.envint.2018.04.042 [DOI] [PubMed] [Google Scholar]
- Ensley S. M. (2018). Pyrethrins and pyrethroids: veterinary toxicology. Basic Clin. Princ. 39 515–520. 10.1016/B978-0-12-811410-0.00039-8 [DOI] [Google Scholar]
- Fai P. B. A., Kinfack J. S. T., Towa Y. J. T. (2017). Acute effects of binary mixtures of Type II pyrethroids and organophosphate insecticides on Oreochromis niloticus. Ecotoxicology 26 889–901. 10.1007/s10646-017-1819-y [DOI] [PubMed] [Google Scholar]
- Fan X., Liu X., Huang R., Liu Y. (2012). Identification and characterization of a novel thermostable pyrethroid-hydrolyzing enzyme isolated through metagenomic approach. Microb. Cell Fact. 11:33. 10.1186/1475-2859-11-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G., Boll M., Heider J. (2011). Microbial degradation of aromatic compounds — from one strategy to four. Nat. Rev. Microbiol. 9 803–816. 10.1038/nrmicro2652 [DOI] [PubMed] [Google Scholar]
- Furihata S., Kasai A., Hidaka K., Ikegami M., Ohnishi H., Goka K. (2019). Ecological risks of insecticide contamination in water and sediment around off-farm irrigated rice paddy fields. Environ. Pollut. 251 628–638. 10.1016/j.envpol.2019.05.009 [DOI] [PubMed] [Google Scholar]
- Gajendiran A., Abraham J. (2018). An overview of pyrethroid insecticides. Front. Biol. 13:79–90. 10.1007/s11515-018-1489-z [DOI] [Google Scholar]
- Gammon D. W., Liu Z., Chandrasekaran A., El-Naggar S. F., Kuryshev Y. A., Jackson S. (2019). Pyrethroid neurotoxicity studies with bifenthrin indicate a mixed Type I/II mode of action. Pest Manag. Sci. 75 1190–1197. 10.1002/ps.5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangola S., Joshi S., Kumar S., Pandey S. (2019). “Comparative analysis of fungal and bacterial enzymes in biodegradation of xenobiotic compounds,” in Smart Bioremediation Technologies, (Cambridge, MA: Academic Press), 169–189. 10.1016/B978-0-12-818307-6.00010-X [DOI] [Google Scholar]
- Gangola S., Sharma A., Bhatt P., Khati P., Chaudhary P. (2018). Presence of esterase and laccase in Bacillus subtilis facilitates biodegradation and detoxification of cypermethrin. Sci. Rep. 8:12755. 10.1038/s41598-018-31082-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazouani L., Feriani A., Mufti A., Tir M., Baaziz I., Mansour H. B., et al. (2019). Toxic effect of alpha cypermethrin, an environmental pollutant, on myocardial tissue in male wistar rats. Environ. Sci. Pollut. Res. Int. 10.1007/s11356-019-05336-2 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Giddings J. M., Barber I., Warren-Hicks W. (2009). Comparative aquatic toxicity of the pyrethroid insecticide lambda-cyhalothrin and its resolved isomer gamma-cyhalothrin. Ecotoxicology 18 239–249. 10.1007/s10646-008-0277-y [DOI] [PubMed] [Google Scholar]
- Giddings J. M., Wirtz J., Campana D., Dobbs M. (2019). Derivation of combined species sensitivity distributions for acute toxicity of pyrethroids to aquatic animals. Ecotoxicology 28 242–250. 10.1007/s10646-019-02018-0 [DOI] [PubMed] [Google Scholar]
- Gong T., Xu X., Dang Y., Kong A., Wu Y., Liang P., et al. (2018). An engineered Pseudomonas putida can simultaneously degrade organophosphates, pyrethroids and carbamates. Sci. Total Environ. 628-629 1258–1265. 10.1016/j.scitotenv.2018.02.143 [DOI] [PubMed] [Google Scholar]
- Grant R. J., Daniell T. J., Betts W. B. (2002). Isolation and identification of synthetic pyrethroid-degrading bacteria. J. Appl. Microbiol. 92 534–540. 10.1046/j.1365-2672.2002.01558.x [DOI] [PubMed] [Google Scholar]
- Guo P., Wang B., Hang B. J., Li L., Ali S. W., He J., et al. (2009). Pyrethroid-degrading Sphingobium sp. JZ-2 and the purification and characterization of a novel pyrethroid hydrolase. Int. Biodeterior. Biodegrad. 63 1107–1112. 10.1016/j.ibiod.2009.09.008 [DOI] [Google Scholar]
- Gupta S., Bhatt P., Chaturvedi P. (2018). Determination and quantification of asiaticoside in endophytic fungus from Centella asiatica (L.) Urban. World J. Microbiol. Biotechnol. 34:111. 10.1007/s11274-018-2493-9 [DOI] [PubMed] [Google Scholar]
- Harms H., Schlosser D., Wick L. Y. (2011). Untapped potential: exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 9 177–192. 10.1038/nrmicro2519 [DOI] [PubMed] [Google Scholar]
- He B., Wang X., Zhu J., Kong B., Wei L., Jin Y., et al. (2019). Autophagy protects murine macrophages from β-cypermethrin-induced mitochondrial dysfunction and cytotoxicity via the reduction of oxidation stress. Environ. Pollut. 250 416–425. 10.1016/j.envpol.2019.04.044 [DOI] [PubMed] [Google Scholar]
- Heidari R., Devonshire A. L., Campbell B. E., Dorrian S. J., Oakeshott J. G., Russell J. R. (2005). Hydrolysis of pyrethroids by carboxylesterases from Lucilia cuprina and Drosophila melanogaster with active sites modified by in vitro mutagenesis. Insect. Biochem. Mol. Biol. 35 597–609. 10.1016/j.ibmb.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Hongsibsong S., Prapamontol T., Dong J. X., Bever C. S., Xu Z. L., Gee S. J. (2019). Exposure of consumers and farmers to organophosphate and synthetic pyrethroid insecticides in Northern Thailand. J. Consum. Protec. Food Saf. 14 17–23. 10.1007/s00003-019-01207-7 [DOI] [Google Scholar]
- Hu G. P., Zhao Y., Song F. Q., Liu B., Vasseur L., Douglas C., et al. (2014). Isolation, identification and cyfluthrin-degrading potential of a novel Lysinibacillus sphaericus strain, FLQ-11-1. Res. Microbiol. 165 110–118. 10.1016/J.RESMIC.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Hu W., Lu Q., Zhong G., Hu M., Yi X. (2019). Biodegradation of pyrethroids by a hydrolyzing carboxylesterase EstA from Bacillus cereus BCC01. Appl. Sci. 9:477 10.3390/app9030477 [DOI] [Google Scholar]
- Hu Y., Beach J., Raymer J., Gardner M. (2004). Disposable diaper to collect urine samples from young children for pyrethroid pesticide studies. J. Expo. Anal. Environ. Epidemiol. 14 378–384. 10.1038/sj.jea.7500334 [DOI] [PubMed] [Google Scholar]
- Huang H., Ottea J. A. (2004). Development of pyrethroid substrates for esterases associated with pyrethroid resistance in the tobacco budworm, Heliothis virescens (F.). J. Agric. Food Chem. 52 6539–6545. 10.1021/jf0493472 [DOI] [PubMed] [Google Scholar]
- Ila H. B., Topaktas M., Rencuzogullari E., Kayraldiz A., Donbak L., Daglioglu Y. K. (2008). Genotoxic potential of cyfluthrin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 656 49–54. 10.1016/j.mrgentox.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Ismail M. F., Mohamed H. M. (2012). Deltamethrin-induced genotoxicity and testicular injury in rats: comparison with biopesticide. Food Chem. Toxicol. 50 3421–3425. 10.1016/j.fct.2012.07.060 [DOI] [PubMed] [Google Scholar]
- Jin Y., Liu J., Wang L., Chen R., Zhou C., Yang Y., et al. (2012). Permethrin exposure during puberty has the potential to enantioselectively induce reproductive toxicity in mice. Environ. Int. 42 144–151. 10.1016/j.envint.2011.05.020 [DOI] [PubMed] [Google Scholar]
- Kalwasinska A., Kesy J., Wilk I., Donderski W. (2011). Neustonic versus epiphytic bacteria of eutrophic lake and their biodegradation ability on deltamethrin. Biodegradation 22 699–707. 10.1007/s10532-010-9414-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamai E. M., McElrath T. F., Ferguson K. K. (2019). Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environ. Health. 18:43. 10.1186/s12940-019-0480-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamita S. G., Mulligan S., Cornell A. J., Hammock B. D. (2016). Quantification of GST and esterase activities in pyrethrin-resistant mosquitoes using pyrethroid-like fluorescent substrates. Int. J. Pest. Manag. 62 276–283. 10.1080/09670874.2016.1175685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P., Yadav G. S., Chauhan R., Kumari B. (2011). Persistence of cypermethrin and decamethrin residues in/on brinjal fruits. Bull. Environ. Contam. Toxicol. 87 693–698. 10.1007/s00128-011-0395-8 [DOI] [PubMed] [Google Scholar]
- Kocaman A. Y., Topaktaş M. (2009). The in vitro genotoxic effects of a commercial formulation of α-cypermethrin in human peripheral blood lymphocytes. Environ. Mol. Mutagen. 50 27–36. 10.1002/em.20434 [DOI] [PubMed] [Google Scholar]
- Kondo M., Miyata K., Nagahori H., Sumida K., Osimitz T. G., Cohen S. M., et al. (2019). Involvement of peroxisome proliferator-activated receptor-alpha in liver tumor production by permethrin in the female mouse. Toxicol. Sci. 168 572–596. 10.1093/toxsci/kfz012 [DOI] [PubMed] [Google Scholar]
- Kuivila K. M., Hladik M. L., Ingersoll C. G., Kemble N. E., Moran P. W., Calhoun D. L., et al. (2012). Occurrence and potential sources of pyrethroid insecticides in stream sediments from seven U.S. metropolitan areas. Environ. Sci. Technol. 46 4297–4303. 10.1021/es2044882 [DOI] [PubMed] [Google Scholar]
- Lan W. S., Gu J. D., Zhang J. L., Shen B. C., Jiang H., Mulchandani A., et al. (2006). Coexpression of two detoxifying pesticide-degrading enzymes in a genetically engineered bacterium. Int. Biodeterior. Biodegrad. 58 70–76. 10.1016/j.ibiod.2006.07.008 [DOI] [Google Scholar]
- Lauretta R., Sansone A., Sansone M., Romanelli F., Appetecchia M. (2019). Endocrine disrupting chemicals: effects on endocrine glands. Front. Endocrinol. 10:178 10.3389/fendo.2019.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Lee J. H., Hwang E. J., Lee H. J., Kim J. H., Heo J. B., et al. (2016). Characterization of biological degradation cypermethrin by Bacillus amyloliquefaciens AP01 Bacillus amyloliiquefaciens AP01. J. Appl. Biol. Chem. 59 9–12. 10.3839/jabc.2016.003 [DOI] [Google Scholar]
- Li G., Wang K., Liu Y. (2008). Molecular cloning and characterization of a novel pyrethroid-hydrolyzing esterase originating from the Metagenome. Microb. Cell Fact. 7:38. 10.1186/1475-2859-7-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu J., Lu M., Ma Z., Cai C., Wang Y., et al. (2016). Bacterial expression and kinetic analysis of carboxylesterase 001D from Helicoverpa armigera. Int. J. Mol. Sci. 17:493. 10.3390/ijms17040493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang X., Toms L. M. L., He C., Hobson P., Sly P. D., et al. (2019). Pesticide metabolite concentrations in Queensland pre-schoolers – exposure trends related to age and sex using urinary biomarkers. Environ. Res. 176:108532. 10.1016/j.envres.2019.108532 [DOI] [PubMed] [Google Scholar]
- Li Z., Zhang Z., Zhang L., Leng L. (2009). Chemosphere isomer- and enantioselective degradation and chiral stability of fenpropathrin and fenvalerate in soils. Chemosphere 76 509–516. 10.1016/j.chemosphere.2009.03.015 [DOI] [PubMed] [Google Scholar]
- Liang W. Q., Wang Z. Y., Li H., Wu P. C., Hu J. M., Luo N., et al. (2005). Purification and characterization of a novel pyrethroid hydrolase from Aspergillus niger ZD11. J. Agric. Food Chem. 53 7415–7420. 10.1021/jf051460k [DOI] [PubMed] [Google Scholar]
- Ligocki I. Y., Munson A., Farrar V., Viernes R., Sih A., Connon R. E., et al. (2019). Environmentally relevant concentrations of bifenthrin affect the expression of estrogen and glucocorticoid receptors in brains of female western mosquitofish. Aquat. Toxicol. 209 121–131. 10.1016/j.aquatox.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Lin Q. S., Chen S. H., Hu M. Y., Ul Haq M. R., Yang L., Li H. (2011). Biodegradation of cypermethrin by a newly isolated actinomycetes HU-S-01 from wastewater sludge. Int. J. Environ. Sci. Technol. 8 45–56. 10.1007/BF03326194 [DOI] [Google Scholar]
- Liu C. H., Pan J., Ye Q., Xu J. H. (2013). Enzymatic production of Cilastatin intermediate via highly enantioselective hydrolysis of methyl (±)-2,2-dimethylcyclopropane carboxylate using newly isolated Rhodococcus sp. ECU1013. Appl. Microbiol. Biotechnol. 97 7659–7667. 10.1007/s00253-013-5038-z [DOI] [PubMed] [Google Scholar]
- Liu W., Gan J., Schlenk D., Jury W. A. (2005). Enantioselectivity in environmental safety of current chiral insecticides. Proc. Natl. Acad. Sci. U.S.A. 102 701–706. 10.1073/pnas.0408847102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Liang M., Liu Y., Fan X. (2017). Directed evolution and secretory expression of a pyrethroid-hydrolyzing esterase with enhanced catalytic activity and thermostability. Microb. Cell Fact. 16:81. 10.1186/s12934-017-0698-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Barr D. B., Pearson M. A., Walker L. A., Bravo R. (2009). The attribution of urban and suburban children’s exposure to synthetic pyrethroid insecticides: a longitudinal assessment. J. Expo. Sci. Environ. Epidemiol. 19 69–78. 10.1038/jes.2008.49 [DOI] [PubMed] [Google Scholar]
- Lu X. (2013). Enantioselective effect of bifenthrin on antioxidant enzyme gene expression and stress protein response in PC12 cells. J. Appl. Toxicol. 33 586–592. 10.1002/jat.1774 [DOI] [PubMed] [Google Scholar]
- Lu Z., Gan J., Cui X., Moreno L. D., Lin K. (2019). Understanding the bioavailability of pyrethroids in the aquatic environment using chemical approaches. Environ. Int. 129 194–207. 10.1016/j.envint.2019.05.035 [DOI] [PubMed] [Google Scholar]
- Luo X., Zhang D., Zhou X., Du J., Zhang S., Liu Y. (2018). Cloning and characterization of a pyrethroid pesticide decomposing esterase gene, Est3385, from Rhodopseudomonas palustris PSB-S. Sci. Rep. 8:7384. 10.1038/s41598-018-25734-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Zhang D., Zhou X., Zhnag S., Liu Y. (2019). Biodegradation of fenpropathrin by Rhodopseudomonas sp. strain PSB07-21 cultured under three different growth modes. J. Basic Microbiol. 59 591–598. 10.1002/jobm.201800490 [DOI] [PubMed] [Google Scholar]
- Maloney S. E., Maule A., Smith A. R. (1988). Microbial transformation of the pyrethroid insecticides: permethrin, deltamethrin, fastac, fenvalerate, and fluvalinate. Appl. Environ. Microbiol. 54 2874–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney S. E., Maule A., Smith A. R. W. (1993). Purification and preliminary characterization of permethrinase from a pyrethroid-transforming strain of Bacillus cereus. Appl. Environ. Microbiol. 59 2007–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool Z., Hussain S., Imran M., Mahmood F., Shahzad T., Ahmed Z., et al. (2016). Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: a critical review. Environ. Sci. Pollut. Res. 23 16904–16925. 10.1007/s11356-016-7003-8 [DOI] [PubMed] [Google Scholar]
- Mense S. M., Sengupta A., Lan C., Zhou M., Bentsman G., Volsky D. J., et al. (2006). The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicol. Sci. 93 125–135. 10.1093/toxsci/kfl046 [DOI] [PubMed] [Google Scholar]
- Mestre A. P., Amavet P. S., Vanzetti A. I., Moleón M. S., Parachú Marcó M. V., Poletta G. L., et al. (2019). Effects of cypermethrin (pyrethroid), glyphosate and chlorpyrifos (organophosphorus) on the endocrine and immune system of Salvator merianae (Argentine tegu). Ecotoxicol. Environ. Saf. 169 61–67. 10.1016/j.ecoenv.2018.10.057 [DOI] [PubMed] [Google Scholar]
- Morales M. M., Cutillas V., Alba A. R. F. (2019). Supercritical fluid chromatography and gas chromatography coupled to tandem mass spectrometry for the analysis of pyrethroids in vegetable matrices: a comparative study. J. Agric. Food. Chem. 10.1021/acs.jafc.9b00732 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Morgan M. K., MacMillan D. K., Zehr D., Sobus J. R. (2018). Pyrethroid insecticides and their environmental degradates in repeated duplicate-diet solid food samples of 50 adults. J. Expo. Sci. Environ. Epidemiol. 28 40–45. 10.1038/jes.2016.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee I., Mittal A. (2007). Dissipation of β-cyfluthrin by two fungi Aspergillus nidulans var. dentatus and Sepedonium maheswarium. Toxicol. Environ. Chem. 89 319–326. 10.1080/02772240601010089 [DOI] [Google Scholar]
- Muzinic V., Ramic S., Zeljezic D. (2018). Chromosome missegregation and aneuploidy induction in human peripheral blood lymphocytes in vitro by low concentrations of chlorpyrifos, imidacloprid and α-cypermethrin. Environ. Mol. Mutagene 60 72–84. 10.1002/em.22235 [DOI] [PubMed] [Google Scholar]
- Nakagawa L. E., do Nascimento C. M., Coasta A. R., Pollato R., Papini S. (2019). Persistence of indoor permethrin and estimation of dermal and non-dietary exposure. J. Expos. Sci. Environ. Epidem. 10.1038/s41370-019-0132-7 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Navarrete-Meneses M. D. P., Pérez-Vera P. (2019). Pyrethroid pesticide exposure and hematological cancer: epidemiological, biological and molecular evidence. Rev. Environ. Helath 34 197–210. 10.1515/reveh-2018-0070 [DOI] [PubMed] [Google Scholar]
- Nishi K., Huang H., Kamita S. G., Kim I., Morisseau C., Hammock B. D. (2006). Characterization of pyrethroid hydrolysis by the human liver carboxylesterases hCE-1 and hCE-2. Arch. Biochem. Biophys. 2445 115–123. 10.1016/j.abb.2005.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paingankar M., Jain M., Deobagkar D. (2005). Biodegradation of allethrin, a pyrethroid insecticide, by an Acidomonas sp. Biotech. Lett. 27 1909–1913. 10.1007/s10529-005-3902-3 [DOI] [PubMed] [Google Scholar]
- Pan C., Liu Y. P., Li Y. F., Hu J. X., Zhang J. P., Wang H. M., et al. (2012). Effects of cypermethrin on the ligand-independent interaction between androgen receptor and steroid receptor coactivator-1. Toxicology 299 160–164. 10.1016/j.tox.2012.05.022 [DOI] [PubMed] [Google Scholar]
- Paravani E. V., Simoniello M. F., Poletta G. L., Casco V. H. (2019). Cypermethrin induction of DNA damage and oxidative stress in zebrafish gill cells. Ecotoxicol. Environ. Saf. 173 1–7. 10.1016/j.ecoenv.2019.02.004 [DOI] [PubMed] [Google Scholar]
- Paravani E. V., Simoniello M. F., Poletta G. L., Zolessi F. R., Casco V. H. (2017). Cypermethrin: oxidative stress and genotoxicity in retinal cells of the adult zebrafish. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 826 25–32. 10.1016/j.mrgentox.2017.12.010 [DOI] [PubMed] [Google Scholar]
- Piner P., Üner N. (2012). Oxidative and apoptotic effects of lambda-cyhalothrin modulated by piperonyl butoxide in the liver of Oreochromis niloticus. Environ. Toxicol. Pharmacol. 33 414–420. 10.1016/j.etap.2012.01.001 [DOI] [PubMed] [Google Scholar]
- Popovic A., Hai T., Tchigvintsev A., Hajighasemi M., Nocek B., Khusnutdinova A. N., et al. (2017). Activity screening of environmental metagenomic libraries reveals novel carboxylesterase families. Sci. Rep. 7:44103. 10.1038/srep44103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor S. P., Maule A. L., Heaton K. J., Cadarette B. S., Guerriere K. I., Haven C. C., et al. (2019). Permethrin exposure from wearing fabric-treated military uniforms in high heat conditions under varying wear-time scenarios. J. Expo. Sci. Environ. Epidemiol. 10.1038/s41370-019-0120-y [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot A. T. (2005). Poisoning due to pyrethrins. Toxicol. Rev. 24 107–113. 10.2165/00139709-200524020-00003 [DOI] [PubMed] [Google Scholar]
- Qi X., Zheng M., Wu C., Wang G., Feng C., Zhou Z. (2012). Urinary pyrethroid metabolites among pregnant women in an agricultural area of the Province of Jiangsu. China. Int. J. Hyg. Environ. Health 215 487–495. 10.1016/j.ijheh.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Qin S., Budd R., Bondarenko S., Liu W., Gan J. (2006). Enantioselective degradation and chiral stability of pyrethroids in soil and sediment. J. Agric. Food. Chem. 54 5040–5045. 10.1021/jf060329p [DOI] [PubMed] [Google Scholar]
- Qin S., Gan J. (2006). Enantiomeric differences in permethrin degradation pathways in soil and sediment. J. Agric. Food Chem. 54 9145–9151. 10.1021/jf061426l [DOI] [PubMed] [Google Scholar]
- Richardson J. R., Fitsanakis V., Westerink R. H. S., Kanthasamy A. G. (2019). Neurotoxicity of pesticides. Acta Neuropathol. 10.1300/J096v12n01_03 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi D. A., Palermo-Neto J. (2005). Effects of type II pyrethroid cyhalothrin on peritoneal macrophage activity in rats. Toxicology 212 98–106. 10.1016/j.tox.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Romero A., Ramos E., Castellano V., Martínez M. A., Ares I., Martínez M., et al. (2012). Cytotoxicity induced by deltamethrin and its metabolites in SH-SY5Y cells can be differentially prevented by selected antioxidants. Toxicol. Vitro 26 823–830. 10.1016/j.tiv.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Ross M. K., Borazjani A., Edwards C. C., Potter P. M. (2006). Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases. Biochem. Pharmacol. 71 657–669. 10.1016/j.bcp.2005.11.020 [DOI] [PubMed] [Google Scholar]
- Saikia N., Das S. K., Patel B. K. C., Niwas R., Singh A. (2005). Biodegradation of beta-cyfluthrin by Pseudomonas stutzeri strain S1. Biodegradation 16 581–589. 10.1007/s10532-005-0211-4 [DOI] [PubMed] [Google Scholar]
- Saikia N., Gopal M. (2004). Biodegradation of β-Cyfluthrin by Fungi. J. Agric. Food Chem. 52 1220–1223. 10.1021/jf0349580 [DOI] [PubMed] [Google Scholar]
- Sakamoto H., Sakai E., Fumimoto R., Yamaguchi Y., Fukuma Y., Nishishita K., et al. (2012). Deltamethrin inhibits osteoclast differentiation via regulation of heme oxygenase-1 and NFATc1. Toxicol. Vitro 26 817–822. 10.1016/j.tiv.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Segura M. E. C., Arroyo S. G., Eslava J. C., Valenzuela C. M., Vazquez L. H. M., Lopez M. S., et al. (2018). In vitro cytotoxicity and genotoxicity of Furia®180 SC (zeta-cypermethrin) and Bulldock 125SC (β-cyfluthrin) pyrethroid insecticides in human peripheral blood lymphocytes. Toxicol. Mech. Methods 28 268–278. 10.1080/15376516.2017.1402977 [DOI] [PubMed] [Google Scholar]
- Selvam A. D. G., Thatheyus A. J., Vidhya R. (2013). Biodegradation of the synthetic pyrethroid, fenvalerate by Pseudomonas viridiflava. Am. J. Microbiol. Res. 1 32–38. 10.12691/ajmr-1-2-4 [DOI] [Google Scholar]
- Sharma D., Singh S. B. (2012). Persistence of bifenthrin in sandy loam soil as affected by microbial community. Bull. Environ. Contam. Toxicol. 88 906–908. 10.1007/s00128-012-0618-7 [DOI] [PubMed] [Google Scholar]
- Sogorb M. A., Vilanova E. (2002). Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol. Lett. 128 215–228. 10.1016/S0378-4274(01)00543-4 [DOI] [PubMed] [Google Scholar]
- Song H., Zhou Z., Liu Y., Deng S., Xu H. (2015). Kinetics and mechanism of Fenpropathrin biodegradation by a newly isolated Pseudomonas aeruginosa sp. strain JQ-41. Curr. Microbiol. 71 326–332. 10.1007/s00284-015-0852-4 [DOI] [PubMed] [Google Scholar]
- Starks S. E., Gerr F., Kamel F., Lynch C. F., Alavanja M. C., Sandler D. P., et al. (2012). High pesticide exposure events and central nervous system function among pesticide applicators in the agricultural health study. Int. Arch. Occup. Environ. Health 85 505–515. 10.1007/s00420-011-0694-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strungaru S. A., Plavan G., Ciobica A., Nicoara A., Robea M. A., Solcan C., et al. (2019). Toxicity and chronic effects of deltamethrin exposure on zebrafish (Danio rerio) as a reference model for freshwater fish community. Ecotoxicol. Environ. Saf. 171 854–862. 10.1016/j.ecoenv.2019.01.057 [DOI] [PubMed] [Google Scholar]
- Sullivan K. M., Poffley A., Funkhouser S., Driver J., Ross J., Ospina M., et al. (2019). Bioabsorption and effectiveness of long-lasting permethrin-treated uniforms over three months among North Carolina outdoor workers. Parasit. Vectors 12 2–9. 10.1186/s13071-019-3314-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram S., Das M. T., Thakur I. S. (2013). Biodegradation of cypermethrin by Bacillus sp. in soil microcosm and in-vitro toxicity evaluation on human cell line. Int. Biodeterior. Biodegr. 77 39–44. 10.1016/j.ibiod.2012.11.008 [DOI] [Google Scholar]
- Tallur P. N., Megadi V. B., Ninnekar H. Z. (2008). Biodegradation of cypermethrin by Micrococcus sp. strain CPN 1. Biodegradation 19 77–82. 10.1007/s10532-007-9116-8 [DOI] [PubMed] [Google Scholar]
- Tang A. X., Liu H., Liu Y. Y., Li Q. Y., Qing Y. M. (2017). Purification and characterization of a novel β-cypermethrin-degrading aminopeptidase from Pseudomonas aeruginosa GF31. J. Agric. Food Chem. 65 9412–9418. 10.1021/acs.jafc.7b03288 [DOI] [PubMed] [Google Scholar]
- Tang J., Hu Q., Liu B., Lei D., Chen T. T., Sun Q., et al. (2019). Efficient biodegradation of 3-phenoxybenzoic acid and pyrethroid pesticides by a novel strain Klebsiella pneumoniae BPBA052. Can. J. Microbiol. 10.1007/s00253-010-3035-z [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Tang W., Wang D., Wang J., Wu Z., Li L., Huang M., et al. (2018). Pyrethroid pesticide residues in the global environment: an overview. Chemosphere 191 990–1007. 10.1016/j.chemosphere.2017.10.115 [DOI] [PubMed] [Google Scholar]
- Tendulkar M., Kulkarni A. (2012). Cypermethrin-induced toxic effect on glycogen metabolism in estuarine clam, Marcia opima (Gmelin, 1791) of Ratnagiri coast, Maharashtra. J. Toxicol. 2012 9–11. 10.1155/2012/576804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatheyus A. J., Selvam A. D. G. (2013). Synthetic pyrethroids: toxicity and biodegradation. Appl. Ecol. Environ. Sci. 1 33–36. 10.12691/aees-1-3-2 [DOI] [Google Scholar]
- Toppw E., Akhtar M. H. (1991). Identification and characterization of a Pseudomonas strain capable of metabolizing phenoxybenzoatest. Appl. Environ. Microbiol. 57 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima H., Suzuki Y., Imai K., Yoshinaga J., Shiraishi H., Mizumoto Y., et al. (2012). Endocrine disrupting chemicals in urine of Japanese male partners of subfertile couples: a pilot study on exposure and semen quality. Int. J. Hyg. Environ. Health 215 502–506. 10.1016/j.ijheh.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Touylia S., Ali M., Abdellhafidh K., Bejaoui M. (2019). Permethrin induced oxidative stress and neurotoxicity on the freshwater beetle Laccophilus minutus. Chem. Ecol. 35 459–471. 10.1080/02757540.2019.1567718 [DOI] [Google Scholar]
- Ullah S., Li Z., Zuberi A., Zain M. A. U., Mirza M. F. A. B. (2019). Biomarkers of pyrethroid toxicity in fish. Environ. Chem. Lett. 17 945–973. 10.1007/s10311-018-00852-y [DOI] [Google Scholar]
- Vasquez M. E., Holstege D. M., Tjeerdema R. S. (2011). Aerobic versus Anaerobic Microbial degradation of etofenprox in a California rice field soil. J. Agric. Food Chem. 59 2486–2492. 10.1021/jf1037773 [DOI] [PubMed] [Google Scholar]
- Wang B., Guo P., Hang B., Li L., He J., Wang B., et al. (2009). Cloning of a novel pyrethroid-hydrolyzing carboxylesterase gene from Sphingobium sp. strain JZ-1. Appl. Environ. Microbiol. 75 5496–5500. 10.1128/AEM.01298-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B. Z., Ma Y., Zhou W. Y., Zheng J. W., Zhu J. C., He J., et al. (2011). Biodegradation of synthetic pyrethroids by Ochrobactrum tritici strain pyd-1. World J. Microbiol. Biotechnol. 27 2315–2324. 10.1007/s11274-011-0698-2 [DOI] [Google Scholar]
- Wang C., Chen Q., Wang R., Shi Chao, Yan X., Jian H., et al. (2014). A novel angular dioxygenase gene cluster encoding 3-phenoxybenzoate 1,2-dioxygenase in Sphingobium wenxiniae JZ-1. Appl. Environ. Microbiol. 80 3811–3818. 10.1128/AEM.00208-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zhang Q., Zhang X. F., Liu J., Liu W. P. (2010). Understanding the endocrine disruption of chiral pesticides: the enantioselectivity in estrogenic activity of synthetic pyrethroids. Sci. China Chem. 53 1003–1009. 10.1007/s11426-010-0143-7 20097460 [DOI] [Google Scholar]
- Wang D., Zou L., Jin Q., Hou J., Ge G., Yang L. (2018). Human carboxylesterases: a comprehensive review. Acta Pharm. Sin. B 8 699–712. 10.1016/j.apsb.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Hu C., Zhang R., Sun A., Li D., Shi X. (2019). Mechanism study of cyfluthrin biodegradation by Photobacterium ganghwense with comparative metabolomics. Appl. Microbiol. Biotechnol. 103 473–488. 10.1007/s00253-018-9458-7 [DOI] [PubMed] [Google Scholar]
- Wei B., Mohan K. R., Weisel C. P. (2012). Exposure of flight attendants to pyrethroid insecticides on commercial flights: urinary metabolite levels and implications. Int. J. Hyg. Environ. Health 215 465–473. 10.1016/j.ijheh.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Task Group on Environmental Health Criteria for Permethrin United Nations Environment Programme International Labour Organisation World Health Organization and International Program on Chemical Safety (1990). d-Permethrin. Geneva: World Health Organization. [Google Scholar]
- Williams R. L., Bernard C. E., Krieger R. I. (2003). Human exposure to indoor residential cyfluthrin residues during a structured activity program. J. Expo. Anal. Environ. Epidemiol. 13 112–119. 10.1038/sj.jea.7500257 [DOI] [PubMed] [Google Scholar]
- Wolansky M. J., Harrill J. A. (2008). Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol. Teratol. 30 55–78. 10.1016/j.ntt.2007.10.005 [DOI] [PubMed] [Google Scholar]
- Wu P. C., Liu Y. H., Wang Z. Y., Zhang X. Y., Li H., Liang W. Q., et al. (2006). Molecular cloning, purification, and biochemical characterization of a novel pyrethroid-hydrolyzing esterase from Klebsiella sp. strain ZD112. J. Agric. Food Chem. 54 836–842. 10.1021/jf052691u [DOI] [PubMed] [Google Scholar]
- Xiao Y., Chen S., Gao Y., Hu W., Hu M., Zhong G. (2015). Isolation of a novel beta-cypermethrin degrading strain Bacillus subtilis BSF01 and its biodegradation pathway. Appl. Microbiol. Biotechnol. 99 2849–2859. 10.1007/s00253-014-6164-y [DOI] [PubMed] [Google Scholar]
- Xie W. J., Zhou J. M., Wang H. Y., Chen X. Q. (2008). Effect of nitrogen on the degradation of cypermethrin and its metabolite 3-phenoxybenzoic acid in soil. Pedosphere 18 638–644. 10.1016/S1002-0160(08)60058-2 [DOI] [Google Scholar]
- Xu H., Li W., Schilmiller A. L., Eekelen H. V., de Vos R. C. H., de Vos R. C. H., et al. (2019). Pyrethric acid of natural pyrethrin insecticide: complete pathway elucidation and reconstitution in Nicotiana benthamiana. New Phytol. 223 751–765. 10.1111/nph.15821 [DOI] [PubMed] [Google Scholar]
- Yang J., Feng Y., Zhan H., Liu J., Yang F., Zhang K., et al. (2018). Characterization of a pyrethroid-degrading Pseudomonas fulva strain P31 and biochemical degradation pathway of D-phenothrin. Front. Microbiol. 9:1003. 10.3389/fmicb.2018.01003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Ghatge S., Hur H. G. (2019). Characterization of a novel thermostable carboxylesterase from thermoalkaliphilic bacterium Bacillus thermocloaceae. Biosci. Biochem. Biotech. 83 882–891. 10.1080/09168451.2019.1574555 [DOI] [PubMed] [Google Scholar]
- Yao G., Gao J., Zhang C., Jiang W., Wang P., Liu X., et al. (2018). Enantioselective degradation of the chiral alpha-cypermethrin and detection of its metabolites in five plants. Environ. Sci. Pollut. Res. 26 1558–1564. 10.1007/s11356-018-3594-6 [DOI] [PubMed] [Google Scholar]
- Yu Y. L., Fang H., Wang X., Wu X. M., Shan M., Yu J. Q. (2006). Characterization of a fungal strain capable of degrading chlorpyrifos and its use in detoxification of the insecticide on vegetables. Biodegradation 17 487–494. 10.1007/s10532-005-9020-z [DOI] [PubMed] [Google Scholar]
- Zartarian V., Xue J., Glen G., Smith L., Tulve N., Tornero-Velez R. (2012). Quantifying children’s aggregate (dietary and residential) exposure and dose to permethrin: application and evaluation of EPA’s probabilistic SHEDS-Multimedia model. J. Expo. Sci. Environ. Epidemiol. 22 267–273. 10.1038/jes.2012.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y., Li K., Song J., Shi Y., Yan Y. (2012). Molecular cloning, purification and biochemical characterization of a novel pyrethroid-hydrolyzing carboxylesterase gene from Ochrobactrum anthropi YZ-1. J. Hazard. Mater. 221–222, 206–212. 10.1016/j.jhazmat.2012.04.031 [DOI] [PubMed] [Google Scholar]
- Zhan H., Wang H., Liao L., Feng Y., Fan X., Zhang L., et al. (2018). Kinetics and novel degradation pathway of permethrin in Acinetobacter baumannii ZH-14. Front. Microbiol. 9:98. 10.3389/fmicb.2018.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Jia L., Wang S., Qu J., Li K., Xu L., et al. (2010). Biodegradation of beta-cypermethrin by two Serratia spp. with different cell surface hydrophobicity. Bioresour. Technol. 101 3423–3429. 10.1016/j.biortech.2009.12.083 [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang S., Yan Y. (2011). Isomerization and biodegradation of beta-cypermethrin by Pseudomonas aeruginosa CH7 with biosurfactant production. Bioresour. Technol. 102 7139–7146. 10.1016/j.biortech.2011.03.086 [DOI] [PubMed] [Google Scholar]
- Zhang S., Yin L., Liu Y. (2011). Cometabolic biotransformation of fenpropathrin by Clostridium species strain ZP3. Biodegradation 22 869–875. 10.1007/s10532-010-9444-y [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhu W., Zheng Y., Yang J., Zhu X. (2008). The antiandrogenic activity of pyrethroid pesticides cyfluthrin and β-cyfluthrin. Reprod. Toxicol. 25 491–496. 10.1016/j.reprotox.2008.05.054 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhang Y., Du J., Zhao M. (2017). Environmentally relevant levels of λ-cyhalothrin, fenvalerate, and permethrin cause developmental toxicity and disrupt endocrine system in zebrafish (Danio rerio) embryo. Chemosphere 185 1173–1180. 10.1016/j.chemosphere.2017.07.091 [DOI] [PubMed] [Google Scholar]
- Zhang X., Hao X., Huo S., Lin W., Xia X., Liu K., et al. (2019). Isolation and identification of the Raoultella ornithinolytica-ZK4 degrading pyrethroid pesticides within soil sediment from an abandoned pesticide plant. Arch. Microbiol. 1–11. 10.1007/s00203-019-01686-0 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Geng Y., Chen L., Tao K., Hou T. (2013). Biodegradation of cypermethrin by a novel Catellibacterium sp. strain CC-5 isolated from contaminated soil. Can. J. Microbiol. 59 311–317. 10.1139/cjm-2012-0580 [DOI] [PubMed] [Google Scholar]
- Zhao J., Chi Y., Liu F., Jia D., Yao K. (2015). Effects of two surfactants and beta-cyclodextrin on beta-cypermethrin degradation by Bacillus licheniformis B-1. J. Agric. Food Chem. 63 10729–10735. 10.1021/acs.jafc.5b04485 [DOI] [PubMed] [Google Scholar]
- Zhao J., Chi Y., Liu F., Jia D., Yao K. (2016). Co-metabolic degradation of β-cypermethrin and 3-phenoxybenzoic acid by co-culture of Bacillus licheniformis B-1 and Aspergillus oryzae M-4. PLoS One 11:e0166796. 10.1371/journal.pone.0166796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Chi Y., Liu F., Jia D., Yao K. (2018). Characterization of co-culturing microorganisms for simultaneous degradation of beta-cypermethrin and 3-phenoxybenzoic acid. Fresen. Environ. Bull. 27 4249–4257. 10.1080/02757540.2019.1567718 [DOI] [Google Scholar]
- Zhao J., Jia D., Du J., Chi Y., Yao K. (2019). Substrate regulation on co-metabolic degradation of β-cypermethrin by Bacillus licheniformis B-1. AMB Express 9:83. 10.1186/s13568-019-0808-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang R., Chen H., Yao J., Li Z., Burnet J. E., Choi M. M. F. (2011). Impact of beta-cypermethrin on soil microbial community associated with its bioavailability: a combined study by isothermal microcalorimetry and enzyme assay techniques. J. Hazard. Mater. 189 323–328. 10.1016/j.jhazmat.2011.02.034 [DOI] [PubMed] [Google Scholar]


